Abstract
To report the safety and efficacy of anti-tumor necrosis factor α (TNFα) therapy in severe and refractory neuro-Behçet disease (NBD) patients.
Observational, multicenter study including 17 BD patients (70.6% of male, with a median age of 39.3 [24–60] years), with symptomatic parenchymal NBD, refractory to previous immunosuppressant and treated with anti-TNFα (infliximab 5 mg/kg [n = 13] or adalimumab [n = 4]). Complete remission was defined by the disappearance of all neurological symptoms and by the improvement of radiological abnormalities at 12 months.
Overall improvement following anti-TNF was evidenced in 16/17 (94.1%) patients including 6 (35.3%) complete response and 10 (58.8%) partial response. The median time to achieve remission was 3 months (1–6). The median Rankin score was 2 (1–4) at the initiation of anti-TNFα versus 1 (0–4) at the time of remission (P = 0.01). Corticosteroids have been stopped in 4 (23.5%) patients, and reduced by more than 50% as compared with the dosage at baseline in 10 (58.8%) patients. Side effects occurred in 23.5% of patients and required treatment discontinuation in 17% of cases.
TNF blockade represents an effective therapeutic approach for patients with severe and refractory NBD, a difficult to treat population.
Key Messages
Overall improvement following anti-TNFα was evidenced in 94.1% of patients with severe and refractory neuro-Behcet disease.
The Rankin score decreased significantly with the use of anti-TNFα.
Anti-TNFα had a significant steroids sparing effect.
INTRODUCTION
Behçet disease (BD) is a chronic and relapsing vasculitis, including recurrent oral aphthous ulcers, along with genital ulcerations, skin lesions, and uveitis. Patients may also present with arthralgia, venous and arterial thrombosis, and neurological involvement. BD affects mainly young patients, with a peculiar geographic distribution (Mediterranean and Eastern countries). Neurologic involvement occurs in 5.3% to 59% of patients.1–3 These lesions are typically described as “parenchymal” and “extraparenchymal.” Although the clinical and imaging features of neurological involvement of BD have been extensively described, few studies have reported on the long-term outcome and treatment of neuro-BD (NBD). The treatment of parenchymal lesions of NBD is based on high doses of corticosteroids and immunosuppressants such as cyclophosphamide and azathioprine.4 We have recently shown that cyclophosphamide tended to be more efficient than azathioprine in severe NBD patients.5 Neurological involvement is 1 of the main cause of disability in BD. Up to 25% of our patients with neuro-BD had moderate-to-severe disabling sequelae (persistent Rankin score ≥3) or died after a median follow-up of 73 months.5 There is an unmet need for less toxic and more effective immunosuppressive treatments in the management of severe and/or refractory neuro-BD patients. Many studies have shown the rapidity of action and the effectiveness of anti-tumor necrosis factor α (TNFα) in severe uveitis of BD.6,7 However, only case reports and compiled data from literature reviews are available for NBD and these have shown very encouraging results with the use of anti-TNFα.8–10 The aim of the present multicenter observational study was to analyze the safety and efficacy of anti-TNFα therapy in 17 severe and refractory neurological BD patients with parenchymal involvement.
METHODS
We conducted a multicenter observational study, including 17 patients followed in 6 internal medicine, and rheumatology referral centers between 2001 and 2015. All patients with symptomatic and refractory NBD were treated with anti-TNFα antibodies, followed in the participating centers were enrolled. All patients fulfilled the international criteria for BD.11 The study was approved by the local ethics committee. The diagnosis of NBD was based on objective neurological symptoms not explained by any other known disease or therapy associated with neuroimaging findings suggestive of BD-related central nervous system (CNS) involvement12 and sometimes with cerebrospinal fluid (CSF) findings showing aseptic inflammation. NBD patients treated with anti-TNFα antibodies for neurological symptoms and specific cerebral parenchymal lesions on magnetic resonance imagery (MRI) were included. Patients with isolated recurrent meningitis or cerebral venous thrombosis without parenchymal NBD lesions were excluded. All patients were refractory and/or intolerant to at least 1 immunosuppressant or high doses of corticosteroids before anti-TNFα initiation. All patients have been treated with immunosuppressants (n = 16) and/or high doses of corticosteroids (n = 17) before anti-TNFα initiation. Immunosuppressive treatments included azathioprine (n = 13, median dosage of 150 mg daily), cyclophosphamide (n = 9), interferon (n = 3), mycophenolate mofetil (n = 2), chlorambucil (n = 2), ciclosporine (n = 1), and methotrexate (n = 1). Patients had received a median of 2 (0; 4) immunosuppressants before anti-TNFα initiation. Corticosteroid pulses were given in 8 patients.
