Abstract
Irisin, a novel exercise-induced myokine, has been suggested to regulate energy homeostasis and insulin sensitivity. However, it remains unclear whether circulating irisin plays a role in the development of DM in human. We investigated the possible association between circulating irisin levels and incident DM in a 2.6-year longitudinal study of a population-based cohort comprised of rural Korean subjects.
We conducted a longitudinal study within the Korean Genome and Epidemiology Study on Atherosclerosis Risk of Rural Areas in the Korean General Population (KoGES-ARIRANG) study from November 2005 to January 2008. Cases (n=85) were patients with incident DM during the follow-up period and controls (n = 85) were matched to incident DM cases based on sex and age at baseline. The relative risk of serum irisin/adiponectin level for incident DM was analyzed using conditional logistic regression analysis.
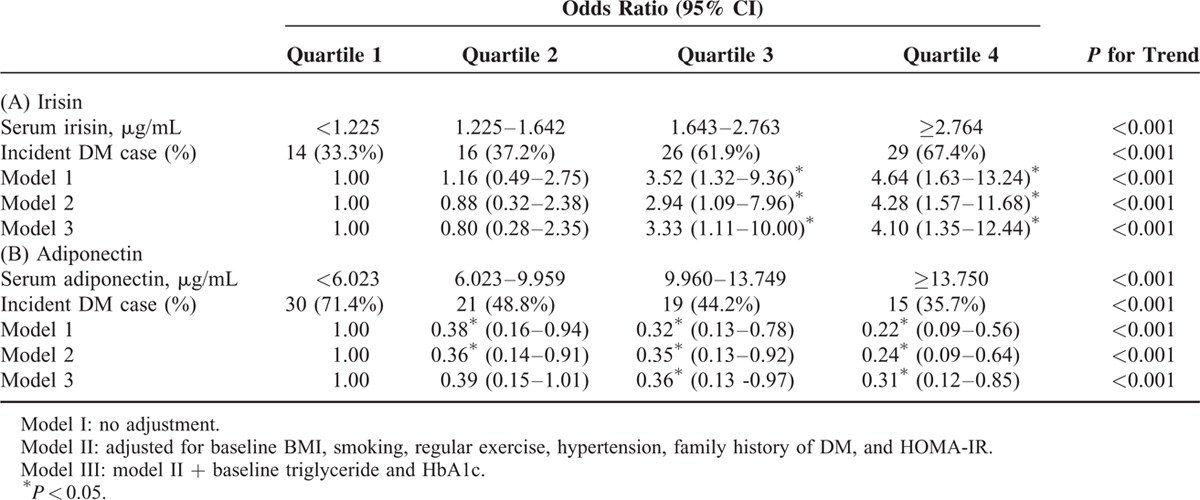
Baseline irisin_ENREF_1 levels were significantly higher in subjects who developed DM than in subjects who did not. The serum irisin level was positively associated with glycated hemoglobin (HbA1c) and postprandial glucose. Irisin was negatively associated with adiponectin (R = –0.189, P = 0.014). After adjustment for potential confounders, including body mass index, the odds ratios [95% confidence intervals] for incident DM increased in a graded manner as the serum irisin level increased (Quartile 1 vs Quartile 2 vs Quartile 3 vs Quartile 4 = 1 vs 0.80 [0.28–2.35] vs 3.33 [1.11–10.00] vs 4.10 [1.35–12.44], respectively), whereas the odds ratios for incident DM decreased in a graded manner as the serum adiponectin level increased.
High serum irisin was independently associated with the development of DM, indicating that irisin may be a useful predictor of DM in Korean adults.
