Abstract
Screening endoscopy is recommended for early detection of esophageal varices (EVs) in cirrhotic patients with portal hypertension. However, this approach is limited by its invasiveness and cost. The aim of the study was to determine if platelet count can predict the presence of EVs, especially large (grade III, IV) EVs in need of prophylactic therapy, in a cohort of Egyptian patients with liver cirrhosis. In all, 110 patients with cirrhosis were prospectively analyzed. The presence of medium or large EVs was correlated with patients’ platelet count and FIB-4. Esophageal varices were present in 87 (79.09%) patients. Among those with thrombocytopenia (platelet level below 150,000), 25.97% (20 patients) and 27.27% (21 patients) had EV grade II and EV grade III or IV, respectively. Whereas in patients in whom the platelet count was above 150,000, only 21.21% (7 patients) and 9.09% (3 patients) of patients had grade II EV and EV grade III or IV, respectively. A platelet count cut-off value of 149,000 was found to have specificity of 82% and sensitivity 39% for detection of presence of varices. A FIB-4 cut-off value of 3.175 was found to have an 83.3% sensitivity and 39.5% specificity in detecting large (grade III, IV) EVs. Platelet count is a noninvasive parameter with high accuracy for prediction of EVs. Cirrhotic patients with normal platelet counts (above 150,000), especially in financially deprived developing countries, can avoid screening endoscopy as they are at a low risk for variceal bleeding, and presence of large EVs in these patients is much less common than in those with thrombocytopenia. A 3.175 cut-off value of FIB-4 could be useful as a noninvasive predictor of large varices requiring prophylactic banding in cirrhotic patients.
Keywords: cirrhosis, esophageal varices, FIB-4, platelets, portal hypertension
1. Introduction
Portal hypertension is a common complication of liver cirrhosis that can lead to development of esophageal varices (EVs), which are abnormally dilated veins within the wall of the esophagus that may lead to haemorrhage.[1] The majority of patients with cirrhosis will develop EV at some point, and about a third of these patients will have at least 1 bleeding episode due to rupture of a varix.[2] For this reason, screening endoscopy for detection of the presence of EV is part of the diagnostic work-up in patients with cirrhosis. This is a very important preventive step for identification of those patients with variceal bleeding risk and furthermore, identification of patients in urgent need for prophylactic treatment.[3]
Guidelines stress on screening endoscopy for early detection of EVs in cirrhotic patients with portal hypertension.[3] However, this is a rather unpleasant method that carries a certain risk of complications.[4]
Recent research has focused on the use of noninvasive methods to detect patients with the intention of avoiding endoscopy in low-risk cases.[5] Thrombocytopenia (platelet count <150,000/μL) is a common complication in patients of chronic liver disease (CLD).[6] The exact pathogenesis of thrombocytopenia in patients with CLD is multifactorial and includes decreased production of thrombopoietin, splenic sequestration of platelets, and myelosuppression of platelet production due to hepatitis C virus (HCV).[7] So, we formulated this study on a cohort of Egyptian patients with liver cirrhosis to determine whether platelet count can predict the presence and size of EVs, because presence of medium and large-sized varices are an indication for prophylactic therapy. The aim was to assess the possibility of utilizing the platelet count to spare patients at low risk for variceal bleeding from endoscopic screening.
2. Methods
2.1. Study design and settings
This open-label trial was conducted at the Tanta University Hospital from October 2014 to March 2015. Our study was approved by the Tanta Faculty of Medicine ethical committee, Tanta University. The research team recruited potential participants, and explained to each patient the aim of the research. A written consent was obtained from all participants in the study.
2.2. Study subjects
Subjects were eligible if they had a diagnosis of cirrhosis based on history, physical examination, laboratory tests, ultrasound scans, and liver biopsy in some cases. Patients with hepatocellular carcinoma, portal vein thrombosis, or parenteral drug addiction were excluded from the study to avoid further factors affecting the platelet count, and also those taking beta-blockers. Clinical and demographic data, prescribed medication, physical examination findings, and severity of liver disease, as assessed by the Child–Pugh classification, were recorded. All patients were asked about history of alcohol intake, intravenous (i.v.) drug abuse, and tested for hepatitis B and hepatitis C viral markers to determine the cause of liver cirrhosis. Parenteral drug addicts were identified through history, clinical suspicion, and skin manifestations, for example, skin tracks along the length of the veins.
