Abstract
Acute cellular rejection after liver transplantation (LT) can be treated with steroid pulse therapy, but there is no ideal treatment for steroid-resistant acute rejection (SRAR). We aimed to determine the feasibility and potential complications of rabbit anti-thymocyte globulin (rATG) application to treat SRAR in liver transplant recipients. We retrospectively reviewed medical records of 429 recipients who underwent LT at Severance Hospital between January 2010 and March 2015. We compared clinical features and graft survival between patients with steroid-sensitive acute rejection (SSAR; n = 23) and SRAR (n = 11). We also analyzed complications and changes in laboratory findings after 2.5 mg/kg rATG treatment in patients with SRAR for 6 to 10 days. There were no significant differences in gender, age, model for end-stage liver disease score, Child–Turcotte–Pugh score, or original liver diseases between patients with SSAR and SRAR, although deceased donors were more frequently associated with the SRAR group (P = 0.004). All SRAR patients responded positively to rATG treatment; after treatment, the patients’ median AST levels decreased from 138 to 63 IU/L, and their median ALT levels dropped from 327 to 70 IU/L 1 day after rATG treatment (P = 0.022 and 0.017, respectively). Median aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin levels significantly decreased 1 month post-treatment (P = 0.038, 0.004, and 0.041, respectively). Median survival after LT was 23 months, and median survival after rATG was 22 months in patients with SRAR. Adverse effects included hepatitis C virus (HCV) reactivation, fungemia, and cytomegalovirus (CMV) infection. Nine SRAR patients survived with healthy liver function, 1 died from a traffic accident during follow-up, and 1 died from graft-versus-host disease and fungemia. Administration of rATG is an effective therapeutic option for SRAR with acceptable complications in liver transplant recipients. However, the occurrence of HCV reactivation and CMV infection in LT patients should be monitored after rATG treatment in these patients.
Keywords: anti-thymocyte globulin, liver transplant, rescue treatment, steroid-resistant acute rejection
1. Introduction
Liver transplantation (LT) is generally regarded as the treatment of choice for end-stage liver disease. In 2013, a total of 5921 adult liver transplants were performed in the United States.[1] Although vascular and biliary complications can be dramatically decreased with surgery,[2,3] acute rejection is common after LT.[4] Steroid pulse therapy is a useful treatment for this complication[4] but is not a preferred treatment for steroid-resistant acute rejection (SRAR).[5–7] Preventing rejection in these cases is important because it can result in graft failure, the need for re-transplantation, or mortality.[8]
Anti-thymocyte globulin (ATG) is a polyclonal antibody commonly used in induction therapy[9] and to treat rejection in solid-organ transplantation.[10] A typical dosing strategy of rabbit ATG (rATG; Thymoglobluin, Sanofi, France) is 1.5 mg/kg over 0 to 3 days for induction therapy,[11] which has lowered incidence and severity of acute rejection more than basiliximab in kidney transplant recipients.[9] Treatment with rATG is also used to minimize delayed graft function and to enable steroid or calcineurin inhibitor sparing immunosuppressant use in kidney recipients.
In contrast to its use in kidney transplant recipients, rATG is not commonly used to treat rejection after LT. Few studies with only small numbers of patients have reported the efficacy of rATG in liver transplant recipients with SRAR.[6,12,13] Thus, we wanted to determine the feasibility and possible complications of administering rATG for treating SRAR in these patients.
2. Methods
2.1. Patients
We retrospectively reviewed medical records of recipients who underwent LT at Severance Hospital, Yonsei University Health System in Seoul, Korea between January 2010 and March 2015. Of 429 cases, who underwent LT during the study period, we excluded 8 patients with other transplanted organs. Two hundred forty-five patients were living donor recipients, 166 were deceased donor recipients, 5 were second transplantations, 1 was a third transplantation, and 38 were pediatric transplantations. Sixty-one patients (14.5%) experienced either clinical or biopsy-proven rejection, including 34 cases of steroid-sensitive acute rejection (SSAR; n = 23) and SRAR; (n = 11). All of the patients with SRAR underwent rATG treatment following steroid pulse therapy. From the records, we compared clinical features and graft survival rates between SSAR and SRAR patients and analyzed changes in laboratory findings and complications after rATG treatment in SRAR patients (Fig. 1). Our study protocol was approved by the independent Institutional Review Board of Yonsei University College of Medicine (IRB No.: 4–2015–1111).
Figure 1.