Data Collection and Outcome Measurement
The following data were collected: age, gender, date of BD criteria and of NBD diagnosis, and clinical manifestations of BD (mucocutaneous lesions, eyes, joint, and vascular involvement). The neurological symptoms and the CNS MRI imaging at diagnosis were also reported. The data regarding the therapeutic modalities (drug, dosage, and duration) were collected.
The following terms were used to describe the NBD course: acute form disease course (including single episodes and relapsing-remitting course) or chronic progressive course. To describe the initial status and the outcome under treatment, the Rankin score was used as a marker of disability status.13
Study Endpoints
For each patient, we evaluated the clinical and radiological response after anti-TNFα initiation, the time to obtain remission, the occurrence of relapse, and side effects.
Complete remission was defined by the disappearance of all neurological symptoms and by the improvement of radiological abnormalities related to NBD at 12 months after anti-TNFα initiation. Partial remission was defined by improvement of neurological symptoms and of radiological abnormalities at 12 months after anti-TNFα initiation and/or by a decrease of more than 50% of the corticosteroids dose as compared with baseline. Other patients were considered as nonresponders.
The relapse was defined by the recurrence of objective neurological symptoms not explained by any other known disease or therapy. The Rankin score was evaluated at the initiation of anti-TNFα and at time of remission.
Statistical Analysis
Continuous variables are presented as median (range or interquartile range as appropriate), and continuous variables before and after anti-TNF were compared between using Wilcoxon rank test. Categorical variables are presented as count (percentage).
RESULTS
Clinical Features
Seventeen BD patients (70.6% of male gender, with a median age of 39.3 [24–60] years) with neurological parenchymal involvement were included. Geographic origin included 8 Caucasian, 5 North Africans, and 3 sub-Saharan Africans patients. All had oral ulcers, 11 (64.7%) skin involvement, 11 (64.7%) genital ulcers, 8 (47%) ocular involvement, 5 (29.4%) joint involvement, 5 (29.4%) venous lesions (i.e., superficial thrombosis [n = 1], deep thrombosis of lower limb [n = 3], and pulmonary embolism [n = 1]), 3 (17.6%) gastrointestinal involvement, 1 (5.9%) arterial occlusion, 1 (5.9%) arterial aneurysm, and 1 (5.9%) pericarditis.
Neurological Involvement
Characteristics and outcome of the 17 patients are summarized in Table 1 . All patients had parenchymal NBD, associated with meningitis in 10 patients, optic neuritis in 1, and cerebral thrombophlebitis in 1. Parenchymal lesions involved spinal cord (n = 4), brainstem (n = 8), and/or supra-tentorial region (deep [n = 5], cortical or subcortical [n = 7]).
TABLE 1.
