During retinogenesis, lamin B1 is critical to maintaining the nuclear integrity of embryonic retinal neurons, whereas lamin B2 is not. The latter is required for postnatal retinal lamination and synaptogenesis. In adult photoreceptors, lamin B1 and lamin B2 have very long half-lives but are dispensable for cone photoreceptor survival and function.
Abstract
Lamin B1 and lamin B2 are essential building blocks of the nuclear lamina, a filamentous meshwork lining the nucleoplasmic side of the inner nuclear membrane. Deficiencies in lamin B1 and lamin B2 impair neurodevelopment, but distinct functions for the two proteins in the development and homeostasis of the CNS have been elusive. Here we show that embryonic depletion of lamin B1 in retinal progenitors and postmitotic neurons affects nuclear integrity, leads to the collapse of the laminB2 meshwork, impairs neuronal survival, and markedly reduces the cellularity of adult retinas. In stark contrast, a deficiency of lamin B2 in the embryonic retina has no obvious effect on lamin B1 localization or nuclear integrity in embryonic retinas, suggesting that lamin B1, but not lamin B2, is strictly required for nucleokinesis during embryonic neurogenesis. However, the absence of lamin B2 prevents proper lamination of adult retinal neurons, impairs synaptogenesis, and reduces cone photoreceptor survival. We also show that lamin B1 and lamin B2 are extremely long-lived proteins in rod and cone photoreceptors. OF interest, a complete absence of both proteins during postnatal life has little or no effect on the survival and function of cone photoreceptors.
INTRODUCTION
The nuclear lamina is a filamentous meshwork located adjacent to the inner nuclear membrane. It is composed of A-type lamins (lamins A and C) and B-type lamins (lamins B1 and B2), all of which are type V intermediate filament proteins (Dechat et al., 2008; Worman et al., 2009). A-type lamins are alternatively spliced products of a single gene, LMNA, whereas lamins B1 and B2 are encoded by separate genes, LMNB1 and LMNB2. LMNB1 and LMNB2 are expressed in all nucleated cells from the earliest stages of development, whereas LMNA is expressed at very low levels until late in embryonic development (Stewart and Burke, 1987; Rober et al., 1989; Coffinier et al., 2011).
A-type lamins have attracted considerable interest, largely because LMNA mutations have been implicated in a host of human genetic diseases, including muscular dystrophy, cardiomyopathy, partial lipodystrophy, and progeroid syndromes (Worman et al., 2009). Hundreds of mutations in LMNA have been documented, and most cause dominantly inherited disease. When Lmna is inactivated in mice, embryonic development is not affected, a finding consistent with the negligible levels of Lmna expression during development (Stewart and Burke, 1987; Rober et al., 1989; Coffinier et al., 2011). However, Lmna-deficient mice succumb to muscular dystrophy and cardiomyopathy within weeks after birth. The synthesis of both lamin A and C is not required to prevent the emergence of disease phenotypes; lamin A–only mice and lamin C–only mice are healthy and entirely free of disease (Fong et al., 2006; Coffinier et al., 2010c; Davies et al., 2010).
In comparison to A-type lamins, B-type lamins were neglected for many years. One of the reasons for the reduced interest in B-type lamins is a paucity of disease-causing mutations in LMNB1 and LMNB2. LMNB1 gene duplications have been shown to cause an adult-onset leukoencephalopathy (Padiath et al., 2006; Padiath and Fu, 2010), but, in contrast to the situation with LMNA, no missense or null mutations in LMNB1 have been linked to human disease. In the case of LMNB2, a homozygous missense mutation was uncovered in two siblings with progressive myoclonus epilepsy (Damiano et al., 2015). A second reason for the relative neglect of B-type lamins is that they were simply considered to be “housekeeping proteins” with unique and essential functions in the cell nucleus, including DNA transcription and the assembly of the mitotic spindle (Tsai et al., 2006; Dechat et al., 2008; Shimi et al., 2008). However, similar to A-type lamins, B-type lamin depletion induces significant defects of nuclear structure both in vitro (Shimi et al., 2008) and in vivo (Vergnes et al., 2004; Coffinier et al., 2010a, 2011).
During the past few years, interest in B-type lamins has grown, primarily because of new insights from knockout models. Mice lacking both B-type lamins in keratinocytes and hepatocytes have no obvious disease phenotypes (Yang et al., 2011a, b), casting considerable doubt on the notion that B-type lamins play unique and essential roles in the cell nucleus. Of greater importance, Coffinier and coworkers created Lmnb1- and Lmnb2-knockout mice and discovered that deficiencies in either protein lead to neuronal layering abnormalities in the cerebral cortex, a consequence of impaired migration of neurons from the ventricular zone to the cortical plate during development (Coffinier et al., 2010a, b, 2011; Young et al., 2012). Those studies uncovered a role for B-type lamins in the brain, but key issues were left unresolved. One was an understanding of distinct effects of lamin B1 deficiency and lamin B2 deficiency in the development of the CNS. A second was whether B-type lamins are important only during CNS development or remain equally crucial in the CNS of adult mice after development is complete.
To elucidate further the functions of B-type lamins in the development and homeostasis of the CNS, we created new knockout models that lacked lamin B1 and/or lamin B2 at different stages of retinal development. We chose to examine the retina for several reasons. First, like other regions of the CNS, retinal development is initiated from asymmetric divisions of retinal progenitor cells (RPCs) on the apical side of the outer neuroblast layer (ONBL), generating postmitotic neurons that migrate toward the inner neuroblast layer (INBL). Second, the retina is a nonessential region of the CNS; thus one can avoid the early postnatal death of conventional Lmnb1- and Lmnb2-knockout mice (Vergnes et al., 2004; Coffinier et al., 2010a, 2011; Kim et al., 2011). Third, standard electrophysiological methods make it possible to quantify the effects of genetic modifications on retinal function. Finally, retinal development involves the same nucleokinesis processes that are essential for the development of the cerebral cortex. The first of these nucleokinesis processes is interkinetic nuclear migration (INM), which is essential for the birth of new neurons (Kosodo, 2012; Strzyz et al., 2015). During INM, the nucleus of neuronal progenitor cells moves basally during G1 and apically during G2. When nuclear movement during INM is impaired, cell cycle progression does not occur, preventing the birth of new neurons. A second nucleokinesis process takes place as newborn neurons migrate to their proper laminar position. In this process, cytoplasmic motors, acting along microtubules, pull the nucleus into the leading edge of the cell. When nucleokinesis is defective, neuronal migration is also defective, leading to a neuronal lamination defect (Ayala et al., 2007; Zhang et al., 2009).
