Abstract
Five birds in a captive zebra finch research colony were diagnosed with systemic amyloidosis within a 7-mo period by means of postmortem Congo red staining and green birefringence under polarized light. The liver was the most frequently and usually the most seriously affected organ, followed by the spleen and then the kidney. All 5 birds had been clinically affected with various inflammatory, infectious, and neoplastic conditions associated with amyloid A (AA) amyloidosis in humans and animals. Immunohistochemistry using antisera against duck AA protein revealed that tissues from 2 of the 5 birds were positive for the presence of AA protein and systemic inflammation-associated amyloidosis. Although the development of AA amyloidosis has been associated with chronic inflammation, trauma, and various infectious and neoplastic diseases as well as possible genetic predispositions and stresses linked to overcrowding, the root causes for individual cases of AA amyloidosis are incompletely understood. As far as we know, this report is the first description of AA amyloidosis in captive, research zebra finches.
Abbreviations: AA, amyloid A; AF, acid-fast; CR, Congo red; GBR, green birefringence; SAA, serum amyloid A
Amyloidosis is a disease caused by extracellular amyloid deposits that often lead to organ dysfunction and death in humans and animals.39,47,64 These deposits are generated from various soluble precursor proteins that misfold under conditions that might involve defective intracellular protein quality control, extracellular chaperones and matrix components, proteases, and mutated precursor proteins.3,6,39,66 X-ray diffraction, electron microscopy, and infrared spectroscopy of the different amyloid proteins have shown that they share a common structure formed from rigid, insoluble, nonbranching fibrils, 7.5 to 10 nm in diameter and of variable length with a high proportion of antiparallel, β-pleated sheets.6,19,52 Other components of amyloid fibrils include amyloid P protein, apolipoprotein E, and the proteoglycan perlecan.6 Currently, there are 31 known extracellular amyloid fibril proteins in humans, 9 of which have also been identified in animals.52 In localized amyloidosis, amyloid deposits form at the site of precursor protein synthesis, whereas in systemic disease, amyloid deposition occurs distant from the site of precursor protein secretion.3
Amyloid deposits from different, misfolded protein precursors have been associated with Alzheimer disease, some transmissible spongiform encephalopathies, type II diabetes mellitus, multiple myeloma, familiar amyloid polyneuropathy, senile systemic amyloidosis, and inflammation-associated amyloidosis.33,43,52 Inflammation-associated amyloidosis—also referred to as amyloid A (AA), acquired, secondary, or reactive amyloidosis—occurs systemically in susceptible humans and animals when excessive amounts of serum AA (SAA) protein are produced, primarily by the liver, as part of the acute-phase response to chronic inflammation.14,47,64 However, AA amyloidosis does not occur in all individuals with abundant SAA protein and chronic inflammation.6,47,51 Under currently undefined intracellular conditions and perhaps species-specific or individual genetic predisposition, SAA protein transforms into AA protein fibrils, which tend to accumulate in the liver, spleen, and kidneys but also have been found in virtually all tissues except the brain parenchyma.3,29,47,64 Both the SAA and AA proteins have been shown to be well conserved among humans and animal species.18,25,31,33,59
Avian amyloidosis frequently affects waterfowl (Anseriformes, Gruiformes, Phoenicopteriformes); and ducks (Anatidae) seem particularly prone to this condition.11,12,33,57 Amyloidosis has also been documented in domestic poultry, captive birds in parks and zoological collections, and free-living birds.7,11,12,26,33,34,57 Pekin ducks (Anas platyrhynchos domesticus) and brown layer hens (Gallus gallus domesticus) are important research models of AA amyloidosis.13,32,33,48 Amyloidosis has also been reported in captive, research black-bellied seedcrackers (Pyrenestes ostrinus) diagnosed with mycobacteriosis and other diseases21 and a captive Gouldian finch (Erythrura gouldiae) diagnosed with Mycobacteria peregrinum.62 No sex-associated predilection for AA amyloidosis has been reported in avian species.33
Historically, an association between AA amyloidosis and mycobacterial infections in humans, birds, and other animals has been recognized.11,13,33,40,46,50,69 Other predisposing conditions include neoplasia, trauma, other infections (bacteria, parasites, possibly some viruses), inflammatory conditions, and stress.11,24,33,46 In addition, AA amyloidosis can be idiopathic, without apparent predisposing conditions.46,47,58,64 There appears to be a genetic predisposition to AA amyloidosis in various species of ducks, felines, canines, mice, and Syrian hamsters, among others, as well as in humans.9,31,33,49,60,64 Recently, convincing evidence supports oral, fecal, and parenteral intra- and interspecies prion-like transmissibility of AA fibrils in particular68 and amyloid fibrils in general.36,64,67
Of the systemic amyloid diseases, only AA amyloidosis has been described and verified in avian species.18,25,31,33,70 There has been one report of a companion zebra finch that presented with a Leydig cell tumor and systemic amyloidosis diagnosed using Congo red staining and green birefringence but without specific confirmation of the amyloid A protein deposited during inflammation–associated amyloidosis.45 In the current report, we diagnosed amyloidosis by means of postmortem Congo red stains and green birefringence under polarized light in 5 zebra finches that had clinical conditions known to cause elevated SAA protein levels and AA disease in susceptible individuals. We also confirmed the presence of AA protein and systemic inflammation-associated amyloidosis in tissue samples from 2 of the 5 birds using immunohistochemistry with antisera against duck AA protein. As far as we know, this report is the first description of AA amyloidosis in captive, research zebra finches.
Case Report
Materials and Methods
Colony.
Five hundred to 650 male and female zebra finches were conventionally housed in an open colony. They belonged to a breeding protocol for an IACUC-approved study for the generation of transgenic animals to be used for investigations of vocal learning. Breeding cycles were 6 wk on and 6 wk off. Nonactive breeders were housed as same-sex groups in metal, plastic-coated flight cages (21 × 60 × 24 in. [0.53 × 1.15 × 0.61 m] if undivided; 21 × 30 × 24 in. [0.53 × 0.76 × 0.61 m] if divided by using partition boards). At any given time, undivided flight cages contained 22 to 30 birds and divided flight cages contained 11 to 15 birds. Breeders were housed in stainless steel cages (12 × 18 × 15 in. [0.31 × 0.46 × 0.38 m]), with 2 to 8 birds (family groups) per cage. Most clinically ill birds were transferred into breeding-size cages for observation and treatment.
Health status.
During 2013 through 2014, randomly selected and clinically ill birds tested negative for Chlamydophila psittaci by using quantitative PCR of choanal swabs and selected tissue samples. Some clinically ill and dead birds tested positive for Mycobacterium genavense using quantitative PCR testing of selected tissue samples. The entire colony was negative for feather and skin mites.
Housing and husbandry.
