Abstract
The objectives of the study were as follows: to examine the national trend of pediatric atypical antipsychotic (AAP) use in the United States; to identify primary mental disorders associated with AAPs; to estimate the strength of independent associations between patient/provider characteristics and AAP use. Data are from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey. First, average AAP prescription rates among 4 and 18-year-old patients between 1993 and 2010 were estimated. Second, data from 2007 to 2010 were combined and analyzed to identify primary mental disorders related to AAP prescription. Third, a multivariate logistic regression model was developed having the presence of AAP prescription as the dependent variable and patient/provider characteristics as explanatory variables. Adjusted odds ratios (AORs) with associated 95% confidence intervals (CIs) were estimated. Outpatient visits including an AAP prescription among 4 to 18-year-old patients significantly increased between 1993 and 2010 in the United States, and over 65% of those visits did not have diagnoses for US Food and Drug Administration-approved AAP indications. During 2007 to 2010, the most common mental disorder was attention-deficit hyperactivity disorder, accounting for 24% of total pediatric AAP visits. Among visits with attention-deficit hyperactivity disorder diagnosis, those with Medicaid as payer (AOR 1.66, 95% CI 1.01–2.75), comorbid mental disorders (e.g., psychoses AOR 3.34, 95% CI 1.35–8.26), and multiple prescriptions (4 or more prescriptions AOR 4.48, 95% CI 2.08–9.64) were more likely to have an AAP prescription. The off-label use of AAPs in children and adolescents is prevalent in the United States. Our study raises questions about the potential misuse of AAPs in the population.
Keywords: adolescents, attention-deficit hyperactivity disorder, atypical antipsychotics, children, off-label
1. Introduction
Antipsychotic medications have long been used for the treatment of mental disorders including psychosis, schizophrenia, and bipolar disorder. These medications can be broadly categorized into 2 classes: conventional antipsychotics, also known as first-generation antipsychotics or typical antipsychotics, which were discovered in the 1950s[1]; and atypical antipsychotics (AAPs) (also known as second-generation antipsychotics), which were introduced during the 1990. Compared with the conventional antipsychotics, AAPs were marketed as reducing adverse side effects such as extrapyramidal symptoms. As a result, AAPs were extensively used not only for the US Food and Drug Administration (FDA)-approved indications but also for other conditions not approved. Off-label use is controversial given the uncertainty regarding the effectiveness and safety of AAPs.[2–5] Several postmarketing clinical trials reported serious adverse side effects, including metabolic syndrome, cardiovascular events, or death in AAP users.[6–8] Nevertheless, AAPs are one of the top-selling classes of pharmaceuticals in the United States. In fact, antipsychotic medications generated about $18.2 billion total revenue in 2011, with 3 individual AAP agents accounting for 65% of the total revenue.[9] In children and adolescents in the United States, AAPs are probably among the most increasingly used classes of prescription drugs.[10,11] A study by Patel et al[11] reported that AAP use in children and adolescents increased 1.5 to 3-fold between 1996 and 2001 in Medicaid programs. Olfson et al[12] reported a 5-fold increase during 1993 to 2002 in antipsychotic prescription for young patients. However, to the best of our knowledge, there are no studies to analyze the recent trends in pediatric off-label AAP use and to evaluate the role of patient and provider characteristics associated with it. Therefore, this study was aimed to examine the historical trend of AAP use among 4 to 18-year-old patients in the United States; to assess the characteristics of AAP use by identifying primary mental disorders; and to estimate the strength of independent association of patient/provider characteristics with AAP prescription among pediatric (4–18-year-old patients) attention-deficit hyperactivity disorder (ADHD) visits.
2. Materials and Methods
2.1. Data source
Data sources for this study were the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital Ambulatory Medical Care Survey (NHAMCS). The NAMCS and NHAMCS are national surveys that collect cross-sectional data on outpatient visits to nonfederal employed, office-based physicians who are primarily engaged in direct patient care and outpatient departments of noninstitutional general and short-stay hospitals. We intended to estimate the national trend of nonemergent visits relevant to an AAP prescription. For this reason, we excluded data collected from hospital emergency departments and ambulatory surgery centers.
The NAMCS/NHAMCS data include patient sociodemographic characteristics, physician information, up to 3 diagnoses relevant to the visit, and drugs prescribed. Diagnoses are recorded using the “International Classification of Diseases, Ninth Revision, Clinical Modification” (ICD-9-CM). There were up to 6 prescribed drugs recorded per visit during 1995 to 2002, and up to 8 after 2003. To avoid overestimating the prescribing rate and based on recommendations from the National Center for Health Statistics (NCHS),[13] we only included the first 6 drugs listed per visit during our study period—1995 to 2010.
Data from 1993 to 2010 were used to compute the average annual rate of pediatric AAP prescriptions. Data from 2007 to 2010 were used to identify primary mental disorders related to AAP use and to estimate independent association of patient/provider characteristics with AAP prescription among ADHD visits. Sample weights to account for sampling techniques were applied in all analyses using Stata statistical software (version 12). For this study, ethical approval was not necessary because it used public use data sets.