RESULTS
Lamin B1 is required for retinogenesis
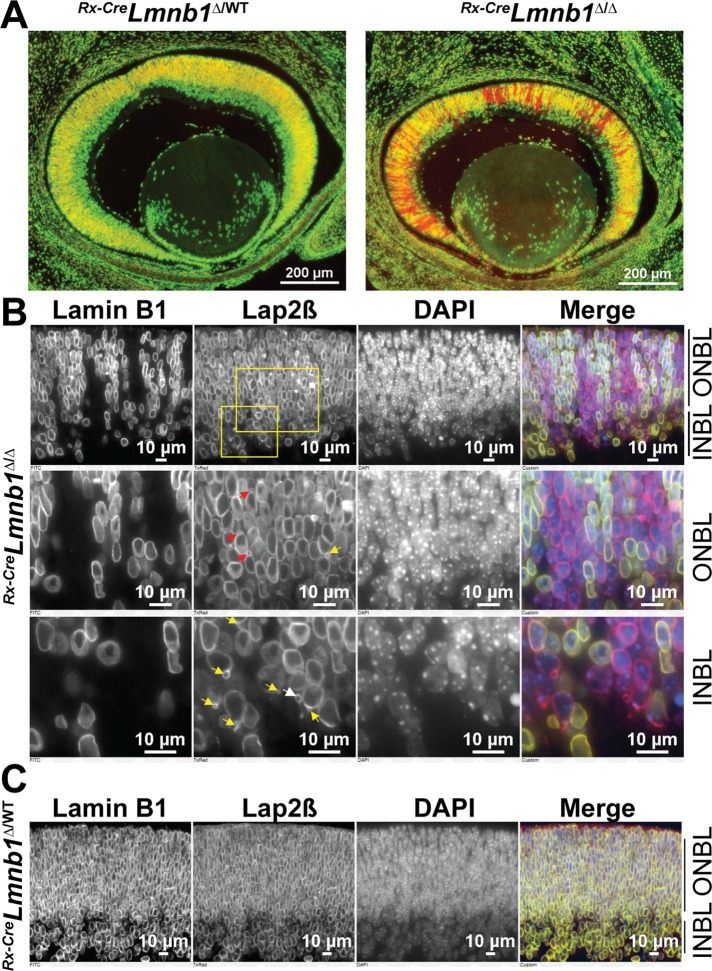
To examine the respective contributions of lamin B1 and lamin B2 in embryonic and postnatal retinal development, we inactivated Lmnb1 and/or Lmnb2 in the retina at different developmental time points. In our initial studies, mice harboring floxed alleles of Lmnb1 or Lmnb2 (Coffinier et al., 2011; Yang et al., 2011a) were bred with Rx-Cre transgenic mice (Swindell et al., 2006). The Rx-Cre transgene is expressed in a variegated manner in the developing eye field, beginning at embryonic day 9.5 (E9.5). As a result of the variegation in Cre expression, the retinas of E14.5 Rx-CreLmnb1fl/fl mice (here called Rx-CreLmnb1Δ/Δ mice) had columns of RPCs expressing lamin B1 adjacent to columns of RPCs devoid of lamin B1 expression (Figure 1A). Immunostaining of the retina for Lap2β, an inner nuclear membrane protein, revealed numerous nuclear blebs in lamin B1–deficient RPCs (Figure 1B). In the ONBL (distinguished by tightly packed cells and intense 4′,6-diamidino-2-phenylindole [DAPI] staining), the nuclear blebs were found on either the apical or the basal side of RPC nuclei (Figure 1B, middle). In the INBL (located beneath the ONBL), the nuclear blebs were found on the basal side of adjacent nuclei (Figure 1B, bottom). In the INBL, micronuclei were often observed near a nucleus, likely representing nuclear blebs that had detached from the nucleus (Figure 1B, bottom, white arrow, and Supplemental Figure S1A). These micronuclei contained chromatin and were often topped with a Lap2β-positive thread (Supplemental Figure S1, A and B). No micronuclei were detected in retinas of Rx-CreLmnb1Δ/WT littermate control mice (Figure 1C). Thus lamin B1 depletion in neurons in the ONBL and INBL results in striking morphological abnormalities in the cell nucleus.
FIGURE 1:
Lamin B1 deficiency in mouse embryonic retina results in the formation of nuclear blebs. (A) Lamin B1 immunofluorescence pattern (green) in E14.5 Rx-CreLmnb1Δ/WT (left) and Rx-CreLmnb1Δ/Δ (right) embryonic retinas counterstained with DAPI (pseudocolored in red). Owing to the variegated expression of the Rx-Cre transgene, there is a mosaic pattern of Lmnb1 inactivation in the retina, resulting in columns of lamin B1–expressing and lamin B1–deficient cells. (B) Immunostaining of E14.5 Rx-CreLmnb1Δ/Δ retinas with antibodies for lamin B1 and Lap2β. In the merged image, lamin B1 is green and Lap2β is red; DNA was stained with DAPI (blue). Top, overview; middle, zoomed-in view of ONBL; bottom, zoomed-in view of inner INBL. Red and yellow arrows point to apical and basal nuclear blebs, respectively. White arrow points to a solitary bleb within the INBL. See also Supplemental Figure S1. (C) Identical immunohistochemistry studies on retinas from E14.5 Rx-CreLmnb1Δ/WT littermate control mice.
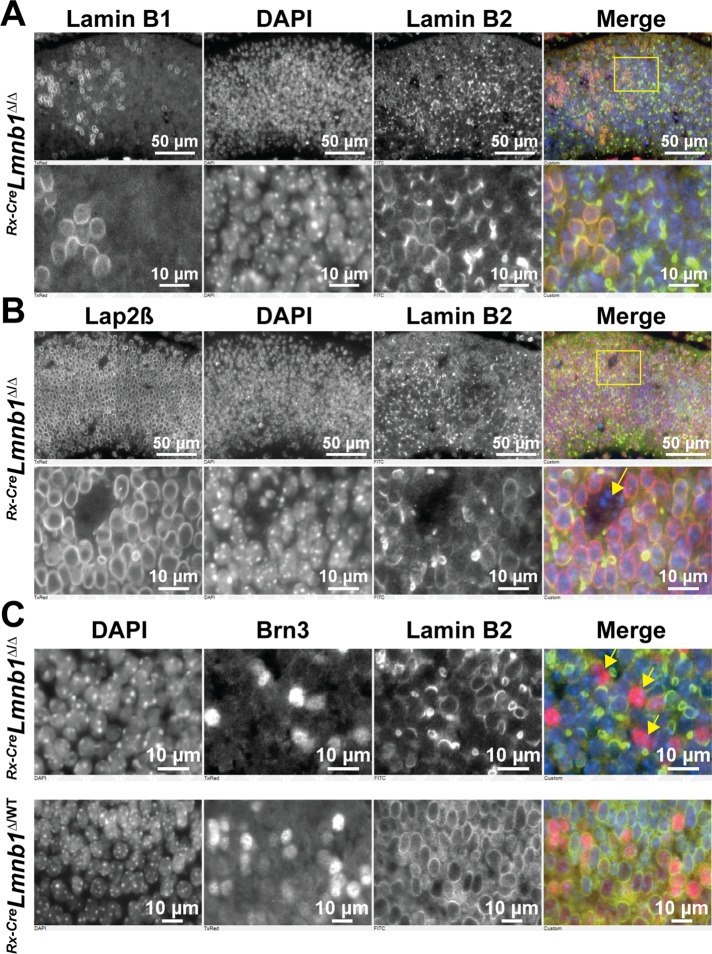
We next examined lamin B2 localization in E14.5 Rx-CreLmnb1Δ/Δ retinas (Figure 2A). In cells lacking lamin B1, most of the lamin B2 meshwork was located asymmetrically in one segment of the nuclear rim and often was found exclusively in a large nuclear bleb, suggesting that the lamin B2 meshwork had simply collapsed into one segment of the nucleus. In contrast, Lap2β was symmetrically distributed along the entire circumference of the nucleus (Figure 2B). In a few cells, small amounts of lamin B1 persisted and could be detected in nuclear blebs (Supplemental Figure S1A).