The holding room was approximately 353 ft2 (33 m2) and 3192 ft3 (91 m3), with 60 small breeding cages and 36 flight cages (18 if undivided). Each cage contained 4 wooden-dowel perches at 2 levels per divided flight cage, and 2 perches per small breeding cage; in addition, one natural-shaped tanzanite perch was provided per cage. Seed diet (LM Animal Farms Pet Bird Diet for canaries and finches; Hartz Mountain Corporation, Secaucus, NJ) was provided free choice and was replaced as needed Monday through Friday. One tablespoon of high-performance protein mash (spirulina), hardboiled eggs, and water were combined and provided Monday through Friday in one bowl per breeding cage and 2 bowls per divided flight cage. Untreated water was available continuously in polysulfone bottles that were replaced and autoclaved once each week. Birds were provided with plain-water baths (1/4 cup each) 3 times each week, and cages were misted with water Monday through Friday. Cuttlebones (1 to 3) were hung on every cage, and opaque, plastic nesting boxes filled with coconut husk or sisal fiber nesting material were provided for breeding cages. Pan liners were changed twice weekly. Feed bowls were replaced with fresh bowls once weekly. Cages were sanitized every other month. Room conditions included a 12:12-h light:dark cycle, temperature was maintained between 65 to 82 °F (18.3 to 27.8 °C; set point, 75 °F [23.9 °C]), and relative humidity was 45% to 50%. Exhaust filters were changed 1× per week. Room air-supply filters were changed quarterly. The differential air pressure into the holding room was negative.
Histopathology.
A complete necropsy was performed on each of the 5 birds. Tissues were collected, immersed in 10% neutral-buffered formalin, paraffin-embedded, and sectioned (thickness, 5 µm) for histologic analysis. All tissue sections were stained routinely by using hematoxylin and eosin. Congo red (CR) staining of tissue sections for amyloid was accomplished by using the AFIP Congo Red (Bennhold) Microwave for Amyloid procedure whereas acid-fast (AF) staining was performed by using Ziehl–Neelsen methodology. CR-stained tissue sections were evaluated for green birefringence (GBR) by using polarizing filters. Histopathology was performed by a board-certified veterinary pathologist.
Microscopy and images.
All sections were examined by microscopy (model BX40, Olympus, Tokyo, Japan). CR-stained tissue sections were evaluated for GBR by using polarizing filters, and photomicrographs were obtained (QICAM Fast1394, QImaging, Surrey, British Columbia, Canada).
Molecular biology.
Postmortem tissue samples (various) and antemortem swabs (cloacal for Mycobacterium spp.; choanal for C. psittaci) were shipped to a commercial laboratory (Animal Genetics, Tallahassee, FL) for quantitative PCR testing.
Immunohistochemistry.
Antisera (generated in rabbits) against duck amyloid A protein was graciously donated by Dr JT Guo (Drexel University College of Medicine, Philadelphia, PA). Formalin-fixed paraffin-embedded tissues from a Siamese cat with breed-specific AA-type amyloidosis (graciously donated by Dr Amy Durham, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA) served as controls for optimization of the immunohistochemistry technique. For this study, endogenous peroxidase activity in deparaffinated sections was blocked by incubating the slides for 5 min in a commercial reagent (Intellipath FLX blocking reagent, Biocare Medical, Concord, CA). Sections were subsequently incubated for 60 min at room temperature with the rabbit antisera (dilution, 1:600), rinsed in wash buffer (PBS Plus, BioCare Medical), and incubated for 20 min with an antirabbit secondary detection system (catalog no. RMR622, BioCare Medical). The chromogenic reaction was performed by using a 3,3′-diaminobenzidine chromogen solution for 5 min. Resulting sections were evaluated for brown reaction product with an Olympus BX40 microscope.
Results
Adult birds (n = 5; age, 2 to 4 y) presented with clinical conditions or were found dead in their cages over approximately 7 mo from September 2013 through April 2014. Bird 5 was genetically modified with a transgene targeted to the brain and vocal learning; the rest were wild-type.
Birds 1 through 5: Clinical history, necropsy findings, and corresponding histopathology.
Bird 1.
This bird displayed severe, bilateral blepharoconjuctivitis. The morbidity of the left eye was more severe, with yellow crusts completely occluding the palpebrae; the right conjunctiva was notably hyperemic, with epiphora, chemosis, and blepharospasm. Moderate to marked feather loss on the face and head secondary to self-mutilation was present and presumably due to pruritis and discomfort. The bird was treated daily with systemic and topical antibiotics but was unresponsive and found dead in its cage after 7 wk of monitoring and treatment.
Gross necropsy revealed marked wasting of pectoral muscles with extensive feather loss on the face and head. The left eyelids were sealed closed (ankyloblepharon), and microscropically the left eye was characterized by chronic blepharoconjunctivitis. AF-positive bacilli associated with granulomatous infiltrates consistent with Mycobacterium spp. were present in a small focus of the gizzard. Other microscopic findings included periarteritis involving the aorta, dermatitis with feather follicle atrophy involving the neck and leg skin, and chronic serositis in sections of the gizzard.
Bird 2.
This bird demonstrated marked swelling, redness, and irritation of the right pes, and the ipsilateral leg band was tightly adhered to the skin around the tibiotarsal joint. The foot swelling interfered with normal perching. In addition, a 1- to 2-mm mass was present above the left eye, adjacent to the upper beak. The tibiotarsal bone fractured during removal of the leg band, and the bird promptly was euthanized with CO2 gas.
Necropsy revealed moderate wasting of the pectoral muscles, with patchy feather loss on the back of the neck and upper back. The right tibiotarsal bone was fractured above the ankle joint, with missing skin where the adherent leg band was removed. The plantar surface of the right pes was enlarged and bulbous, with firm, reddish-pink tissue and superficial excoriations. Microscopically, the necropsy findings in the right pes correlated with the presence of severe pododermatitis and osteomyelitis as characterized by the presence of dramatic mixed inflammatory cell infiltrates of the skin with chondromalacea and lysis and sequestration of underlying phylangeal bones.
A 3- to 4-mm, round, slightly raised, reddish brown, and smooth subcutaneous mass was present in the left wing web. This mass was composed of a well-circumscribed nodule of well-differentiated adipocytes admixed with leukocytes and consistent with myelolipoma. The mass above the left eye was subcutaneous, firm, yellow, and discrete and due to the presence of focal hyperkeratosis. The liver was reddish brown and friable, with one yellowish area due to the presence of amyloid. Fibrinous epicarditis also was noted in this bird.
Bird 3.
This bird was found dead in its cage. Necropsy revealed marked wasting of the pectoral muscles, and autolysis of the internal organs was noted. Adhesions were observed among some portions of the intestines, and peritonitis, associated with a perforating, proventricular ulcer, was noted microscopically. Overgrowth of elongated, rod-shaped organisms, suggestive of Macrorabdus ornithogaster was observed in the proventriculus as well as the gizzard. The kidneys were enlarged, pale, and tan, correlating with the presence of amyloid. This bird was negative for Mycobacterium infection according to quantitative PCR testing of tissues taken from the liver, kidney, and gizzard, and no AF-positive organisms were observed in the tissues examined.
Bird 4.
