Abstract
Stem cells are being widely investigated for a wide variety of applications in tissue engineering due to their ability to differentiate into a number of cells such as neurons, osteoblasts, and fibroblasts. This ability of stem cells to differentiate into different types of cells is greatly based on mechanical and chemical cues received from their three-dimensional environments. All organs are formed by a number of cells linked together via an extracellular matrix (ECM). The ECM is a complex network of proteins and carbohydrates, which occupies intercellular spaces and regulates cellular activity by controlling cell adhesion, migration, proliferation, and differentiation. The ECM is composed of two main types of macromolecules, namely, polysaccharide glycosaminoglycans, which are covalently attached to proteins in the form of proteoglycans and fibrous proteins belonging to two functional groups, structural (collagen and elastin) and adhesive (fibronectin, laminin, vitronectin, etc). Tissue engineering is a multidisciplinary field that aims to develop biomimetic scaffolds that emulate properties of the ECM to help repair or regenerate diseased or damaged tissue. This study introduces one of these matrices, XanoMatrix, as an optimal scaffold for tissue engineering applications, in particular, for stem cell research, based on its composition, nanofibrous structure, and porosity. Results of this study suggest that XanoMatrix scaffolds are promising for stem cell tissue engineering applications and as improved cell culture inserts for studying stem cell functions (compared to traditional Corning and Falcon cell culture plates) and, thus, should be further studied.
Keywords: stem cells, cell culture inserts, tissue engineering, nanofibrous matrices
Introduction
Tissue engineering is a multidisciplinary research field that has gained popularity over the past few years. It involves the repair/regeneration of diseased/damaged tissue to restore such tissue to partial or full functionality.1–4 Tissue engineering scaffolds are often designed for a variety of functions and have versatile properties that enable them to perform those functions. In general, all tissue engineering scaffolds must be porous; biocompatible; must promote the attachment, migration, proliferation, and differentiation of cells in a natural way; must promote the secretion of extracellular matrix (ECM) proteins in a natural way; and must degrade into nontoxic materials that can be safely eliminated from the body. In addition to these characteristics, tissue engineering scaffolds must have a large volume fraction of interconnected pores to facilitate the migration of cells as well as diffusion of growth factors and nutrients. The pore size must be tailored according to specific cell morphologies, they must have sufficient mechanical integrity to be handled easily during surgery, and finally they must degrade at a controllable rate so that the targeted tissue becomes fully formed via ECM deposition as the scaffold is fully resorbed.
Of course, most tissues are three dimensional (3D) for proper cell functions and are responsible for providing cells with a specific growth environment, structure, water diffusion, as well as cytokine, growth factor, and nutrient availability.5 Therefore, in order to expedite cell growth and proliferation for tissue formation and regeneration, it is necessary to develop a 3D scaffold.5–7 In addition, if designed correctly, these scaffolds can help in the development of new vasculature and innervation,7,8 and they could actively participate in the tissue regenerative process through the release of growth/differentiation factors, present in their structure.9
The next step after selecting an adequate biodegradable polymer is to develop or choose an adequate fabrication process. In order to do so, and to be sure that all the scaffold characteristics are fulfilled, the chosen processing technique should not adversely affect the biocompatibility and chemical properties of the implant and it should be consistent with regard to maintaining porosity, pore distribution, pore size, and interconnectivity.10 Furthermore, scaffold properties should be highly repeatable when more than one scaffold is produced from the same batch with the same processing technique.
Thus, based on all this information, despite their popularity, it is clear that traditional cell culture substrates are poor replicates of the complex 3D in vivo complex ECM environment insufficiently allowing cells to communicate with each other and their environment via cytokine release. In traditional two-dimensional (2D) cell culture systems, cells are often grown on a flat surface that lacks all these characteristics and are not true representatives of the natural in vivo system.11 The growth of a cellular monolayer is significantly different than a 3D cellular structure.12 This 2D confinement is far removed from the aforementioned 3D complexities of living tissue, yet such systems are still widely used today across all aspects of in vitro assays.13 Such problems are exacerbated when conducting stem cell research and put into question stem cell research that is conducted on nonbiologically inspired 2D cell culture plates.