Demographic, Neurological Characteristics and Outcome of the 17 Patients With BD With Refractory Neurological Involvement Treated With Anti-TNFα Antibodies
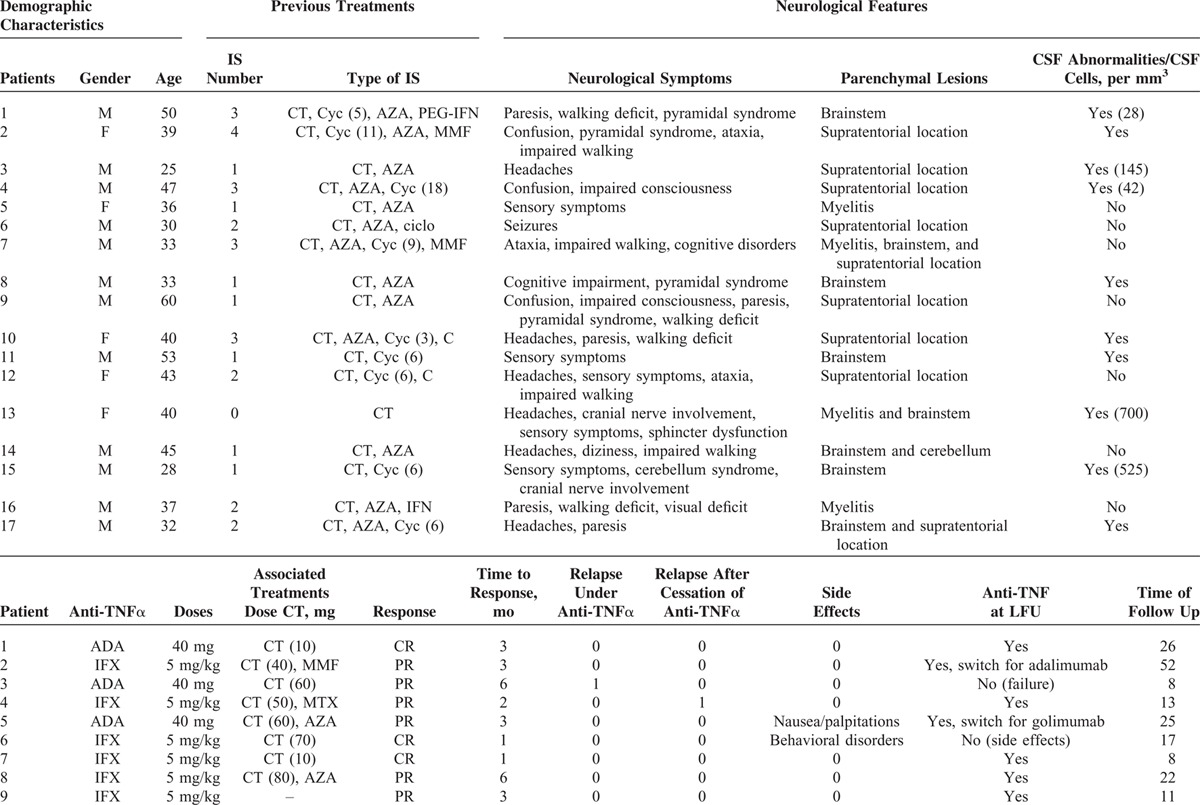
TABLE 1 (Continued).
Demographic, Neurological Characteristics and Outcome of the 17 Patients With BD With Refractory Neurological Involvement Treated With Anti-TNFα Antibodies

Main symptoms included: walking disorders (n = 8), headaches (n = 6), paresis (n = 5), sensory symptoms (n = 5), confusion/cognitive disorders (n = 5), pyramidal syndrome (n = 3), ataxia (n = 3), impaired consciousness (n = 2), cranial nerve involvement (n = 2), cerebellum syndrome (n = 1), and seizures (n = 1). Symptoms were acute in 88.9% of patients. Among the 17 patients, 3 had also fever and 15 had associated involvements with neurological symptoms (ocular involvement [n = 5], skin or mucosal lesions [n = 11], gastrointestinal [n = 1], articular symptoms [n = 3], aortic aneurysm [n = 1], and cardiac involvement [n = 1]). No patients required management in an intensive care unit.
The median (range) level of C-reactive protein was 18 (3–40) mg/dL. The median number of cells and proteins level in the CSF was 145 per mm3 (28–700) and 0.6 (0.55–1.00), respectively, in patients with meningitis.
Treatment and Outcome
Anti-TNFα antibodies included infliximab (5 mg/kg [n = 13]) or adalimumab (40 mg/14 days [n = 3], 40 mg/7 days [n = 1]). The median duration of disease before the initiation of anti-TNFα was 4.6 (6; 284) months. Besides anti-TNFα, all but 1 also received corticosteroids (median initial daily dose 50 mg [5–80], pulses [n = 5]) and 9 received immunosuppressants (azathioprine [AZA], n = 4; methotrexate [MTX], n = 4; and mycophenolate mofetil, n = 1) (Table 1 ). Overall improvement following anti-TNF was evidenced in 16/17 (94.1%) patients. The response was complete in 5 (29.4%) and partial in 11 (64.7%) patients. One patient was nonresponder and was switched to tocilizumab with favorable outcome. The median time to achieve remission was 3 (1–6) months (Table 1 ). Four patients (23.5%) had a Rankin score ≥ 3 at the initiation of anti-TNF therapy. The median Rankin score was 2 (1–4) at the initiation of anti-TNFα versus 1 (0–4) at the time of remission (P = 0.01) (Figure 1). After anti-TNF therapy (at last follow-up), 3 patients (17.6%) had moderate-to-severe disabling sequelae (persistent Rankin score ≥ 3; i.e., severe walking deficit). Four patients experienced a relapse of NBD including 2 over anti-TNFα therapy and 2 after cessation of anti-TNFα agents (2 and 12 months after stopping anti-TNFα). Anti-TNFα were stopped because of side effects in 1 and poor compliance to treatment in 1 patient. Radiological abnormalities improved in 73.3% were stable in 20% and worsened in 6.7% of patients. No significant difference was found with respect to the efficacy of anti-TNF used as monotherapy or in association with an immunosuppressive agent (AZA, MTX) (Table 1 ). After a median follow-up of 17.1 (3–163) months, 13 (76.5%) were still receiving anti-TNFα agents. The initial anti-TNFα treatment was discontinued in 5 patients because of side effects (n = 3), treatment failure (n = 1), and relapse (n = 1).