2.2. Definition of an AAP visit
An outpatient visit was regarded as an AAP visit if one or more following medications were prescribed: risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, paliperidone, asenapine, and iloperiodone. For the period 1993 to 2005, AAPs were identified using generic drug names as provided by the NCHS; for after 2006, AAP use was identified based on Multum Lexincon NAMCS/NHAMCS. Furthermore, AAP visits were categorized into 2 groups based on the presence of one or more diagnosis codes that corresponded to an US FDA-approved condition for the prescription. During 1993 to 2010, AAPs were approved by US FDA for the following 4 conditions: (1) schizophrenia (ICD-9-CM, 295); (2) bipolar disorder (ICD-9-CM, 296.0; 296.1; 296.4–296.8); (3) depression (ICD-9-CM, 296.2; 296.3; 300.4; 311.X); and (4) autism (ICD-9-CM; 299.0). Figure 1 depicts US FDA-approved indications for each AAP agent throughout the study period. The US FDA approved olanzapine for the manifestations of psychoses (ICD-9-CM; 290.XX-299.XX) between 1996 and 2000. In 2000, the US FDA changed the indication for olanzapine to schizophrenia and bipolar disorder.
Figure 1.
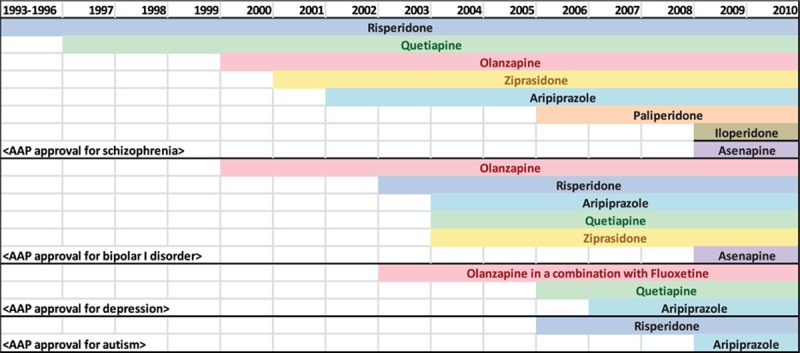
US FDA-approved indications for atypical antipsychotics. US FDA, US Food and Drug Administration.
2.3. National trend of AAP visit (1993–2010)
As the first objective of the study, we examined the national trend of AAP prescriptions by calculating average AAP visit rates among 4 to 18-year-old patients for each survey year between 1993 and 2010. Based on significant changes related to AAP use that occurred during the study period, we combined survey years and formed 3 phases. Specifically, each phase began when US FDA approved an additional indication for AAP use: (1) phase I between 1993 and 1999, (2) phase II between 2000 and 2002, and (3)phase III between 2003 and 2010. For each phase, we examined whether there were newly available AAP agents or additional US FDA warnings.
2.4. Mental diagnoses related with AAP visit (2007–2010 combined)
As the second objective of the study, we identified primary mental disorders related to AAP use. For each AAP visit, we examined (1) whether any mental diagnosis (ICD-9-CM, 290.XX-310.XX) was present, (2) whether the mental diagnosis was for an US FDA-approved indication (as defined above), and (3) what was the first-listed mental diagnosis for the visit. For mental health visits without an US FDA-approved indication, the first-listed mental diagnosis was classified into following categories: (1) psychoses with origin specific to childhood (“psychoses” hereafter, ICD-9CM, 299.X); disturbances (ICD-9CM, 312.XX; 313), (3) neurotic disorders (ICD-9CM, 300.0X; 300.1X, 300.2X, 300.3; 300.5; 300.8X; 300.9), and (4) other mental disorders (ICD-9-CM, other codes between 290.XX-310.XX).
2.5. Factors associated with an AAP prescription in pediatric ADHD visits (2007–2010 combined)
As the third objective of the study, the strength of independent association of patient/provider characteristics with AAP prescription among ADHD visits was estimated. Data from 2007 to 2010 were combined and analyzed to assess independent associations of patient demographic/socioeconomic characteristics (age, sex, race, region of residence, household income/education level based on ZIP code, and payer source), physician characteristics (provider type, metropolitan statistical area located), and patients’ health information (presence of hyperactivity, number of non-AAP drugs, other comorbidities) with an AAP prescription among pediatric ADHD visits. A multivariate logistic regression model was developed including these covariates, and adjusted odds ratios (AORs) with associated 95% confidence intervals (CIs) were estimated.