There are a variety of technologies available that enable the growth of cells in a 3D environment, but these technologies have not necessarily been developed for performing general cell culture assays in day-to-day laboratory experiments. These include naturally occurring materials as well as products fabricated from naturally derived and synthetic polymers. Natural substrates (such as alginate, which is a seaweed-derived material) have been used to support cell growth in a number of ways including cell encapsulation.14 It is true that this technology enables the growth of cells in a 3D environment, but this spherical mass is not necessarily very useful as the distribution of cells throughout this mass could be different with different rates of mass transfer. An alternative, and one of the most early successful approaches, has been the culture of cells on biodegradable polymers such as poly(glycolic acid), poly(lactic acid), and their copolymers poly(lactic-co-glycolic acid).15 Hydrogels are also common and have been successfully used to support ex vivo 3D stem cell growth for a variety of systems, such as bone, cartilage, and nervous system tissues.16
Through the years, a series of processing techniques (such as solvent casting, phase inversion, fiber bonding, melt-based technologies, high pressure-based methods, freeze drying, and rapid prototyping technologies) were developed with the aim of producing scaffolds with adequate properties for tissue engineering, but all these processes have significant variabilities making them hard to monitor for consistency and are also economically less viable.
This study introduces a new method, XanoShear™, which is a novel manufacturing method capable of creating nanofibers through a combination of shear forces and polymer phase separation without the use of nozzles or spinnerets. It is significantly different from its counterpart, electrospinning, where an electrical charge draws individual fibers to a grounded surface. In addition, this technique uses short staple fibers instead of continuous fibers. These fibers do not have fixed pore sizes of electrospun materials, but have a close biomimetic material of natural ECM.
A polymer solvent, which is miscible with the medium but still precipitates in the medium, is chosen, and the bulk polymer is introduced into the viscous medium under shear. The ultralow interfacial tension between the polymer droplets and the medium gives rise to thin individual fibers with a high degree of stretching and great surface area. The shear force causes the solvent to be highly stretched in parallel as the solvent diffuses out of the droplets leaving behind polymer fibers in a diameter range of 50 nm to 2 µm. This process can be used to draw nanofibers from a variety of different materials. Functional groups of interest can also be added to the material by simply adding them to the polymer solvent. Due to the aforementioned and the relatively unstudied application of nanofibers prepared in this manner for stem cell culture applications, the objective of the current in vitro study was to determine the functions of stem cells on a biologically inspired nanofibrous XanoMatrix compared to traditional tissue culture polystyrene dishes.
The XanoMatrix tested here was made with biocompatible materials (polyethylene terephthalate) and cellulose acetate (CA). Polyethylene terephthalate, also known as Dacron, has been widely used as a prosthetic vascular graft and has shown excellent mechanical strength and good biocompatability.17 CA is an industrially important cellulose ester with good mechanical and wetting properties. CA nanofibers have also been increasingly used in tissue engineering.18 XanoMatrix scaffolds offer the advantage of mimicking the natural stem cell environment while combining the advantage of nanofibered tortuous beds and supports. XanoMatrix scaffolds can be easily placed in traditional six-well, 48-well, and 96-well formats as well as 10″×11″ sheets for custom shapes and sizes, which makes them excellent candidates for improved stem cell culture.
Materials and methods
Surface characterization
Scanning electron microscopy
A Hitachi 4800-S scanning electron microscopy with a voltage of 5.0 kV and a magnification of 250× was used to visualize the surface of the samples.
Contact angle analyses
The wettability of the samples was determined using a Pioneer contact angle 300 goniometer. The tests were performed using three different liquids with different viscosities. The liquids used were distilled water, polyethylene glycol, and also 70% ethanol.
Cell assays
Cell culture
This study was approved by the ethics committee of Northeastern University. Mesenchymal stem cells, purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA; catalog number PCS-500-012™, population numbers 1–3), were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 17.5% fetal bovine serum (ATCC® 30-2300™) and 1% penicillin (HyClone; Thermo Fisher Scientific, Waltham, MA, USA). The samples were sterilized with 70% ethanol for 20 minutes and then rinsed thrice with phosphate-buffered saline. An MTS (CellTiter 96® AQueous One Solution Cell Proliferation Assay, G3581; Promega Corporation, Fitchburg, WI, USA) assay was used to determine cell adhesion and proliferation after 1 day, 7 days, and 14 days of culture. The cells were seeded at 5,000 cells/cm2 and 3,500 cells/cm2 for the adhesion and proliferation assays, respectively. For the proliferation assay, the media were changed every other day. The MTS reagent (1:5 ratio with cell culture media) was added to each well and incubated for 3 hours on the day of the measurement. Absorbance from each well was measured by a SpectraMax M3(MT05412) at 490 nm, and a color change from pink to dark brown was seen. A standard curve was created with known number of cells to correlate absorbance to cell numbers.