FIGURE 1.
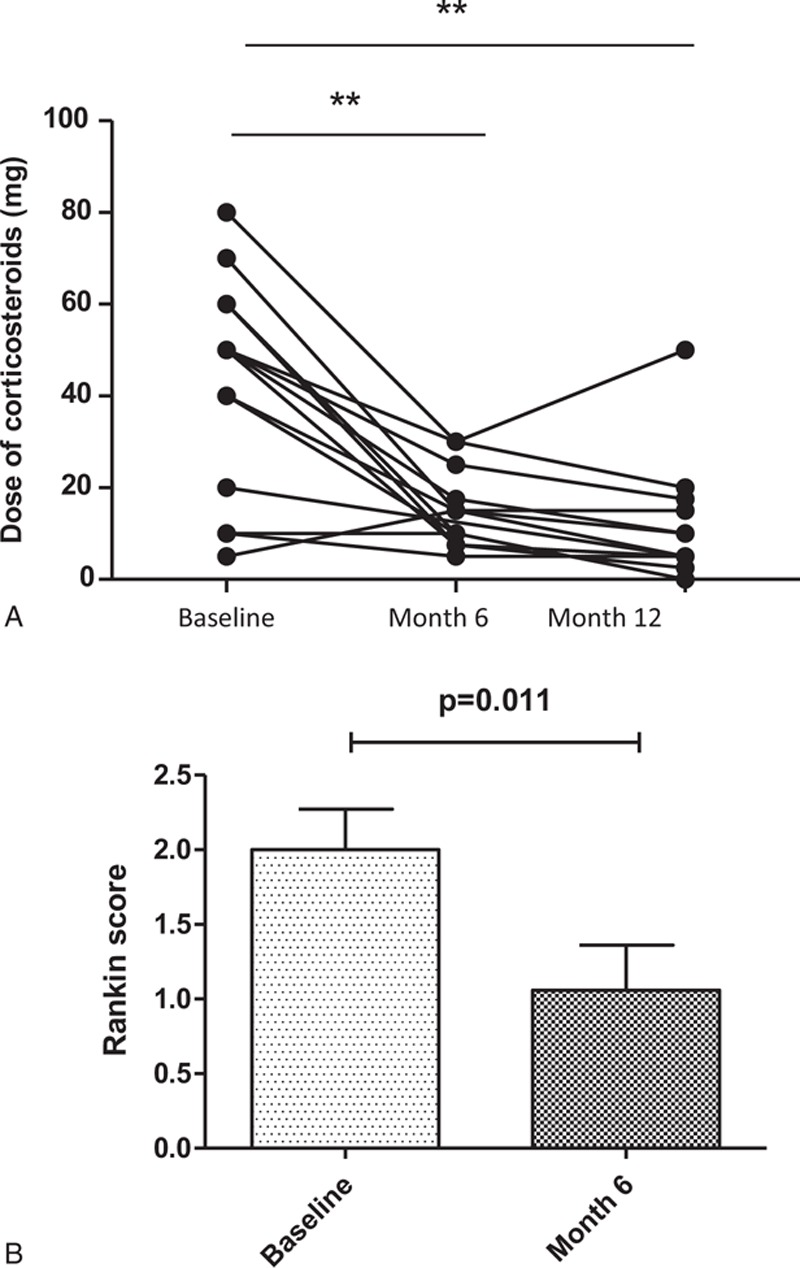
Outcome of patients with BD with severe and refractory neurological involvement treated with anti-TNFα. (A) Rankin score at the initiation of anti-TNFα and at the time of remission. (B) Course of corticosteroids daily dose (mg) after initiation of anti-TNFα therapy. BD = Behçet disease, TNF = tumor necrosis factor.