Routine laboratory tests were performed for all patients, and these included the following: complete liver function tests, complete blood count, fasting and postprandial blood glucose, serum creatinine, antinuclear antibody, immunoglobulin G (IgG), HBsAg, HCV antibody test, and polymerase chain reaction (PCR) for hepatitis B virus (HBV)-DNA and HCV-RNA. Tests for other causes of cirrhosis, for example, serum ceruloplasmin and slit lamp examination for Wilson disease, autoantibodies for autoimmune liver disease (antismooth muscle antibodies [SMA], antiliver/kidney microsomal autoantibodies [LKM-1], and antiliver/kidney microsomal autoantibodies [LKM-1]) and iron studies for hemochromatosis were carried out only if history and clinical findings were suggestive, such as presence of diabetes mellitus, impotence, hyperpigmentation, arthritis suggestive for hemochromatosis, or neurological disturbances, and marked unexplained elevations of INR, transaminases, or bilirubin suggestive for Wilson disease. Coexistence of other diseases with immune or autoimmune features, for example, immune thrombocytopenic purpura, myasthenia gravis, thyroiditis, or serum IgG >2.5, was considered suggestive for autoimmune liver disease. None of the patients tested positive for autoantibodies.
The FIB-4 score was calculated for all patients using the formula: FIB-4 = age ([years] × aspartate aminotransferase [AST] [U/L])/((PLT [109/L]) × (alanine aminotransferase [ALT] [U/L]) [1/2]).[8]
Abdominal ultrasonography with Duplex-Doppler ultrasound was performed for all patients using a Siemens G60 Ultrasound System with a convex probe 3.5 MHz. Patients were examined after overnight fasting in supine position. Aquatic gel was spread as a film on the abdomen of the patient to prevent interposition of air between the transducer and the skin. Survey screening was done through several longitudinal, oblique, and transverse cuts. Measurements were taken in quiet respiration. Ultrasonography evaluation included the appearance of the liver as regards size, echo pattern of the liver, established cirrhosis signs, uneven hepatic margins, increased parenchymatous reflectivity, coarseness, increased echographic contrast between right lobe of liver and right kidney, hypertrophied caudate lobe, and attenuated hepatic veins. The presence of hepatic focal lesions and portal vein patency and diameter were noted. Splenic size was recorded. Portal vein, hepatic artery, and splenic artery flow and patency were assessed.
Endoscopy was carried out for all enrolled patients. Patients were requested to fast overnight and received premedication in the form of xylocaine local spray above the tongue and nasopharynx. Midazolam 3 to 5 mg i.v. was also given before the procedure. Endoscopy was performed using Pentax EG-2985 endoscopes.
When EVs were visualized, the size was graded as I to IV using the Paquet grading system.[9] On the basis of platelet count measured by Sysmex XS 500 apparatus, patients were divided into 4 groups; group I with a platelet count below 50,000/μL, group II 51,000 to 99,000/μL, group III 100,000 to 150,000/μL, and group IV with a platelet count above 150,000/μL.[10] Correlation of severity of thrombocytopenia with the grading of EVs was assessed.
2.3. Statistical analysis
The data were expressed as the mean ± standard deviation (SD), compared using 1-way analysis of variance (ANOVA) test and Tukey test as a post-hoc test. Spearman correlations were used to test for the associations of EV grades (parametric data) with platelet count and FIB-4 score (numerical data). Multivariate analysis was done to test relation between EV as dependent variable and other factors using PLUM-ordinal regression test, and all the analyses were performed using Graph Pad Instat, 32 bit for win 95/NT (Version 3.05). A receiving-operating characteristic (ROC) curve was constructed using the slandered level of thrombocytopenia (150,000/mL) and level of significant fibrosis of FIB-4 (3.175) as cut-off points of platelet count and FIB-4, respectively. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and test accuracy were calculated accordingly. For all used tests, a P value <0.05 was considered statistically significant.
3. Results
This open-label trial was conducted in Tanta University Hospital from October 2014 to March 2015. In all, 172 cirrhotic patients were invited to share in the study. However, 62 patients were excluded (22 patients had HCC, 3 patients had portal vein thrombosis, and 37 patients refused to share in the study). Finally, a total of 110 cirrhotic patients were enrolled in the study. Their mean age was 54.39 ± 7.46 years; 73 (66.36%) of them were men and 37 (33.64%) were women.
Our patients were divided into 4 groups; group I with a platelet count below 50,000/μL, group II 51,000 to 99,000/μL, group III 100,000 to 150,000/μL, and group IV with a platelet count above 150,000/μL. Basic demographic, clinical, and laboratory characteristics of the 4 study groups are presented in Table 1.