FIGURE 2:
Inactivation of Lmnb1 during embryonic development causes a collapse of the lamin B2 meshwork but does not prevent the birth of new postmitotic neurons. (A) Immunostaining of E14.5 Rx-CreLmnb1Δ/Δ retinas with lamin B1 and lamin B2. In the merged image, lamin B1 is red and lamin B2 is green; DNA was stained with DAPI (blue). Bottom, zoomed-in views of the yellow boxed area in top. (B) Immunostaining of E14.5 Rx-CreLmnb1Δ/Δ retinas with Lap2β and lamin B2. In the merged image, Lap2β is red and lamin B2 is green; DNA was stained with DAPI (blue). Bottom, zoomed-in views of the yellow boxed area in the top. The yellow arrow points to apoptotic nuclei in a gap. (C) Immunostaining of retinas from E14.5 Rx-CreLmnb1Δ/Δ (top) and Rx-CreLmnb1Δ/WT (bottom) mice with antibodies against lamin B2 and Brn3, a marker of RGCs. In the merged image, Brn3 is red and lamin B2 is green; DNA was stained with DAPI (blue). The arrows point to misshapen nuclei of RGCs. These studies show that RGCs devoid of lamin B1 are present and have irregularly shaped nuclei (yellow arrows). See also Supplemental Figure S2.
At E14.5, the ONBL is mostly populated by RPCs (Supplemental Figure S2A) and a population of newborn retinal ganglion cells (RGCs) that are migrating from the apical side of the ONBL toward the INBL. To determine whether RGCs are generated from RPCs in the absence of lamin B1, we stained E14.5 Rx-CreLmnb1Δ/Δ retinas with an antibody against Brn3 (a marker of RGCs) and an antibody against lamin B2. As shown in Figure 2C and Supplemental Figure S2B, RGC nuclei lacking lamin B1 could be detected, but most of them were misshapen. The identification of newborn RGCs lacking lamin B1 demonstrates that lamin B1 is not absolutely essential for exit of neurons from the cell cycle.
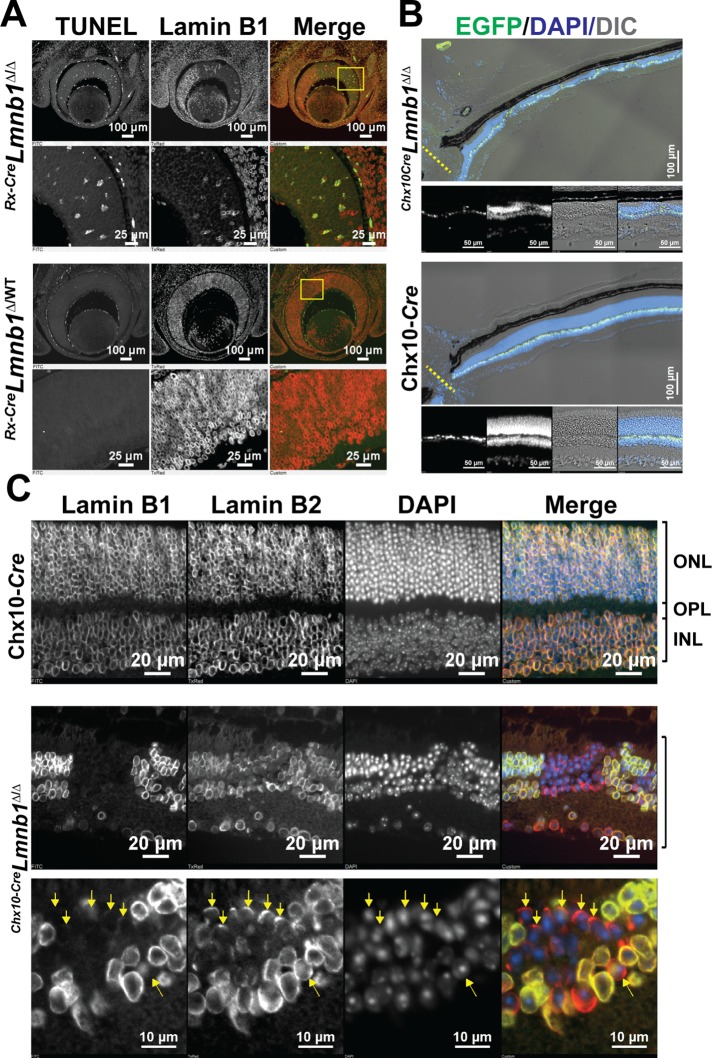
We often observed unstained “gaps” across Rx-CreLmnb1Δ/Δ retinas. These gaps contained condensed chromatin, as judged by DAPI staining, raising the possibility that they contained apoptotic cells (Figure 2B, bottom merge). To assess the possibility of increased apoptosis, we performed terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining assays. Those studies revealed more apoptosis in lamin B1–deficient zones of E14.5 Rx-CreLmnb1Δ/Δ retinas (Figure 3A, top). In contrast, TUNEL staining was negligible in E14.5 Rx-CreLmnb1Δ/WT littermate retinas (Figure 3A, bottom, and Supplemental Figure S3).
FIGURE 3:
Inactivation of Lmnb1 during embryonic development leads to increased apoptosis and reduced retinal cellularity in adult mice. (A) TUNEL labeling of E14.5 Rx-CreLmnb1Δ/Δ (top) and Rx-CreLmnb1Δ/WT (bottom) mice; specimens were also stained for lamin B1. Note the significant increase in apoptosis in retinal patches devoid of lamin B1. See also Supplemental Figure S3. (B) Low-power images of half-retinas from a Chx10-CreLmnb1Δ/Δ mouse (top) and a Chx10-Cre transgenic control mouse (bottom) stained with DAPI (blue). Cre recombinase expression in retinal bipolar cells is evident from EGFP expression (green). Bottom views emphasize regions of the retina that are devoid of photoreceptors. (C) Coimmunostaining for lamin B1 and lamin B2 of paraffin-embedded retinal sections from a Chx10-Cre transgenic mouse (top) and a Chx10-CreLmnb1Δ/Δ mouse (bottom). Note the asymmetric distribution of lamin B2 in a few cells with little or no lamin B1 (yellow arrows).