This bird had a discrete mass on its right cheek, below the eye and adjacent to the commissures of the beak. The bird was euthanized with CO2 gas after the mass had progressively enlarged over several weeks.
At necropsy, this bird was in good body condition. The facial mass present clinically was noted at necropsy to lie within the subcutis, 3- to 4-mm in diameter, firm and round, smooth, and mostly yellow. This mass was diagnosed as a hemangiosarcoma. No abnormalities were noted in the coelomic organs of this bird.
Bird 5.
This bird was found dead in its cage. Necropsy revealed marked wasting of the pectoral muscles and autolysis of internal organs. Microscopically, there were widespread granulomatous infiltrates involving the liver, gizzard, kidney, aorta (media), and crop wall; all of these infiltrates were associated with AF-positive bacilli consistent with Mycobacterium spp. In addition, noteworthy periarteritis was present in the aorta.
Diagnosis and histopathology of amyloidosis (Figure 1).
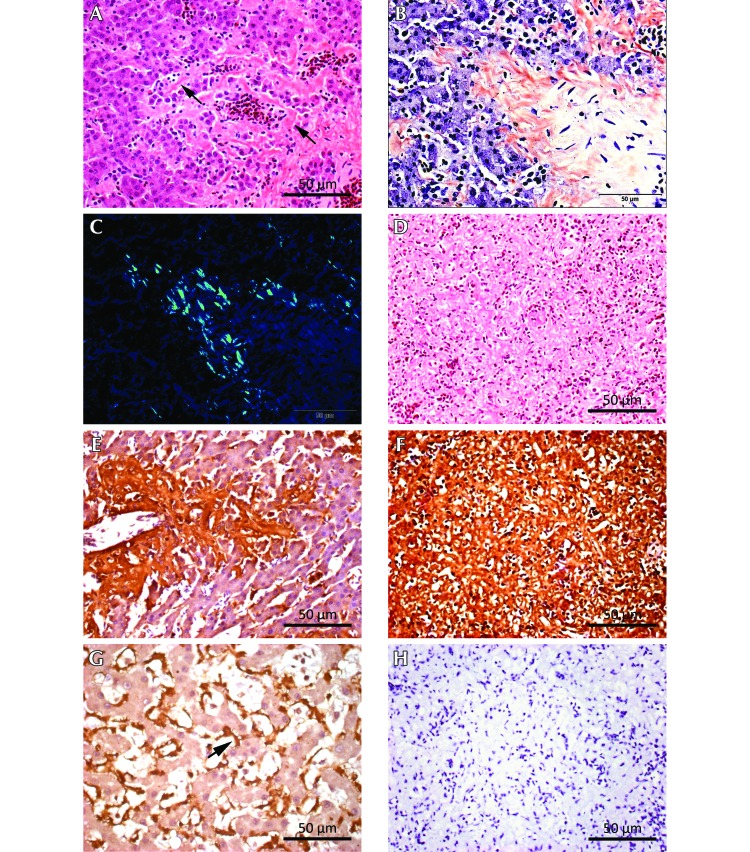
Figure 1.
Amyloid lesions in birds 1 through 5.
Extracellular deposits of acellular, eosinophilic material were observed in hematoxylin- and eosin-stained sections from multiple tissues from birds 1 through 5 (Figure 2 A); and amyloidosis was diagnosed due to the presence of congophilia and GBR under polarized light (Figure 2 B and C). Green birefringence was strong in most tissues with some weaker reactions in a few tissues. Congophilia and strong GBR were evident in at least one tissue type from every bird (liver) and in 2 tissue types in birds with more than one positive organ.
Figure 2.
Histopathology and immunohistochemstry for birds 3 and 4, with positive-control tissue from a Siamese cat. (A) Bird 3, liver. Diffuse, acellular eosinophilic material within the hepatic sinusoids (space of Disse, black arrows). Hematoxylin and eosin stain; scale bar, 50 µm. (B) Bird 3, liver. Salmon-stained areas (congophilia) correspond to diffuse sinusoidal amyloid deposits visible in the hematoxylin- and eosin-stained tissue shown in panel A. Congo red stain; scale bar, 50 µm. (C) Bird 3, liver. The Congo-red–stained section shows green birefringence (GBR), denoting amyloid protein, under polarized light. Scale bar, 50 µm. (D) Bird 3, spleen. Diffuse amyloid deposition involving both red and white pulp, with extensive loss of normal architecture. Hematoxylin and eosin stain; scale bar, 50 µm. (E) Bird 4, liver. Immunohistochemical localization of amyloid A (AA) protein shows copious brown reaction product within the hepatic sinusoids, with distribution similar to that of the amyloid detected by using congophilia and GBR. Antisera against duck AA protein, 1:600 dilution; scale bar, 50 µm. (F) Bird 3 spleen. Immunohistochemical localization of AA protein shows copious brown reaction product, with diffuse distribution similar to that of amyloid detected by using congophilia and GBR. Antisera against duck AA protein, 1:600 dilution; scale bar, 50 µm. (G) Siamese cat, liver. Positive control for immunohistochemical detection of AA protein shows brown reaction product (black arrow) within the sinusoids. Antisera against duck AA protein, 1:600 dilution; scale bar, 50 µm. (H) Bird 3, spleen. Omission of the incubation step from the staining procedure with primary antisera against duck AA protein shows no staining for AA protein. Scale bar, 50 µm.
In all 5 birds, amyloid deposits present in the livers involved the sinusoids (space of Disse) and perivascular zones (portal regions and sublobar hepatic veins; Figure 2 A). Liver involvement with amyloid was associated with extensive parenchymal atrophy in birds 1 and 2. The spleen was affected in all animals except bird 5, and amyloid deposition was multifocal to diffuse in deposition. Diffuse amyloid deposition in the spleen involving both the red and white pulp led to extensive loss of the normal architecture (birds 3 and 4; Figure 2 D). Renal amyloid deposits were identified in birds 1, 2, and 3, with multifocal to diffuse distribution invariably involving the interstitium. In addition, birds 1 and 2 had intestinal amyloid (large or small intestine or both) that was present in the lamina propria. Tissues that were affected by amyloid depositions in single animals were the ovarian stroma (bird 1), perivascular areas of the pancreas (bird 2), sinusoids of the adrenal glands (bird 3), and the lamina propria of the proventriculus (bird 4).