Table 1.
Basic demographic, clinical, and laboratory characteristics of the studied groups.
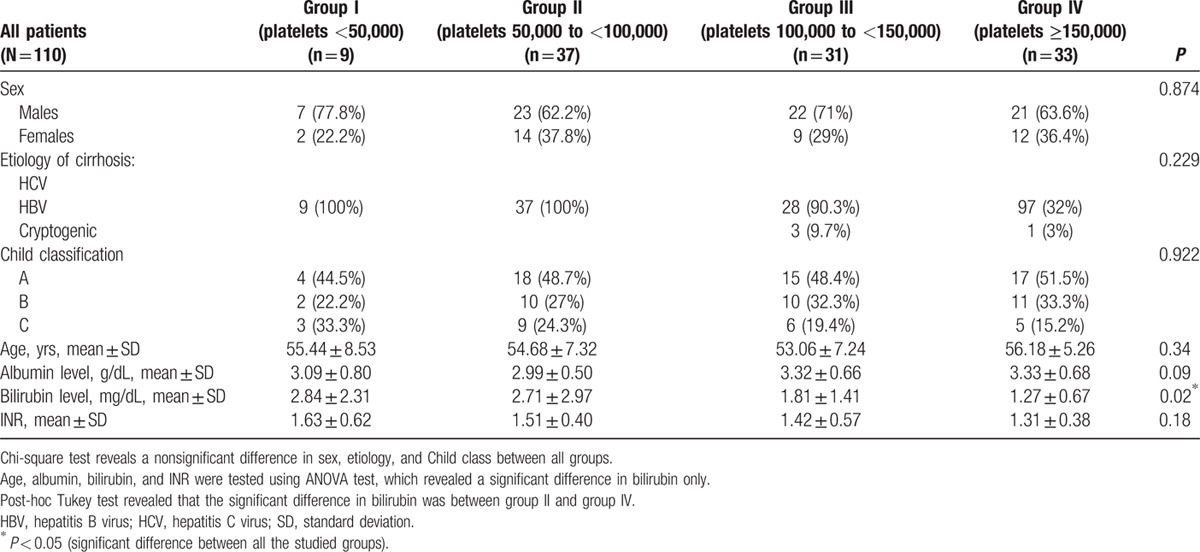
The etiology of cirrhosis was determined as hepatitis C in the majority of patients (107 [97.27%]); hepatitis B was the cause in only 1 patient (0.91%); and 2 (1.82%) had cryptogenic cirrhosis. EVs were present in 87 (79.09%) patients. The mean albumin level was 3.16 ± 0.63, mean bilirubin level was 2.18 ± 2.1, and the mean INR was 1.42 ± 0.43. As regards the Child–Pugh classification, 54 (49.1%) patients were classified as class A, 33 (30%) as class B, and 23 (20.9%) as class C.
We recorded the presence and grade of varices in the different Child classes and found that among the Child A patients; 27.8% had no varices, 38.9% had EV grade I, 20.4% had EV grade II, and 12.9% had EV grade III or IV. Whereas in Child B patients, 15.15% had no varices, 33.33% had EV grade I, 24.24% had EV grade II, and 27.27% had EV grade III or IV. In Child C patients, 13.04% had no varices, 17.39% had EV grade I, 34.79% had EV grade II, and 34.79% had EV grade III or IV. Incidence of EVs in patient groups divided according to the platelet count is demonstrated in Table 2.
Table 2.
Occurrence of esophageal varices (EVs) in all studied groups.
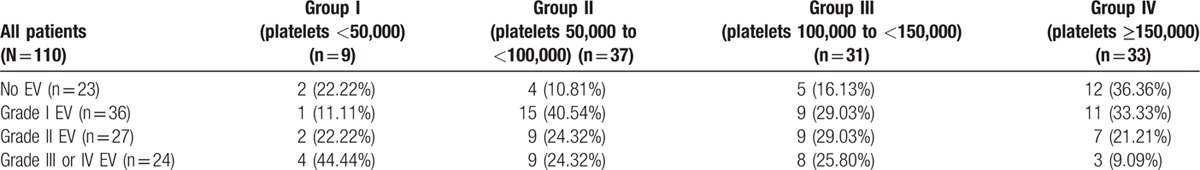
Among our 77 patients with thrombocytopenia (platelet level below 150,000), 11 had no varices and 66 showed varices on endoscopy. On the other hand, among the 33 patients with normal platelet counts (above 150,000), 12 had no varices and 21 had EVs. The difference between nonthrombocytopenic and thrombocytopenic patients was significant (P = 0.019), indicating that thrombocytopenia is associated with occurrence of EVs (Table 3).