Among 4 to 18-year-old ADHD patient visits during 2007 to 2010, 4% had missing observations for variables that were based on patient ZIP code, such as median household income and percent of bachelor degree or higher. We did not include missing observations in the analysis. However, we used imputed data for observations missing a race variable. There were 29% missing observations for the race variable, and we used NAMCS/NHAMCS provided imputation values for those missing observations. The method used by NAMCS/NHAMCS for 2007 and 2008 data to impute the race value was to use the patient's locality (ZIP code or state/county of residence), physician locality, specialty, or 3-digit ICD-9-CM code for primary diagnosis. If all failed to assign the race value, the imputation was done based on a randomly selected record. For 2009 and 2010 data, race was imputed using a model-based, single, sequential regression imputation method. The model for imputing race is described in more detail in the 2009 to 2010 NAMCS/NHAMCS Public Use Data File Documentation.[14]
3. Results
3.1. National trend of AAP prescription
From 1993 to 2010, the overall AAP use showed an increasing pattern (Fig. 2). Starting from 1995, the rate of AAP prescription increased gradually until 1999. Between 1999 and 2000, the average AAP prescription rate doubled, increasing from 0.4 per 100 visits to 0.9 per 100 visits. A similar pattern occurred between 2002 and 2003 when the average rate increased from 0.8 per 100 visits to 1.6 per 100 visits; after 2003, it showed a more fluctuating pattern.
Figure 2.
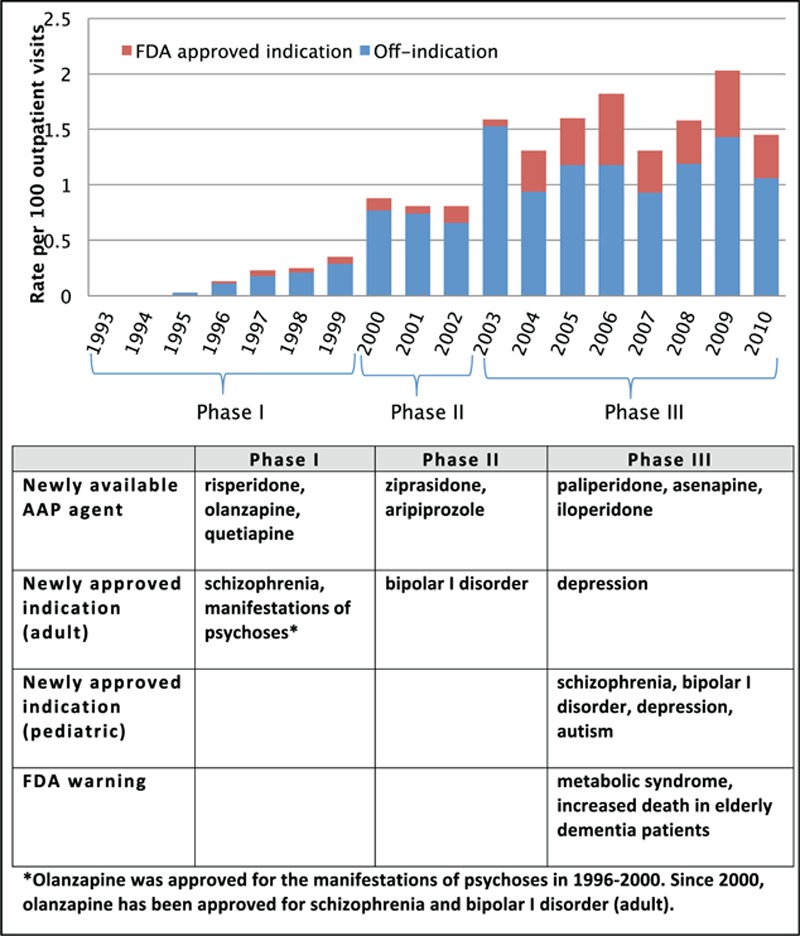
Atypical antipsychotic use in children and adolescents (age 4–18 years).
It seems that the rates of AAP prescription increased significantly when the US FDA approved the drugs for additional indications. During phase I (1993–1999), AAPs were approved by the US FDA to treat schizophrenia; the average AAP prescription rate for phase I was 0.15 per 100 visits (95% CI 0.1–0.21 per 100 visits). During phase II (2000–2002), bipolar I disorder was added as a new indication of AAPs; the average AAP prescription rate increased significantly to 0.81 per 100 visits (95% CI 0.54–1.21 per 100 visits). During phase III (2003–2010), the US FDA approved AAPs for more indications, including depression and autism. The average AAP prescription rate during phase III was 1.59 per 100 visits (95% CI 1.37–1.83), which is a statistically significant increase from phase II.
Throughout the study period, a majority of AAP visits did not include a diagnosis for US FDA-approved indications (referred to as “off-indication” in Fig. 2). The off-indication visits accounted for approximately 86% of pediatric AAP visits during 1995 to 2003, and 71% during 2004 to 2010. A statistically significant increase for US FDA-approved AAP use was observed between 2003 and 2004 (P < 0.001) when the US FDA approved 3 AAP agents (aripiprazole, quetiapine, and ziprasidone) for bipolar disorder in addition to their previously approved indication, schizophrenia.
3.2. Mental diagnoses related to AAP visit
The estimated number of total AAP visits for pediatric patients during 2007 to 2010 was 8,380,436 (weighted count), which accounted for approximately 2% of the total pediatric outpatient visits in the United States. Of those, 34% included one or more diagnoses of US FDA-approved indications (Fig. 3). Within this group, a majority of visits had diagnoses of bipolar disorder or depression (16% and 14% of total pediatric AAP visits, respectively), followed by autism and schizophrenia (5% and 1% of total pediatric AAP visits, respectively). Approximately 2% of total pediatric AAP visits had 2 or more diagnoses of US FDA-approved indications.