Confocal microscopy
The data gathered by the MTS assays were verified by using confocal microscopy (LSM 700; Carl Zeiss Meditec AG, Jena, Germany) after fixing the cells with glutaraldehyde followed by successive dehydration using 50%, 70%, 90%, and 100% ethanol and then staining with a 20 nm Syto 9 dye.
Alkaline phosphatase assay
Alkaline phosphatase (ALP) is an enzyme produced by osteoblasts (bone-forming cells) to release phosphate groups for the formation of calcium phosphate in bone. A BioAssay QuantiChrom™ Alkaline Phosphatase Assay Kit (DALP-250) was used for the colorimetric kinetic determination of serum ALP activity. After equilibrating reagents to room temperature, the working solution was prepared by mixing, for each 96-well assay, 200 µL of the assay buffer, 5 µL of an acetate solution (final 5 mM), and 2 µL of the p-nitrophenylphosphate liquid substrate (10 mM). Two hundred microliters of distilled water (H2O) and 200 µL of a calibrator solution were transferred into separate wells of a clear bottom 96-well plate. Then, 5–50 µL samples were carefully transferred into other wells, and 150–195 µL of the working solution was pipetted into sample wells. The final reaction volume in the sample wells was 200 µL. The optical density was measured at 405 nm (t=0) and again after 4 minutes (t=4 minutes) on a plate reader. ALP measurements were normalized to the reference plate after day 1 and expressed as ALP-fold induction.
Statistical analysis
All experiments were conducted in triplicate and were repeated at least three different times with analysis of variance (ANOVA) followed by Student’s t-tests to determine statistical differences between mean values.
Results and discussion
In vitro cells can behave very differently depending on the growth substrate employed. Conventional tissue culture is carried out on 2D surfaces with the assumption that cells adopt natural morphologies and communicate efficiently with their neighbors under such an environment. However, this 2D confinement is far removed from the 3D complexities of living tissue. Engineering the cell culture micro- and nanoenvironment to create growth conditions that more accurately stimulate the in vivo behavior of stem cells is an essential step for improving predictive accuracy during pharmaceutical development/clinical trials.
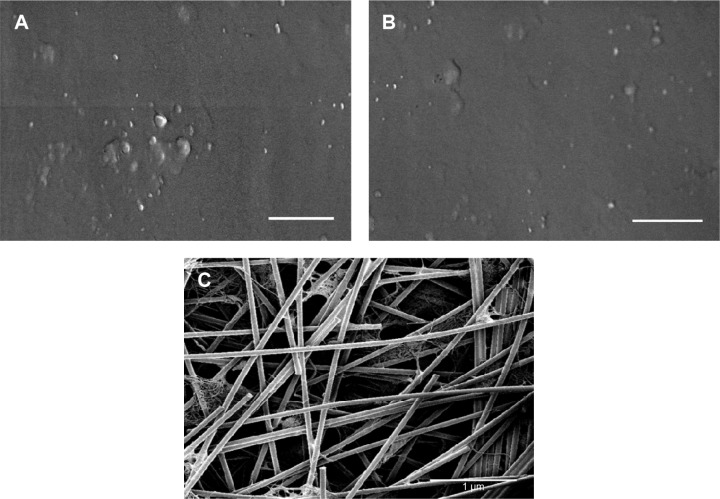