Table 3.
Difference between occurrence of EV in thrombocytopenic and nonthrombocytopenic patients.
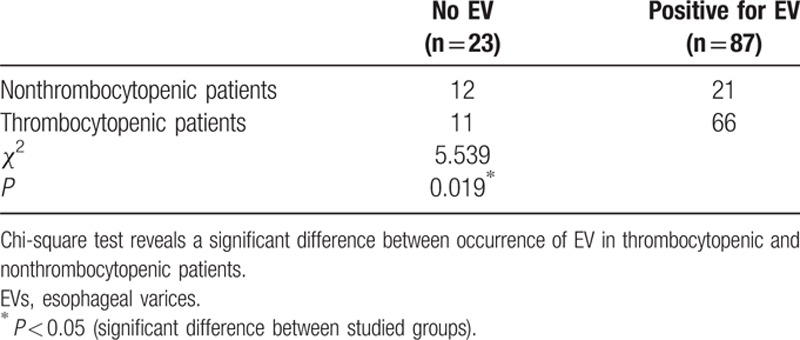
We found that among our patients with thrombocytopenia (platelet level below 150,000), 14.28% (11 patients) had no varices, 32.46% (25 patients) had small (grade I varices), 25.97% (20 patients) had medium-sized EVs (grade II), and 27.27% (21 patients) had large-sized EVs (grade III and IV). Whereas in patients whose platelet count was above 150,000, 12 patients (36.36%) had no varices, 11 (33.33%) had small (grade I) varices, 21.21% had medium-sized (grade II) EVs, and only 9.09% of patients had large grade III or IV varices.
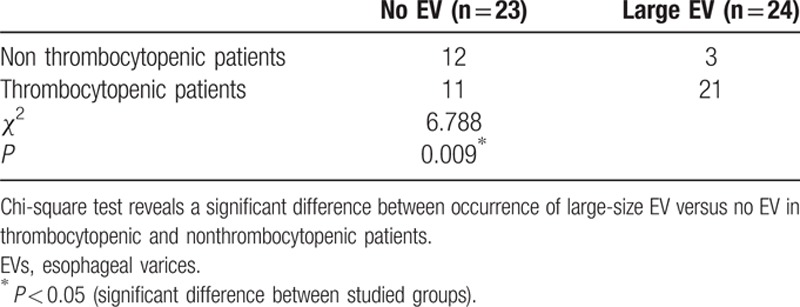
Patients with thrombocytopenia had significantly higher frequency of large varices (grades III and IV) compared with patients with normal platelet counts (P < 0.009) (Table 4).
Table 4.
Difference between occurrence of large-size EV versus no EV in thrombocytopenic and nonthrombocytopenic patients.
Although thrombocytopenia is associated with variceal occurrence, the degree of thrombocytopenia does not affect their occurrence as demonstrated in Table 5
Table 5.
Difference between occurrence of EV at different levels of platelet count in thrombocytopenic patients (N = 77).
Grading of EVs showed a negative significant correlation with platelet count, whereas it was directly proportional to the FIB-4 index. Correlation between EV grade and these variables is shown in Table 6.
Table 6.
Correlation between esophageal varices grade with platelet count and FIB-4 score.
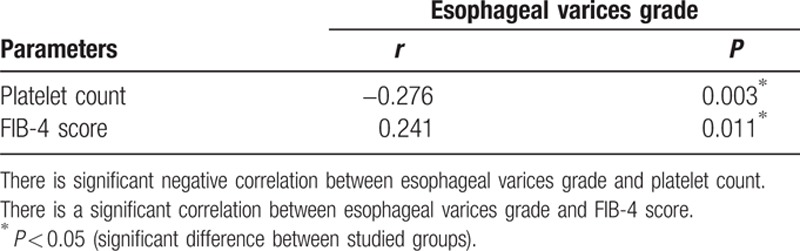
A platelet count cut-off value >149,000 (normal platelet count) was accurate in detecting absence of varices with 39% sensitivity, 82% specificity, 72% PPV, 54% NPV, and accuracy of 59%. This is evident in the ROC curve shown in Fig. 1. When the platelet count was used to predict large varices (grades III and IV), the area under the ROC curve was less than 0.5 and therefore of no significance.