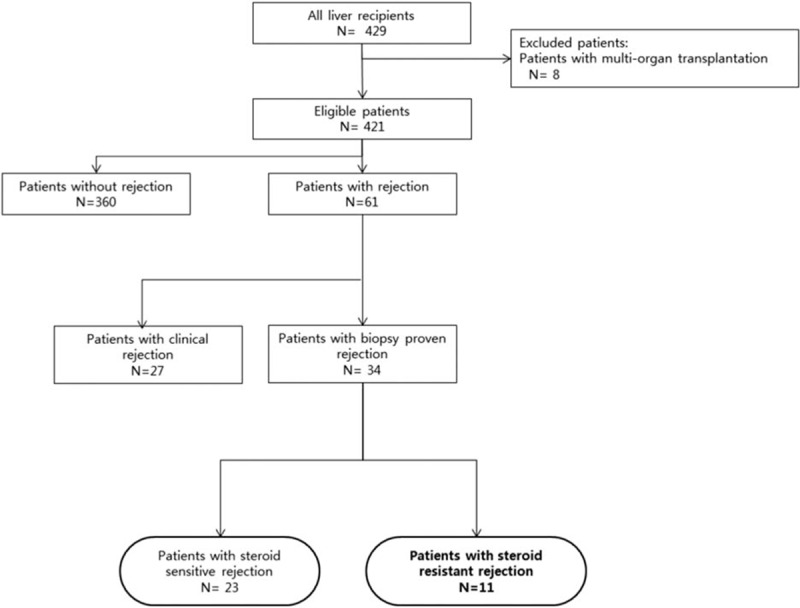
Study design and overview of patient population.
2.2. Rejection and treatment
We defined clinical rejection as graft dysfunction, evidenced by increased transaminase and/or bilirubin (by ≥50%), their persistent initial elevation at least 3 times the upper normal limit without infection, or vascular or biliary complications.[14] We identified vascular and biliary complications with Doppler ultrasound, computed tomography scans, and magnetic resonance cholangiopancreatography and biopsy-proven rejection using BANFF criteria.[15] Early chronic rejection was differentiated from acute cellular rejection by perivenular hepatocyte dropout, potentially reversible central perivenulitis, and mild portal inflammation with bile duct atrophy.[16]
The first rejection episode of each patient was usually treated with methyl prednisolone (MP; 500 mg IV for 3 consecutive days). If patients did not respond to MP pulses and manifested aggravated liver dysfunction, we considered rATG treatment for SRAR. We investigated the possibility of infection by chest x-ray, complete blood count (CBC), and urinary analysis and culture. Infection, drug reaction, and pancytopenia were initial relative contraindications.
Pre-treatment consisted of 1 mg/kg MP and 650 mg acetaminophen twice daily to prevent cytokine release syndrome. We usually decreased the calcineurin inhibitor dosage by one-half and stopped mycophenolate mofetil (MMF) treatment to avoid over-immunosuppression. The patients received 1.5 to 2.5 mg/kg rATG with one-half saline (250 cc) for 12 hours via a central venous line. We then monitored their CBC, routine chemistry, and CD3 count. Fig. 2 schematically shows the protocol of SRAR treatments used in our study.
Figure 2.
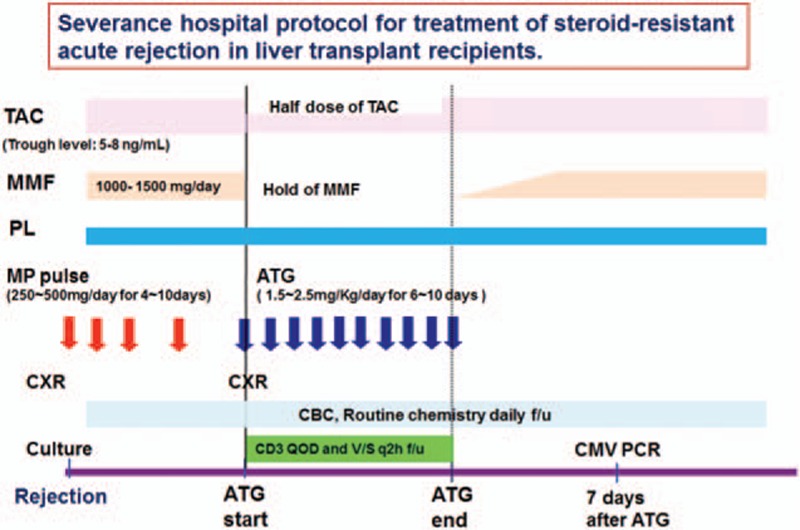
Protocol for treatment of steroid-resistant rejection in liver recipients. ATG, anti-thymocyte globulin; TAC, tacrolimus; MMF, mycophenolate mofetil; PL, prednisolone; MP, methylprednisolone; CXR; chest x-ray; CBC, complete blood count; f/u, follow-up; GVHD, graft-versus-host disease; QOD, every other day; q2 h, every 2 hours; PCR, polymerase chain reaction.