Figure 3.
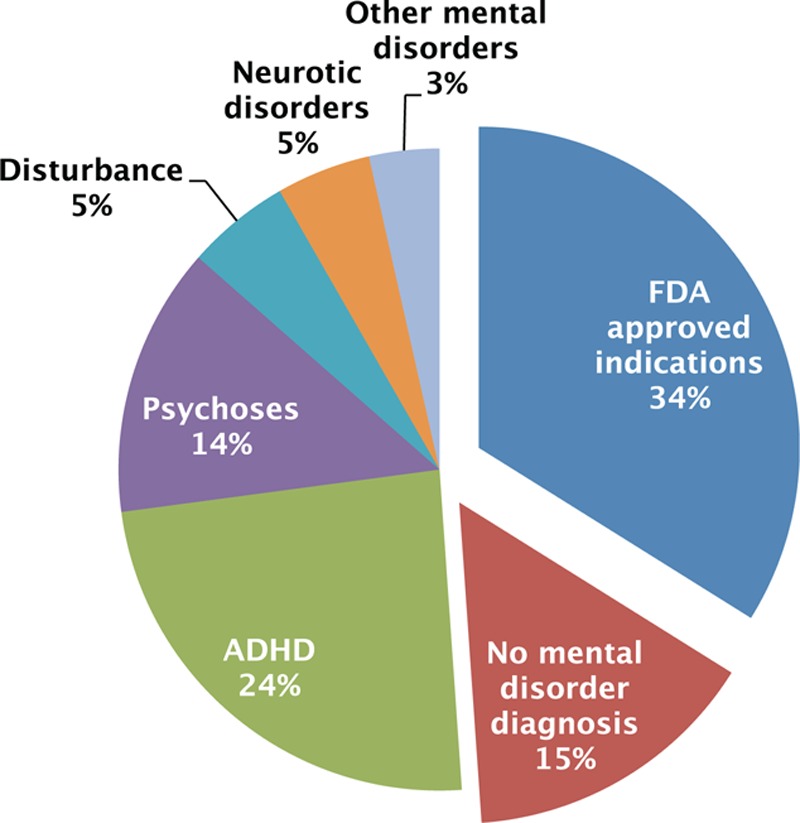
Mental diagnoses related to AAP visits. AAP, atypical antipsychotic.
Among those without any US FDA-approved indications, ADHD was the most common primary mental diagnosis (24% of total pediatric AAP visits), followed by psychoses (14% of total pediatric AAP visits). Disturbances and neurotic disorders took up about 5% of total pediatric AAP visits, respectively. Approximately 15% of total pediatric AAP visits did not include any mental disorder diagnosis.
3.3. Factors associated with an AAP prescription in pediatric ADHD visits
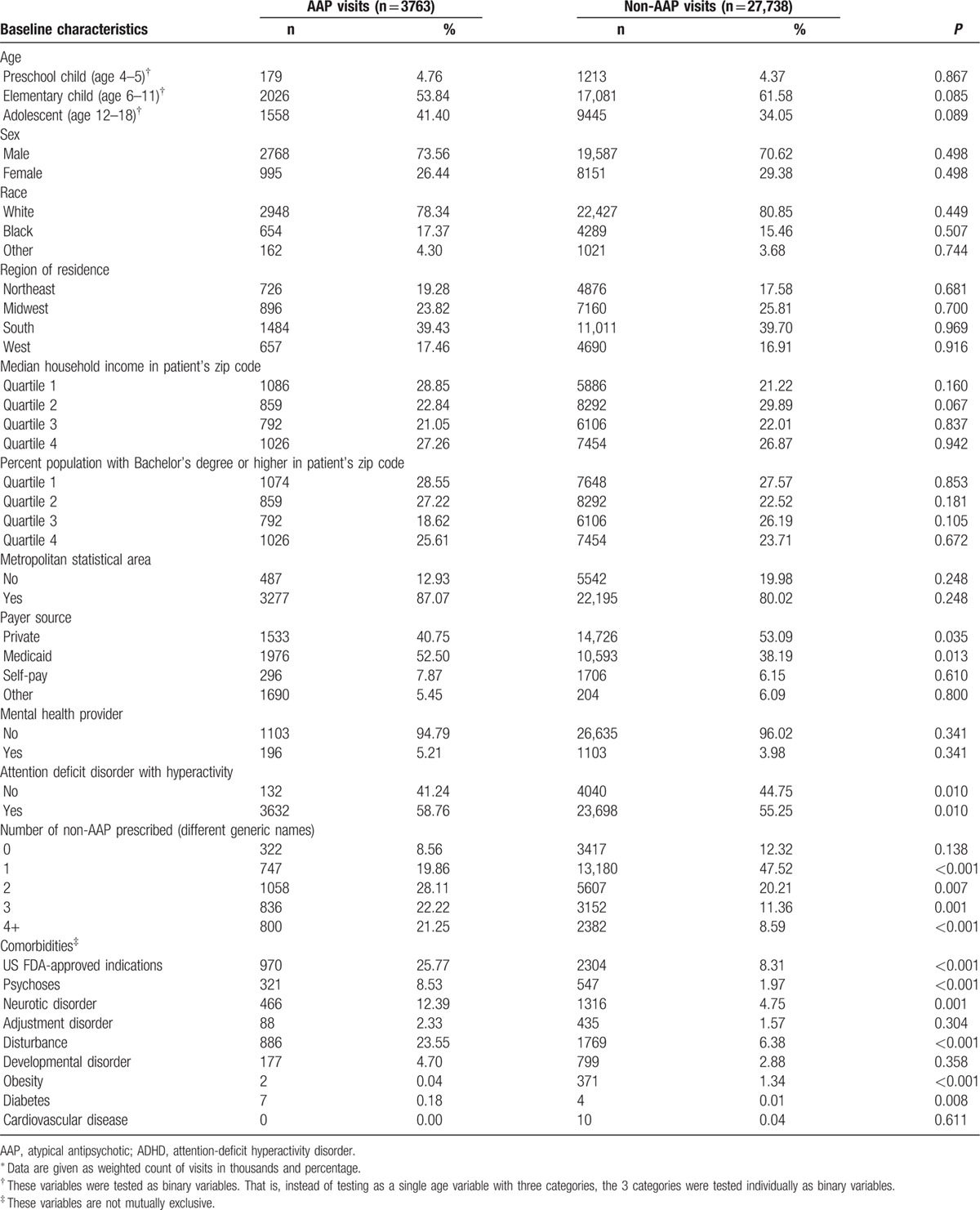
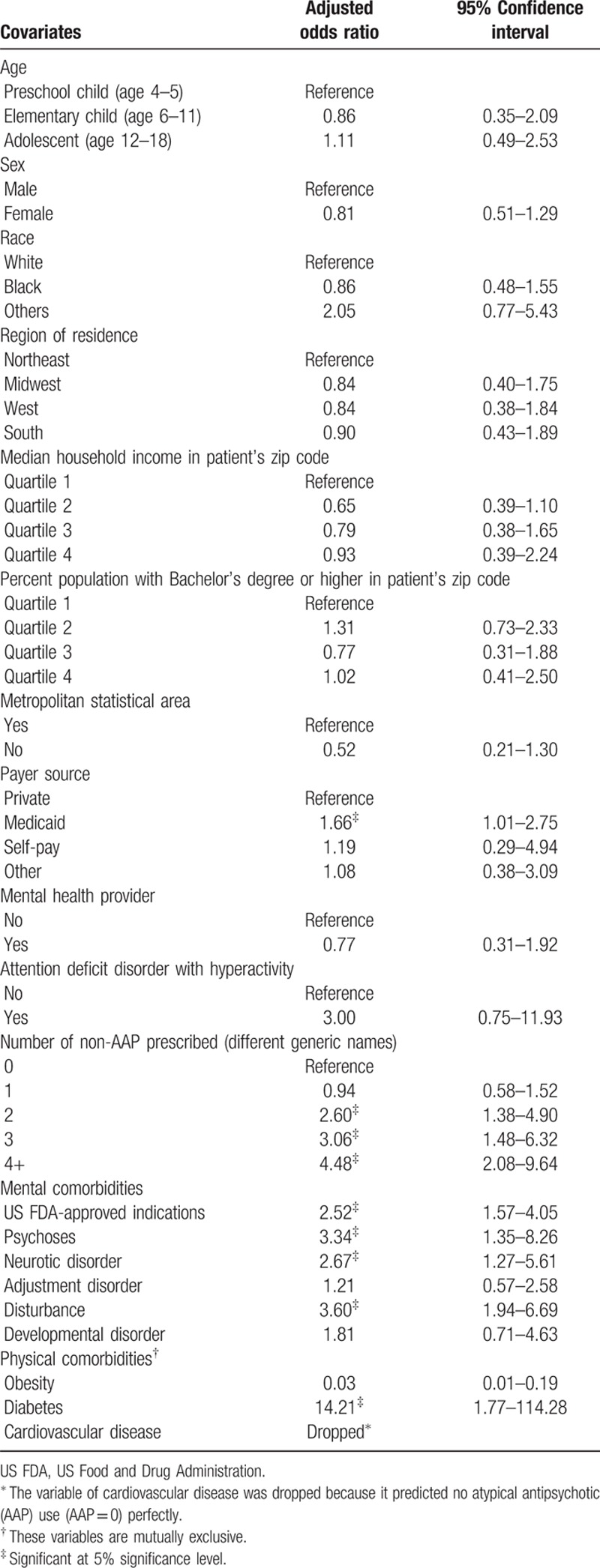
During 2007 to 2010, the total number of pediatric ADHD visits was estimated to be 31,501,209 (weighted count). Of those, 12% included 1 or more AAP prescriptions. Characteristics of pediatric ADHD visits are summarized in Table 1. Patient demographics and healthcare provider characteristics were not statistically different between AAP visits and non-AAP visits. A significantly larger proportion of AAP visits had Medicaid as the primary source of payment (52.50% vs 38.19%; P = 0.0135). Regarding ADHD characteristics, AAP visits were more likely to have attention-deficit disorder with hyperactivity compared with those without hyperactivity (58.76% vs 55.25%; P = 0.010). More drugs (excluding AAPs) were prescribed during AAP visits as compared with non-AAP visits. Comorbidity profile was also different in a way that AAP visits had more comorbid conditions listed, including US FDA-approved AAP indications, psychoses, neurotic disorder, disturbance, and diabetes.