Figure 1.
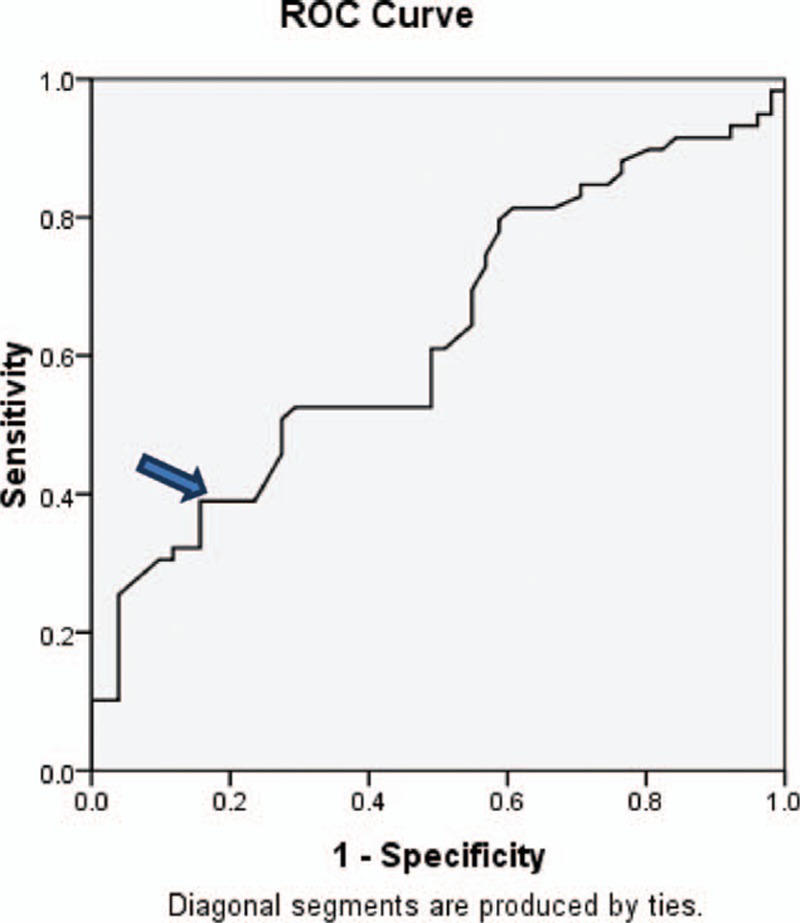
The ROC curve for detection of cut-off value of platelet count. ROC, receiving-operating characteristic. Area under curve (0.627), confidence interval (CI) 95 % (0,523–0.731), P value (0.022).
We further tested FIB-4 and found that at a cut-off value of 3.175, it has 78.4% sensitivity,45% specificity, 78.4% PPV, 45.7% NPV, and 61% accuracy in detecting large (grade III and IV) EVs. Area under the curve was 0.647. The 95% interval for the test was (0.520–0.774; P = 0.028). The ROC curve is shown in Fig. 2
Figure 2.
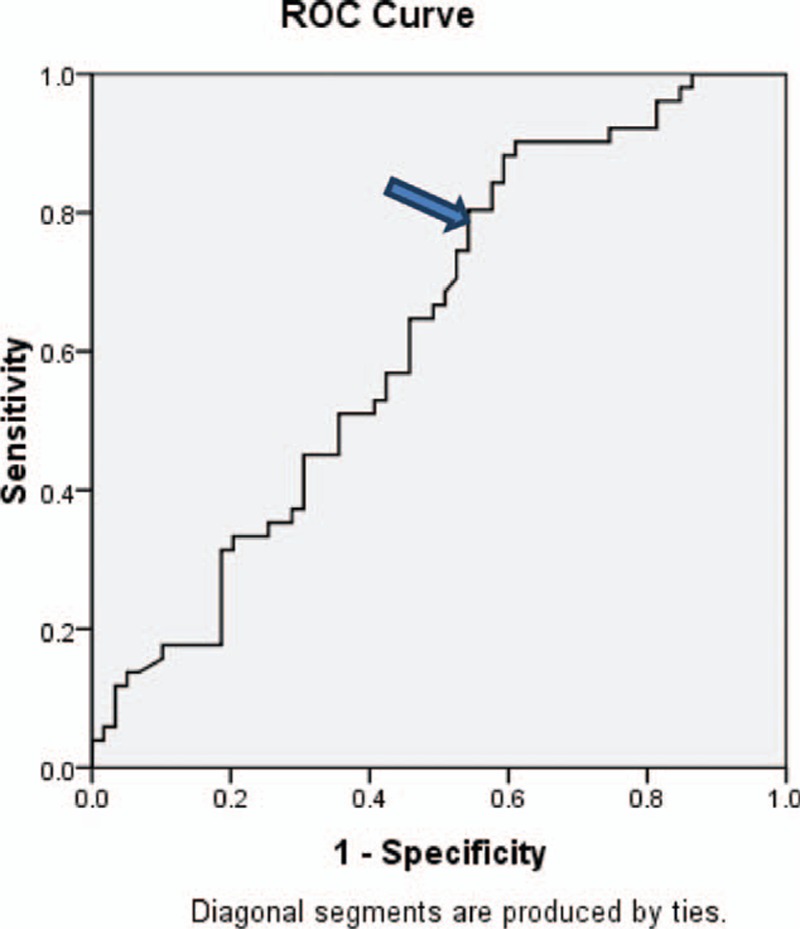
The ROC curve for detection of cut-off value of FIB-4. ROC, receiving-operating characteristic. AUC = 0.627 , with 95% CI (0.523–0.731) and P value = 0.022.