INTRODUCTION
There is increasing evidence that skeletal muscle acts as a secretory organ. During or immediately after physical activity, skeletal muscles release a variety of cytokines, termed myokines, which mediate the beneficial effects of exercise on metabolism.1 Bostrom et al reported that irisin is a muscle-derived factor secreted from muscle after shedding of the extracellular portion of the type I membrane protein fibronectin type III domain-containing protein 5 precursor gene.2 After its release, irisin signals adipose tissue to become more similar to brown-like adipocytes using a pathway involving peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1 α). The proposed beneficial effects include the browning of white adipose tissue and increased thermogenesis, which promotes insulin sensitivity, body weight, and glucose tolerance in mice.2,3 Irisin has been proposed to improve glucose homeostasis by increasing fatty acid oxidation and utilizing glucose via the AMPK signaling pathway in diabetic mice.4
Although several in vitro and in vivo studies have demonstrated the benefits of irisin on glucose homeostasis and body weight, there has been controversy regarding the role of irisin on glucose/energy homeostasis in humans. Park et al reported that irisin is associated with a higher risk of metabolic syndrome and cardiovascular disease in humans, indicating either increased secretion by muscle tissue and/or a compensatory increase in irisin to overcome underlying irisin resistance in metabolically unhealthy subjects.5 Furthermore, 1 study suggested that irisin is inversely associated with the insulin sensitivity index in human, suggesting increased release by adipose/muscle tissue in response to deterioration of insulin sensitivity, whereas other recent studies also have demonstrated that serum irisin levels were lower in patients with type 2 diabetes mellitus (DM) compared with nondiabetic patients.6,7 However, almost all these studies were cross-sectional and could not address the cause-effect relationship between serum irisin and DM. In addition, there is evidence that circulating irisin levels are involved in the development of DM in adults.
In the present study, we investigated the prospective association between serum irisin levels and incident DM in a 2.6-year follow-up study involving a population-based cohort comprising relatively healthy Korean subjects. We also assessed in similar models the associations between the same parameters and adiponectin, an established biomarker of lower risk of DM.
METHODS
Study Participants
We analyzed the data obtained from the Korean Genome and Epidemiology Study on Atherosclerosis Risk of Rural Areas in the Korean General Population (KoGES-ARIRANG), a community-based prospective cohort study, which is supported by the Korea National Institute of Health. The aim of KoGES-ARIRANG was to investigate the genetic and environmental etiology of metabolic disorders (i.e., DM, hypertension, obesity, metabolic syndrome, and cardiovascular disease) and to develop comprehensive and applicable health care guidelines for common metabolic disorders in Korean rural adults.8,9 This cohort consists of relatively healthy adults aged 40 to 70 years at baseline in rural areas of Wonju and Pyengchang in South Korea. The baseline examination was carried out from November 2005 to January 2008, including 5178 participants (2127 men and 3051 women). All study participants were invited to the first follow-up survey (April 2008 to January 2011) and 3862 (74.6%) attended. In the present study, we excluded patients with a history of previous antidiabetic medication usage and those with newly detected DM at baseline (N = 362). Finally, 3500 participants were enrolled in this study. In our study, incident DM was defined as a fasting glucose concentration ≥126 mg/dL or HbA1c ≥ 6.5% or taking antidiabetic medication during the follow-up period based on the World Health Organization criteria.10 During the mean 2.6-year follow-up period, 85 incident DM cases were confirmed. A total of 85 subjects were selected from the same cohort of patients who did not develop DM. These nonincident DM subjects were matched to each incident DM individuals by age and sex. All study subjects were informed that they had been randomly chosen to participate in this cohort study with the right to refuse involvement in further analyses, and they signed informed consents. The present study was approved by the institutional review board of the Yonsei University, Wonju College of Medicine (CR105024–026), and this study was carried out in accordance with the ethical standards of the Helsinki Declaration.
