Abstract
Engineering of large articular cartilage tissue constructs remains a challenge as tissue growth is limited by nutrient diffusion. Here, a novel strategy is investigated, generating large constructs through the assembly of individually cultured, interlocking, smaller puzzle-shaped subunits. These constructs can be engineered consistently with more desirable mechanical and biochemical properties than larger constructs (~4-fold greater Young's modulus). A failure testing technique was developed to evaluate the physiologic functionality of constructs, which were cultured as individual subunits for 28 days, then assembled and cultured for an additional 21-35 days. Assembled puzzle constructs withstood large deformations (40-50% compressive strain) prior to failure. Their ability to withstand physiologic loads may be enhanced by increases in subunit strength and assembled culture time. A nude mouse model was utilized to show biocompatibility and fusion of assembled puzzle pieces in vivo. Overall, the technique offers a novel, effective approach to scaling up engineered tissues and may be combined with other techniques and/or applied to the engineering of other tissues. Future studies will aim to optimize this system in an effort to engineer and integrate robust subunits to fill large defects.
Keywords: articular cartilage, tissue engineering, scaling up, puzzle, nutrient limitations
1. Introduction
Articular cartilage has a limited ability to regenerate, making damage to the tissue especially debilitating (Buckwalter and Mankin, 1998; Sherman et al., 2014). Osteoarthritis (OA), a degenerative disease, affects nearly 27 million Americans, leading to an estimated annual economic cost of $89.1 billion (Bitton, 2009; Lawrence et al., 2008; Leigh et al., 2001). Acute injury to articular cartilage is common and may lead to OA if untreated (Buckwalter and Mankin, 1998; Flanigan et al., 2010), especially when defects become large (Moisio et al., 2009).
Osteochondral allografting is the most common treatment used for large (>2-3 cm2) articular cartilage defects (Cole et al., 2009; Gortz and Bugbee, 2006; Sherman et al., 2014) in the US. It is a biomimetic technique, used as a long-term solution clinically for over 30 years (Gross et al., 2005; Gross et al., 1975). Allografting shows numerous advantages over alternative techniques. Autologous chondrocyte implantation (ACI) leads to donor site morbidity, requires multiple surgeries, is costly, and is most effective in young, active patients (Lee et al., 2000; Mandelbaum et al., 2007; Sherman et al., 2014; Whittaker et al., 2005). Osteochondral autografting is also associated with donor site morbidity (Lee et al., 2000; Reddy et al., 2007) and consistently successful outcomes are limited to young patients with relatively small (<2 cm2) defects.
Despite their benefits, allografts are insufficient in supply to meet clinical demand (Mow et al., 1991; Paige and Vacanti, 1995). As an alternative, tissue engineering may provide a cell-based strategy for developing additional grafts for cartilage repair (Johnstone et al., 2013; Lima et al., 2008a). Our laboratories have demonstrated ability to utilize juvenile (Mauck et al., 2000) or adult chondrocytes (Bian et al., 2009a; Kelly et al., 2013; Ng et al., 2010; Nover et al., 2015b) in an agarose hydrogel scaffold system to engineer chondral and osteochondral articular cartilage grafts (Lima et al., 2008a; Lima et al., 2004; Nover et al., 2015a) with native or near-native mechanical and biochemical properties.
Still, in these cases, the engineered grafts were relatively small in size (~∅4 mm). In order to increase the clinical relevance of engineered grafts, it is critical to scale up their size (Hung et al., 2003). With larger grafts, loads affecting the graft may not be shouldered by surrounding tissue as they are with smaller grafts, making the graft strength even more critical.
A challenge for successful scale up is the inherent diffusion of nutrients and other chemical factors required to support tissue growth. According to Fick's law, diffusion time is proportional to the distance squared (Atkins, 1994). This transport limitation may be exacerbated by nutrient consumption (Buckley et al., 2009) and increased transport resistance arising from dense elaborated tissue within the engineered construct (Leddy et al., 2004). Areas of decreased tissue growth in engineered constructs have been attributed to gradients in various internal nutrient levels including glucose and oxygen (Cigan et al., 2013; Heywood et al., 2006a, b; Nims et al., 2015; Obradovic et al., 1999; Sengers et al., 2005; Zhou et al., 2008). Even in small constructs, this effect has resulted in preferential matrix deposition in peripheral rather than central regions (Kelly et al., 2009; Kelly et al., 2006).
Various methods have been investigated for overcoming this diffusion limitation, including dynamic loading (Albro et al., 2008; Albro et al., 2010; Bian et al., 2010; Hung et al., 2004; Lima et al., 2007; Mauck et al., 2000; Mauck et al., 2003a; Mauck et al., 2003b), perfusion (Davisson et al., 2002), dynamic laminar flow (Vunjak-Novakovic et al., 1999), and orbital motion (Cigan et al., 2014) utilized to increase convection; microbubbles used to create internal reservoirs (Lima et al., 2012); and channels introduced within the tissue to increase surface area and decrease the nutrient path length (Bian et al., 2009a; Buckley et al., 2009; Cigan et al., 2014; Nims et al., 2015).
In the current study, we take a unique approach to this problem by investigating a strategy in which small, interlocking tissue engineered constructs are individually cultured to achieve robust tissue properties, and then assembled to provide a graft capable of covering large defects of variable size and shape in a modular fashion. Proof-of-principle studies are presented that explore a single puzzle piece shape (Fig. 1). Puzzle assemblies are compared to equivalently sized large constructs cultured under the same conditions as puzzle pieces.
Fig. 1.
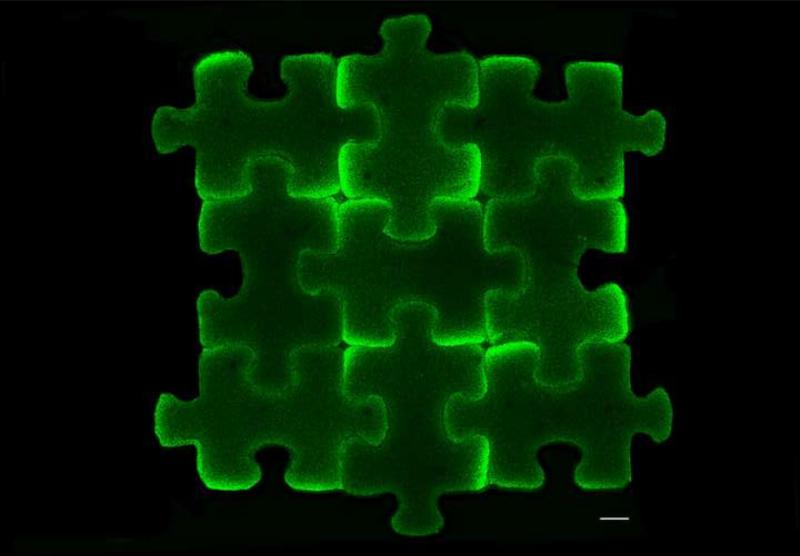
Schematic of puzzle piece strategy. Cell-seeded agarose stained with calcein for cell visualization. Scale bar: 1 mm.
2. Methods
2.1. Engineering of puzzle-shaped constructs
Custom punches were designed using Illustrator (Adobe) and SolidWorks (Dassault Systèmes), and then 3D printed (Objet24, Stratasys). An interlocking puzzle-shaped punch was designed to produce a construct with volume similar to that of a ∅6 mm disk, but with greater surface area. A square-shaped punch was designed to produce single large constructs with equivalent size to an assembly of nine (3×3) puzzle pieces to serve as a control.