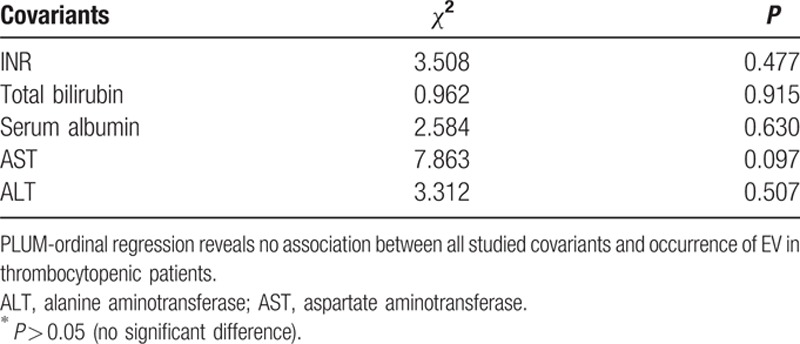
A multivariate analysis performed between EVs and INR, total bilirubin level, serum albumin, AST, and ALT as covariants, revealed that none of these parameters had a significant effect on the results as shown in Table 7.
Table 7.
Multivariate analysis between presence of esophageal varices and study parameters.
4. Discussion
The development of gastro-EVs is a common complication of portal hypertension, and bleeding from it is a frequent cause of mortality and morbidity. Esophagogastroduodenoscopy is the standard method to diagnose the presence of esophagogastric varices and to estimate the risk of bleeding. It is recommended that all patients undergo endoscopic screening for varices at the time when cirrhosis is diagnosed.[11] However, many previous studies have shown a good predictive value of different nonendoscopic variables for the presence or absence of gastro-EVs .[12]
Spider nevi—a low-albumin and low-platelet count—were shown to be independent risk factors for the presence of varices in a study by Garcia-Tsao et al[13] in 1997.
Hepatitis C was the underlying cause of cirrhosis in the 97.3% of our patients. This is because it is the major cause of liver cirrhosis in our country, because Egypt has the highest prevalence rate of HCV in the world.[14–16]
The main limitation of platelet count in prediction of EVs is that it can depend on other factors rather than portal hypertension in liver cirrhosis. To overcome this limitation, Giannini et al[17] in 2003 introduced a noninvasive test based on platelet count/spleen diameter ratio, and the results were impressive. It was surprising in their study that the discriminative power of platelets/spleen diameter ratio was nearly the same as the discriminative power of platelet count alone in their population. Therefore, the excellent results of platelet count/spleen diameter ratio in their study are not explained by the discriminative power of their index, but are mainly related to the discriminative power of platelet count alone in their series. The main explanation behind this is the high rate of viral related cirrhosis in their patients where the platelet count is less liable to other variations occurring with cirrhosis due to other causes, for example, as in alcoholic cirrhosis.
For this reason, we excluded patients with liver cancer, portal vein thrombosis, and drug addiction to avoid other variations that can affect the platelet count other than portal hypertension in liver cirrhosis.
In our study, presence of EVs was significantly less frequent in patients with normal platelet count compared with thrombocytopenic patients (P < 0.019). This is in accordance with Yang et al,[18] who stated that presence of EV in cirrhotic patients was predicted by low platelet count. Our findings are also in accordance with those of Lahmidani et al,[19] who state that low platelet count (< or equal 100,000) is associated with the presence of varices in viral cirrhotic patients.