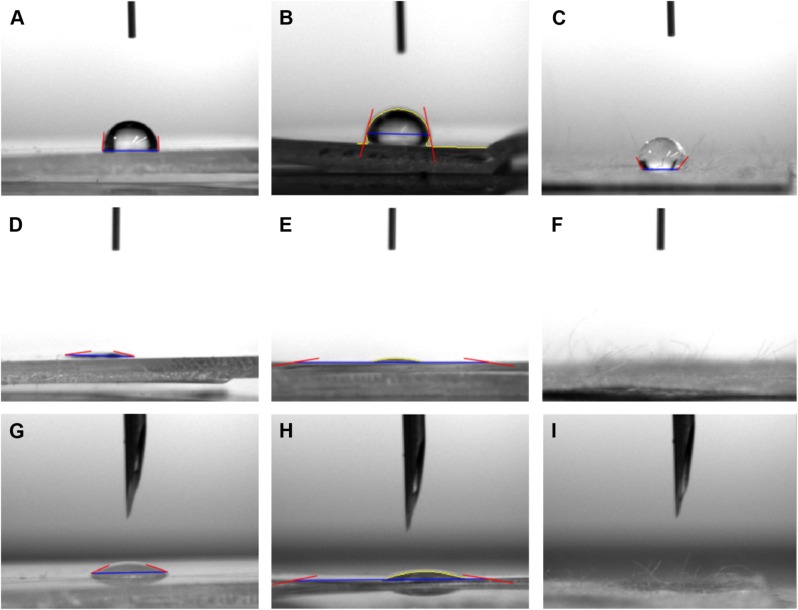
Results of the current study showed that, as expected, the surface of the XanoMatrix scaffold possessed nanofibers and was 3D in nature as compared to the 2D Corning and Falcon petri dishes (Figure 1). Thus, the XanoMatrix scaffold was more replicative of a biologically inspired in vivo environment for stem cell growth and proliferation. In addition, results showed that the XanoMatrix surface was more hydrophobic as compared to the Corning and Falcon petri dishes (Figure 2).
Figure 1.
SEM images of the surfaces of the (A) Corning; (B) Falcon; and (C) XanoMatrix samples.
Note: Scale bar =1 µm.
Abbreviation: SEM, scanning electron microscopy.
Figure 2.
Contact angle images of the surfaces of (A) Corning (81.53°, distilled water); (B) Falcon (76.724°, distilled water); (C) XanoMatrix (121.593°, distilled water); (D) Corning (12.54°, 70% ethanol); (E) Falcon (10.42°, 70% ethanol); (F) XanoMatrix (0°, 70% ethanol); (G) Corning (21.7°, PEG); (H) Falcon (10.4484°, PEG); and (I) XanoMatrix (0°, PEG).
Abbreviation: PEG, polyethylene glycol.
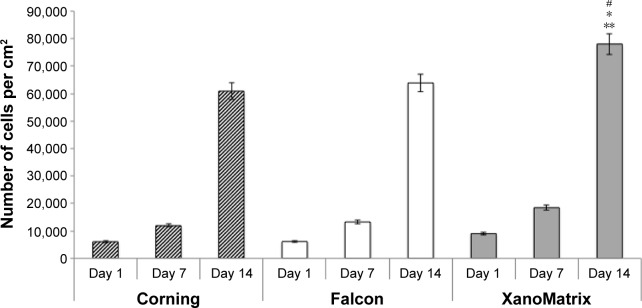
As expected, it was observed for the first time in this study that the XanoMatrix cell culture well plates increased stem cell adhesion and proliferation as compared to the traditional Corning and Falcon cell culture petri dishes, since they offered a more biologically inspired 3D nanofiber structure (Figure 3). In addition, the ALP assay indicated increased ALP activity by stem cells on the XanoMatrix surface compared to the other surfaces (Figure 4), suggesting their differentiation into osteoblasts (bone-forming cells), although more studies are needed to confirm this (Figure 5). There was almost a four times increase in ALP on the 14th day of stem cell culture on the XanoMatrix samples as compared to the Corning and Falcon samples.
Figure 3.
Stem cell adhesion and proliferation on Corning, Falcon, and XanoMatrix cell culture petri dishes after 1 day, 7 days, and 14 days.
Notes: Data are expressed as the mean ± standard error of the mean; N=4; *P<0.01 as compared to Corning and Falcon petri dishes on the seventh day of culture; **P<0.01 as compared to the Corning and Falcon petri dishes on the 14th day of culture; and #P<0.01 as compared to XanoMatrix cell culture petri dishes on the first day of cell culture.
Figure 4.