Chondrocytes were isolated from articular cartilage harvested from juvenile bovine knees via an 11 hour digestion with 390 U/mL collagenase type IV (Worthington) and slight agitation. Chondrocytes were passaged twice (P2) in Dulbecco's Modified Eagle's Media (DMEM, Invitrogen) containing 10% FBS (Atlanta Biologicals), 1 ng/mL transforming growth factor-beta-1 (TGF-β1, Invitrogen), 5 ng/mL fibroblast growth factor-2 (FGF-2, Invitrogen), and 1% antibiotics/antimycotics (Invitrogen). Chondrocytes were encapsulated in 2% w/v agarose (Type VII, Sigma-Aldrich) at a concentration of 30 × 106 cells/mL, and cast between two glass panes, yielding slabs of 2.3 mm thickness. Constructs were excised from slabs using the 3D printed punches (Groups: “Puzzle” and “Square”).
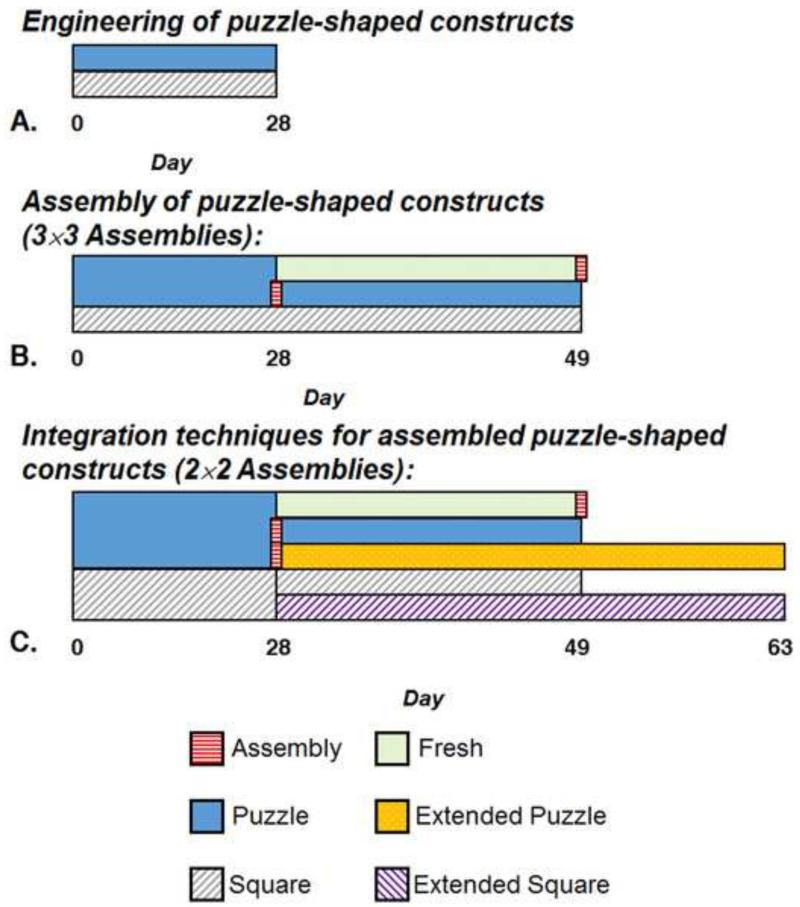
Constructs were cultured in chemically-defined chondrogenic media: DMEM containing 50 μg/mL L-Proline (Sigma-Aldrich), 100 μg/mL sodium pyruvate (Sigma-Aldrich), 1% ITS+ premix (BD Biosciences), 100 nM dexamethasone (Sigma-Aldrich), 1% antibiotics/antimycotics, and 50 μg/mL ascorbic acid (Sigma-Aldrich). TGF-β3 (R&D Systems) was supplemented for the first 14 days of culture. Per group, medium was scaled by construct volume. Day 0 constructs were evaluated as a baseline. Media was changed 3X/week for 28 days, and then constructs were evaluated (Fig. 2A).
Fig. 2.
Experimental Flowcharts. A. Engineering of puzzle-shaped constructs (Section 2.1): Puzzle and Square constructs cultured individually for 28 days. This experiment was repeated independently (Studies 1 and 2). B. Assembly of puzzle-shaped constructs (Section 2.2): Day 49 Evaluation of: Puzzle constructs assembled on day 28; Fresh constructs assembled on Day 49; and Square construct controls. Puzzle and Square constructs stem from Studies 1 and 2; Fresh constructs stem from Study 1. All assemblies are 3×3. C. Integration techniques for assembled puzzle-shaped constructs (Section 2.3): Puzzle constructs assembled on day 28 evaluated at days 49 and 63 (Extended Puzzle); Fresh constructs assembled and evaluated on day 49; and Square construct controls evaluated on days 49 and 63 (Extended Square). All assemblies are 2×2. All constructs stem from Study 1.
Local equilibrium Young's modulus (EY) was obtained at a central location by curve-fitting the load response of a spherical tip indentation stress-relaxation test (10% strain at 0.5 μm/s, S∅ 2.31 mm indenter) using the open-source finite element code FEBio (www.febio.org) (Maas et al., 2012). Constructs were modeled as a biphasic material (Mow et al., 1980) with a solid matrix consisting of a neo-Hookean elastic solid (EY, and Poisson's ratio, ν), representing the proteoglycan content and a continuous spherical (isotropic) distribution of fibers (fiber modulus ξ and power law exponent β), representing the collagen content (Huang et al., 2012). For reference, spatially-averaged mechanical properties of whole Puzzle constructs were obtained in unconfined compression as previously described (Soltz and Ateshian, 1998), yielding EY at 10% unconfined compressive strain and the dynamic modulus (G*) at 0.01 Hz at 1% strain amplitude superposed over the equilibrated 10% strain configuration. Note that square constructs were not evaluated in this manner due to their large size. In all cases, construct surface area was measured from images using ImageJ (NIH).
For biochemical analysis, constructs were halved along a line of symmetry, and weighed wet (WW), lyophilized, and weighed dry (DW). Constructs were digested in 0.5 mg/mL proteinase-K (MP Biomedicals) for 16 hours at 56 °C. A PicoGreen assay (Invitrogen) was used to quantify DNA content (McGowan et al., 2002). A 1,9-dimethylmethylene blue (Sigma-Aldrich) dye-binding assay was used to quantify glycosaminoglycan (GAG) content (Farndale et al., 1982). A colorimetric assay was used to determine orthohydroxyproline (OHP) content (Stegemann and Stalder, 1967). Collagen content was calculated assuming a 1:7.64 OHP-to-collagen mass ratio (Hollander et al., 1994). Biochemical measures were normalized to WW, DW, and DNA content.
Viability was assessed by imaging halved constructs stained with a LIVE/DEAD Assay Kit (Invitrogen) using a confocal microscope (Fluoview FV1000, Olympus).
Halved constructs were fixed for histological analysis using a 5% acetic acid, 3.7% formaldehyde, and 70% ethanol solution for 24 hours, and then stored in 70% ethanol. Fixed samples were serially dehydrated in ethanol, embedded in paraffin for visualization of the construct cross-section, sectioned (5 μm), and then mounted on glass slides. Samples were dewaxed, rehydrated, and stained with Fast Green/Safranin O (Sigma-Aldrich) and Picrosirius Red (following 0.5 mg/mL testicular hyaluronidase treatment, Sigma-Aldrich) to visualize GAG and collagen, respectively.