Table 1.
Characteristics of pediatric ADHD visits.a
In the multivariable logistic regression analysis, having Medicaid as the primary payment source (AOR 1.66, 95% CI 1.01–2.75), more non-AAP prescription medications (4 or more prescriptions AOR 4.48, 95% CI 2.08–9.64), comorbid mental disorders such as US FDA-approved AAP indications (AOR 2.52, 95% CI 1.57–4.05), psychoses (AOR 3.34, 95% CI 1.35–8.26), neurotic disorder (AOR 2.67, 95% CI 1.27–5.61), disturbance (AOR 3.60, 95% CI 1.94–6.69), and diabetes (AOR 14.21, 95% CI 1.77–114.28) significantly increased the odds of having an AAP prescription in a pediatric ADHD-related visit (Table 2). However, having comorbid obesity was negatively associated with an AAP prescription (AOR 0.03, 95% CI 0.01–0.19).
Table 2.
Factors associated with atypical antipsychotic prescription.
4. Discussion
The purpose of this study was to examine the national trend of pediatric AAP use in an outpatient treatment setting in the United States. We estimated the average annual AAP prescription rates between 1993 and 2010, and explored events related to AAPs. Then, we identified mental diagnoses related to AAP prescription for the period of 2007 to 2010. Lastly, we estimated the strength of independent association of patient/provider characteristics with AAP prescription among pediatric ADHD visits.
From 1993 and 2010, the overall AAP prescription rates in pediatric outpatient visits showed an increasing pattern. There was approximately 5-fold significant increase from phase I (1993–1999) to phase II (2000–2002), and 2-fold increase from phase II to phase III (2003–2010). As more AAP agents became available and the US FDA approved more indications, AAP prescription rates increased (Fig. 2). Also, it seemed that sudden increases in AAP prescription rates were associated with an US FDA approval for an additional AAP indication. During 1993 to 2010, all AAP agents entered the market by obtaining US FDA approval for the treatment of schizophrenia. Other indications including bipolar disorder, depression, and autism were added to the label a few years later. Interestingly, during the year of 2000 when bipolar disorder was first indicated, there was an abrupt increase in AAP prescription rates. Also, olanzapine was first approved for treatment of depression in 2003 and there was another abrupt increase in AAP prescription rates. Autism was added to the label of risperidone in 2006, and the rate increase was the highest since 2003. However, it is difficult to argue that such increased prescription rates are mostly to treat the additionally approved indication because the rate increased not only for on-label but also for off-label. More specifically, from phase I to phase II, AAP visits for US FDA-approved indications increased only 0.09 per 100 pediatric outpatient visits, whereas AAP visits for off-label uses increased 0.57 per 100 pediatric outpatient visits. Similarly, from phase II to phase III, the magnitude of increase in off-label visits was larger than for US FDA-approved indication visits (Fig. 2). Perhaps, having approval for additional AAP indications impacted the AAP therapy decision-making process in a way that AAPs were regarded as useful for conditions other than currently approved indications. Our analysis only intended to capture the national trend, and further investigation using carefully designed models is needed to clarify the association of a specific event with AAP prescription rates.
Our analysis for identifying mental diagnoses that are related to AAP prescriptions revealed that approximately 66% of total pediatric AAP visits did not include a diagnosis for US FDA-approved indications. Of those, ADHD was the most common primary mental diagnosis. This finding is consistent with several previous studies that examined pediatric AAP use. Pathak et al[7] assessed the dispensing pattern of AAPs using Arkansas Medicaid claims data and reported ADHD as the most common condition for children and adolescents to be prescribed AAPs. Cooper et al[15] reported the same finding from the NAMCS/NHAMCS data between 1995 and 2002. In the study, approximately 15% of total pediatric AAP visits did not include any mental diagnosis. In a study by Staller et al, 77% of the outpatient antipsychotic visits did not include a mental diagnosis. They collected medical and prescription data from 8 outpatient clinics in Central New York in 2002. The fact that they had a much higher proportion of psychiatric visits without a mental diagnosis than our study could be explained by a number of factors, including different sampling method, number of recorded diagnoses, or inclusion criteria in defining antipsychotic visits. Nonetheless, our study, with previous studies, raises a major issue about current antipsychotic prescription pattern in which antipsychotic medications could frequently be misused in pediatric population.