Data Collection
All study participants completed a medical history including medication use and lifestyle factors and underwent a comprehensive health examination according to a site visit schedule. Body weight and height were measured using standardized techniques and equipment. Skeletal muscle mass and fat mass were measured using multifrequency BIA (In-Body 720; Biospace, Seoul, Korea). Blood pressure was measured on 2 occasions, 5 min apart using a standard mercury sphygmomanometer (Baumanometer, Copiague, NY). Baseline information on smoking status and current alcohol intake was collected with a self-reported questionnaire (yes/no). Subjects who answered yes to the question: “Do you regularly perform physical exercise that makes you sweat?” were assigned to the regular exercise group. After fasting for ≥12 hours or overnight, venous blood samples were drawn from study participants. Irisin was measured by colorimetric ELISA (EK-067–52; Phoenix Pharmaceuticals) with intra-assay and inter-assay coefficients of variation < 10% and <15%, respectively. The serum concentrations of adiponectin were measured by radioimmunoassay (RIA) (LINCO Research, Inc.,) with intra-assay and inter-assay coefficients of variation ranging from 2.9 to 6.6% for adiponectin. Fasting glucose was measured by a glucose oxidase-based assay and fasting insulin was measured by a double-antibody radioimmunoassay (Biosource Europe SA, Nivelles, Belgium). The plasma concentrations of lipid profile, and liver eynzyme were measured using enzymatic methods (Advia 1650, Siemens, Tarrytown, NY). High sensitivity C-reactive protein (hsCRP) was determined by the Denka Seiken (Tokyo, Japan) assay. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting plasma glucose (milligrams per deciliter) × fasting insulin (milli-international units per milliliter))/22.5.11 The skeletal muscle index was calculated using the bioelectrical impedance analysis equation of Janssen et al.12