2.3. Immunosuppression
During the study period, all liver transplant recipients were given 20 mg basiliximab as induction therapy on days 0 and 4 post-transplantation. Intravenous MP (1000 mg on day 0, 500 mg on day 1, and 250 mg on day 2 post-transplantation) was also administered. The maintenance immunosuppressive regimens for LT recipients included a triple regimen (tacrolimus, steroids, and MMF) or double regimen (tacrolimus and steroids). In some patients, we used an m-TOR inhibitor instead of MMF and cyclosporine instead of tacrolimus. The trough level of tacrolimus was 6 to 12 ng/mL during the first month and 5 to 10 ng/mL thereafter. No protocol-mandated biopsy was performed, but an ultrasound-guided liver biopsy was performed when graft dysfunction was obvious.
2.4. CMV infection and disease
Infection and subsequent CMV disease were defined according to American Society of Transplantation Recommendations[17] and guidelines reported by Ljungman et al.[18] CMV infection was defined as evidence of CMV replication, such as a positive CMV antigen assay or CMV polymerase chain reaction (PCR), regardless of symptoms. CMV disease was defined based on histopathological evidence of CMV in an end organ (e.g., intranuclear inclusion bodies upon biopsy) and the presence of signs and symptoms related to CMV infection. Prophylactic ganciclovir was not routinely used in our institution, but if the serological status of the donor and recipient were at high risk, such as donor +/recipient –, prophylaxis was mandatory. If not, treatment depended on the clinical situation, such as atypical pneumonia, hepatitis of unknown origin, or elderly or pediatric age. After collection, blood samples of donors and recipients were immediately centrifuged, and sera were frozen for screening pre-transplantation. CMV specific-IgG and -IgM determination was performed by Vidas (bioMerieux Vitek, Inc., Italy) via enzyme-linked fluorescence assay. After LT, recipients were tested for CMV infection using a quantitative real-time PCR kit (Bio-Core Inc., Seoul, Korea).
2.5. Statistical analysis
Statistical analysis was performed using SPSS 20 (SPSS Inc., Chicago, IL). Data were expressed as the median (range) for continuous variables and number (proportion) for categorical variables. Differences in demographic and clinical data between SSAR and SRAR cases were compared by the Mann–Whitney U test for continuous variables and Fisher's exact test for categorical variables. Differences in laboratory data pre- and post-rATG treatment were compared by the Wilcoxon signed-rank test. Graft survival rates were calculated using the Kaplan–Meier method, and the log-rank test was used to evaluate statistical significance. A P-value <0.05 indicated statistical significance.
3. Results
3.1. Patients
We saw no significant difference in gender, age, model for end-stage liver disease (MELD) score, Child–Turcotte–Pugh score (CTP), or original liver diseases between SSAR and SRAR patients. The proportion of deceased donors in SRAR cases was higher than in SSAR cases (P = 0.004). The number of human leukocyte antigen (HLA) mismatches in SRAR patients was also higher, but the difference between the groups was not statistically significant. Demographic and clinical data of SSAR and SRAR patients are summarized in Table 1.
Table 1.
Demographic and clinical data of liver recipients diagnosed with biopsy-proven acute rejection.
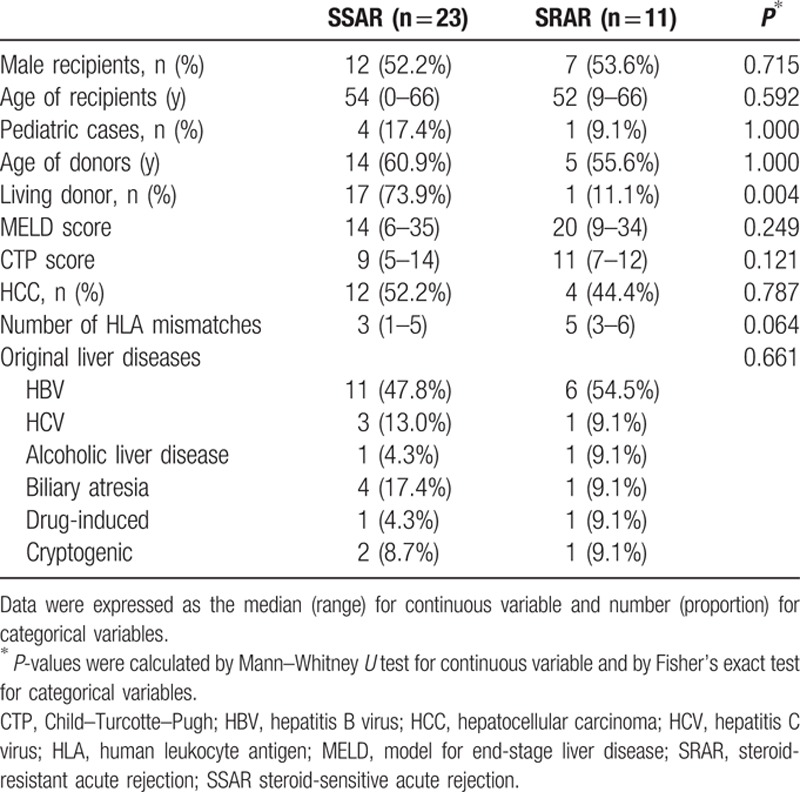
Seven males and four females received rATG for biopsy-proven SRAR after LT at our institution during the study period. Their median age was 52 years (range 9–66 years). Ten of the SRAR patients received livers from deceased donors. Underlying liver diseases included hepatitis B virus (HBV; n = 6), hepatitis C virus (HCV; n = 1), alcoholic cirrhosis (n = 1), drug-induced fulminant hepatitis (n = 1), biliary atresia (n = 1), and cryptogenic (n = 1).