Corticosteroids Sparing
Doses of corticosteroids decreased significantly at 6 and 12 months after anti-TNFα initiation (median daily dose at baseline of 50 mg vs 15 mg at month 6 [P = 0.004] vs 5 mg at month 12 [P = 0.006]), respectively (Figure 1). Corticosteroids have been stopped in 4 (23.5%) patients, and reduced by more than 50% as compared with the dosage at baseline in 10 (58.8%) patients. At the end of follow-up the median daily dose of corticosteroids was 6.25 mg.
Side Effects
Side effects occurred in 4 (23.5%) patients (i.e., nausea/palpitations [n = 1], cardiac insufficiency [n = 1], pulmonary infection [n = 1], behavioral disorder [n = 1]). Side effects required treatment discontinuation in 3 patients. Among them, 2 received another anti-TNFα agent (adalimumab [n = 1] and golimumab [n = 1]), with a recurrence of dyspnea requiring treatment cessation in 1.
DISCUSSION
In the present study, we report the largest cohort of severe and refractory parenchymal NBD treated by anti-TNFα therapy. To the best of our knowledge, only case reports and small series (i.e., <8 patients) have reported the outcome of NBD patients treated with anti-TNFα.9 Pipitone et al9 included 8 NBD patients (3 with new onset NBD), and all patients had partial clinical and radiological improvement after anti-TNFα initiation. A literature review reporting anti-TNFα efficacy in BD has shown a 90% response rate in NBD treated with infliximab.10 However, these data concerned case reports published in literature, leading to obvious bias (heterogeneous patients and management and selection bias of patients with good response after anti-TNFα treatment). Moreover, accurate data on clinical features and response in NBD patients were not available in this study. Thus data relative to the efficacy of anti-TNFα in NBD are lacking. Neurological involvement is 1 of the main causes of disability in BD accounting for 25% of moderate-to-severe disabling sequelae.5 There is an unmet need for less toxic and more effective immunosuppressive treatments in the management of severe and/or refractory NBD patients. In BD, the efficacy of anti-TNFα has been largely demonstrated mainly in uveitis.3,4 Arida et al reported uveitis improvement in 89% and 100% of patients with IFX and ADA, respectively.10 In an open label, multicenter study of 124 BD patients, intraocular inflammation, macular thickness, and visual acuity, the sparing effect of corticosteroids and immunosuppression load showed a rapid and maintained improvement. Consistently, experts recommend to use anti-TNFα antibodies, as first-line therapy, in BD patients with severe ocular involvement.4,14
We have shown that anti-TNFα antibodies may be efficient in severe and refractory NBD patients. Overall improvement following anti-TNFα was evidenced in 94.1% of patients and complete response was achieved in one-third of cases. The onset of action was fast as the median time to achieve remission was of 3 months. The proportion of NBD patients with moderate-to-severe neurological disability could be reduced by 50% and the Rankin score decreased significantly with the use of anti-TNFα therapy. Lastly, anti-TNFα had a significant steroids sparing effect as they can be stopped in 23.5% of patients, and reduced by more than 50% as compared with the dosage at baseline in up to 60% of cases. Taken together, these results are likely to be clinically meaningful.
Herein, we included NBD patients with severe neurological involvement as, 41.2% had brainstem lesions, 24% had myelitis, and 58.8% had Rankin score ≥2 at anti-TNFα initiation. Along this line, the presence of brainstem lesions is an independent risk factor of death and/or persistent Rankin score ≥ 3 in NBD.5 Moreover, our NBD patients had received a median of 2 immunosuppressants before the use of anti-TNFα therapy.
The safety profile was acceptable and comparable to that observed in patients with chronic inflammatory arthritis or Crohn disease. Side effects occurred in 23.5% of patients and required treatment discontinuation in 17% of cases.
We acknowledge some limitations in our study. We were unable to collect complete longitudinal data on patients who were seen only on an intermittent basis. Prospective enrollment and data collection from the time of diagnosis would have been ideal but is more difficult to achieve with rare diseases. Small size of patient cohort treated with adalimumab does not allow us to compare them to the infliximab cohort or to make further definitive conclusions. However, anti-TNFα therapy was associated with a beneficial response in 94% of our patients who were resistant to conventional therapies.