From our logistic regression model estimating the association between several factors and AAP prescription in ADHD patients, patient demographics and healthcare provider characteristics did not show a significant relationship with AAP prescription. Instead, patients’ medical profiles showed much stronger associations with AAP prescription. More specifically, having more coprescribed medications (i.e., other than AAPs) and comorbid mental disorders, including US FDA-approved AAP indications, psychoses, neurotic disorder, and disturbance, increased the likelihood of having an AAP prescription. This result indicates that an AAP is more likely to be prescribed to ADHD patients when multiple health conditions are present. Medicaid being a significant factor could be explained with this result, because chronic illness and other health risk factors are more prevalent among Medicaid enrolees compared with those who are covered by private insurance.[16–18]
Some limitations should be noted. First, the survey may not capture sufficient information to estimate the AAP prescription rates and characteristics of visits. We used 6 first-listed medications and 3 diagnosis codes for the study period. Such limited availability of medical/pharmacy records may have misrepresented the true estimates in the study. For example, it is possible that some AAP-treated patients had a severe physical illness in addition to mental disorders, and due to the limited space for the number of diagnosis codes on the survey form, their healthcare providers were only able to record diagnoses for physical illness. In this case, the visit data would have been classified as an AAP visit with no mental disorder diagnosis code, although the visit had a mental disorder diagnosis. Second, the NAMCS/MHAMCS are designed to obtain the national/regional estimate of outpatient healthcare service measures. State-level estimates during the study period are unreliable, and we were unable to assess the association of particular states with AAP prescription rates. Third, due to the nature of micro visit level data, the temporal relationship between explanatory variables and AAP prescriptions was not identifiable. In other words, it is not possible to conclude that having comorbid conditions triggered the AAP use. Instead, we only observed that comorbidities were associated with AAP use. Fourth, variables of obesity, diabetes, and cardiovascular disease in our logistic regression model had only a few observations, making the estimated values unstable.
The findings of this study are from nonemergent outpatient visits. Because our data source does not have hospital inpatient data, we are apt to miss serious illness cases. This limitation could lead to bias if hospitalized patients are exposed to AAPs at a rate different from what is seen in the outpatient setting. For example, we found that ADHD patients with another mental disorder comorbidity were more likely to receive an AAP than those without. Meanwhile, patients with comorbid mental disorders such as schizophrenia or a mood disorder are more frequently hospitalized,[19] indicating a possibility that hospitalized ADHD patients may use AAPs more often than outpatient patients. Thus, without inpatient visit data, our analysis would have underestimated the true association.
To minimize potential confounding, we reported AORs after controlling for a number of mental and physical comorbidities. However, our regression is limited to variables that are observable and measurable from the data source. Also, we grouped comorbid mental disorders into smaller categories, such as US FDA-approved indications, to estimate the overall impact of having those conditions, but we did not estimate the impact of more specific disorders such as bipolar disorder or autism spectrum disorder.
The identification of mental disorders in this study was done through ICD-9-CM, although “Diagnostic and Statistical Manual of Mental Disorders” (DSM) serves as a more reliable resource for prescribing clinicians in the United States. For ADHD, ICD criteria seem to be more stringent than DSM criteria, as ICD criteria do not recognize some subtypes of ADHD.[20,21] As a result, the prevalence of ADHD would be much lower for an ICD-based diagnosis than for DSM-based diagnosis.[22,23] Such discrepancies between ICD and DSM relate to a potential misclassification. In the absence of DSM-based diagnosis information, it is likely that the number of ADHD cases from ICD-based records (reported as 24% of total pediatric AAP visits) underestimates the number identified in real practice.
Although our study covers the national trend until 2010, several changes occurred in antipsychotic therapy and mental disorder diagnosis during recent years. Several antipsychotic agents were newly approved after 2010, and the introduction of these agents would be expected to influence off-label antipsychotic use. Also, mental disorder diagnostic criteria were revised in DSM-V, which was published in 2013. One of the changes made in the DSM-V, unlike the previous version (DSM-IV), is that ADHD can be diagnosed alongside autism spectrum disorder. Previously, the presence of autism spectrum disorder ruled out ADHD. This change may further inflate the rate of AAP prescription in ADHD patients. Nonetheless, caution is required in implementing the findings of this study, because it may not represent the current trend. Further investigation of recent changes in psychiatry and the impact on AAP use is an area for further investigation.
In conclusion, we show that AAP prescription rates in 4 to 18-year-old patients significantly increased between 1993 and 2010 in the United States, and over 65% of those visits did not have a diagnosis for US FDA-approved AAP indications. During 2007 to 2010, the most common mental disorder was ADHD, accounting for 24% of total pediatric AAP visits. Among visits with ADHD diagnosis, those with comorbid mental disorders such as psychoses, neurotic disorder, and disturbance were more likely to have an AAP prescription.