Because of the greater amounts of TUNEL staining in E14.5 Rx-CreLmnb1Δ/Δ retinas, we predicted that we would find few viable lamin B1–negative neurons in adult Rx-CreLmnb1Δ/Δ retinas. Indeed, in adult Rx-CreLmnb1Δ/Δ retinas, we observed few viable lamin B1–negative neurons, and those cells were found in shrunken and highly dysmorphic regions of the retina (unpublished data). To assess further the effect of embryonic deficiency of lamin B1 on the morphology of adult retinas, we bred Lmnb1fl/fl mice harboring a Chx10-Cre-eGFP transgene (Chx10-CreLmnb1Δ/Δ), which expresses an enhanced green fluorescent protein (EGFP)–tagged Cre recombinase in a high percentage of embryonic retinal progenitors and later in retinal bipolar cells (Rowan and Cepko, 2004). Retinas of adult Chx10-CreLmnb1Δ/Δ mice were dysmorphic and exhibited markedly reduced cellularity (Figure 3B). Labeling of Chx10-CreLmnb1Δ/Δ retinas with a lamin B1 antibody revealed only small patches of lamin B1–deficient retina cells. Similar to findings in E14.5 Rx-CreLmnb1Δ/Δ retinas, the lamin B1–deficient nuclei displayed an asymmetric distribution of lamin B2 at the nuclear rim (Figure 3C). Taken together, these results show that lamin B1 expression in RPCs and newborn neurons is crucial for the survival of retinal neurons.
Lamin B2 is dispensable for retinogenesis but required for postnatal retina lamination and photoreceptor survival
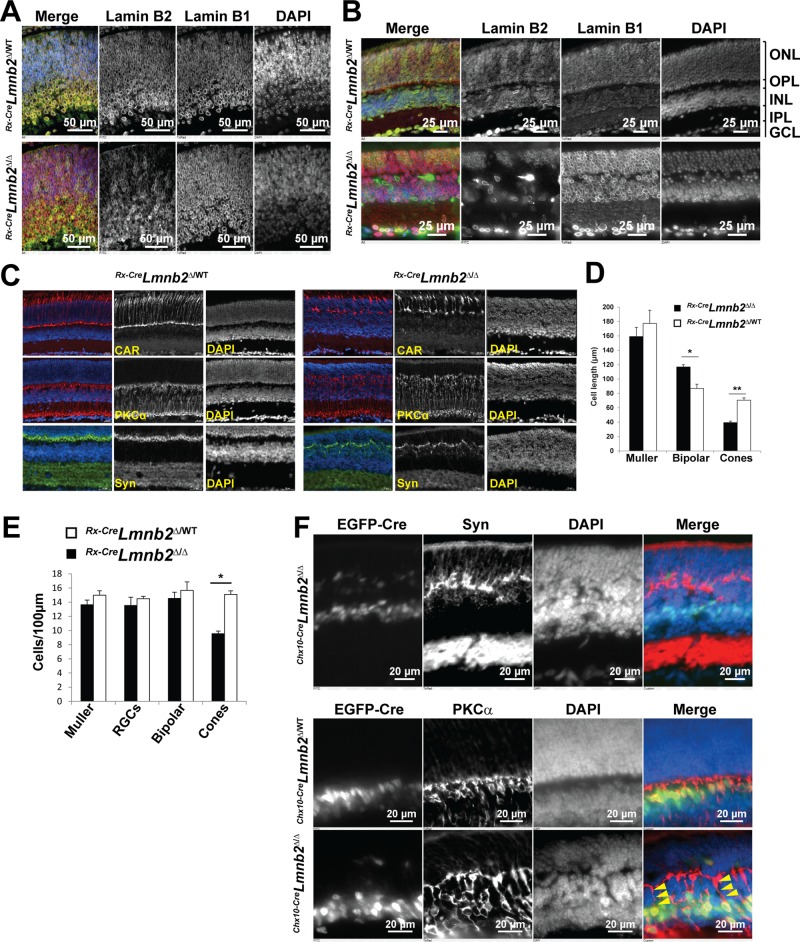
In contrast to the situation with lamin B1 deficiency, lamin B2 deficiency during development had no apparent adverse effects on nuclear morphology in RPCs (Figure 4A). We did not observe misshapen nuclei in E14.5 Rx-CreLmnb2Δ/Δ retinas, and lamin B1 decorated the entire nuclear rims. In adult Rx-CreLmnb2Δ/Δ mice, all retinal cell types were present, and lamin B1 was normally positioned at the nuclear rim in lamin B2–deficient retinal cells (Figure 4B). However, an absence of lamin B2 caused severe abnormalities in retinal organization; the outer plexiform layer (OPL) was not sharply delineated due to intermixing of cells from the inner and outer nuclear layers (INLs and ONLs, respectively). There was a significant shortening of cone photoreceptor length that was counterbalanced by an increased length of bipolar cells, as judged by cone arrestin (CAR) and protein kinase Cα (PKCα) staining (Figure 4, C and D). In addition, labeling of Rx-CreLmnb2Δ/Δ retinas with synaptophysin, a marker for presynaptic processes, revealed ectopic synaptic connections between photoreceptors and bipolar neurons (Figure 4C). Population counts revealed a substantial loss of cone photoreceptors in P25 Rx-CreLmnb2Δ/Δ retinas, whereas numbers of Muller, ganglion, and bipolar cells were unaltered (Figure 4E). Retinas from Chx10-CreLmnb2Δ/Δ mice displayed the same neuronal lamination and synaptogenesis defects (Figure 4F, top). The expression of EGFP-tagged Cre recombinase in bipolar cell nuclei in Chx10-CreLmnb2Δ/Δ retinas further indicated that the lengthening of lamin B2–deficient bipolar cells results from the elongation of their apical axons (Figure 4F, bottom). Taken together, these data indicate that lamin B2 depletion affects neither the localization of lamin B1 nor the production of different retinal cell types but is required for proper lamination of retinal neurons and cone photoreceptor survival.