Statistical Analyses
All statistical analyses were performed with PASW statistics software (version 20.0; SPSS Inc., Chicago, IL). Continuous variables between 2 groups were compared using the independent t-test and Mann–Whitney U test. Categorical variables are expressed as frequencies with percentages, and compared using the chi-square test or Fisher exact test, as appropriate. Associations between irisin and baseline metabolic parameters were evaluated using Pearson correlation analysis. As the irisin values were not normally distributed, it was logarithmically transformed in correlation analysis. A conditional logistic regression was used to estimate the odds ratio (OR) for incident DM with subjects in the lowest quartiles of irisin and adiponectin as the reference category. Multivariable models were developed by individually adding covariates to the model. Because cases and controls were matched, their median values, proportions, and all risk estimates should be interpreted as adjusted for the matching factors (age and sex). Results were expressed as ORs with 95% confidence intervals (CI). P-values <0.05 were considered statistically significant.
RESULTS
Participants Characteristics
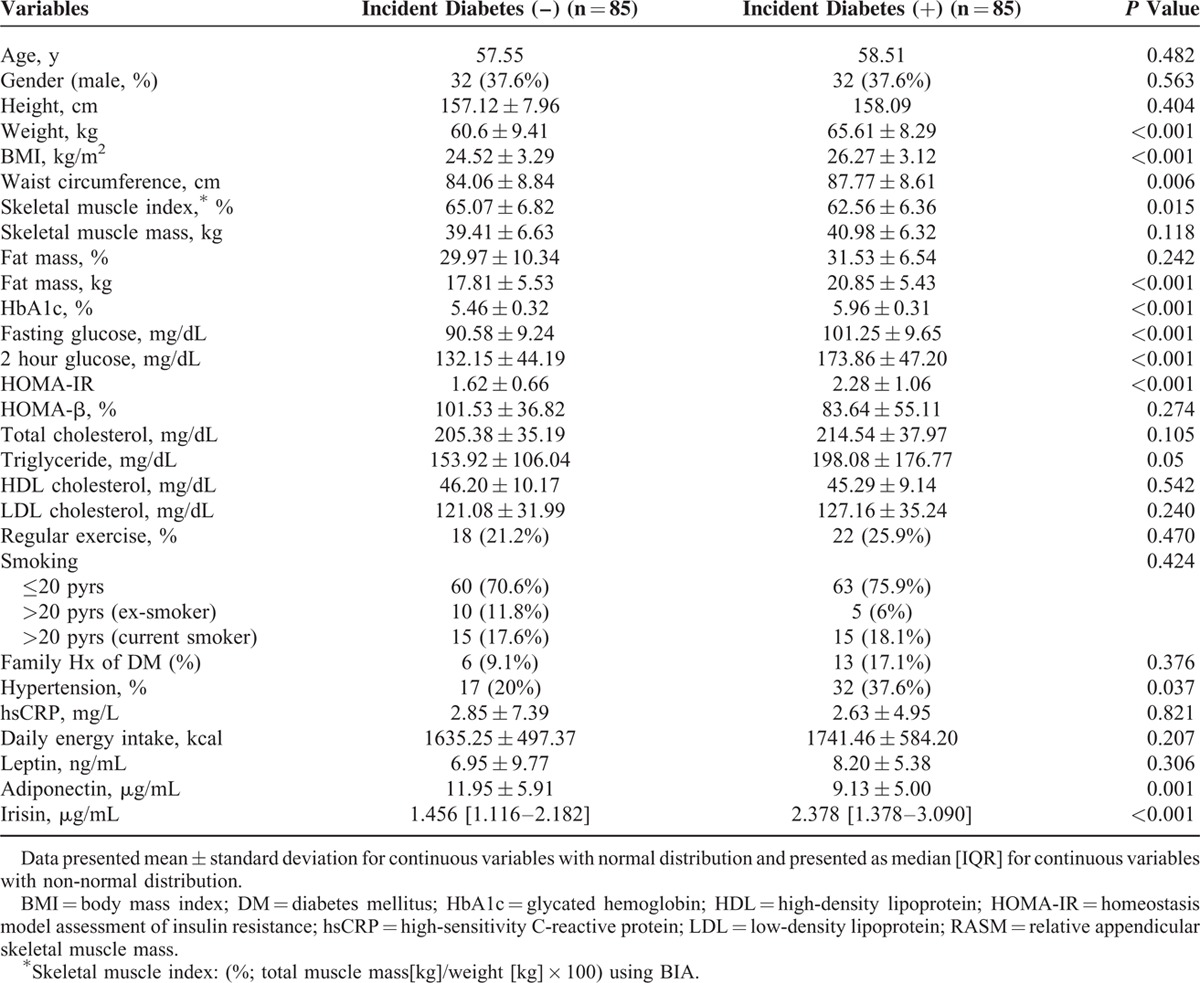
The baseline characteristics of participants based on of the incident DM over a 2.6-year period are shown in Table 1. The incidence of DM during this period was 2.43 % (N = 85). Because the 2 groups were initially matched by age and sex, there were no differences in age and sex between the 2 groups. Subjects who developed DM had higher baseline BMI, HbA1c, fasting glucose, HOMA-IR, and TG than those who did not. The skeletal muscle index was lower in incident DM group compared with those in the nonincident DM group. Serum irisin levels were higher in the incident DM group, whereas adiponectin levels were lower in the incident DM group.
TABLE 1.