Immunohistochemistry using the rabbit antisera against duck amyloid A protein was performed on spleen sections from bird 3 and spleen and liver sections from bird 4. Copious brown reaction product consistent with immunohistochemical detection of AA-type amyloid was demonstrated in all tested specimens from these 2 birds and was similar in distribution to the amyloid detected by using congophilia and GBR (Figure 2 E and F). The liver of the Siamese cat with breed-specific AA-type amyloidosis had a similar staining pattern and thus similar crossreactivity of the rabbit antisera against duck amyloid A protein (Figure 2 G). The test specimens from birds 3 and 4 and from the Siamese cat were negative for amyloid staining when the incubation step with primary antisera was omitted from staining procedures (Figure 2 H)
Discussion
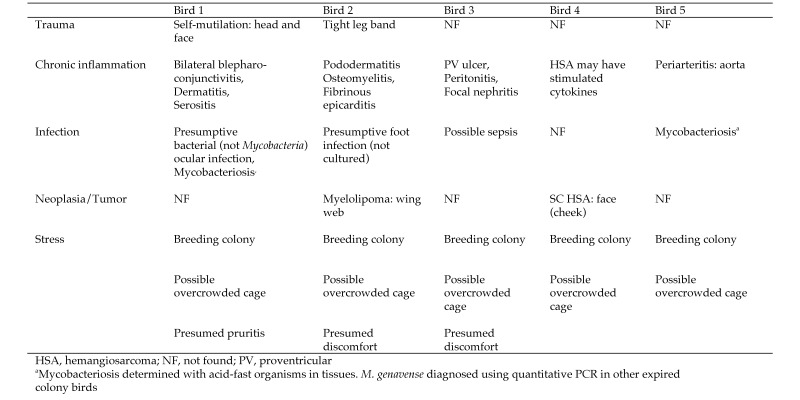
Over a period of less than 7 mo, 5 zebra finches in a research colony were diagnosed with amyloidosis. The deposition of amyloid protein was confirmed with congophilia of infiltrated tissues that showed the characteristic apple-green birefringence19,33 when viewed microscopically through polarizing filters. Only systemic AA protein has been diagnosed in avian species by using a combination of CR staining, immunohistochemistry, and amino acid sequencing.18,23,31,33,70 Among the 5 cases described in the current report, the presence of AA protein was confirmed in 2 birds by using antisera against duck amyloid A on selected tissue sections. All 5 birds were ill with chronic inflammatory or infectious conditions, which have been acknowledged as predisposing factors for the development of AA amyloidosis in humans and animals (Figure 3)3,33,46,64 . In addition, the preponderance of amyloid deposits in the liver, spleen, and kidneys of all 5 birds correlated with the primary sites of AA deposition in other species. In light of these similarities, we deduced that the systemic amyloidosis in the other 3 birds also consisted primarily of AA protein. Amyloid L protein, accumulated systemically in humans with multiple myeloma and rarely in animals with abnormal proliferation of monoclonal plasma cells,3,52,64 has not been documented in avian species; and there were no indications of these types of illnesses in the birds of the current report. Potassium permanganate pretreatment methods used historically to differentiate AA from AL amyloidoses61,65 are not foolproof70 and have become obsolete due to the advent of molecular techniques for differentiating different types of amyloid.
Figure 3.
Comorbidity classification system for birds with amyloidosis.
In the current study, the liver was the most frequently and usually most severely affected organ, showing amyloid deposition in all 5 animals. The liver was the only organ affected by amyloid in bird 5, although mild autolysis of the coelomic organs may have hindered the observation of amyloid deposits in the other tissues. During the 7 mo when the 5 amyloidosis cases were diagnosed, there were a total of 22 necropsies of birds in which the livers were examined microscopically. Using the liver as a reliable indicator thus reveals an amyloidosis incidence of roughly 23% in sick colony animals. However, not every bird that died was necropsied during this timeframe, and it is possible that amyloid deposits were missed in hematoxylin- and eosin-stained tissue sections from incipient cases. Therefore the actual incidence of amyloidosis in the colony has not been determined.
AF-positive organisms suggestive of Mycobacterium infection were present in the tissues from 2 birds with amyloidosis. Only a small focus of AF-positive organisms was found in the gizzard of bird 1, whereas bird 5 showed numerous AF organisms in many tissues, with granulomatous lesions similar to those described in birds diagnosed with M. genavense.27 During the timeframe of the current report, M. genavense infection was specifically diagnosed in additional birds in the colony according to quantitative PCR analysis of various tissues. Although a definitive association between amyloidosis and mycobacteriosis has been noted in birds, humans, and other mammals,11,33,43,46,50,69 this association may be incidental or secondary in some situations, given that other factors (genetics, environment, stress, other infections) might contribute to the generation of an amyloid response.11,12
The extensive and diffuse deposition of hepatic amyloid in bird 1 compared with the multifocal and moderate deposition in bird 5 may reflect the fact that bird 1 was clinically ill with severe, bilateral, blepharoconjunctivitis and inflammation in other organs, whereas bird 5 was primarily affected by mycobacteriosis. The etiology of the ocular disease in bird 1 was presumed infectious but unrelated to mycobacteriosis, and the clinical signs of marked conjunctival swelling, chemosis, and epiphora with severe palpebral crusting were consistent with an exuberant inflammatory reaction. This case might represent an early and mild mycobacterial infection, and perhaps the systemic serum AA response was primarily triggered by the bilateral conjunctival and palpebral morbidity. Another 8 colony birds had blepharoconjunctivitis of varying severity, but all of those cases were unilateral and less severe than the presented case. The presented case was the only one with amyloidosis, even though 4 of the additional 8 birds with ocular disease also tested positive for systemic M. genavense. Generally, colony birds that presented with bilateral conjunctivitis or blepharoconjunctivitis had more severe morbidity and worse prognoses than did those with unilateral conditions. The feather-follicle atrophy in bird 1 revealed its debilitated condition; and the self-mutilation of the head and face connoted another layer of dysregulated homeostasis and stress.
The diffuse amyloidosis in the liver and spleen, with multifocal deposition in the kidney, intestines, and ovary in bird 1 suggested chronicity of the amyloid process. However, experimentally induced amyloidosis progresses rapidly in some animal models when exogenous agents are inoculated;20,37 and spontaneous amyloid deposits developed within days in wild-caught Herring gulls (Larus argentatus) held in captivity.26 Other influences, perhaps related to genetics, stress, hygiene, disease severity, self-mutilation, and age, might have contributed to and enhanced the amyloidogenic response in bird 1 and the other cases. Age might correlate with an increased likelihood for chronic conditions, duration of corresponding inflammatory responses, and reduced compensatory physiologic reserves.16 One report noted that amyloidosis was more prevalent in older—but not the oldest—birds in a zoological collection.11 The susceptibility of Pekin ducks to spontaneous AA amyloidosis increases with age,43 but ducklings with spontaneous and experimentally induced amyloidosis have also been described.25,48
Severe pododermatitis (‘bumblefoot’) that progressed to osteomyelitis was associated with amyloidosis in the liver, kidney, spleen, and some intestinal organs in bird 2. Amyloid deposits did not occur in a colony bird with locally extensive pododermatitis without osteomyelitis or in another colony bird with osteomyelitis of the tibiotarsus and necrosis of the ipsilateral foot, again highlighting the apparently individual, sporadic, and unpredictable nature of the amyloidogenic process. Perhaps the fibrinous epicarditis observed incidentally in bird 2 also factored into the overall inflammatory and amyloid response, although bumblefoot has been specifically linked to AA amyloidosis in other bird species, particularly waterfowl.33,43,57 In one report, more than 75% of a group of mute swans (Cygnus olor) living in an open park developed AA amyloidosis, and almost all of them were diagnosed with bumblefoot.57
It is difficult to know whether the benign tumor observed incidentally in bird 2 contributed to the pathophysiology of the amyloidosis. Neoplasia is a recognized predisposing factor for AA amyloidosis in humans and animals;33,46,64 and one report described systemic amyloidosis associated with an encapsulated Leydig cell tumor in a companion zebra finch.45 Usually Leydig cell tumors in animals are benign, but the documented necrosis and hemorrhage that accompanied the zebra finch mass may have aggravated an acute-phase response in that animal. AA amyloidosis has been reported in humans with Hodgkin lymphoma, renal cell carcinoma, and adenocarcinoma of the lung and gut.46 In addition, hepatic adenoma and renal carcinoma without metastasis have been associated with amyloidosis in humans;2,46 so an invasive or metastatic condition is not an essential predisposing factor for amyloid disease, although perhaps the affected organ plays a role.