2.2 Assembly of puzzle-shaped grafts
Following 28 days of culture, Puzzle constructs were assembled in a 3×3 arrangement of 9 subunits (Fig. 1). These assemblies and the Square controls were cultured for an additional 21 days (Fig. 2B).
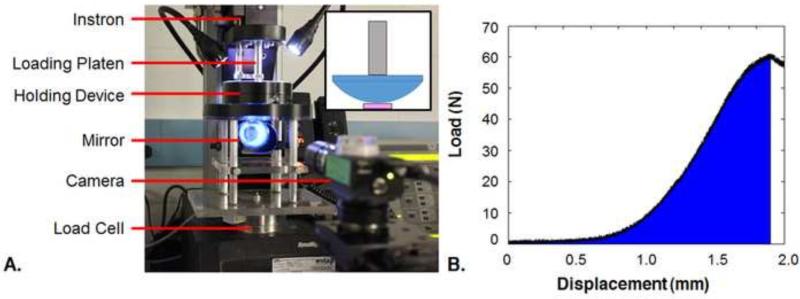
On day 49, Square and assembled Puzzle constructs were evaluated for functionality using compression to failure at a rate of 0.3% of the construct thickness per second (0.3% strain/s at the center point, Fig. 3A) (Tan et al., 2010) using a plano-convex lens (∅50.8 mm planar end, S∅65 mm convex surface, Newport Corporation) within a tabletop material testing device (Instron). This novel testing configuration was selected to simulate physiological compression between congruent articular surfaces as the lens would contact nearly the entire construct surface area when its apex penetrated halfway through the construct thickness. Additionally, Puzzle constructs that had been cultured individually for the full 49 days were tested after being assembled fresh prior to testing (Fresh). The energy to failure (ETF) was calculated using a custom MATLAB (Mathworks) program to integrate the area under the load-displacement curve from start to failure (Fig. 3B). ETF values were normalized by tissue volume (ImageJ). The failure response of the construct was visualized using a custom testing chamber consisting of an elevated reservoir with a transparent base, allowing sample imaging via a right angle prism. Failure tests were recorded using a Grasshopper2 camera (Point Grey Research) controlled by the open-source μManager acquisition software (https://micro-manager.org/) to capture images at 1 frame/s (Edelstein et al., 2001; Edelstein et al., 2014). For visualization, constructs were speckled with Verhoeff's stain using an airbrush. Groups are referenced as “Puzzle,” “Square,” and “Fresh.” Viability and histology of assembled Puzzle constructs were also assessed (Section 2.1).
Fig. 3.
A. Labeled schematic of failure testing setup. Inset: A plano-convex lens (blue) is attached to the loading platen (gray). A 16 mm width, 3 mm thickness square construct is shown beneath the lens (pink). B. Example of load-displacement curve with ETF demarcated in blue.
2.3 Integration techniques for assembled puzzle-shaped constructs
In an additional series of tests, Puzzle and Square constructs were generated as described in Section 2.1 and cultured for 28 days to investigate scenarios for increasing integration strength between assembled pieces (Fig. 2C). For this evaluation, assembled Puzzle constructs with a 2×2 arrangement and respective Square constructs of similar size were studied. Three experiments were performed: (1) as described above, Puzzle constructs were assembled on day 28 followed by culturing the assembly for an additional 21 days (Day 49) - “Puzzle”; (2) Puzzle constructs were maintained separately in culture and then assembled together fresh on day 49 - “Fresh”; and (3) a group of constructs assembled on day 28 was cultured for another 35 days (until Day 63) - “Extended Puzzle.” At the end of the experiment, each puzzle construct group and their respective square control (“Square” and “Extended Square”) were evaluated via failure testing (Section 2.2). Puzzle, Square, Fresh, and Extended Puzzle groups were evaluated histologically (Section 2.1).
2.4 Evaluation of biocompatibility
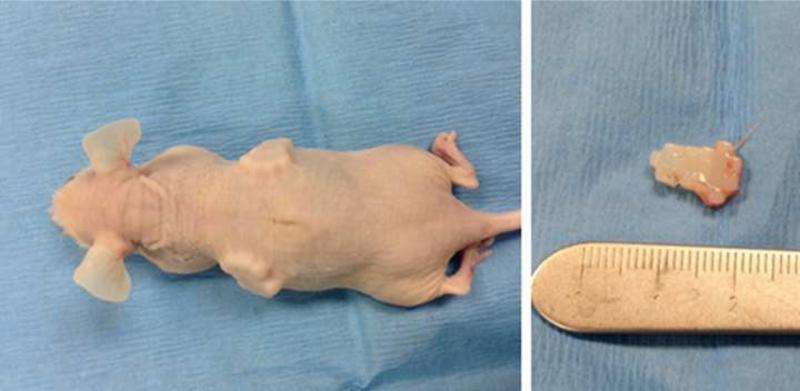
Pairs of puzzle constructs (n=4 pairs) cultured for 42 days were assembled and implanted subcutaneously in the dorsum of nude mice (n=2) for 28 days. Mice were sacrificed on day 28, implants were harvested and assessed grossly and histologically, and complete necropsy was performed on each mouse. All research was approved by the University of Missouri Institutional Animal Care and Use Committee.
2.5 Statistics
Statistica (Statsoft) was used to perform one-way analysis of variance (ANOVA) tests with Tukey's honest significant difference (HSD) post hoc test (α=0.05) to determine significance (p≤0.05) among mean values. Sample size per group was at minimum three (n≥3). Results are reported as mean ± standard deviation.
3. Results
3.1 Engineering of puzzle-shaped constructs
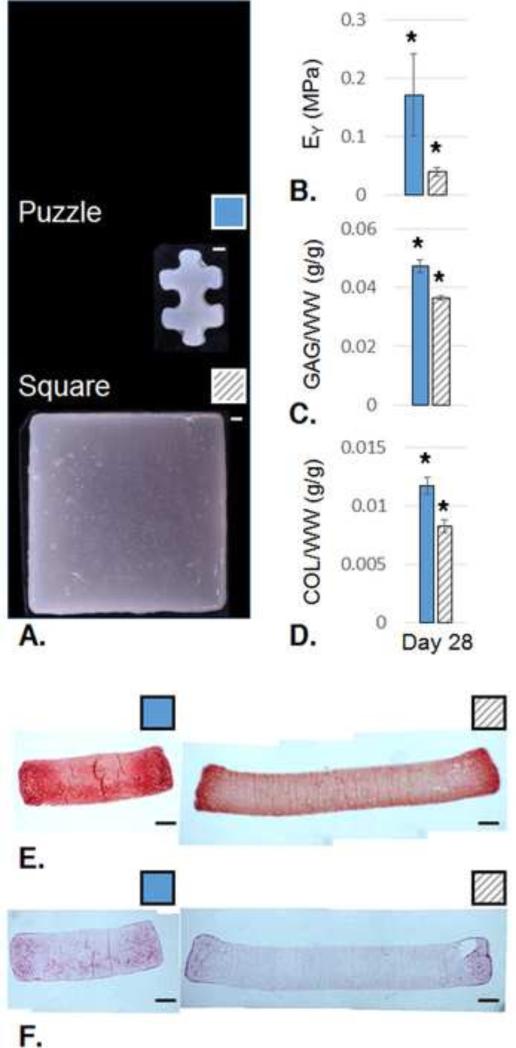
Two independent studies were conducted, repeating experimental conditions. In the first study (Study 1), at day 28, Puzzle constructs were opaque across their entire area, while Square constructs showed opacity only at their periphery (Fig. 4A). At the center, Puzzle constructs displayed a 4.2-fold increase in EY over the square group (p<0.05, Fig. 4B), based on FEBio curve fits of spherical indentation responses (R2>0.96). For reference, whole Puzzle construct EY based on unconfined compression was 0.27±0.03 MPa and G* was 1.2±0.2 MPa (n=4). On day 0, the cell-seeded hydrogel had EY=9.2±1.2 kPa and G*=47±3 kPa (n=3). GAG content and collagen content were significantly higher for Puzzle constructs normalized to WW, DW, and DNA (p<0.001, Fig. 4C-D). Histology confirmed quantitative biochemical assays and gross morphological appearance. GAG and collagen were distributed more consistently throughout the Puzzle constructs than the respective Square control constructs (Fig. 4E-F). Live/Dead staining confirmed viability of both groups (not shown).