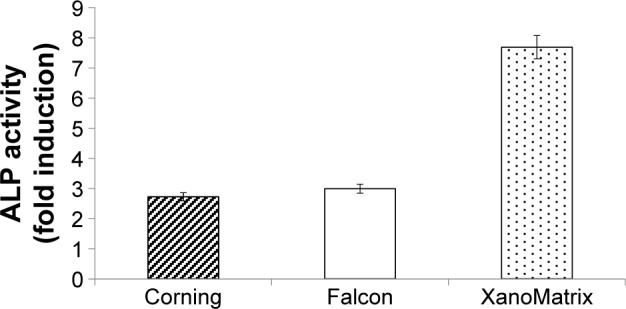
ALP activity fold induction as observed on the Corning, Falcon, and XanoMatrix surfaces after 14 days of culture.
Notes: Results suggest stem cell differentiation into osteoblast on the XanoMatrix scaffold. ALP activity is significantly (P<0.01) greater on XanoMatrix than Corning and Falcon.
Abbreviation: ALP, alkaline phosphatase activity.
Figure 5.
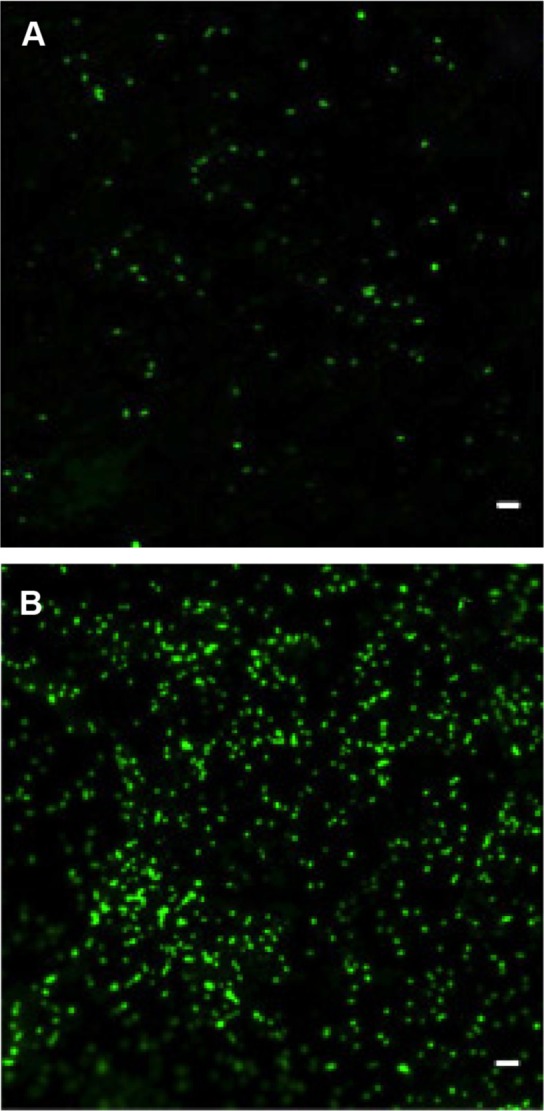
Cell density on XanoMatrix surfaces at different time points.
Notes: (A) and (B) MSC cell density on XanoMatrix surfaces at 1 day and 14 days, respectively; scale bar =60 µm.
Abbreviation: MSC, mesenchymal stem cell.
As to possible reasons why, in addition to a more biologically inspired 3D surface, the XanoMatrix surface had increased hydrophobicity as compared to the other control surfaces used in this study. Alteration in surface energy, as observed here, can change initial protein interactions (such as adsorption and bioactivity), which will influence stem cell responses. The authors hypothesize that the increasing hydrophobicity of the scaffold causes some conformational changes in the pendant side chains of the scaffold material, which causes differential binding of proteins to the scaffold surface, in turn, affecting the adhesion and proliferation of cells. Further studies are being performed to confirm this theory.