Bird 4 presented with a subcutaneous hemangiosarcoma that was localized and apparently nonmetastatic. Subcutaneous hemangiosarcoma found in other colony birds also did not display metastatic behavior in tissues examined. The neoplastic cells of the hemangiosarcoma in bird 4, as a form of chronic antigen stimulation, might have induced cytokines that promoted the production of hepatic SAA,8,56with eventual accumulations of AA in the liver, spleen, and intestines. Another 6 birds with neoplastic or benign tumors that were necropsied during the 7-mo time period did not have discernable amyloid deposits in the liver or other tissues examined. These findings reinforce the notion that unknown factors or combinations thereof contribute to the development of amyloidosis in susceptible animals. In addition to an increased pool and possibly genetic abnormalities of the SAA protein, proposed mechanisms for spontaneous amyloidogenesis include the introduction of an amyloid-enhancing factor (which is believed to be the amyloid fibril itself),36,38,54 altered basement membrane protein metabolism, exposure to glycosaminoglycans which provide a scaffolding for polymerization, malfunctioning extracellular chaperones that lead to protein misfolding, and the presence of certain inorganic ions such as calcium and sulphate.25,33,46,64,66
The normal body condition of bird 4 implied overall robust health, unlike the wasting and general debilitation in the 4 other cases. The severe wasting observed in bird 3 was most likely due to the perforating proventricular ulcer and peritonitis noted on gross and microscopic examinations. Both postmortem and antemortem quantitative PCR testing of this bird were negative for Mycobacterium spp., and postmortem testing was also negative for C. psittaci. Organisms bearing a morphologic resemblance to M. ornithogaster (avian gastric yeast) were observed in the proventriculus and gizzard; and an association between M. ornithogaster and proventricular ulcers has been reported in some bird species.30 The ulceration, peritonitis, and chronic fibrosis suggested significant prolonged inflammation with accompanying stress, likely precipitating the diffuse amyloidosis which might also have contributed to the wasting condition. In addition, the focal nephritis noted might have been an incidental finding or developed ancillary to the peritonitis. Renal amyloidosis has been documented in captive cheetahs (Acinonyx jubatus) affected with chronic lymphoplasmacytic gastritis.64,68
The anatomic sublocation of the amyloid deposits was mostly analogous to the distributions reported for other bird and mammalian species.29,33,48,70 Liver deposits tended to be extensive within the sinusoids (space of Disse), similar to the descriptions of hepatic amyloid in the companion zebra finch,45caged wild birds, and domestic poultry.33 In addition, the perivascular deposits noted in birds 3, 4, and 5 were comparable to those of hepatic amyloid in Pekin ducks.17 In the current study, splenic amyloid was located within the walls of small arteries and within the red and white pulp, akin to the splenic distribution reported in ducks and other bird species.7,45,48,70 In the kidney, amyloid accumulated in the interstitium but not the glomeruli, unlike the pattern in some waterfowl, which had both interstitial and glomerular infiltrates.7,17,57,70 Glomerular deposition has been reported in most animal species.29 Furthermore, amyloid deposition in the renal blood-vessel walls was noted in bird 2 and has been described in mute swans57 and a flamingo.7 In the intestines, amyloid deposits were mostly confined to the lamina propria, similar to findings in chickens and ducks.42,48 Amyloid infiltration of the adrenal gland was noted only in bird 3 but has been reported to occur in waterfowl, caged wild birds, and domestic poultry.33,57 Pancreatic infiltration only occurred in bird 2 and has also been reported in a hooded merganser (Lophodytes cucullatus).50
Two reports speculated that colony density might have been a factor in the development of amyloidosis in ducks and other waterfowl residing in a zoological setting.11,12 A prospective study using Pekin ducks showed that flocked ducks developed more amyloid deposits than did isolated or paired ducks, with the combination of space and animal numbers producing an effect.13 Social hierarchy and dominance behaviors might play a role in these situations.13 The colony density at our facility might have been stressful for subordinate or ill birds and might have been influential in the development of the various morbidities that predisposed the birds to amyloidosis. Neither the Guide28 nor Animal Welfare Act Regulations1 include recommended cage population densities for zebra finches, and the housing densities at our facility resulted from overall space availability and best-practice standards when compared with zebra finch housing densities at other research-animal facilities.
Other stresses, such as intensive breeding, handling, and noise, might have affected the health of some of our birds. In one study, inbred Khaki Campbell ducks exposed to intravenous inoculations and blood sampling displayed a significant increase in amyloidosis compared with that of control animals.55 Corticosterone concentrations increase in birds in response to confinement and to stressors such as blood sampling, water deprivation, immobilization, osmolar and thermal stress and vigorous exercise.26 Studies show that glucocorticoids can enhance hepatic production of SAA by inducing cytokines such as IL6 and IL1.59 However, glucocorticoids also help regulate the acute-phase response by providing negative feedback on cytokine production.59 Therefore, the association between increased glucocorticoid levels and amyloidosis cannot be simply defined.
Evidence supporting the intra- and interspecies prion-like fecal, oral, and parenteral transmission of amyloid fibrils is increasing.36,37,41,43,54,67,68 Fecal transmission of AA protein has been verified in captive cheetahs manifesting spontaneous AA amyloidosis.68 Experimental induction of AA amyloidosis in chickens occurred after both intravenous and oral inoculation of AA fibrils, with intravenous inoculation resulting in amyloid deposits distributed homogeneously in all organs, whereas oral inoculation resulted exclusively in severe splenic deposits.43 Another report suggested that apolipoprotein AII amyloid disease was propagated within 3 mo in young mice that ate feces containing apolipoprotein AII fibrils from geriatric mice that were housed in the same cage.66 Generally, the transmitted fibrils are thought to act as seeds that obviate the lag time normally required for the development of an amyloid nucleus which triggers the process of continued polymerization of misfolded amyloid proteins.45,66 With these factors in mind, horizontal transmission of amyloidosis cannot be ruled out in our zebra finch colony, even though the affected birds were not housed in close physical proximity, given that the cages were all open to the same room and exposed to the same ambient air and environmental conditions.