Fig. 4.
A. Gross morphology at day 28. B. local EY, (Puzzle: n=5; Square: n=3; p<0.05); C. GAG content per wet weight, (Puzzle: n=8; Square: n=3; p<0.001); D. Collagen (COL) content per wet weight (Puzzle: n=9; Square: n=3; p<0.001). E. Safranin O/Fast Green staining for GAG visualization (Puzzle: n=2; Square: n=1); F. Picrosirius Red staining for collagen visualization (Puzzle: n=2; Square: n=1); Solid blue bars: Puzzle; Hatched gray bars: Square. All scale bars: 1 mm.
In the independent repeat study of the above (Study 2), similar results were observed. Here, Puzzle constructs achieved EY=0.12±0.02 MPa and G*=0.62±0.10 MPa (n=7) from an initial day 0 level of EY=5.0±3.1 kPa and G*=40±10 kPa (n=3). Indentation showed centers of Puzzle constructs as having a 3.8X greater EY than Square constructs (p<0.05; fit R2>0.82; Puzzle: n=6; Square: n=4). GAG/WW and GAG/DNA were significantly higher for Puzzle than Square constructs (p<0.001). Collagen content was significantly higher for Puzzle constructs than square constructs across all normalizations (p<0.001). Gross morphology also showed consistent opacity in Puzzle constructs and increased peripheral opacity in Square constructs.
3.2 Assembly of puzzle-shaped constructs
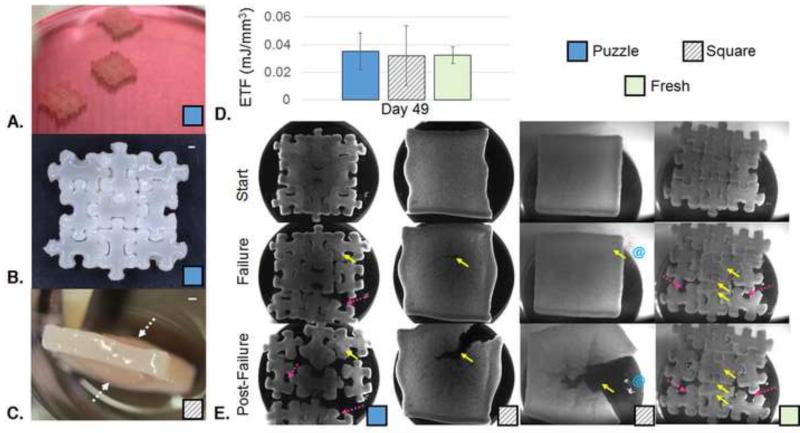
Puzzle constructs could be assembled successfully on day 28 despite their growth (Fig. 5A). On day 49, assembled Puzzle constructs appeared opaque throughout (Fig. 5B). Data from the Puzzle and Square groups were pooled from the two independent studies. Data for the Fresh group stem from one study (Study 1). In the repeat study showing greater tissue growth (Study 1), square constructs developed an internal fluid-filled region or sac due to osmotic swelling of proteoglycans and reduced collagen deposition at the center of the construct (Fig. 5C).
Fig. 5.
A. Photo of Puzzle constructs assembled and placed in culture at Day 28. B. Gross morphology assembled 3×3 arrangement of 9 subunit Puzzle pieces at Day 49 (28 days individual + 21 days assembled culture). Scale bar: 1 mm; C. Gross morphology of Square construct showing a fluid-filled sac (marked by dotted white arrows). D. Energy to Failure (Puzzle: n=9; Square: n=6; Fresh: n=3; no statistically significant difference; Puzzle and Square groups contain values from two independent studies, Fresh group contains values from one study). E. Representative images of puzzle, square, and fresh constructs at the start strain, the failure strain, and a strain past failure. Failure of the square construct is shown in the case of with (right) and without (left) fluid filled sacs. Constructs speckled with Verhoeff stain for visualization; Solid yellow arrows mark areas of tearing. Dotted pink arrows mark areas of minimal damage separation. Blue @ symbols indicate exudation of fluid from internal sacs. The corresponding videos for these representative failure test images are compiled in Supplemental Video 1.
Failure testing showed no significant differences in ETF across groups (Fig. 5D), however failure modes varied (Fig. 5E). Puzzle and Fresh groups failed by a combination of tearing and separating. In many cases, the subunits that separated suffered minimal damage. Tearing was not limited to the “male” portion of the Puzzle constructs. Square constructs without fluid-filled sacs failed by tearing. Square constructs with the sacs failed as the sac burst, releasing fluid, and then eventually tore.
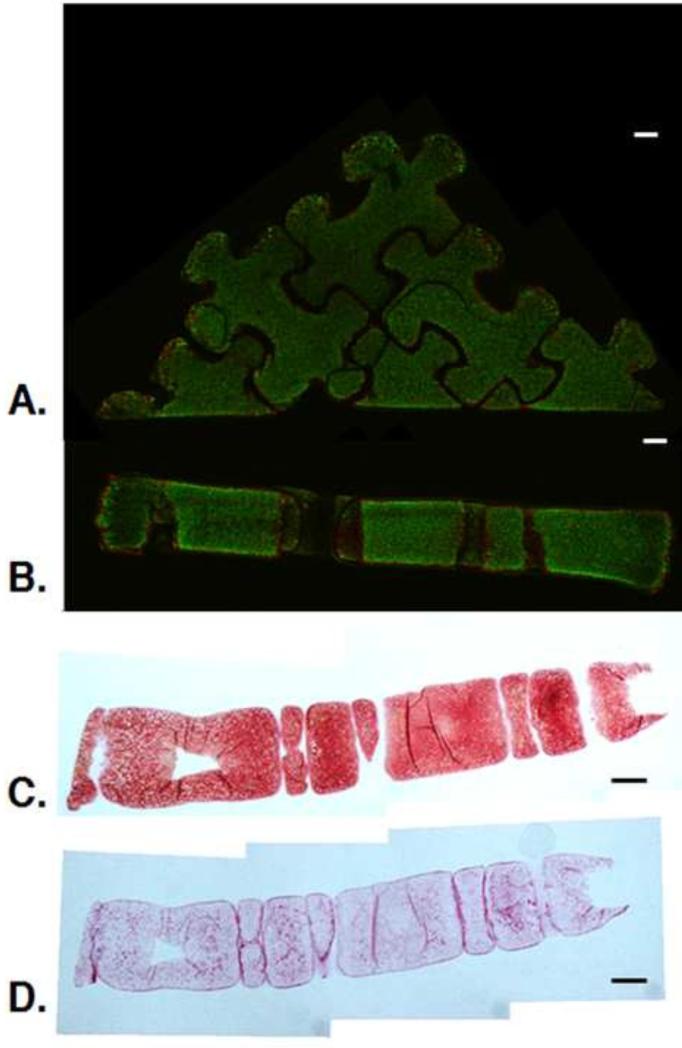
Assembled Puzzle constructs contained viable cells throughout (Fig. 6A). Histological analysis showed intense staining throughout the assembled construct (Fig. 6B-C). Collagen staining appeared more intense at the borders between Puzzle construct subunits.