Baseline Characteristics of the Subjects
Association Between Irisin and Metabolic Parameters
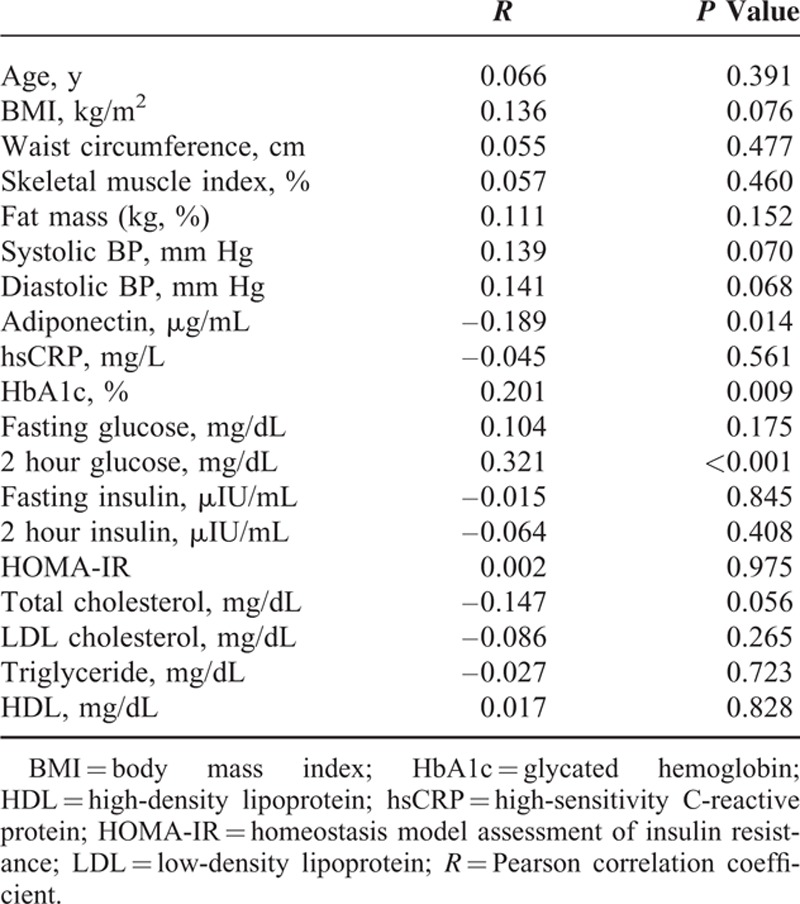
We performed correlation analysis results between irisin and metabolic parameters at baseline (Table 2). The irisin levels were positively associated with HbA1c, and postprandial (2 hour) glucose. There was a significantly negative association between irisin and adiponectin (R = –0.189, P = 0.014). The significant negative association between irisin and adiponectin still remained even after adjustment for age and gender (partial correlation coefficient: −0.241, P = 0.002). There was no significant association between irisin and body composition indices such as BMI, waist circumference skeletal muscle index, and total fat mass.
TABLE 2.
Association Between Baseline Anthropometric, Metabolic Parameters, and Irisin
The Risk for Incident DM According to the Baseline Irisin Level
The risk of incident DM according to irisin and adiponectin quartiles is shown in Table 3. Higher concentrations of serum irisin at baseline were positively and significantly associated in a level-dependent manner with the development of DM in both crude and adjusted conditional logistic regression models (Table 3A). After adjustment for baseline BMI, smoking status, regular exercise, hypertension, family history of DM and HOMA-IR, the ORs for incident DM increased gradually as the irisin level increased. This trend remained statistically significant even after further adjustment of baseline TG and HbA1c. On the other hand, serum levels of adiponectin were negatively associated with the odds of incident DM (Table 3B).
TABLE 3.
Conditional Logistic Regression Analysis of Baseline Irisin and Adiponectin Level for Developing New-Onset Type 2 Diabetes
DISCUSSION
In this population-based prospective study, we demonstrated that higher circulating irisin levels are associated with the development of DM during a 2.6-year period. Furthermore, the circulating irisin levels were an independent predictor for incident DM, regardless of BMI and systemic insulin resistance. Our study also replicated well-known negative associations between adiponectin and incident DM. To the best of our knowledge, it is the first prospective study to show a relationship between irisin and incident DM. Our results suggest that irisin itself may increase the risk of diabetes and that circulating irisin levels might be applied as a simple clinical predictor for incident DM.
Irisin is a hormone that has been proposed to play a significant role in energy homeostasis and obesity.13–15 Irisin exerts beneficial effects on overall metabolism by inducing the browning of white adipocytes. Moreover, some studies have revealed an additional role of irisin in glucose homeostasis, increasing the expression of glucose transporter 4 and mitochondrial biogenesis.16,17 However, despite the expectation that irisin has potential benefits in regulating body weight, insulin sensitivity, and glucose homeostasis, studies in humans have led to conflicting results. In addition, the role of irisin in DM development remains to be clarified. Several cross-sectional design studies found a significantly lower level of circulating irisin in type 2 diabetes patients compared to nondiabetic controls. However, because above previous studies were performed cross-sectional design, they could not establish a cause-effect relationship between irisin and DM. Moreover, they did not take into account other potential confounders such as baseline insulin resistance status (reflected by HOMA-IR) and physical activity status, so they could not clarify the independent impact of irisin on incident DM. Thus, we aimed to assess whether irisin contributes to the development of DM independently of individual lifestyle status and insulin resistance in Korean adults.