FIGURE 4:
Lamin B2 is not required for neurogenesis but adversely affects lamination of retinal neurons. (A) Immunostaining of retinas from E14.5 Rx-CreLmnb2Δ/WT (top) and Rx-CreLmnb2Δ/Δ (bottom) mice with antibodies for lamin B1 and lamin B2. In the merged image, lamin B1 is red and lamin B2 is green; DNA was stained with DAPI (blue). Note the normal localization of lamin B1 in progenitors lacking lamin B2. (B) Immunostaining of retinas from P25 Rx-CreLmnb2Δ/WT (top) and Rx-CreLmnb2Δ/Δ (bottom) mice with antibodies against lamin B1 and lamin B2. Lamin B1 was normally positioned at the nuclear rim in all cells lacking lamin B2. In addition, in the DAPI-stained retina, note the intermixing of nuclei from the INL and ONL in patches of retina lacking lamin B2. IPL, inner plexiform layer; GCL, ganglion cell layer. (C) Immunostaining of retinas from P25 Rx-CreLmnb2Δ/WT (left) and Rx-CreLmnb2Δ/Δ (right) littermate mice with antibodies against CAR, PKCα (a marker of retinal bipolar cells), and synaptophysin (Syn). These images show abnormalities in the length of cone photoreceptors, length of bipolar cells, and ectopic synaptogenesis in retinas lacking lamin B2. (D) Length of Muller, bipolar, and cone photoreceptor cells in retinas of mice with the indicated genotypes. Measurements were performed in retinas of at least three mice per genotype. Data are represented as mean ± SEM. (E) Population counts of retinal cell types in retinas of P25 mice of indicated genotypes. Counts were performed on sections stained with cell type–specific markers (see Materials and Methods) using at least three retinas per genotype from at least two different litters. Data are represented as mean ± SEM. (F) Overview of frozen sections from Chx10-CreLmnb2Δ/Δ retinas immunostained with synaptophysin (top). Bottom, PKCα immunostaining of Chx10-CreLmnb2Δ/WT (top) and Chx10-CreLmnb2Δ/Δ (bottom) retinas, revealing abnormal elongation of apical axons from bipolar cells expressing EGFP-tagged Cre recombinase (arrowheads).
B-type lamins are long-lived proteins
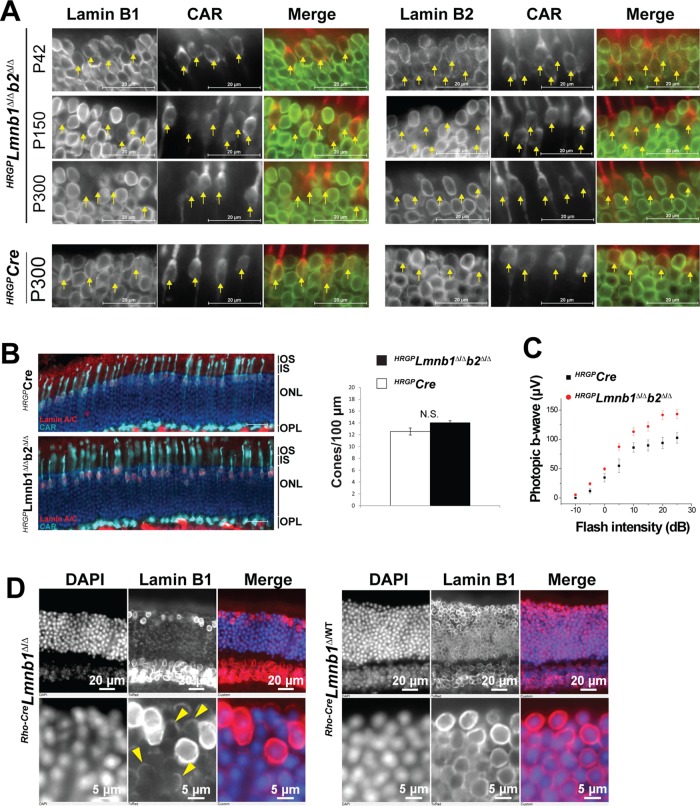
We previously demonstrated that LINC complexes mediate the apical migration of cone photoreceptor nuclei during postnatal retinal development, and we further showed that this process does not depend on A-type lamins (Razafsky et al., 2012). To test the involvement of B-type lamins in this process, we inactivated B-type lamins in postnatal cone photoreceptors by breeding Lmnb1fl/flLmnb2fl/fl mice that harbored an HRGP-Cre transgene. The HRGP-Cre transgene is expressed in cone photoreceptors beginning at postnatal day 6 (P6) and has been highly effective in inactivating other floxed genes (Le et al., 2006; Razafsky et al., 2012). When we embarked on these studies, we expected that we would find a complete absence of lamins B1 and B2 in cone photoreceptors in 6-wk-old HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ mice. However, both proteins were easily detectable at that age (Figure 5A, top). At 5 mo of age, lamin B2 was almost undetectable in cone photoreceptors, but lamin B1 could still be detected at the nuclear rim. By 10 mo, lamin B1 was undetectable in HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ cone photoreceptors. In control experiments with age-matched HRGP-Cre transgenic mice, we found that the expression of both B-type lamins was robust in photoreceptor cells (Figure 5A, bottom).
FIGURE 5:
B-type lamins are long-lived proteins that are dispensable for cone photoreceptor viability and function. (A) Top, Coimmunostaining of retinas from P42, P150, and P300 HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ (HRGPLmnb1Δ/Δb2Δ/Δ) mice with antibodies against CAR and either lamin B1 (left) or lamin B2 (right). Yellow arrows point to cone photoreceptor nuclei labeled with CAR. Scale bars, 20 μm. Bottom, same experiment performed on retinas from P300 HRGP-Cre transgenic mice. (B) Coimmunostaining of retinas from 9-mo-old HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ and age-matched HRGP-Cre transgenic mice with antibodies against lamin A/C and CAR; these studies allowed us to count cone photoreceptor populations and assess cone photoreceptor morphology. The number of cones photoreceptors was determined in at least three fields of two retinas from two 9-mo-old mice of the indicated genotype. Note the scattering of nuclei in cone photoreceptors in HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ retinas. (C) An absence of B-type lamins in cone photoreceptors does not inhibit cone photoreceptor function. ERGs were performed on each eye from 10-mo-old HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ (n = 12) and three age-matched HRGP-Cre transgenic mice (n = 6). (D) Lamin B1 immunostaining of retinas from P300 Rho-CreLmnb1Δ/Δ mice (left) and Rho-CreLmnb1Δ/WT (right) littermate mice. Note the significant but incomplete down-regulation of lamin B1 expression in P300 rod photoreceptors (arrowheads).
B-type lamins are dispensable for the viability and function of cone photoreceptors
Next we assessed the effect of B-type lamin deficiencies in retinas of 10-mo-old HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ mice. When we stained cone photoreceptor nuclei with antibodies against lamin C and CAR, we did not find any major alterations in cone photoreceptor morphology and numbers in comparison to age-matched HRGP-Cre transgenic mice (Figure 5B). In addition, in vivo electroretinography (ERG) did not show any decrease of photopic b-wave amplitude, a measure of bipolar cell activation, when cone photoreceptors are stimulated with light flashes of increasing intensity (Figure 5C). In fact, b-wave amplitudes were slightly higher in retinas of HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ mice than in retinas from HRGP-Cre transgenic mice. The only mild phenotype that we observed in HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ retinas was a subtle but significant defect in the positioning of nuclei in cone photoreceptors relative to the apical side of the ONL (Figure 5B; unpublished data).