Scaffolds and polymers used for cell culture are not inert, and various factors such as surface topography, chemical composition, and surface energy/wettability control their interactions with cells and the surrounding environment.19 Hence, an improvement in the biocompatibility of scaffolds/materials is dependent on these factors.20 The 3D growth process of cells offers the advantage of mimicking true 3D nutrient exchange, cell migration, and invasion by cells and this is widely used for developing synthetic materials for cancer, drug delivery, tissue engineering, and stem cell research. Nanofibers clearly mimic native tissue, offer high shape flexibility and batch-to-batch consistency, and are not derived from animal-based products as opposed to hydrogels. As shown here for the first time, XanoMatrix scaffolds combine all of these advantages to offer a true 3D platform for testing cell growth and proliferation. Future studies will have to elucidate why stem cells respond this way to XanoMatrix, since numerous material properties, including nanoscale surface features, energy, and chemistry alter cell functions. Nonetheless, this study introduces a new material, XanoMatrix, as a promising scaffold that requires further study for stem cell interactions for improved cell culture and/or tissue engineering applications.21
Conclusion
This study shows that replacing the use of traditional tissue culture-treated polystyrene petri dishes with more biologically inspired XanoMatrix scaffolds provided a better environment for the adhesion and growth of mesenchymal stem cells, thus making it a suitable choice for studying tissue engineering materials. In this manner, this study shows that cell culture dishes that mimic features of natural tissues should be continually studied for a wide range of applications in which mimicking natural stem cell functions is important.
Acknowledgments
The authors would like to thank Xanofi for providing the materials for the study and for funding.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Teti A. Regulation of cellular functions by extracellular matrix. J Am Soc Nephrol. 1992;2(10 Suppl):S83–S87. doi: 10.1681/ASN.V210s83. [DOI] [PubMed] [Google Scholar]
- 2.Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60(2):184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29(13):1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(Suppl 1):SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 5.Kneser U, Schaefer DJ, Munder B, Klemt C, Andree C, Stark GB. Tissue engineering of bone. Minim Invasive Ther Allied Technol. 2002;11(3):107–116. doi: 10.1080/136457002320174177. [DOI] [PubMed] [Google Scholar]
- 6.Laurencin CT, Ambrosio AM, Borden MD, Cooper JA., Jr Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 7.Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21(24):2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 8.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculosk-eletal tissue engineering. J Biomed Mater Res. 2001;55(2):141–150. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003;24(13):2363–2378. doi: 10.1016/s0142-9612(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 11.Bhadriraju K, Chen CS. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov Today. 2002;7(11):612–620. doi: 10.1016/s1359-6446(02)02273-0. [DOI] [PubMed] [Google Scholar]
- 12.Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116(Pt 12):2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltman DJ, Przyborski SA. Developments in three-dimensional cell culture technology aimed at improving the accuracy of in vitro analyses. Biochem Soc Trans. 2010;38(4):1072–1075. doi: 10.1042/BST0381072. [DOI] [PubMed] [Google Scholar]
- 14.de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27(32):5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated 3-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14(5):323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- 16.Blackshaw SE, Arkison S, Cameron C, Davies JA. Promotion of regeneration and axon growth following injury in an invertebrate nervous system by the use of three-dimensional collagen gels. Proc Biol Sci. 1997;264(1382):657–661. doi: 10.1098/rspb.1997.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewez JL, Doren A, Schneider YJ, Rouxhet PG. Competitive adsorption of proteins: key of the relationship between substratum surface properties and adhesion of epithelial cells. Biomaterials. 1999;20(6):547–559. doi: 10.1016/s0142-9612(98)00207-5. [DOI] [PubMed] [Google Scholar]
- 18.Arbeitman CR, del Grosso MF, Ibañez I, et al. Irradiation of polystyrene and polypropylene to study NIH 3T3 fibroblasts adhesion. Nucl Instrum Methods Phys Res B. 2010;268(19):3059–3062. [Google Scholar]
- 19.Li Y, Ma T, Yang ST, Kniss DA. Thermal compression and characterization of three-dimensional nonwoven PET matrices as tissue engineering scaffolds. Biomaterials. 2001;22(6):609–618. doi: 10.1016/s0142-9612(00)00224-6. [DOI] [PubMed] [Google Scholar]
- 20.Ali S, Khatri Z, Oh K, Kim I-S, Kim S. Zein/cellulose acetate hybrid nanofibers: electrospinning and characterization. Macromol Res. 2014;22(9):971–977. [Google Scholar]
- 21.Dalby MJ, Gadegaard N, Oreffo RO. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13(6):558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]