We recorded that patients with thrombocytopenia had significantly higher frequency of large grade III and IV EVs compared with patients with normal platelet counts (P < 0.009). These findings are in agreement with those of Ding et al,[20] who demonstrated that the combination of liver stiffness measurement (LSM) ≤25 kPa and platelet count ≥100 can be used in clinical practice to exclude the presence of high-risk gastro-EVs in patients with Child–Pugh class A cirrhosis.
Grading of EVs was inversely correlated with platelet count in our study. This is in agreement with the findings of Abbasi et al,[21] who stated that the severity of thrombocytopenia increased as the grading of EVs increased.
We report a platelet count cut-off value of 149,000 for presence of varices in our patients, with the specificity of 82% and the 95% confidence interval (CI) for the test being 0.523 to 0.731 (P = 0.022). Our cut-off value is higher than that recorded by other studies. Schepis et al[22] found that presence of EVs was independently predicted by platelet count less than 100 × 109/L (odds ratio [OR] 2.83, 95% CI 1.27–6.28).
Agha et al[23] found that median platelet count (82,000 vs 172,000/μL; P < 0.0001) in cirrhotic patients correlated with the presence or absence of EV, respectively.
Tafarel et al[24] revealed that factors independently associated with EVs were: thrombocytopenia (<92,000/mm3; P < 0.01) and AST higher than 1.47 × upper normal limit (UNL) (P = 0.03). A platelet count lower than 92,000/mm3 had sensitivity of 65.7%, specificity of 57.9%, and an area under the ROC curve of 0.62 for the presence of EV that needs prophylactic therapy.
We found that a cut-off value of 3.175 FIB-4 has 83.3% sensitivity and 39.5% specificity in detecting presence of large (grade III and IV) EVs. Our findings are congruent with those of Hassan et al,[25] who recorded that the diagnostic accuracy of FIB-4 for prediction of large varices was 70% at a cut-off value of 3.3.
On the other hand, Morishita et al[26] recorded a higher FIB-4 cut-off value of 7.70 for detection of high-risk varices with a sensitivity of 67% and specificity of 78%.
Whereas a normal platelet count of ≥150,000 was useful in determining absence of EV, a FIB-4 ≥3.175 was useful in screening and prediction of large varices. Calculation of FIB-4 requires use of a mathematical equation by the physician. Therefore, we believe that platelet count is a simple and useful tool for ruling out the presence of varices in cirrhotic patients. A 3.175 cut-off value of FIB-4 could be useful as a noninvasive predictor of large varices requiring prophylactic banding in cirrhotic patients.
One of the limitations of our study may be that most of patients were post hepatitis C cirrhotic patients. However, this is a reflection of the high prevalence of HCV in our country, because Egypt has the highest prevalence rate of HCV in the world.[14–16]
Other limitations were that we did not study platelet indices such as mean platelet volume (MPV), platelet distribution width (PDW), or plateletcrit (PCT). We did not measure 24-hour urinary copper excretion before and after challenge with penicillamine, so the possibility of Wilson disease was not fully excluded. Further studies on larger numbers of patients are needed to confirm the results, because we had a relatively small number of patients.
5. Conclusions
In conclusion, in this study, we have demonstrated that the platelet count is a noninvasive parameter with high accuracy for prediction of EV. Cirrhotic patients with normal platelet counts (above 150,000), especially in financially deprived developing countries, can avoid screening endoscopy, because they are at low risk for a variceal bleed and presence of large EV in these patients is much less common than in those with thrombocytopenia. A 3.175 cut-off value of FIB-4 could also be useful as a noninvasive predictor of large varices requiring prophylactic banding in cirrhotic patients.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, CI = confidence interval, CLD = chronic liver diseases, DM = diabetes mellitus, EV = esophageal varices, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, IgG = immunoglobulin G, IV = intra-venous, LSM = liver stiffness measurement, MPV = mean platelet volume, PCR = polymerase chain reaction, PCT = plateletcrit, PDW = platelet distribution width, PLT = platelets, ROC = receiving-operator characteristic, SD = standard deviation, UNL = upper normal limit, US = ultrasonography.
The authors confirm that there is no prior manuscript publication or presentation.