3.2. rATG treatment responses
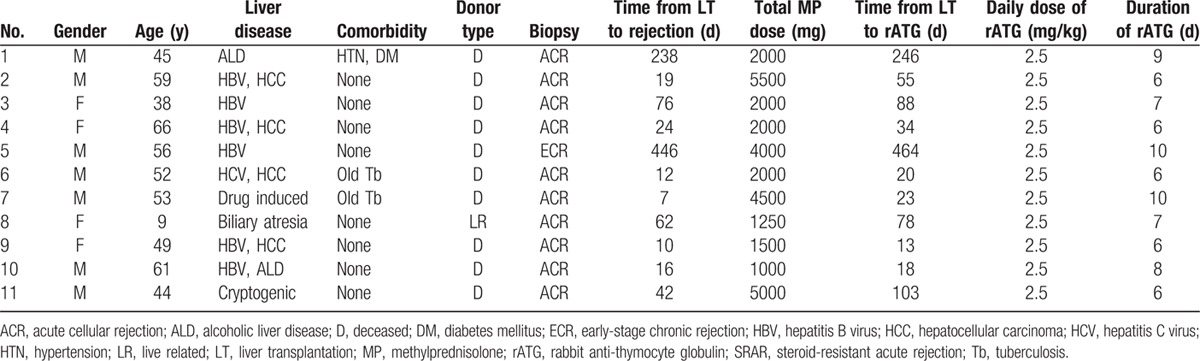
Of the SRAR patients, 10 were diagnosed with acute cellular rejection and 1 patient was diagnosed with early stage chronic rejection. The median time from LT to rejection was 24 days (range 7–446 days), and the median total dose of MP was 2000 mg (range 1000–5500 mg). The median time from LT to rATG treatment was 55 days (range 13–464 days). All 11 SRAR patients received 2.5 mg rATG/kg/day for a median of 7 days (range 6–10 days). Detailed demographic and clinical data of SRAR patients are provided in Table 2.
Table 2.
Detailed demographic data of liver transplant recipients who underwent ATG therapy for SRAR after LT.
All SRAR patients responded positively to rATG treatment, but 1 died because of graft-versus-host disease (GVHD) and fungemia. With respect to pre- versus post-rATG treatment, the patients’ median aspartate aminotransferase (AST) levels decreased from 138 to 63 IU/L (P = 0.013), and their alanine aminotransferase (ALT) levels dropped from 327 to 70 IU/L (P = 0.006) 1 day after rATG treatment. Actual lymphocyte counts and the proportion of CD3 also declined from 390 to 190 (P = 0.022) and 77.3% to 7.4% (P = 0.017), respectively. Although median total bilirubin, direct bilirubin, alkaline phosphatase (ALP), and gamma-glutamyl transpeptidase (GGT) levels seemed to decline 1 day after rATG treatment, the decrease was not statistically significant. Detailed laboratory values from SRAR patients pre- and post-rATG treatment are shown in Table 3. Moreover, total median bilirubin levels declined from 6.5 to 1.8 (P = 0.041), AST levels dropped from 138 to 25 (P = 0.038), and ALT levels decreased from 327 to 41 (P = 0.004) in patients 1 month after rATG treatment and continued to decline 3 to 6 months post-treatment (Fig. 3).
Table 3.
Biochemical values before and 1 day after rATG for SRAR.