To examine lamin B1 turnover in rod photoreceptors, we bred Lmnb1fl/fl mice harboring a Rho-Cre transgene (which results in Cre expression in rod photoreceptors at P6). In 10-mo-old Rho-CreLmnb1Δ/Δ retinas, lamin B1 expression was much lower in rods than in cone photoreceptors (Figure 5D); however, weak lamin B1 expression could still be detected, indicating that the turnover of lamin B1 is extremely slow in rods. Thus our data indicate that B-type lamins are very long-lived proteins in rod and cone photoreceptors. In the case of cone photoreceptors, we can also conclude that the B-type lamins are dispensable for cell survival and phototransduction.
DISCUSSION
In the present studies, we show that lamin B1 and lamin B2 have crucial but distinct functions in the development of the retina. Lamin B1 is extremely important for the survival of retinal neurons. When lamin B1 expression is inactivated at midgestation, the nuclear integrity of embryonic retinal neurons is severely compromised. By adulthood, few lamin B1–deficient neurons survive, and the retina is small and highly dysmorphic. In contrast, when lamin B2 is inactivated at midgestation, the effect on the genesis of retinal neurons is minimal, but the lamination of retinal neurons is profoundly abnormal, and there are reduced numbers of cone photoreceptors in adult mice. Another significant finding from this study is the long half-lives of B-type lamins in rod and cone photoreceptors. However, in contrast to their vital importance during development of the retina, depletion of B-type lamins in cone photoreceptors does not significantly affect cone survival, phototransduction, or morphology. Thus the present studies show that B-type lamins are crucial for retina development but far less important for the homeostasis of postmitotic cone photoreceptors in adult mice.
The morphological abnormalities in the developing retina after the inactivation of Lmnb1 were both intriguing and informative. First, we found that the majority of retinal progenitor and postmitotic cells had a large, solitary nuclear bleb. We suspect that the solitary nuclear blebs in retinal progenitor cells relate, at least in part, to forces pulling on the nucleus during INM in retinal progenitors and nuclear translocation in migrating retinal neurons. In the absence of lamin B1, forces applied on the cell nucleus during nucleokinesis are not appropriately relayed to the whole cell nucleus and instead simply elicit a nuclear bleb. Apical and basal nuclear blebs in progenitor cells could correspond to apical and basal forces that translocate the nucleus during INM, respectively. Nuclear blebs in the INBL cells were always located basally, very likely a consequence of basal forces applied on the cell nucleus as RGCs migrate toward the INBL.
Embryonic neurons devoid of lamin B1displayed a profound mislocalization of lamin B2. Instead of being evenly distributed along the entire nuclear periphery, lamin B2 was located asymmetrically in one segment of the nucleus and very often was located entirely within the large, solitary nuclear bleb. In the absence of lamin B1, the lamin B2 meshwork is apparently incapable of remaining affixed to the nuclear rim, particularly in the setting of forces applied during nucleokinesis. We suspect that this collapse of the lamin B2 meshwork in lamin B1–deficient retinal progenitor cells relates to its inability to withstand forces involved in nucleokinesis. In contrast, retinal neurons lacking lamin B2 did not display nuclear blebs, and lamin B1 localization was normal. Thus lamin B1 by itself appears perfectly capable of generating a lamina meshwork that can withstand the deformational forces that are applied during nucleokinesis. We strongly suspect that the differences in the ability of the lamin B1 and lamin B2 meshworks to withstand the stresses of nucleokinesis underlie most of the phenotypical differences in lamin B1– and lamin B2–deficient embryonic retinas.
The cellularity of lamin B1–deficient retinas was far lower than in lamin B2–deficient retinas. The reduced numbers of retinal neurons is caused, at least in part, by higher levels of apoptosis. In E14.5 Rx-CreLmnb1Δ/Δ mice, apoptotic neurons were prevalent in patches of the retina that lacked lamin B1. It is possible, however, that reduced production of neurons contributes to diminished retinal cellularity. Neurogenesis in the developing retina is utterly dependent on INM. When apical migration of the nucleus during INM falls short, even by a few micrometers, neurogenesis is blocked (Hu et al., 2013). It is conceivable that INM and neuronal cell birth are adversely affected in the setting of lamin B1 deficiency, although we know that the birth of new neurons is not completely blocked because we found newborn RGCs in the embryonic retina and small numbers of lamin B1–deficient retinal neurons in adult mice. Lamin B2 deficiency had a less pronounced effect on numbers of retinal neurons during development, but we suspect that it does lead to reduced survival of certain retinal cell types because the numbers of cone photoreceptors in adult Rx-CreLmnb2Δ/Δ mice was reduced by 35%. The latter observations are consistent with observations by Coffinier et al. (2011), who found that forebrain-specific Lmnb2 knockout had minimal effect on the cellularity of the cerebral cortex during development but led to a substantial reduction in forebrain cellularity within a few months after birth.
An impaired nuclear barrier in embryonic neurons lacking lamin B1 is most likely at the root of apoptosis induction. Because these cells do not express lamin A/C and display a gross mislocalization of lamin B2, we suspect that the absence of a lamina adversely affects nuclear integrity, particularly when the nucleus is subjected to mechanical stresses (i.e., forces on the cell nucleus during the nucleokinesis processes underlying CNS development). An absence of nuclear lamina in peripheral cell types impairs nuclear integrity; when keratinocytes lack all of the nuclear lamins, one can find rough endoplasmic reticulum within the nuclear chromatin (Jung et al., 2014). In the present studies, we found chromatin-containing micronuclei that were detached from the nucleus. We suspect that these abnormalities relate to an impaired nuclear barrier and lead inexorably to apoptotic cell death.
Although an absence of lamin B2 did not appear to cause grossly misshapen nuclei, it nevertheless caused a striking defect in the lamination of retinal neurons. For example, the OPL separating the ONL from the INL was not sharply delineated when lamin B2 was absent. In addition, lamin B2 deficiency induced a marked shortening of cone photoreceptor neurons, a lengthening of retinal bipolar cells, and ectopic synaptogenesis. The abnormalities in synaptogenesis are quite intriguing. Whereas those findings could simply be secondary to impaired neuronal migration, it is also possible that lamin B2 plays a more direct role in synaptic plasticity. In that regard, extranuclear functions for lamin B2 in axonal integrity have been proposed (Yoon et al., 2012). In any case, this role of lamin B2 needs more attention, particularly in light of a proposed linkage between a LMNB2 missense mutation and a progressive form of epilepsy (Damiano et al., 2015).