Whether a genetic susceptibility to AA amyloidosis exists in zebra finches or arose in our colony is unknown. Only bird 5 was genetically manipulated. Systemic AA amyloidosis in the absence of known preexisting inflammatory, infectious, or neoplastic conditions is well documented in humans, domestic ducks, Syrian hamsters, and some domestic feline breeds.4,47,49,60,64 In addition, systemic AA amyloidosis in black-footed cats (Felis nigripes) and captive Siberian tigers (Panthera tigris altaica) is thought to be idiopathic and probably genetic in nature.33,58,64 Familiar amyloidosis, in which the genetic mutation occurs in genes expressing nonamyloid proteins52 and represented by familial Mediterranean fever syndrome in humans, is a well-known problem in Shar Pei dogs, which demonstrate a similar febrile syndrome.15,35 It is also acknowledged that some isotypes of SAA are more amyloidogenic than others in humans and mice.5,22,24,46,64 Both ducks and chickens have only a single isotype of SAA which is expressed from a single gene, compared with the multiple isotypes found in mammalian species.25,55,59 One report identified 2 expressed alleles from the single SAA gene in outbred domestic ducks, and both alleles were noted in ducks with severe amyloid disease; however the sample size was quite small.25 The report investigating inbred Khaki Campbell ducks found that only one of the SAA alleles was expressed, but the incidence of AA deposition varied among experimental groups, suggesting that additional, nongenetic factors were involved with the development of amyloidosis in those animals.55 A BLAST search of the zebra finch genome disclosed a single gene for SAA protein.63 An informative next step might be to compare the DNA sequence of the SAA gene in birds diagnosed with amyloidosis with that from unaffected birds.
All 5 cases were diagnosed postmortem by using histopathology. Antemortem diagnostics, consisting primarily of biopsies of relevant tissues and/or abdominal fat or rectal tissues, are available for humans and some animal species35,46,47 but are probably impractical for zebra finches. Clinical signs of the condition depend on the location and extent of the amyloid deposits and might be confused with those due to concurrent disease. But in light of the data in the current report, the index of suspicion for susceptibility to amyloidosis should be elevated for birds with chronic inflammatory conditions, infections, and neoplasia and perhaps for those living under stressful conditions that involve intensive breeding and possible crowding. Preventative or treatment approaches primarily involve diagnosing and treating the predisposing conditions associated with AA amyloidosis. Amyloid A protein has been shown to resorb in humans and animals, once the predisposing conditions have been treated or resolved.47,51 Over the past decade, the incidence of AA amyloidosis in human rheumatoid arthritis and patients with familial Mediterranean fever syndrome has declined dramatically because of treatment with biologics that have dampened inflammatory and SAA responses.6,47 Preemptive treatment with colchicine has improved with quality of life and extended the survival of Shar Pei dogs35and humans with familial Mediterranean fever syndrome47 and decreased amyloid deposits in animal models.24 Antibodies against serum amyloid P have reduced amyloid deposits in some human patients.47
Whether zebra finches would be an informative laboratory model for AA amyloidosis is unclear. Zebra finches are housed and handled more easily than are domestic ducks or brown layer hens but have much smaller organs and blood volumes and fewer accessible venipuncture sites. Unlike the obvious, tan wax-like amyloid deposits grossly visible in the livers and kidneys of affected ducks and some other animals,7,17,19 the amyloid-infiltrated livers of our zebra finches were predominantly normal in color and, although sometimes enlarged and friable, were often normal in size and consistency. Therefore the livers of zebra finches may not accumulate the extensive amounts of amyloid observed in other experimental species, although exogenous amyloid-enhancing factor or additional inflammatory agents might induce more abundant deposits. Inbred and outbred mice repeatedly injected with AgNO3, casein, or other irritants have historically been used as models to study AA amyloidosis.10,22,24,64 The genetic backgrounds of laboratory mice are generally well understood and readily amenable to manipulation. One mouse model used dose-dependent, doxycycline-inducible transgenic expression of SAA to show that amyloid deposition can occur independent of inflammation.51 Another genetically modified mouse model sustained consistently high levels of human IL6 produced under the control of the MT1 promoter and developed amyloidosis by 3 mo of age without the use of exogenous agents such as amyloid-enhancing factor and inflammatory stimuli.53
Interest in amyloid-related research has intensified over the past decade as the number of serious amyloid-associated illnesses in humans and animals has become apparent. Further investigation into possible variants of the zebra finch SAA gene and perhaps knowledge of the amino acid sequence of the zebra finch AA protein might provide more insight into its usefulness as a model for AA amyloidosis in particular or amyloidosis in general. A potential horizontal mode of transmission is another intriguing area for inquiry. However, researchers using zebra finches as models for other diseases and veterinarians overseeing these animals should be aware that AA amyloidosis can occur in captive, colony birds and presents a welfare concern.
Acknowledgments
We thank Dr. Ju-Tau Guo for providing the antisera to duck amyloid A protein, and Dr. Amy Durham for providing the Siamese cat amyloid A controls. We thank Dr. James Stanley for his technical assistance. This case study was supported with funding by the Department of Animal Medicine, University of Massachusetts Medical School, Worcester, Massachusetts.
References
- 1.Animal Welfare Act as Amended. 2008. 7 USC §2131–2156.