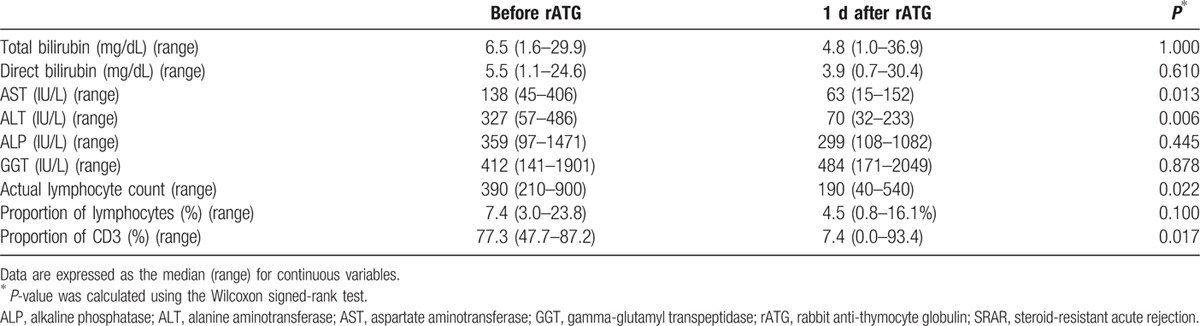
Figure 3.
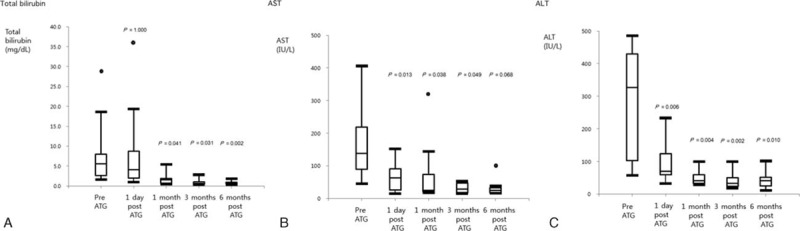
Surrogate laboratory markers pre- and post-ATG treatment for SRAR. (A) Total bilirubin, (B) AST, (C) ALT. †P-values were calculated by the Wilcoxon signed-rank test to compare changes in laboratory values pre- and post-rATG. ATG, anti-thymocyte globulin; SRAR, steroid-resistant acute rejection; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
3.3. Complications and outcomes
One patient developed a fungal infection and 3 patients developed CMV infections. In 1 patient with HCV, the HCV PCR levels increased from 1.26 × 104 to 3.94 × 106 over 20 days. One patient died because of GVHD and fungemia, and 1 patient was lost during follow-up because of a traffic accident. Nine patients are alive with normal liver function. The patient with GVHD and fungemia tested positive for Candida albicans in urine and blood cultures and was treated with amphotericin B. However, the patient died because of multi-organ failure caused by sepsis and GVHD 20 days after diagnosis with the fungal infection.
The median survival time after LT was 23 months (range 3–49 months) and median survival after rATG was 22 months (range 1–46 months) in SRAR patients. Detailed complications and survival outcomes are given in Table 4. Graft survival rates in SRAR patients were lower than those with SSAR, but no statistically significant differences in survival rates were found during this study (95.7 vs. 90.9% at 1 year and 85.8 vs. 68.2% at 3 years; P = 0.594; Fig. 4).
Table 4.
Complications and survival after rATG for SRAR treatment.
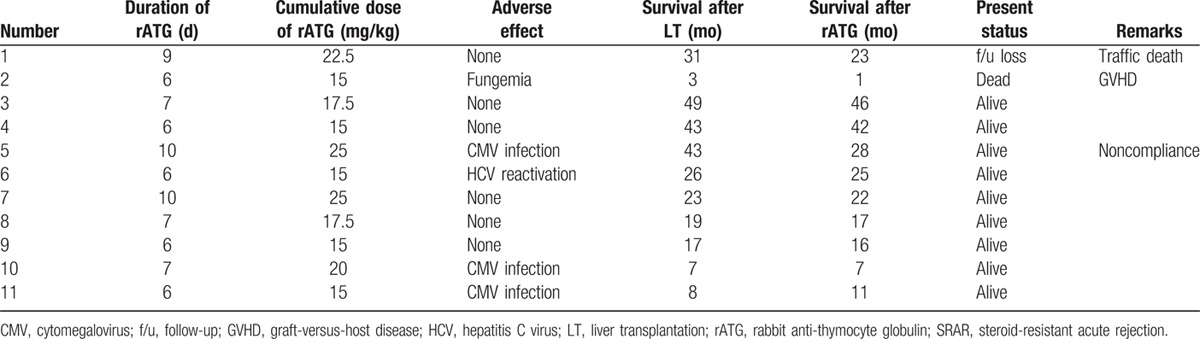
Figure 4.