In this study, we provide the first evidence that baseline irisin levels are significantly higher in subjects who progressed to DM at 2.6 years compared with subjects who did not. This observed link between higher circulating irisin levels and incident DM can be explained by several mechanisms. Increased baseline secretion of irisin in participants who developed DM may be the result of a compensatory increase in irisin levels in response to insulin resistance and obesity, similarly to leptin resistance in obesity and metabolic disorders. A recent study reported that under certain conditions of elevated oxidative stress, inflammation, and high levels of free fatty acids, such as in obesity, metabolic syndrome or DM, the expression of PGC1-α in muscle could be stimulated increasing fibronectin type III domain-containing protein 5 and subsequently irisin levels, which stimulate the production of brown adipocytes, increasing UCP1 expression and thermogenesis and decreasing insulin resistance.18 From this background, our results suggest that, similar to the elevated insulin and leptin levels in insulin and leptin resistance associated with obesity and metabolic abnormalities, circulating irisin levels were also elevated, and irisin may play a compensatory role with respect to regulating energy expenditure and glucose metabolism in obese and/or glucose intolerance status. Similarly, although our results were adjusted for baseline BMI, HOMA-IR and fasting glucose level in the final analysis, the BMI, HOMA-IR, and fasting glucose levels were higher in participants who developed DM in our study. Furthermore, we also demonstrated that baseline circulating irisin levels were positively correlated with blood pressure and BMI as well as with glucose parameters. These findings support the hypothesis of “irisin resistance” regarding a compensatory increase in the secretion of irisin in the case of obesity and insulin resistance. However, in our study, we found that the significance of the association between higher baseline irisin levels and incident DM remained unchanged even after adjustment of the obesity index assessed by BMI and insulin resistance status assessed by HOMA-IR. Thus, other potential mechanisms supporting this independent relationship should be elucidated by further mechanistic studies.
Interestingly, our study clearly demonstrated that the risk of incident DM increased in a graded manner as the serum irisin level increased. Our results also demonstrated that baseline circulating irisin levels are significantly positively correlated with metabolic parameters. Our results are in line with those of previous studies that have reported a positive association between irisin and BMI, fasting glucose, and blood pressure.19,20 These findings further support the notion that elevation in circulating irisin levels occurs with impaired glucose tolerance status or insulin resistance status, well before the diagnosis of DM. From these findings, we suggest that the circulating irisin level can be a good predictor of the development of DM in individuals. Because measuring irisin levels is simple, irisin can be used as a marker for predicting the presence of insulin resistance and incident DM. Irisin can also be applied in clinical practices to predict and prevent metabolic disorders in addition to DM.
The major strength of our study is that it is the first prospective study that extensively investigated the potential role of irisin in the pathogenesis of DM. The other strength of this study is that it provided validity in terms of the methodology used here by replicating expected associations between adiponectin and incident DM. We replicated the finding that higher adiponectin levels are correlated with a decreased risk of incident DM, consistent with previous studies.21,22 Our study also has internal validity, as both the cases and controls were derived from the same cohort, eliminating selection bias. However, this study had several limitations. First, this study included a relatively small sample size (n = 170). In addition, because the follow-up period of our cohort was too short, the number of incident DM cases was too small (n = 85). Therefore, we could not analyze data in a large sample. Second, we did not measure other cytokines, such as IL-6 and TNF-α, which may be related to serum irisin. Third, because we did not measure appendicular skeletal muscle using dual energy absorptiometry, we could not clearly provide information for the association between circulating irisin levels and body composition. Fourth, in defining incident DM, we did not use results of 75 g oral glucose tolerance test. Finally, because our study participants were rural Korean adults, our results may not be applied to other ethnic groups. Future studies with larger sample sizes and examining irisin in various conditions and populations will be needed.