References
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014; 383:1749–1761. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44:217–231. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007; 102:2086–2102. [DOI] [PubMed] [Google Scholar]
- 4.Grgurević I, Jukić I, Sokol S, et al. low specificity of platelet to spleen ratio for noninvasive prediction and characterization of esophageal varices in patients with alcoholic liver cirrhosis. Acta Med Croatica 2014; 68:353–360. [PubMed] [Google Scholar]
- 5.Chiodi D, Hernández N, Saona G, et al. Noninvasive diagnosis of esophageal varices in cirrhotic patients. Acta Gastroenterol Latinoam 2014; 44:108–113. [PubMed] [Google Scholar]
- 6.Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol 2008; 48:1000–1007. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi H, Beppu T, Shirabe K, et al. Management of thrombocytopenia due to liver cirrhosis: a review. World J Gastroenterol 2014; 20:2595–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 9.Paquet KJ. Prophylactic endoscopic sclerosing treatment of esophageal wall in varices: a prospective controlled trial. Endoscopy 1982; 14:4–5. [DOI] [PubMed] [Google Scholar]
- 10.Li P, Chen F, Zhao X, et al. Occurrence and clinical significance of in-hospital acquired thrombocytopenia in patients undergoing transcatheter device closure for congenital heart defect. Thromb Res 2012; 130:882–888. [DOI] [PubMed] [Google Scholar]
- 11.De Franchis R, Dell’Era A, Primignani M. Diagnosis and monitoring of portal hypertension. Dig Liver Dis 2008; 40:312–317. [DOI] [PubMed] [Google Scholar]
- 12.D’ Amico G, Morabito A. Noninvasive markers of esophageal varices: another round, not the last. Hepatology 2004; 39:30–34. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Tsao G, Escorsell A, Zakko M, et al. Predicting the presence of significant portal hypertension and varices in compensated cirrhotic patients. Hepatology 1997; 26:360A. [Google Scholar]
- 14.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005; 5:558–567. [DOI] [PubMed] [Google Scholar]
- 15.Sievert W, Altraif I, Razavi HA, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011; 31 Suppl 2:61–80. [DOI] [PubMed] [Google Scholar]
- 16.Strickland GT. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology 2006; 43:915–922. [DOI] [PubMed] [Google Scholar]
- 17.Giannini E, Botta F, Borro P, et al. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut 2003; 52:1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YY, Hou MC, Lin MW, et al. Combined platelet count with sCD163 and genetic variants optimizes esophageal varices prediction in cirrhotic patients. J Gastroenterol Hepatol 2013; 28:112–121. [DOI] [PubMed] [Google Scholar]
- 19.Lahmidani N, El Fakir S, Benyachou B, et al. Noninvasive predictors of presence and grade of esophageal varices in viral cirrhotic patients. Pan Afr Med J 2015; 20:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding NS, Nguyen T, Iser DM, et al. Liver stiffness plus platelet count can be used to exclude high-risk oesophageal varices. Liver Int 2015; 25. [DOI] [PubMed] [Google Scholar]
- 21.Abbasi A, Butt N, Bhutto AR, et al. Correlation of thrombocytopenia with grading of esophageal varices in chronic liver disease patients. J Coll Phys Surg Pakistan 2010; 20:369–372. [PubMed] [Google Scholar]
- 22.Schepis F, Camma C, Niceforo D, et al. Which patients with cirrhosis should undergo endoscopic screening for esophageal varices detection? Hepatology 2001; 33:333–338. [DOI] [PubMed] [Google Scholar]
- 23.Agha A, Abdulhadi MM, Marenco S, et al. Use of the platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices in patients with schistosomiasis. Saudi J Gastroenterol 2011; 17:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tafarel JR, Tolentino LH, Correa LM, et al. Prediction of esophageal varices in hepatic cirrhosis by noninvasive markers. Eur J Gastroenterol Hepatol 2011; 23:754–758. [DOI] [PubMed] [Google Scholar]
- 25.Hassan EM, Omran DA, El Beshlawey ML, et al. Can transient elastography, Fib-4, Forns Index, and Lok Score predict esophageal varices in HCV-related cirrhotic patients? Gastroenterol Hepatol 2014; 37:58–65. [DOI] [PubMed] [Google Scholar]
- 26.Morishita N, Hiramatsu N, Oze T, et al. Liver stiffness measurement by acoustic radiation force impulse is useful in predicting the presence of esophageal varices or high-risk esophageal varices among patients with HCV-related cirrhosis. J Gastroenterol 2014; 49:1175–1182. [DOI] [PubMed] [Google Scholar]