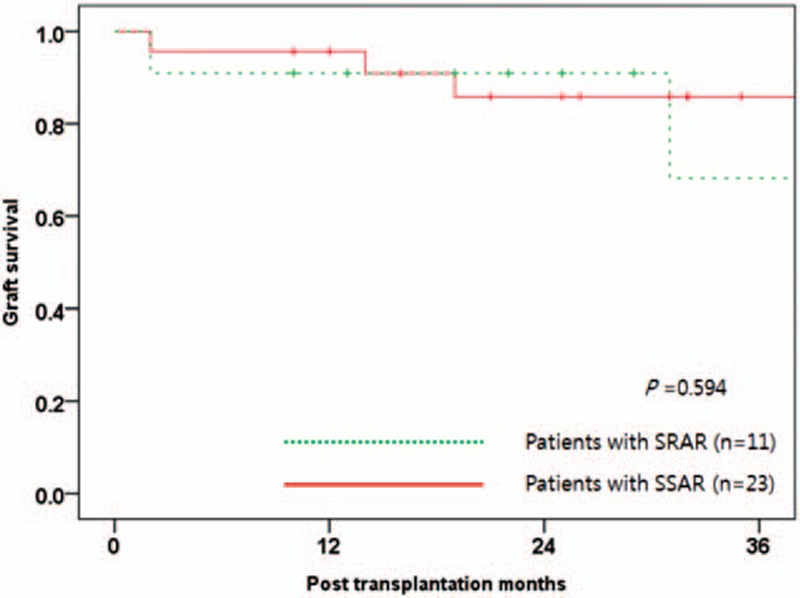
Graft survival according to rejection type. SSAR, steroid-sensitive acute rejection; SRAR, steroid-resistant acute rejection.
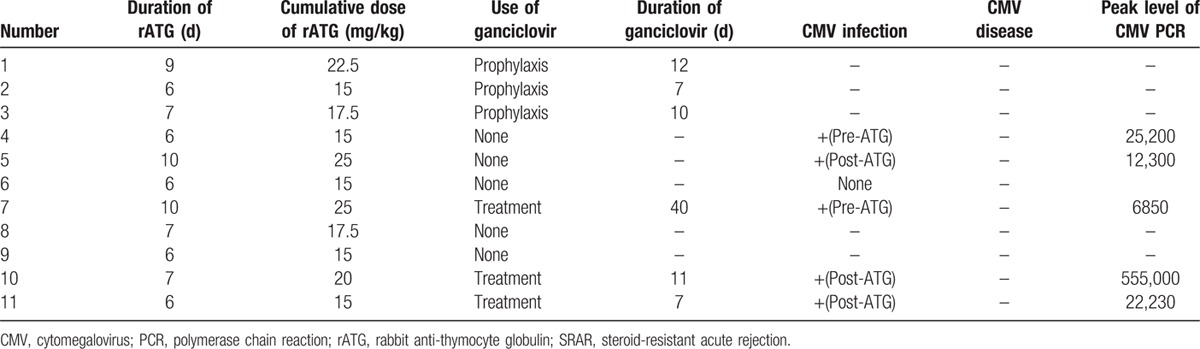
Three patients were given prophylactic intravenous ganciclovir, and 1 was treated for CMV infection with rATG. Of the 7 patients who did not receive ganciclovir, 3 developed CMV viremia. Other CMV diseases were not detected in these patients. These patients also had negative conversion by iv ganciclovir during 7 days or more. Detailed data regarding CMV infection in these patients are given in Table 5.
Table 5.
CMV infection and use of ganciclovir in liver transplant recipients who underwent rATG for SRAR.
4. Discussion
Our results corroborate previous studies[6,12,13] on the feasibility of rATG for treatment of SRAR. For example, AST, ALT, and bilirubin levels significantly decreased after rATG treatment and continued to decline months after the patients’ LT.
More than 90% of rejection episodes could be reversible because of steroid bolus treatments.[19] Although the incidence rate of SRAR is low, patients without timely treatment may suffer from graft loss or death. Results of rescue treatments for SRAR vary in the literature, but few are widely accepted.[5] Pfitzmann et al reported that 38 of 47 patients with SRAR experienced liver function normalized by MMF treatment,[20] and Aw et al observed that 21 of 27 pediatric liver patients with SRAR also had positive responses to MMF treatment.[21] In addition, Aw et al[22] and Shigeta et al[7] reported positive efficacy of basiliximab treatment for SRAR in pediatric transplant recipients.