Our study is the first longitudinal design study to investigate the association between higher circulating irisin levels and development of DM in a relatively healthy rural population. The results of this study demonstrate that irisin itself is associated with incident DM, independently of insulin resistance, physical activity, and BMI. These results suggest that irisin may play a possible role in the regulation of glucose metabolism. In addition, we also presented evidence that circulating the irisin level may be a predictive marker for high risk of DM. Further studies with larger sample sizes in various conditions and populations are warranted to elucidate the mechanism for the role of irisin in incident DM.
Footnotes
Abbreviations: BMI = body mass index, DM = diabetes mellitus, HDL = low density lipoprotein, HOMA-IR = Homeostasis model assessment of insulin resistance, LDL = low density lipoprotein, PGC1 α = peroxisome proliferator-activated receptor-γ coactivator 1α, TG = Triglyceride.
Funding: this study was supported in part by the Korea Centers for Disease Control and Prevention (2005-E71013-00, 2006-E71002-00, 2007-E71013-00, 2008-E71004-00, 2009-E71006-00, 2010-E71003-00).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8:457–465. [DOI] [PubMed] [Google Scholar]
- 2.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Enerback S, Smith U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res 2003; 11:1182–1191. [DOI] [PubMed] [Google Scholar]
- 4.Xin C, Liu J, Zhang J, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes (Lond) 2016; 40:443–451. [DOI] [PubMed] [Google Scholar]
- 5.Park KH, Zaichenko L, Brinkoetter M, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab 2013; 98:4899–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YK, Kim MK, Bae KH, et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract 2013; 100:96–101. [DOI] [PubMed] [Google Scholar]
- 7.Liu JJ, Wong MD, Toy WC, et al. Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications 2013; 27:365–369. [DOI] [PubMed] [Google Scholar]
- 8.Jung DH, Kim JY, Kim JK, et al. Relative contribution of obesity and serum adiponectin to the development of hypertension. Diabetes Res Clin Pract 2014; 103:51–56. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Ahn SV, Yoon JH, et al. Prospective study of serum adiponectin and incident metabolic syndrome: the ARIRANG study. Diabetes Care 2013; 36:1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15:539–553. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 12.Janssen I, Heymsfield SB, Baumgartner RN, et al. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 2000; 89:465–471. [DOI] [PubMed] [Google Scholar]
- 13.Polyzos SA, Kountouras J, Shields K, et al. Irisin: a renaissance in metabolism? Metabolism 2013; 62:1037–1044. [DOI] [PubMed] [Google Scholar]
- 14.Villarroya F. Irisin, turning up the heat. Cell Metab 2012; 15:277–278. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Bostrom P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hojlund K, Bostrom P. Irisin in obesity and type 2 diabetes. J Diabetes Complications 2013; 27:303–304. [DOI] [PubMed] [Google Scholar]
- 17.Huh JY, Dincer F, Mesfum E, et al. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) 2014; 38:1538–1544. [DOI] [PubMed] [Google Scholar]
- 18.Sanchis-Gomar F, Perez-Quilis C. The p38-PGC-1alpha-irisin-betatrophin axis: exploring new pathways in insulin resistance. Adipocyte 2014; 3:67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012; 61:1725–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stengel A, Hofmann T, Goebel-Stengel M, et al. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity—correlation with body mass index. Peptides 2013; 39:125–130. [DOI] [PubMed] [Google Scholar]
- 21.Shan T, Liang X, Bi P, et al. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J 2013; 27:1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Hirose H, Saito I, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci 2002; 103:137–142. [DOI] [PubMed] [Google Scholar]