- 2.Azzopardi JG, Lehner T. 1966. Systemic amyloidosis and malignant disease. J Clin Pathol 19:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blancas-Mejia LM, Ramirez-Alvarado M. 2013. Systemic amyloidoses. Annu Rev Biochem 82:745–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce JT, DiBartola SP, Chew DJ, Gasper PW. 1984. Familial renal amyloidosis in Abyssinian cats. Vet Pathol 21:33–38. [DOI] [PubMed] [Google Scholar]
- 5.Buxbaum J. 2006. The genetics of the amyloidoses: interactions with immunity and inflammation. Genes Immun 7:439–449. [DOI] [PubMed] [Google Scholar]
- 6.Buxbaum JN, Linke RP. 2012. A molecular history of the amyloidoses. J Mol Biol 421:142–159. [DOI] [PubMed] [Google Scholar]
- 7.Camus A, Roberts C, Heatley JJ, Pirie G. 2002. What is your diagnosis? J Avian Med Surg 16:69–71. [Google Scholar]
- 8.Chiarella P, Vulcano M, Bruzzo J, Vermeulen M, Vanzulli S, Maglioco A, Camerano G, Palacios V, Fernandez G, Brando RF, Isturiz MA, Dran GI, Bustuoabad OD, Ruggiero RA. 2008. Anti-inflammatory pretreatment enables an efficient dendritic cell-based immunotherapy against established tumors. Cancer Immunol Immunother 57:701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coe JE, Ross MJ. 1990. Amyloidosis and female protein in the Syrian hamster. Concurrent regulation by sex hormones. J Exp Med 171:1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen AS, Shirahama T. 1972. Animal model for human disease: spontaneous and induced amyloidosis. Am J Pathol 68:441–444. [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan DF. 1968. Avian amyloidosis. I. General incidence in zoo birds. Pathol Vet 5:51–58. [PubMed] [Google Scholar]
- 12.Cowan DF. 1968. Avian amyloidosis. II. Incidence and contributing factors in the family anatidae. Pathol Vet 5:59–66. [PubMed] [Google Scholar]
- 13.Cowan DF, Johnson WC. 1970. Amyloidosis in the white Pekin duck. I. Relation to social environmental stress. Lab Invest 23:551–555. [PubMed] [Google Scholar]
- 14.Cray C, Zaias J, Altman NH. 2009. Acute phase response in animals: a review. Comp Med 59:517–526. [PMC free article] [PubMed] [Google Scholar]
- 15.DiBartola SP, Tarr MJ, Webb DM, Giger U. 1990. Familial renal amyloidosis in Chinese Shar Pei dogs. J Am Vet Med Assoc 197: 483–487. [PubMed] [Google Scholar]
- 16.Dobson CM. 2002. Getting out of shape. Nature 418:729–730. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty E, III, Rickard CG, Scott ML. 1963. Subacute and chronic liver diseases of the white Pekin duck. Avian Dis 7:217–234. [Google Scholar]
- 18.Ericsson LH, Eriksen N, Walsh KA, Benditt EP. 1987. Primary structure of duck amyloid protein A. The form deposited in tissues may be identical to its serum precursor. FEBS Lett 218:11–16. [DOI] [PubMed] [Google Scholar]
- 19.Francis RJ. 1990. Amyloid, p 155. In: Bancroft JD, Stevens A. Theory and practice of histological techniques 3 ed. New York (NY):Churchill Livingstone. [Google Scholar]
- 20.Ganowiak K, Hultman P, Engstrom U, Gustavsson A, Westermark P. 1994. Fibrils from synthetic amyloid-related peptides enhance development of experimental AA-amyloidosis in mice. Biochem Biophys Res Commun 199:306–312. [DOI] [PubMed] [Google Scholar]
- 21.Garcia A, Latimer KS, Niagro FD, Norton TM, Campagnoli RP, Harmon BG, Howerth EW, Ritchie BW. 1994. Diagnosis of polyomavirus infection in seedcrackers (Pyrenestes sp.) and bluebills (Spermophaga Haematina) using DNA in situ hybridization. Avian Pathol 23:525–537. [DOI] [PubMed] [Google Scholar]
- 22.Gervais F, Hebert L, Skamene E. 1988. Amyloid-enhancing factor: production and response in amyloidosis-susceptible and resistant mouse strains. J Leukoc Biol 43:311–316. [DOI] [PubMed] [Google Scholar]
- 23.Gorevic PD, Greenwald M, Frangione B, Pras M, Franklin EC. 1977. The amino acid sequence of duck amyloid A (AA) protein. J Immunol 118:1113–1118. [PubMed] [Google Scholar]
- 24.Gruys E, Snel FW. 1994. Animal models for reactive amyloidosis. Baillieres Clin Rheumatol 8:599–611. [DOI] [PubMed] [Google Scholar]
- 25.Guo JT, Aldrich CE, Mason WS, Pugh JC. 1996. Characterization of serum amyloid A protein mRNA expression and secondary amyloidosis in the domestic duck. Proc Natl Acad Sci USA 93:14548–14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman AM, Leighton FA. 1985. Hemograms and microscopic lesions of herring gulls during captivity. J Am Vet Med Assoc 187:1125–1128. [PubMed] [Google Scholar]
- 27.Hoop RK, Bottger EC, Ossent P, Salfinger M. 1993. Mycobacteriosis due to mycobacterium genavense in 6 pet birds. J Clin Microbiol 31:990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 29.Jakob W. 1971. Spontaneous amyloidosis of mammals. Vet Pathol 8:292–306. [DOI] [PubMed] [Google Scholar]
- 30.Jansson DS, Brojer C, Mattsson R, Feinstein R, Morner T, Hard af Segerstad C. 2008. Mycotic proventriculitis in gray partridges (perdix perdix) on 2 game bird farms. J Zoo Wildl Med 39:428–437. [DOI] [PubMed] [Google Scholar]
- 31.Landman WJ. 1999. Amyloid arthropathy in chickens. Vet Q 21: 78–82. [DOI] [PubMed] [Google Scholar]
- 32.Landman WJ, Feberwee A. 2001. Field studies on the association between amyloid arthropathy and mycoplasma synoviae infection, and experimental reproduction of the condition in brown layers. Avian Pathol 30:629–639. [DOI] [PubMed] [Google Scholar]
- 33.Landman WJ, Gruys E, Gielkens AL. 1998. Avian amyloidosis. Avian Pathol 27:437–449. [DOI] [PubMed] [Google Scholar]
- 34.Landman WJ, Sletten K, Koch CA, Tooten PC, Gruys E. 1996. Chicken joint amyloid protein is of the AA-type. I. characterization of the amyloid protein. Scand J Immunol 43:210–218. [DOI] [PubMed] [Google Scholar]
- 35.Loeven KO. 1994. Hepatic amyloidosis in 2 Chinese Shar Pei dogs. J Am Vet Med Assoc 204:1212–1216. [PubMed] [Google Scholar]
- 36.Lundmark K, Westermark GT, Nystrom S, Murphy CL, Solomon A, Westermark P. 2002. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc Natl Acad Sci USA 99:6979–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundmark K, Westermark GT, Olsen A, Westermark P. 2005. Protein fibrils in nature can enhance amyloid protein A amyloidosis in mice: cross-seeding as a disease mechanism. Proc Natl Acad Sci USA 102:6098–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lupi O, Peryassu MA. 2007. An emerging concept of prion infections as a form of transmissible cerebral amyloidosis. Prion 1:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merlini G, Seldin DC, Gertz MA. 2011. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol 29:1924–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montali RJ, Bush M, Thoen CO, Smith E. 1976. Tuberculosis in captive exotic birds. J Am Vet Med Assoc 169:920–927. [PubMed] [Google Scholar]