Given the unmet needs of these patients, the results presented herein may substantiate future recommendations for their use in refractory NBD.
In conclusion, our results suggest that TNF blockade represents an effective therapeutic approach for patients with severe NBD and resistant to standard immunosuppressive regimens. Further studies are warranted to further evaluate their effectiveness in the management of severe manifestations of BD.
Footnotes
Abbreviations: AZA = azathioprine, BD = Behçet disease, CSF = cerebrospinal fluid, MRI = magnetic resonance imagery, MTX = methotrexate, NBD = neuro-Behçet disease, TNF = tumor necrosis factor.
PC and DS are co-authors.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Ideguchi H, Suda A, Takeno M, et al. Neurological manifestations of Behçet's disease in Japan: a study of 54 patients. J Neurol 2010; 257:1012–1020.doi:10.1007/s00415-010-5454-2. [DOI] [PubMed] [Google Scholar]
- 2.Siva A, Kantarci OH, Saip S, et al. Behçet's disease: diagnostic and prognostic aspects of neurological involvement. J Neurol 2001; 248:95–103. [DOI] [PubMed] [Google Scholar]
- 3.Akman-Demir G, Serdaroglu P, Tasçi B. Clinical patterns of neurological involvement in Behçet's disease: evaluation of 200 patients. The Neuro-Behçet Study Group. Brain J Neurol 1999; 122:2171–2182. [DOI] [PubMed] [Google Scholar]
- 4.Hatemi G, Silman A, Bang D, et al. EULAR recommendations for the management of Behçet disease. Ann Rheum Dis 2008; 67:1656–1662.doi:10.1136/ard.2007.080432. [DOI] [PubMed] [Google Scholar]
- 5.Noel N, Bernard R, Wechsler B, et al. Long-term outcome of neuro-Behçet's disease: outcome of neuro-Behçet's disease. Arthritis Rheumatol 2014; 66:1306–1314.doi:10.1002/art.38351. [DOI] [PubMed] [Google Scholar]
- 6.Calvo-Rio V, Blanco R, Beltran E, et al. Anti-TNF-therapy in patients with refractory uveitis due to Behcet's disease: a 1-year follow-up study of 124 patients. Rheumatology 2014; 53:2223–2231.doi:10.1093/rheumatology/keu266. [DOI] [PubMed] [Google Scholar]
- 7.Vallet H, Riviere S, Sanna A, et al. Efficacy of anti-TNF alpha in severe and/or refractory Behçet's disease: multicenter study of 124 patients. J Autoimmun 2015; 62:67–74. [DOI] [PubMed] [Google Scholar]
- 8.Sarwar H, McGrath H, Espinoza LR. Successful treatment of long-standing neuro-Behçet's disease with infliximab. J Rheumatol 2005; 32:181–183. [PubMed] [Google Scholar]
- 9.Pipitone N, Olivieri I, Padula A, et al. Infliximab for the treatment of neuro-Behçet's disease: a case series and review of the literature. Arthritis Rheum 2008; 59:285–290.doi:10.1002/art.23345. [DOI] [PubMed] [Google Scholar]
- 10.Arida A, Fragiadaki K, Giavri E, et al. Anti-TNF agents for Behçet's disease: analysis of published data on 369 patients. Semin Arthritis Rheum 2011; 41:61–70.doi:10.1016/j.semarthrit.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 11.International Team for the Revision of the International Criteria for Behçet's Disease (ITR-ICBD), Davatchi F, Assaad-Khalil S, et al. The International Criteria for Behçet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014;28:338–347. doi:10.1111/jdv.12107. [DOI] [PubMed] [Google Scholar]
- 12.Koçer N, Islak C, Siva A, et al. CNS involvement in neuro-Behçet syndrome: an MR study. Am J Neuroradiol 1999; 20:1015–1024. [PMC free article] [PubMed] [Google Scholar]
- 13.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke J Cereb Circ 2007; 38:1091–1096.doi:10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 14.Levy-Clarke G, Jabs DA, Read RW, et al. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology 2014; 121:785–796.e3.doi:10.1016/j.ophtha.2013.09.048. [DOI] [PubMed] [Google Scholar]