Fig. 6.
A-B. Live/Dead image of surface (A) and cross-section (B) of 3×3 puzzle arrangement at day 49. C-D. Cross-sectional histology of assembled 3×3 puzzle arrangement at day 49: Safranin O/Fast Green for GAG visualization (C) and Picrosirius Red for collagen visualization (D). (n=1). Scale bars: 1 mm. Live/Dead images provided to clarify orientation of histological sections.
3.3 Integration techniques for assembled puzzle-shaped constructs
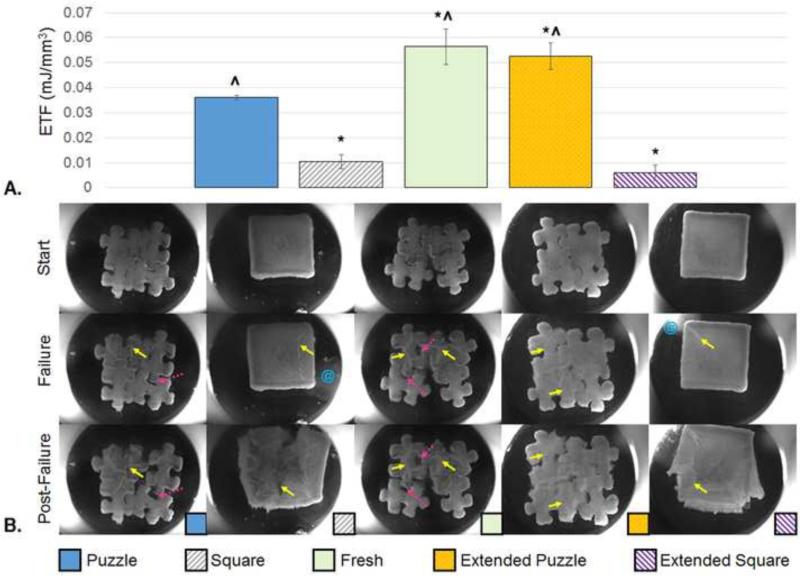
Here, all Square constructs (cast in Study 1) contained the aforementioned fluid-filled sacs past day 28 in culture. Compared to the assembled Puzzle group, Square and Extended Square constructs failed at a significantly lower energy (p<0.001, Fig. 7A). Fresh and Extended Puzzle groups failed at a significantly higher energy than the Puzzle group (p<0.05) and the two square groups (p<0.001). Similar to the larger constructs with fluid-filled sacs described in Section 3.2, the Square and Extended Square groups failed by “bursting,” releasing internal fluid, and then eventually tearing (Fig. 7B). Puzzle, Fresh and Extended Puzzle constructs failed by a combination of tearing and some subunit separation.
Fig. 7.
A. Energy to Failure (n=3). * indicates significant difference from the Puzzle Group (p<0.05), ^ indicates significant difference from the square group (p<0.001). Puzzle, fresh, and square groups evaluated on Day 49. Extended Puzzle and Extended Square groups evaluated on Day 63. B. Representative images of constructs at the start and the failure points. An image of a point past failure is also included to emphasize failure mode. Solid yellow arrows show areas of tearing. Dotted pink arrow show areas of separation. Blue @ symbols indicate fluid release from the construct. The corresponding videos for these representative failure test images are compiled in Supplemental Video 2.
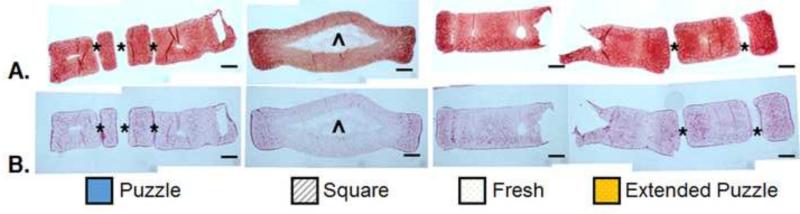
As seen at day 28 (Section 3.1, Fig. 4E-F), staining for GAG and collagen in the Square constructs was more intense towards the periphery (Fig. 8). The Puzzle and Extended Puzzle groups showed more consistent staining throughout the construct cross-section. Collagen staining appeared more intense at each subunit periphery. Likewise, staining of individual Fresh constructs showed consistent staining throughout the cross-section, similar to the individual constructs shown in Fig. 4E-F.
Fig. 8.
A-B. Cross-sectional histology of assembled 2×2 puzzle arrangement at D49: Safranin O/Fast Green for GAG visualization (A) and Picrosirius Red for collagen visualization (B). (Puzzle, Square, Extended Puzzle: n=1; Fresh: n=2). Puzzle, Square, and Fresh groups were evaluated on day 49, while the Extended Puzzle group was evaluated on day 63. Scale bars: 1 mm. * indicates gaps between or within (“female” portion) subunits, ^ indicates area inside the fluid-filled sac.
3.4 Evaluation of Biocompatibility
All puzzle constructs remained assembled and intact in mice for the 28-day study duration (Fig. 9). Complete necropsy showed no gross or histologic pathology in any tissues including liver, kidney, brain, or bone marrow. Retrieved pairs of puzzle pieces showed preserved viability and phenotype in each construct with histologic evidence for fusion between individual pieces. Constructs were joined by fibrovascular tissue with low to moderate cellularity, comprised primarily of mesenchymal and fibroblastic cells.
Fig. 9.
Two pairs of puzzle constructs implanted subcutaneously in the dorsum of nude mice (left) and retrieved (right). Scale bar shows mm.
4. Discussion
Overcoming diffusional limitations to scale up the size of engineered articular cartilage constructs remains a critical challenge for clinical translation. The puzzle piece strategy described here shows promise as a relatively simple technique to address the problem. The technique inverts the channel concept; rather than introducing additional nutrient pathways into a large engineered tissue, it maintains short nutrient pathways in culture, followed by assembly and integration once the subunits are functional.
In concept, filling a large defect with multiple small grafts is most similar to osteochondral autografting such as mosaicplasty, in which multiple cylindrical autografts are harvested from a low weight bearing region, then arrayed to fill the defect (Hangody and Baló, 2011; Robert, 2011). Mosaicplasty is difficult to perform, and though maximum coverage of the defect is desired, the technique is often associated with dead space between cylindrical grafts. This space can be minimized by utilizing grafts of varying sizes or filling with a bone paste (Hangody and Baló, 2011; Robert, 2011). In this context, an interlocking engineered graft system would offer near 100% coverage without the aforementioned donor site morbidity associated with autograft procedures.
Culturing large constructs in static, free-swelling conditions results in tissues with central regions lacking consistent biochemical content, strength, and functional mechanical properties (Fig. 4). The disparity between the central and peripheral tissue properties, especially in the case of rapid growth, can lead to undesired effects. Bian et al. reported cracking at the center of constructs cast at high cell seeding density, positing the effect was due to GAG-induced osmotic swelling forces in constructs with disproportional peripheral and central collagen content (Bian et al., 2009a; Bian et al., 2009b). Here the effect was amplified in the Square constructs (both sizes), which in one of the two batches of constructs developed an internal separation at the center, which then filled with fluid (Fig. 5C). As expected, culturing small subunits minimizes the radial property disparity (Fig. 4). Puzzle constructs punched from the same cell-seeded gel did not develop such cracks. It is expected that mechanical and biochemical functionality would be further increased by engineering even smaller puzzle subunits (here puzzle tissue volume was similar to that of a ∅6 mm disk, larger than the commonly utilized ∅4 mm disk adopted in our laboratory (Kelly et al., 2008; Lima et al., 2008b; Ng et al., 2008; Nover et al., 2015a; Nover et al., 2015b). Assembling the subunits yields a large tissue with more radially consistent GAG and collagen distribution (Fig. 6C-D).