- 41.Munch C, Bertolotti A. 2012. Propagation of the prion phenomenon: beyond the seeding principle. J Mol Biol 421:491–498. [DOI] [PubMed] [Google Scholar]
- 42.Murakami T, Inoshima Y, Sakamoto E, Fukushi H, Sakai H, Yanai T, Ishiguro N. 2013. AA amyloidosis in vaccinated growing chickens. J Comp Pathol 149:291–297. [DOI] [PubMed] [Google Scholar]
- 43.Murakami T, Ishiguro N, Higuchi K. 2014. Transmission of systemic AA amyloidosis in animals. Vet Pathol 51:363–371. [DOI] [PubMed] [Google Scholar]
- 44.Murakami T, Muhammad N, Inoshima Y, Yanai T, Goryo M, Ishiguro N. 2013. Experimental induction and oral transmission of avian AA amyloidosis in vaccinated white hens. Amyloid 20:80–85. [DOI] [PubMed] [Google Scholar]
- 45.Nouri M, Sasani F, Gharagozloo MJ, Jazani MM. 2011. Systemic amyloidosis and testicular interstitial tumor in a zebra finch (taeniopygia guttata): a case report in Iran. Vet Res Forum 2:209–213 . [Google Scholar]
- 46.Obici L, Merlini G. 2012. AA amyloidosis: basic knowledge, unmet needs and future treatments. Swiss Med Wkly 142:w13580. [DOI] [PubMed] [Google Scholar]
- 47.Pepys MB. 2006. Amyloidosis. Annu Rev Med 57:223–241. [DOI] [PubMed] [Google Scholar]
- 48.Rigdon Rh. 1961. Amyloidosis: spontaneous occurrence in white Pekin ducks. Am J Pathol 39:369–378. [PMC free article] [PubMed] [Google Scholar]
- 49.Rudnick CM, Dowton SB. 1993. Serum amyloid P (female protein) of the Syrian hamster. Gene structure and expression. J Biol Chem 268:21760–21769. [PubMed] [Google Scholar]
- 50.Sato Y, Aoyagi T, Matsuura S, Fukui S, Kitazawa I, Nishimori K, Yokomizo Y. 1996. An occurrence of avian tuberculosis in hooded merganser (lophodytes cucullatus). Avian Dis 40:941–944. [PubMed] [Google Scholar]
- 51.Simons JP, Al-Shawi R, Ellmerich S, Speck I, Aslam S, Hutchinson WL, Mangione PP, Disterer P, Gilbertson JA, Hunt T, Millar DJ, Minogue S, Bodin K, Pepys MB, Hawkins PN. 2013. Pathogenetic mechanisms of amyloid A amyloidosis. Proc Natl Acad Sci USA 110:16115–16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Westermark P. 2014. Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid 21:221–224. [DOI] [PubMed] [Google Scholar]
- 53.Solomon A, Weiss DT, Schell M, Hrncic R, Murphy CL, Wall J, McGavin MD, Pan HJ, Kabalka GW, Paulus MJ. 1999. Transgenic mouse model of AA amyloidosis. Am J Pathol 154:1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soto C, Estrada L, Castilla J. 2006. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci 31:150–155. [DOI] [PubMed] [Google Scholar]
- 55.Stepanets V, Vernerova Z, Vilhelmova M, Geryk J, Hejnar J, Svoboda J. 2001. Amyloid A amyloidosis in non-infected and avian leukosis virus–C persistently infected inbred ducks. Avian Pathol 30:33–42. [DOI] [PubMed] [Google Scholar]
- 56.Tamamoto T, Ohno K, Ohmi A, Goto-Koshino Y, Tsujimoto H. 2008. Verification of measurement of the feline serum amyloid A (SAA) concentration by human SAA turbidimetric immunoassay and its clinical application. J Vet Med Sci 70:1247–1252. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka S, Dan C, Kawano H, Omoto M, Ishihara T. 2008. Pathological study on amyloidosis in cygnus olor (mute swan) and other waterfowl. Med Mol Morphol 41:99–108. [DOI] [PubMed] [Google Scholar]
- 58.Terio KA, O'Brien T, Lamberski N, Famula TR, Munson L. 2008. Amyloidosis in black–footed cats (felis nigripes). Vet Pathol 45:393–400. [DOI] [PubMed] [Google Scholar]
- 59.Uhlar CM, Whitehead AS. 1999. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 265:501–523. [DOI] [PubMed] [Google Scholar]
- 60.van der Linde-Sipman JS, Niewold TA, Tooten PC, de Neijs-Backer M, Gruys E. 1997. Generalized AA–amyloidosis in Siamese and oriental cats. Vet Immunol Immunopathol 56:1–10. [DOI] [PubMed] [Google Scholar]
- 61.van Rijswijk MH, van Heusden CW. 1979. The potassium permanganate method. A reliable method for differentiating amyloid AA from other forms of amyloid in routine laboratory practice. Am J Pathol 97:43–58. [PMC free article] [PubMed] [Google Scholar]
- 62.Vitali SD, Eden PA, Payne KL, Vaughan RJ. 2006. An outbreak of mycobacteriosis in Gouldian finches caused by mycobacterium peregrinum. Vet Clin North Am Exot Anim Pract 9:519–522. [DOI] [PubMed] [Google Scholar]
- 63.Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Kunstner A, Searle S, White S, Vilella AJ, Fairley S, Heger A, Kong L, Ponting CP, Jarvis ED, Mello CV, Minx P, Lovell P, Velho TA, Ferris M, Balakrishnan CN, Sinha S, Blatti C, London SE, Li Y, Lin YC, George J, Sweedler J, Southey B, Gunaratne P, Watson M, Nam K, Backstrom N, Smeds L, Nabholz B, Itoh Y, Whitney O, Pfenning AR, Howard J, Volker M, Skinner BM, Griffin DK, Ye L, McLaren WM, Flicek P, Quesada V, Velasco G, Lopez-Otin C, Puente XS, Olender T, Lancet D, Smit AF, Hubley R, Konkel MK, Walker JA, Batzer MA, Gu W, Pollock DD, Chen L, Cheng Z, Eichler EE, Stapley J, Slate J, Ekblom R, Birkhead T, Burke T, Burt D, Scharff C, Adam I, Richard H, Sultan M, Soldatov A, Lehrach H, Edwards SV, Yang SP, Li X, Graves T, Fulton L, Nelson J, Chinwalla A, Hou S, Mardis ER, Wilson RK. 2010. The genome of a songbird. Nature 464:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woldemeskel M. 2012. A concise review of amyloidosis in animals. Vet Med Int 2012:42729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wright JR, Calkins E, Humphrey RL. 1977. Potassium permanganate reaction in amyloidosis. A histologic method to assist in differentiating forms of this disease. Lab Invest 36:274–281. [PubMed] [Google Scholar]
- 66.Wyatt AR, Yerbury JJ, Dabbs RA, Wilson MR. 2012. Roles of extracellular chaperones in amyloidosis. J Mol Biol 421:499–516. [DOI] [PubMed] [Google Scholar]
- 67.Xing Y, Nakamura A, Chiba T, Kogishi K, Matsushita T, Li F, Guo Z, Hosokawa M, Mori M, Higuchi K. 2001. Transmission of mouse senile amyloidosis. Lab Invest 81:493–499. [DOI] [PubMed] [Google Scholar]
- 68.Zhang B, Une Y, Fu X, Yan J, Ge F, Yao J, Sawashita J, Mori M, Tomozawa H, Kametani F, Higuchi K. 2008. Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. Proc Natl Acad Sci USA 105:7263–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zschiesche W, Jakob W. 1989. Pathology of animal amyloidoses. Pharmacol Ther 41:49–83. [DOI] [PubMed] [Google Scholar]
- 70.Zschiesche W, Linke RP. 1989. Immunohistochemical characterization of spontaneous amyloidosis in captive birds as AA–type, using monoclonal and polyclonal anti–AA antibodies against mammalian amyloid. Acta Histochem 86:45–50. [DOI] [PubMed] [Google Scholar]