To explore the importance of B-type lamins in postmitotic neurons in adult mice, we inactivated both Lmnb1 and Lmnb2 in cone photoreceptors long after their embryonic specification. We quickly encountered a surprise: B-type lamins are extremely long-lived proteins. Based on immunofluorescence microscopy, lamin B2 and lamin B1 proteins persist in cone photoreceptors for ∼5 and ∼9 mo, respectively. We encountered similar findings in rod photoreceptors. Metabolic labeling studies also strongly supported the idea that lamin B1 and lamin B2 are long-lived proteins in the brain (Toyama et al., 2013). Surprisingly, the survival of cone photoreceptors lacking both lamin B1 and lamin B2 appeared to be normal. In addition, cone morphology was only minimally perturbed (a subtle mispositioning of the nucleus), and a deficiency of B-type lamins did not affect cone phototransduction as judged by ERGs. Thus B-type lamins appear to be dispensable in cones after development is complete and when cell nuclei are no longer subjected to stresses of nucleokinesis. Yang et al. (2011a, b) found that B-type lamins were dispensable in keratinocytes and hepatocytes. Those cells, like postnatal cone photoreceptors, produce A-type lamins. Thus, when A-type lamins are produced and the stresses of nucleokinesis processes are out of the picture, peripheral cell types and neurons (e.g., cone photoreceptors) within the CNS survive without B-type lamins. In future studies, it will be important to examine the importance of B-type lamins in neurons that naturally lack A-type lamins—for example, rod photoreceptors (Razafsky et al., 2013; Solovei et al., 2013). These sorts of studies will require very lengthy experiments and considerable patience, given that we could detect B-type lamins in rods 10 mo after the onset of Cre expression.
MATERIALS AND METHODS
Mice
Animal protocols used in this study were approved by the Washington University School of Medicine Animal Studies Committee (Animal Welfare Insurance Permit A-3381-01, Protocol 20130225). Conditional knockout alleles for Lmnb1 and Lmnb2 were described previously (Coffinier et al., 2011; Yang et al., 2011a). HRGP-Cre, Rx-Cre, Chx10-Cre (line 2), and Rho-Cre transgenic mice were obtained directly or indirectly from Y. Le, M. Jamrich, C. Cepko, and C. Chen, respectively (Rowan and Cepko, 2004; Li et al., 2005; Le et al., 2006; Swindell et al., 2006).
Preparation of embryonic and adult retinal sections
Immunofluorescence microscopy was performed either on optimal cutting temperature (OCT)– or paraffin-embedded sections of retinas from embryos and adult mice. For paraffin embedding, heads of E14.5 embryos or whole eyes (with the cornea dissected away) were incubated in 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS; Electron Microscopy Sciences, Hatfield, PA) overnight at 4°C. Tissues were then dehydrated for 30 min each in 70% ethanol and 80% ethanol, followed by two 30-min cycles each of 95% ethanol, 100% ethanol, and histological-grade xylenes and four 30-min cycles of Tissue-Prep 2 paraffin. Tissue slices (4 μm thick) were cut on a rotary microtome, floated in a 49°C water bath, collected on poly-l-lysine–coated slides, and air dried overnight. Slides were deparaffinized, and antigen retrieval was carried out in a citrate buffer (pH 6.0) in a pressure cooker for 3 min. For OCT embedding, the cornea of enucleated eyes was cut open, and the whole eye was incubated in 4% PFA for 1 h and then in 30% sucrose overnight. Embryonic heads were submitted to the same protocol, except that the incubation in 4% PFA was performed overnight. Eyes/embryonic heads positioned in OCT-filled cryomolds were then frozen in a bath of dry ice and isopentane. OCT blocks were mounted on a cryostat, and 15-μm sections were cut and collected on Millennia 1000 slides (StatLab, Baltimore, MD).
Immunofluorescence microscopy on embryonic and adult retinas
Paraffin and OCT sections were rinsed three times in PBS, permeabilized in 0.5% Triton X-100/PBS, and incubated with primary antibodies diluted in 10% donkey serum/0.5% Triton X-100 in PBS overnight. Secondary antibodies (Alexa 594 or 488; Thermo Scientific, Waltham, MA) were then added for 1 h in the same solution. After DAPI staining, slices were mounted in fluorescent mounting medium (DAKO, Carpinteria, CA). We used antibodies against lamins A/C (Santa Cruz Biotechnology), lamin B1 (Santa Cruz Biotechnology, Paso Robles, CA), lamin B2 (Thermo Scientific or ProteinTech, Rosemont, IL), cone arrestin (Sigma-Aldrich, St. Louis, MO), Lap2β (BD Transduction Laboratories, San Jose, CA), Brn3 (Santa Cruz Biotechnology), Protein Kinase Cα (Santa Cruz Biotechnology), synaptophysin (Cell Signaling, Danvers, MA), and Ki67 (Thermo Scientific). TUNEL assays were performed according to the recommendation of the manufacturer (Apop Tag Fluorescein Direct In Situ Apoptosis Detection Kit; Sigma-Aldrich).
Image acquisition and quantification
Image acquisition (single field, large scans, and z-stacks) was performed with a Nikon Eclipse Ti coupled to an LED light source (Lumencor, Beaverport, OR) with the NIS-Elements software package (Nikon, Melville, NY) using 20× (numerical aperture [NA] 1.0) or 40× (NA 1.4) objectives. Retinal cell population counts were determined with the quantification tools from NIS-Elements. Specific retinal cell types labeled with appropriate markers (glutamine synthase for Muller cells, PKCα for bipolar cells, Brn3 for ganglion cells, and CAR for cone photoreceptors) were counted in at least two large stitched fields from at least two retina slices that crossed the optic nerve per genotype. The number of a given type of cells was averaged for 100 μm of retinal length. A Student’s t test was used to compare the statistical significance of measurements, with *p < 0.05 and **p < 0.01.
Electrophysiology
In vivo ERG recordings were performed with an LKC ERG system as described (Kolesnikov et al., 2011; Xue et al., 2015). The functional studies of cones lacking B-type lamins were performed on 10-mo-old HRGP-CreLmnb1Δ/ΔLmnb2Δ/Δ mice and age-matched HRGPCre transgenic mice. All animals were free of the rd8 mutation (Mattapallil et al., 2012). The mice were dark-adapted for at least 18 h before the experiment and anesthetized with a ketamine/xylazine cocktail (100/20 mg/kg). Before placing the mouse on a heating pad (37°C), the pupils were dilated with atropine, and contact lens electrodes were placed on the eyes for signal recordings. The mouse was allowed to stabilize and light-adapt for 15 min in 30 cd/m2 background light before start of the recordings. The cone component of the light response was isolated by rod-saturating background light. For each mouse, multiple photopic responses were elicited at flash intensities ranging from −10 to 25 dB relative to the background, and the cone b-wave amplitudes were recorded and averaged.
Supplementary Material
Acknowledgments
We thank Belinda McMahan from our in-house Morphology and Imaging Core and the Mouse Genetics Core for mouse breeding and genotyping. This work was funded by the National Eye Institute (EY022632 to D.H., EY019312 and EY021126 to V.J.K.); the National Heart, Lung, and Blood Institute (HL089781 to L.G.F. and HL126551 to S.G.Y.), a National Eye Institute Center Core Grant (P30EY002687), and an unrestricted grant to the Department of Ophthalmology and Visual Sciences at Washington University from Research to Prevent Blindness.