Footnotes
Abbreviations: AAP = atypical antipsychotic, ADHD = attention-deficit/hyperactivity disorder, AOR = adjusted odds ratios, CI = confidence interval, DSM = Diagnostic and Statistical Manual of Mental Disorders, FDA = Food and Drug Administration, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NAMCS = National Ambulatory Medical Care Survey, NCHS = National Center for Health Statistics, NHAMCS = National Hospital Ambulatory Medical Care Survey.
The authors declare that there are no conflicts of interest to disclose.
References
- 1.Pieters T, Majerus B. The introduction of chlorpromazine in Belgium and the Netherlands (1951-1968); tango between old and new treatment features. Stud History Philos Biol Biomed Sci 2011; 42:443–452. [DOI] [PubMed] [Google Scholar]
- 2.Seida JC, Schouten JR, Boylan K, et al. Antipsychotics for children and young adults: a comparative effectiveness review. Pediatrics 2012; 129:e771–784. [DOI] [PubMed] [Google Scholar]
- 3.Scheltema Beduin A, de Haan L. Off-label second generation antipsychotics for impulse regulation disorders: a review. Psychopharmacol Bull 2010; 43:45–81. [PubMed] [Google Scholar]
- 4.Pathak P, West D, Martin BC, et al. Evidence-based use of second-generation antipsychotics in a state Medicaid pediatric population, 2001-2005. Psychiatric Serv 2010; 61:123–129. [DOI] [PubMed] [Google Scholar]
- 5.Crystal S, Olfson M, Huang C, et al. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Affairs 2009; 28:w770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- 7.Bobes J, Arango C, Aranda P, et al. Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: results of the CLAMORS Study. Schizophr Res 2007; 90:162–173. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JH. Atypical antipsychotics in the elderly with Parkinson disease and the “black box” warning. Neurology 2006; 67:564–566. [DOI] [PubMed] [Google Scholar]
- 9.Lindsley CW. The top prescription drugs of 2011 in the United States: antipsychotics and antidepressants once again lead CNS therapeutics. ACS Chem Neurosci 2012; 3:630–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zito JM, Safer DJ, DosReis S, et al. Psychotropic practice patterns for youth: a 10-year perspective. Arch Pediatr Adolesc Med 2003; 157:17–25. [DOI] [PubMed] [Google Scholar]
- 11.Patel NC, Crismon ML, Hoagwood K, et al. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry 2005; 44:548–556. [DOI] [PubMed] [Google Scholar]
- 12.Olfson M, Blanco C, Liu L, et al. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry 2006; 63:679–685. [DOI] [PubMed] [Google Scholar]
- 13.McCaig LF, Burt CW. Understanding and interpreting the National Hospital Ambulatory Medical Care Survey: key questions and answers. Ann Emerg Med 2012; 60:716–721.e711. [DOI] [PubMed] [Google Scholar]
- 14.Center for Disease Control and Prevention. Ambulatory Health Care Data. Available at: http://www.cdc.gov/nchs/ahcd.htm Accessed April 7, 2014. [Google Scholar]
- 15.Cooper WO, Arbogast PG, Ding H, et al. Trends in prescribing of antipsychotic medications for US children. Ambul Pediatr 2006; 6:79–83. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum S. Medicaid. N Engl J Med 2002; 346:635–640. [DOI] [PubMed] [Google Scholar]
- 17.Shatin D, Levin R, Ireys HT, et al. Health care utilization by children with chronic illnesses: a comparison of Medicaid and employer-insured managed care. Pediatrics 1998; 102:E44. [DOI] [PubMed] [Google Scholar]
- 18.Alaimo K, Olson CM, Frongillo EA., Jr Low family income and food insufficiency in relation to overweight in US children: is there a paradox? Arch Pediatr Adolesc Med 2001; 155:1161–1167. [DOI] [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality. HCUPnet: National statistics on mental health hospitalizations. Available at: http://hcupnet.ahrq.gov Accessed April 14, 2016. [Google Scholar]
- 20.Santosh PJ, Taylor E, Swanson J, et al. Refining the diagnoses of inattention and overactivity syndromes: a reanalysis of the Multimodal Treatment study of attention deficit hyperactivity disorder (ADHD) based on ICD-10 criteria for hyperkinetic disorder. Clin Neurosci Res 2005; 5:307–314. [Google Scholar]
- 21.Prendergast M, Taylor E, Rapoport JL, et al. The diagnosis of childhood hyperactivity. A U.S.-U.K cross-national study of DSM-III and ICD-9. J Child Psychol Psychiatry 1988; 29:289–300. [DOI] [PubMed] [Google Scholar]
- 22.Faraone SV, Sergeant J, Gillberg C, et al. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2003; 2:104–113. [PMC free article] [PubMed] [Google Scholar]
- 23.Döpfner M, Breuer D, Wille N, et al. How often do children meet ICD-10/DSM-IV criteria of attention deficit-/hyperactivity disorder and hyperkinetic disorder? Parent-based prevalence rates in a national sample: results of the BELLA study. Eur Child Adolesc Psychiatry 2008; 17 Suppl 1:59–70. [DOI] [PubMed] [Google Scholar]