The mechanical integrity of assembled puzzle constructs was evaluated using a novel test, which compressed tissues to failure with a convex, yet highly congruent lens. This test was utilized to acquire an understanding of how the large engineered tissue constructs would fare under physiologic conditions without the shouldering of loads by surrounding native tissue. Evaluation of functionality is important, especially since there may be concerns of tissue filling in the holes and stress concentrations in channeled constructs (Nims et al., 2015). Such testing was chosen to yield more functional data, combining aspects of subunit integration as well as overall compressive strength, rather than individual measures of subunit integration (Jeon et al., 2014) or compressive strength (Soltz and Ateshian, 1998).
In the current study, assembled puzzle constructs were able to withstand large strains before failure. Assembled groups of nine puzzle constructs failed at a central strain (maximum strain located at the center of the lens) of 50±9% strain (n=9) and assembled groups of four puzzle constructs failed at a central strain of 40±5%, which is similar to the failure strain of stronger, smaller constructs reported by Tan et al. using a flat platen, both at 0.3% strain/s (Tan et al., 2010). For reference, Park et al. applied dynamic loading at physiologic stress levels to native cartilage, measuring peak compressive strain amplitudes as 15.8±3.4% at 0.1 Hz and 12.7±2.3% at 1 Hz, and reporting curve-fit functions for relating stress and strain (Park et al., 2004). Integrating these functions from 0% to these strain amplitudes yields mean energies of 0.42 mJ/mm3 at 0.1 Hz and 0.26 mJ/mm3 at 1 Hz. In other words, normal physiological loading requires articular cartilage to withstand these levels of stored strain energy density without failure. The ETF of the assembled Puzzle constructs is within one-eighth of these values, which represents an encouraging outcome for this first proof-of-principle, suggesting that future enhancements in individual puzzle properties and puzzle integration may realistically lead to graft survival. As reported in previous studies using this chondrocyte-seeded agarose system (Hung et al., 2004; O'Connell et al., 2014), GAG levels achieved in the constructs (Fig. 4C) are comparable to native levels in the bovine cartilage from which chondrocytes are harvested, whereas collagen levels (Fig. 4D) remain at one-tenth of the native level. Therefore, strategies that enhance synthesized collagen content will likely be highly beneficial for this puzzle assembly strategy.
In this study, assembled puzzle constructs fail through a combination of tearing and separation, which in many instances leaves separated subunits relatively undamaged (Fig. 5 & Fig. 7). The overall functionality of these assembled constructs may be linked to the strength of the individual subunits, their junctions, and attachment between pieces. These puzzle assemblies show a significant advantage over the squares whenever those developed fluid-filled sacs (Fig. 7). No advantage was apparent when this phenomenon did not occur (Fig. 5); despite the weak central region of squares, their border of elaborated matrix seems to hold the large tissues together until a tear propagates outward.
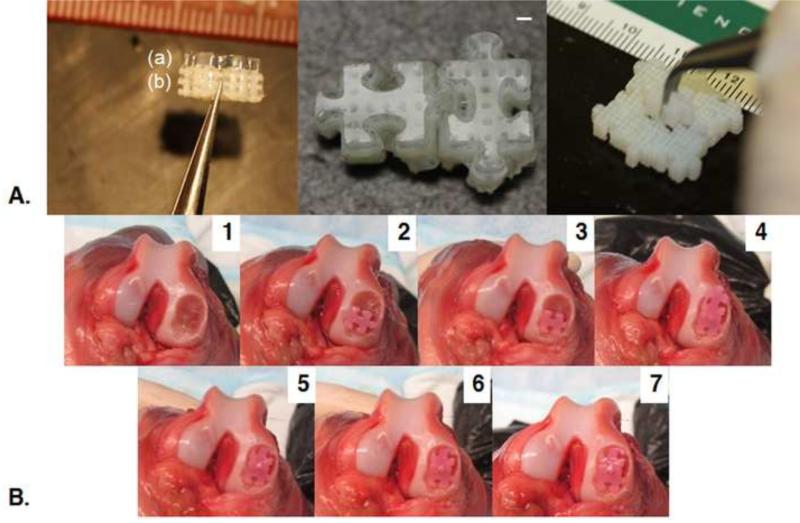
Integration of puzzle constructs may be augmented by a variety of methods. Here, extending the culture of assembled constructs showed a clear advantage in failure properties (Fig. 5). Allowing for increased individual subunit culture time prior to assembly provided mixed results, failing at significantly higher energy in one study (Fig. 7) and at a similar energy in the other (Fig. 5). Subunit integration may also be amplified by caulking the assembled pieces using cell-seeded agarose, fibrin glue, or another biocompatible tissue adhesive (Cascio and Sharma; Englert et al., 2007; Jeon et al., 2014; Jurgensen et al., 1997; Sargeant et al., 2012; Sitterle et al., 2009; Wang et al., 2007). Additionally, it is expected that the incorporation of strong, interlocking, porous bases (such as previously described by our laboratory (Lima et al., 2008a; Nover et al., 2015a)) into the underside of puzzle subunits may further prevent separation (Fig. 10A). Fusion of subunits may also be facilitated post implantation in vivo. Computational modeling may be used to guide alterations to the puzzle shape for improved growth and junctional integration (Nims et al., 2015). Integration of implanted engineered grafts with the surrounding native tissue remains a challenge in the field of regenerative medicine (Tan and Hung, 2011). In addition to attaching individual subunits, the aforementioned biocompatible tissue adhesives and osteochondral bases may help to secure grafts in the defect site.
Fig. 10.
A. Demonstration of acellular osteochondral Puzzle Constructs cast on 3D printed interlocking porous bases: side-view of individual osteochondral construct consisting of (a) gel-region and (b) bone-region (left); paired osteochondral constructs (center); assembly of nine bases (right). Scale bars: 1 mm. B. Demonstration of in situ assembly and trimming of puzzle constructs (42 days cultured) in a canine cadaver femur defect.
Further studies are required to determine the optimal strategy for puzzle implantation. It is envisioned that puzzle subunits could be cultured individually, then assembled and trimmed (Tan et al., 2010) in situ via open joint surgery (Fig. 10B), as currently done for cartilage resurfacing of large defects, or potentially arthroscopically based on the ability to deliver individual pieces to the defect. Alternatively, puzzle constructs could also be cultured, assembled, and then subpunched to the desired size. Incorporating porous bases, as mentioned above, for anchoring and meshing with the underlying bone would require additional shapes to cap or plug the “male” and “female” parts of the puzzle shape or trimming, if permitted by the base material. Ideally, a large anatomically curved articular surface (Hung et al., 2003; Roach et al., 2015) could be assembled from interlocking engineered subunits, then sealed with a bioadhesive.
In summary, the puzzle assembly strategy offers a promising option for circumventing the limitations of nutrient diffusion in the generation of large engineered cartilage grafts. It is anticipated that this strategy could be utilized in the engineering of other tissues as well as combined with other complementary culture strategies.