However, all of the above-mentioned cases relied on traditional strategies for effective immunosuppression. Presently, many institutions use triple immunosuppressant regimens that include tacrolimus, steroids, and MMF and induction immunosuppressants such as basiliximab. Thus, other treatments, such as ATG and OKT3, are needed to treat SRAR in liver transplant recipients with immunosuppression therapy. Previous studies of small patient groups reported the positive use of ATG for SRAR treatment in liver transplant recipients.[6,12,13] In our study, all 11 SRAR patients successfully regained normal liver function after rATG treatment, which was comparable to rates reported in previous studies: Bijleveld et al[6] reported 76.9% (10/13) success, Aydogan et al[13] reported 83.3% (10/12) success, and Schmitt et al[12] reported 100% (13/13) success using rATG treatment for SPAR in liver recipients.
Complications of rATG treatment include cytokine release syndrome, thrombocytopenia, leukopenia, HCV reactivation, and CMV infection. We did not observe cases of cytokine release syndrome in the present study because our protocol which included MP and acetaminophen could prevent this condition. We also saw no cases of severe thrombocytopenia or leukopenia, likely because rATG dosages were adjusted according to daily CBC and CD3 counts. We did note one patient with HCV reactivation and one patient with fungemia. A 4.2% CMV infection rate was reported in patients undergoing kidney transplantation with ATG induction in a United Network for Organ Sharing analysis.[23] However, rATG induction accompanied with CMV prophylaxis resulted in lower CMV infection rates than basiliximab induction alone in kidney transplant recipients,[9] although LT data do not indicate a higher rate of CMV infection using standard regimens.[24] In our study, 3 patients experienced CMV infection, but not CMV disease, and they all showed normal liver function at their last follow-up visit. CMV prophylaxis during rATG administration effectively decreases rates of CMV infection. Although we often began CMV treatment after detection of CMV viremia via PCR, no patients developed CMV disease, which is often fatal in liver recipients. Thus, we checked CMV-PCR on 7 day after rATG treatment and then routinely checked every 3 month (Fig. 2).
Graft survival in SRAR patients was lower than in SSAR patients, but the difference was not statistically significant. We surmise that the presence of hepatocellular carcinoma and MELD scores are major risk factors for decreased graft survival, and our study could not control for these variables because of our small SRAR patient sample size.
Several limitations of our study require consideration. First, the study is limited by its retrospective, single-center study design and small sample size. Second, the feasibility of rATG for SRAR was evaluated only by surrogate laboratory markers. Third, a control group, such as a placebo or alternate treatment group, was not available to compare the efficacy of rATG treatment for SRAR. However, this study does establish the feasibility use of rATG for SRAR, as other studies did not provide accurate information regarding patient outcomes at various times after rATG treatment. This long-term monitoring of outcomes is important because laboratory data after treatments can vary over time. Thus, we compared laboratory values in SRAR patients before rATG treatment and from 1 day to 6 months after rATG treatment and found that levels of relevant biochemical markers, such as AST, ALT, and bilirubin decreased significantly in SRAR patients after treatment.
5. Conclusions
Our findings indicate that rATG is a good therapeutic option for LT patients with SRAR that results in acceptable complications. These complications, including HCV reactivation and CMV infection, should be monitored in these patients after rATG treatment.
Footnotes
Abbreviations: ALD = alcoholic liver disease, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, ATG = anti-thymocyte globulin, CBC = complete blood counts, CMV = cytomegalovirus, CT = computed tomography, CTP = Child–Turcotte–Pugh, GGT = gamma-glutamyl transpeptidase, GVHD = graft-versus-host disease, HBV = hepatitis B virus, HCV = hepatitis C virus, HLA = human leukocyte antigen, LT = liver transplantation, MELD = Model for End-stage Liver Disease, MMF = mycophenolate mofetil, MP = methylprednisolone, MRCP = magnetic resonance cholangiopancreatography, PCR = polymerase chain reaction, rATG = rabbit anti-thymocyte globulin, SRAR = steroid-resistant acute rejection, SSAR = steroid-sensitive acute rejection.
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2010 (6–2010–0072).
The authors have no funding and conflicts of interest to disclose.