Abbreviations used:
- CAR
cone arrestin
- INBL
inner neuroblast layer
- INL
inner nuclear layer
- ONBL
outer neuroblast layer
- ONL
outer nuclear layer
- OPL
outer plexiform layer
- RGC
retinal ganglion cell
- RPC
retinal progenitor cell.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-03-0143) on April 13, 2016.
REFERENCES
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Chang SY, Nobumori C, Tu Y, Farber EA, Toth JI, Fong LG, Young SG. Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc Natl Acad Sci USA. 2010a;107:5076–5081. doi: 10.1073/pnas.0908790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Fong LG, Young SG. LINCing lamin B2 to neuronal migration: growing evidence for cell-specific roles of B-type lamins. Nucleus. 2010b;1:407–411. doi: 10.4161/nucl.1.5.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Jung HJ, Li Z, Nobumori C, Yun UJ, Farber EA, Davies BS, Weinstein MM, Yang SH, Lammerding J, et al. Direct synthesis of lamin A, bypassing prelamin a processing, causes misshapen nuclei in fibroblasts but no detectable pathology in mice. J Biol Chem. 2010c;285:20818–20826. doi: 10.1074/jbc.M110.128835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH, 2nd, Yoshinaga Y, de Jong PJ, Vergnes L, Reue K, et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell. 2011;22:4683–4693. doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano JA, Afawi Z, Bahlo M, Mauermann M, Misk A, Arsov T, Oliver KL, Dahl HH, Shearer AE, Smith RJ, et al. Mutation of the nuclear lamin gene LMNB2 in progressive myoclonus epilepsy with early ataxia. Hum Mol Genet. 2015;24:4483–4490. doi: 10.1093/hmg/ddv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BS, Barnes RH, 2nd, Tu Y, Ren S, Andres DA, Spielmann HP, Lammerding J, Wang Y, Young SG, Fong LG. An accumulation of non-farnesylated prelamin A causes cardiomyopathy but not progeria. Hum Mol Genet. 2010;19:2682–2694. doi: 10.1093/hmg/ddq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong LG, Ng JK, Lammerding J, Vickers TA, Meta M, Cote N, Gavino B, Qiao X, Chang SY, Young SR, et al. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–752. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DJ, Baffet AD, Nayak T, Akhmanova A, Doye V, Vallee RB. Dynein recruitment to nuclear pores activates apical nuclear migration and mitotic entry in brain progenitor cells. Cell. 2013;154:1300–1313. doi: 10.1016/j.cell.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Tatar A, Tu Y, Nobumori C, Yang SH, Goulbourne CN, Herrmann H, Fong LG, Young SG. An absence of nuclear lamins in keratinocytes leads to ichthyosis, defective epidermal barrier function, and intrusion of nuclear membranes and endoplasmic reticulum into the nuclear chromatin. Mol Cell Biol. 2014;34:4534–4544. doi: 10.1128/MCB.00997-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334:1706–1710. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov AV, Tang PH, Parker RO, Crouch RK, Kefalov VJ. The mammalian cone visual cycle promotes rapid M/L-cone pigment regeneration independently of the interphotoreceptor retinoid-binding protein. J Neurosci. 2011;31:7900–7909. doi: 10.1523/JNEUROSCI.0438-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y. Interkinetic nuclear migration: beyond a hallmark of neurogenesis. Cell Mol Life Sci. 2012;69:2727–2738. doi: 10.1007/s00018-012-0952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le YZ, Zheng L, Zheng W, Ash JD, Agbaga MP, Zhu M, Anderson RE. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol Vis. 2006;12:389–398. [PubMed] [Google Scholar]
- Li S, Chen D, Sauve Y, McCandless J, Chen YJ, Chen CK. Rhodopsin-iCre transgenic mouse line for Cre-mediated rod-specific gene targeting. Genesis. 2005;41:73–80. doi: 10.1002/gene.20097. [DOI] [PubMed] [Google Scholar]
- Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012;53:2921–2927. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padiath QS, Fu YH. Autosomal dominant leukodystrophy caused by lamin B1 duplications a clinical and molecular case study of altered nuclear function and disease. Methods Cell Biol. 2010;98:337–357. doi: 10.1016/S0091-679X(10)98014-X. [DOI] [PubMed] [Google Scholar]
- Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptacek LJ, Fu YH. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- Razafsky D, Blecher N, Markov A, Stewart-Hutchinson PJ, Hodzic D. LINC complexes mediate the positioning of cone photoreceptor nuclei in mouse retina. PLoS One. 2012;7:e47180. doi: 10.1371/journal.pone.0047180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky DS, Ward CL, Kolb T, Hodzic D. Developmental regulation of linkers of the nucleoskeleton to the cytoskeleton during mouse postnatal retinogenesis. Nucleus. 2013;4:399–409. doi: 10.4161/nucl.26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rober RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- Rowan S, Cepko CL. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev Biol. 2004;271:388–402. doi: 10.1016/j.ydbio.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–598. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Stewart C, Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987;51:383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- Strzyz PJ, Lee HO, Sidhaye J, Weber IP, Leung LC, Norden C. Interkinetic nuclear migration is centrosome independent and ensures apical cell division to maintain tissue integrity. Dev Cell. 2015;32:203–219. doi: 10.1016/j.devcel.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Bailey TJ, Loosli F, Liu C, Amaya-Manzanares F, Mahon KA, Wittbrodt J, Jamrich M. Rx-Cre, a tool for inactivation of gene expression in the developing retina. Genesis. 2006;44:361–363. doi: 10.1002/dvg.20225. [DOI] [PubMed] [Google Scholar]
- Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, 3rd, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, Zheng Y. A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science. 2006;311:1887–1893. doi: 10.1126/science.1122771. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA. 2004;101:10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–1836. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Shen SQ, Corbo JC, Kefalov VJ. Circadian and light-driven regulation of rod dark adaptation. Sci Rep. 2015;5:17616. doi: 10.1038/srep17616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, de Jong PJ, Fong LG, Young SG. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet. 2011a;20:3537–3544. doi: 10.1093/hmg/ddr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Jung HJ, Coffinier C, Fong LG, Young SG. Are B-type lamins essential in all mammalian cells. Nucleus. 2011b;2:562–569. doi: 10.4161/nucl.2.6.18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon BC, Jung H, Dwivedy A, O’Hare CM, Zivraj KH, Holt CE. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148:752–764. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SG, Jung HJ, Coffinier C, Fong LG. Understanding the roles of nuclear A- and B-type lamins in brain development. J Biol Chem. 2012;287:16103–16110. doi: 10.1074/jbc.R112.354407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.