Supplementary Material
Acknowledgements
This research was supported in part by the National Institutes of Health under the award number NIH 1R01AR060361. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional financial support by Coulter Foundation/Columbia CTV. We thank Kei Kuroki, DVM, PhD, Chantelle C. Bozynski, DVM, and Carin E. Ahner, DVM. (University of Missouri) for their help with the in vivo study. We thank Arthur J. Autz, MS (Columbia University) for his help with fabricating the punches.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions
All authors contributed significantly to the work reported in this manuscript. ABN, BKJ, GAA, and CTH designed the experiments. ABN, BKJ, WTY, DSD, and JDP carried out the experiments and collected the data. ABN, BKJ, GAA, and CTH analyzed the data. CTH, GAA, and JLC supervised and advised the work. ABN, BKJ, JLC, GAA, CTH wrote the paper. All authors have read and approved the final submitted manuscript.
Conflict of interest statement
The authors certify that there is no conflict of interest related to the work presented in this manuscript.
References
- Albro MB, Chahine NO, Li R, Yeager K, Hung CT, Ateshian GA. Dynamic loading of deformable porous media can induce active solute transport. J Biomech. 2008;41:3152–3157. doi: 10.1016/j.jbiomech.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Li R, Banerjee RE, Hung CT, Ateshian GA. Validation of theoretical framework explaining active solute uptake in dynamically loaded porous media. J Biomech. 2010;43:2267–2273. doi: 10.1016/j.jbiomech.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins PW. Physical chemistry. 5th ed. W.H. Freeman; New York: 1994. [Google Scholar]
- Bian L, Angione SL, Ng KW, Lima EG, Williams DY, Mao DQ, Ateshian GA, Hung CT. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthritis Cartilage. 2009a;17:677–685. doi: 10.1016/j.joca.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Crivello KM, Ng KW, Xu D, Williams DY, Ateshian GA, Hung CT. Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A. 2009b;15:2065–2072. doi: 10.1089/ten.tea.2008.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, Hung CT. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781–1790. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15:S230–235. [PubMed] [Google Scholar]
- Buckley CT, Thorpe SD, Kelly DJ. Engineering of large cartilaginous tissues through the use of microchanneled hydrogels and rotational culture. Tissue Eng Part A. 2009;15:3213–3220. doi: 10.1089/ten.TEA.2008.0531. [DOI] [PubMed] [Google Scholar]
- Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instructional course lectures. 1998;47:487–504. [PubMed] [Google Scholar]
- Cascio BM, Sharma B. The Future of Cartilage Repair. Operative Techniques in Sports Medicine. 16:221–224. [Google Scholar]
- Cigan AD, Nims RJ, Albro MB, Esau JD, Dreyer MP, Vunjak-Novakovic G, Hung CT, Ateshian GA. Insulin, ascorbate, and glucose have a much greater influence than transferrin and selenous acid on the in vitro growth of engineered cartilage in chondrogenic media. Tissue Eng Part A. 2013;19:1941–1948. doi: 10.1089/ten.tea.2012.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan AD, Nims RJ, Albro MB, Vunjak-Novakovic G, Hung CT, Ateshian GA. Nutrient channels and stirring enhanced the composition and stiffness of large cartilage constructs. J Biomech. 2014;47:3847–3854. doi: 10.1016/j.jbiomech.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91:1778–1790. [PubMed] [Google Scholar]
- Davisson T, Sah RL, Ratcliffe A. Perfusion increases cell content and matrix synthesis in chondrocyte three-dimensional cultures. Tissue Eng. 2002;8:807–816. doi: 10.1089/10763270260424169. [DOI] [PubMed] [Google Scholar]
- Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer Control of Microscopes Using µManager, Current Protocols in Molecular Biology. John Wiley & Sons, Inc.; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N. Advanced methods of microscope control using μManager software. Journal of Biological Methods. 2014;1(2) doi: 10.14440/jbm.2014.36. (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert C, Blunk T, Muller R, von Glasser SS, Baumer J, Fierlbeck J, Heid IM, Nerlich M, Hammer J. Bonding of articular cartilage using a combination of biochemical degradation and surface cross-linking. Arthritis Res Ther. 2007;9:R47. doi: 10.1186/ar2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Flanigan DC, Harris JD, Trinh TQ, Siston RA, Brophy RH. Prevalence of chondral defects in athletes' knees: a systematic review. Med Sci Sports Exerc. 2010;42:1795–1801. doi: 10.1249/MSS.0b013e3181d9eea0. [DOI] [PubMed] [Google Scholar]
- Gortz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374–1384. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005:79–87. doi: 10.1097/01.blo.0000165845.21735.05. [DOI] [PubMed] [Google Scholar]
- Gross AE, Silverstein EA, Falk J, Falk R, Langer F. The allotransplantation of partial joints in the treatment of osteoarthritis of the knee. Clin Orthop Relat Res. 1975:7–14. doi: 10.1097/00003086-197505000-00003. [DOI] [PubMed] [Google Scholar]
- Hangody L, Baló E. Autologous osteochondral mosaicplasty, Anterior Knee Pain and Patellar Instability. Springer; 2011. pp. 483–494. [Google Scholar]
- Heywood HK, Bader DL, Lee DA. Glucose concentration and medium volume influence cell viability and glycosaminoglycan synthesis in chondrocyte-seeded alginate constructs. Tissue Engineering. 2006a;12:3487–3496. doi: 10.1089/ten.2006.12.3487. [DOI] [PubMed] [Google Scholar]
- Heywood HK, Bader DL, Lee DA. Rate of oxygen consumption by isolated articular chondrocytes is sensitive to medium glucose concentration. Journal of Cellular Physiology. 2006b;206:402–410. doi: 10.1002/jcp.20491. [DOI] [PubMed] [Google Scholar]
- Hollander AP, Heathfield TF, Webber C, Iwata Y, Bourne R, Rorabeck C, Poole AR. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AH, Baker BM, Ateshian GA, Mauck RL. Sliding contact loading enhances the tensile properties of mesenchymal stem cell-seeded hydrogels. Eur Cell Mater. 2012;24:29–45. doi: 10.22203/ecm.v024a03. [DOI] [PubMed] [Google Scholar]
- Hung CT, Lima EG, Mauck RL, Takai E, LeRoux MA, Lu HH, Stark RG, Guo XE, Ateshian GA. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853–1864. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- Jeon O, Samorezov JE, Alsberg E. Single and dual crosslinked oxidized methacrylated alginate/PEG hydrogels for bioadhesive applications. Acta Biomater. 2014;10:47–55. doi: 10.1016/j.actbio.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE, Stoddart MJ. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater. 2013;25:248–267. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- Jurgensen K, Aeschlimann D, Cavin V, Genge M, Hunziker EB. A new biological glue for cartilage-cartilage interfaces: tissue transglutaminase. J Bone Joint Surg Am. 1997;79:185–193. doi: 10.2106/00004623-199702000-00004. [DOI] [PubMed] [Google Scholar]
- Kelly T-AN, Roach BL, Weidner ZD, Mackenzie-Smith CR, O'Connell GD, Lima EG, Stoker AM, Cook JL, Ateshian GA, Hung CT. Tissue-engineered articular cartilage exhibits tension–compression nonlinearity reminiscent of the native cartilage. Journal of Biomechanics. 2013;46:1784–1791. doi: 10.1016/j.jbiomech.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TA, Fisher MB, Oswald ES, Tai T, Mauck RL, Ateshian GA, Hung CT. Low-serum media and dynamic deformational loading in tissue engineering of articular cartilage. Ann Biomed Eng. 2008;36:769–779. doi: 10.1007/s10439-008-9476-1. [DOI] [PubMed] [Google Scholar]