References
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant 2015; 15 Suppl 2:1–28. [DOI] [PubMed] [Google Scholar]
- 2.Balderramo D, Navasa M, Cardenas A. Current management of biliary complications after liver transplantation: emphasis on endoscopic therapy. Gastroenterologia Hepatologia 2011; 34:107–115. [DOI] [PubMed] [Google Scholar]
- 3.Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant 2009; 9:746–757. [DOI] [PubMed] [Google Scholar]
- 4.Andreu H, Rimola A, Bruguera M, et al. Acute cellular rejection in liver transplant recipients under cyclosporine immunosuppression: predictive factors of response to antirejection therapy. Transplantation 2002; 73:1936–1943. [DOI] [PubMed] [Google Scholar]
- 5.Wu L, Tam N, Deng R, et al. Steroid-resistant acute rejection after cadaveric liver transplantation: Experience from one single center. Clinics Res Hepatol Gastroenterol 2014; 38:592–597. [DOI] [PubMed] [Google Scholar]
- 6.Bijleveld CG, Klompmaker IJ, van den Berg AP, et al. Incidence, risk factors, and outcome of antithymocyte globulin treatment of steroid-resistant rejection after liver transplantation. Transplant Int 1996; 9:570–575. [DOI] [PubMed] [Google Scholar]
- 7.Shigeta T, Sakamoto S, Uchida H, et al. Basiliximab treatment for steroid-resistant rejection in pediatric patients following liver transplantation for acute liver failure. Pediatr Transplant 2014; 18:860–867. [DOI] [PubMed] [Google Scholar]
- 8.Candinas D, Gunson BK, Nightingale P, et al. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet 1995; 346:1117–1121. [DOI] [PubMed] [Google Scholar]
- 9.Brennan DC, Daller JA, Lake KD, et al. Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 2006; 355:1967–1977. [DOI] [PubMed] [Google Scholar]
- 10.Casadei DH, del CRM, Opelz G, et al. A randomized and prospective study comparing treatment with high-dose intravenous immunoglobulin with monoclonal antibodies for rescue of kidney grafts with steroid-resistant rejection. Transplantation 2001; 71:53–58. [DOI] [PubMed] [Google Scholar]
- 11.Mohty M, Bacigalupo A, Saliba F, et al. New directions for rabbit antithymocyte globulin (Thymoglobulin®) in solid organ transplants, stem cell transplants and autoimmunity. Drugs 2014; 74:1605–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt TM, Phillips M, Sawyer RG, et al. Anti-thymocyte globulin for the treatment of acute cellular rejection following liver transplantation. Dig Dis Sci 2010; 55:3224–3234. [DOI] [PubMed] [Google Scholar]
- 13.Aydogan C, Sevmis S, Aktas S, et al. Steroid-resistant acute rejections after liver transplant. Exp Clin Transplant 2010; 8:172–177. [PubMed] [Google Scholar]
- 14.Tippner C, Nashan B, Hoshino K, et al. Clinical and subclinical acute rejection early after liver transplantation: contributing factors and relevance for the long-term course. Transplantation 2001; 72:1122–1128. [DOI] [PubMed] [Google Scholar]
- 15.Demetris AJ, Ruppert K, Dvorchik I, et al. Real-time monitoring of acute liver-allograft rejection using the Banff schema. Transplantation 2002; 74:1290–1296. [DOI] [PubMed] [Google Scholar]
- 16.Yu E. Histopathological features of late liver allograft dysfunction. J Korean Soc Transplant 2013; 27:153–159. [Google Scholar]
- 17.Humar A, Michaels M. AIWGoID Monitoring. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant 2006; 6:262–274. [DOI] [PubMed] [Google Scholar]
- 18.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34:1094–1097. [DOI] [PubMed] [Google Scholar]
- 19.Neil DA, Hubscher SG. Current views on rejection pathology in liver transplantation. Transplant Int 2010; 23:971–983. [DOI] [PubMed] [Google Scholar]
- 20.Pfitzmann R, Klupp J, Langrehr JM, et al. Mycophenolatemofetil for immunosuppression after liver transplantation: a follow-up study of 191 patients. Transplantation 2003; 76:130–136. [DOI] [PubMed] [Google Scholar]
- 21.Aw MM, Verma A, Rela M, et al. Long-term outcome of mycophenolate mofetil rescue therapy for resistant acute allograft rejection in pediatric liver transplant recipients. Liver Transplant 2008; 14:1303–1308. [DOI] [PubMed] [Google Scholar]
- 22.Aw MM, Taylor RM, Verma A, et al. Basiliximab (Simulect) for the treatment of steroid-resistant rejection in pediatric liver transpland recipients: a preliminary experience. Transplantation 2003; 75:796–799. [DOI] [PubMed] [Google Scholar]
- 23.Gaber AO, Matas AJ, Henry ML, et al. Antithymocyte globulin induction in living donor renal transplant recipients: final report of the TAILOR registry. Transplantation 2012; 94:331–337. [DOI] [PubMed] [Google Scholar]
- 24.Rostaing L, Saliba F, Calmus Y, et al. Review article: use of induction therapy in liver transplantation. Transplant Rev 2012; 26:246–260. [DOI] [PubMed] [Google Scholar]