- Kelly TA, Ng KW, Ateshian GA, Hung CT. Analysis of radial variations in material properties and matrix composition of chondrocyte-seeded agarose hydrogel constructs. Osteoarthritis Cartilage. 2009;17:73–82. doi: 10.1016/j.joca.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489–1497. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F, National Arthritis Data, W. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy HA, Awad HA, Guilak F. Molecular diffusion in tissue-engineered cartilage constructs: effects of scaffold material, time, and culture conditions. J Biomed Mater Res B Appl Biomater. 2004;70:397–406. doi: 10.1002/jbm.b.30053. [DOI] [PubMed] [Google Scholar]
- Lee CR, Grodzinsky AJ, Hsu HP, Martin SD, Spector M. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2000;18:790–799. doi: 10.1002/jor.1100180517. [DOI] [PubMed] [Google Scholar]
- Leigh JP, Seavey W, Leistikow B. Estimating the costs of job related arthritis. J Rheumatol. 2001;28:1647–1654. [PubMed] [Google Scholar]
- Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima EG, Chao PHG, Ateshian GA, Bal BS, Cook JL, Vunjak-Novakovic G, Hung CT. The effect of devitalized trabecular bone on the formation of osteochondral tissue-engineered constructs. Biomaterials. 2008a;29:4292–4299. doi: 10.1016/j.biomaterials.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima EG, Durney KM, Sirsi SR, Nover AB, Ateshian GA, Borden MA, Hung CT. Microbubbles as biocompatible porogens for hydrogel scaffolds. Acta Biomater. 2012;8:4334–4341. doi: 10.1016/j.actbio.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima EG, Mauck RL, Han SH, Park S, Ng KW, Ateshian GA, Hung CT. Functional tissue engineering of chondral and osteochondral constructs. Biorheology. 2004;41:577–590. [PubMed] [Google Scholar]
- Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshian GA, Cook JL, Hung CT. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng Part A. 2008b;14:1721–1730. doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- Maas SA, Ellis BJ, Ateshian GA, Weiss JA. FEBio: finite elements for biomechanics. J Biomech Eng. 2012;134:011005. doi: 10.1115/1.4005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum B, Browne JE, Fu F, Micheli LJ, Moseley JB, Jr., Erggelet C, Anderson AF. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35:915–921. doi: 10.1177/0363546507299528. [DOI] [PubMed] [Google Scholar]
- Mauck R, Soltz M, Wang C, Wong D, Chao P-H, Valhmu W, T HC, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. Journal of Biomechanical Engineering. 2000;122:9. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003a;125:602–614. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003b;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- McGowan K, Kurtis M, Lottman L, Watson D, Sah R. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen® and Hoechst 33258. Osteoarthritis and cartilage. 2002;10:580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- Moisio K, Eckstein F, Chmiel JS, Guermazi A, Prasad P, Almagor O, Song J, Dunlop D, Hudelmaier M, Kothari A, Sharma L. Denuded subchondral bone and knee pain in persons with knee osteoarthritis. Arthritis and rheumatism. 2009;60:3703–3710. doi: 10.1002/art.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- Mow VC, Ratcliffe A, Rosenwasser MP, Buckwalter JA. Experimental studies on repair of large osteochondral defects at a high weight bearing area of the knee joint: a tissue engineering study. J Biomech Eng. 1991;113:198–207. doi: 10.1115/1.2891235. [DOI] [PubMed] [Google Scholar]
- Ng KW, DeFrancis JG, Kugler LE, Kelly TA, Ho MM, O'Conor CJ, Ateshian GA, Hung CT. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino Acids. 2008;35:433–438. doi: 10.1007/s00726-007-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KW, Lima EG, Bian L, O'Conor CJ, Jayabalan PS, Stoker AM, Kuroki K, Cook CR, Ateshian GA, Cook JL, Hung CT. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041–1051. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nims RJ, Cigan AD, Albro MB, Vunjak-Novakovic G, Hung CT, Ateshian GA. Matrix Production in Large Engineered Cartilage Constructs Is Enhanced by Nutrient Channels and Excess Media Supply. Tissue engineering. Part C, Methods. 2015;21:747–757. doi: 10.1089/ten.tec.2014.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover AB, Lee SL, Georgescu MS, Howard DR, Saunders RA, Yu WT, Klein RW, Napolitano AP, Ateshian GA, Hung CT. Porous titanium bases for osteochondral tissue engineering. Acta Biomater. 2015a;27:286–293. doi: 10.1016/j.actbio.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover AB, Stefani RM, Lee SL, Ateshian GA, Stoker AM, Cook JL, Hung CT. Long-term storage and preservation of tissue engineered articular cartilage. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2015b doi: 10.1002/jor.23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell GD, Nims RJ, Green J, Cigan AD, Ateshian GA, Hung CT. Time and dose-dependent effects of chondroitinase ABC on growth of engineered cartilage. Eur Cell Mater. 2014;27:312–320. doi: 10.22203/ecm.v027a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic B, Carrier RL, Vunjak-Novakovic G, Freed LE. Gas exchange is essential for bioreactor cultivation of tissue engineered cartilage. Biotechnology and Bioengineering. 1999;63:197–205. doi: 10.1002/(sici)1097-0290(19990420)63:2<197::aid-bit8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Paige KT, Vacanti CA. Engineering new tissue: formation of neo-cartilage. Tissue Eng. 1995;1:97–106. doi: 10.1089/ten.1995.1.97. [DOI] [PubMed] [Google Scholar]
- Park S, Hung CT, Ateshian GA. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage. 2004;12:65–73. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Reddy S, Pedowitz DI, Parekh SG, Sennett BJ, Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35:80–85. doi: 10.1177/0363546506290986. [DOI] [PubMed] [Google Scholar]
- Roach BL, Hung CT, Cook JL, Ateshian GA, Tan AR. Fabrication of tissue engineered osteochondral grafts for restoring the articular surface of diarthrodial joints. Methods. 2015;84:103–108. doi: 10.1016/j.ymeth.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert H. Chondral repair of the knee joint using mosaicplasty. Orthop Traumatol Surg Res. 2011;97:418–429. doi: 10.1016/j.otsr.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Sargeant TD, Desai AP, Banerjee S, Agawu A, Stopek JB. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomater. 2012;8:124–132. doi: 10.1016/j.actbio.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Sengers BG, Heywood HK, Lee DA, Oomens CWJ, Bader DL. Nutrient utilization by bovine articular chondrocytes: A combined experimental and theoretical approach. Journal of Biomechanical Engineering-Transactions of the Asme. 2005;127:758–766. doi: 10.1115/1.1993664. [DOI] [PubMed] [Google Scholar]
- Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22:121–133. doi: 10.5435/JAAOS-22-02-121. [DOI] [PubMed] [Google Scholar]
- Sitterle VB, Nishimuta JF, Levenston ME. Photochemical approaches for bonding of cartilage tissues. Osteoarthritis Cartilage. 2009;17:1649–1656. doi: 10.1016/j.joca.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Tan AR, Dong EY, Ateshian GA, Hung CT. Response of engineered cartilage to mechanical insult depends on construct maturity. Osteoarthritis Cartilage. 2010;18:1577–1585. doi: 10.1016/j.joca.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AR, Hung CT. Engineering Functional Cartilage Grafts. In: Bernstein HS, editor. Tissue Engineering in Regenerative Medicine. Humana Press; 2011. pp. 237–250. [Google Scholar]
- Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, Richardson JB. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87:179–183. doi: 10.1302/0301-620x.87b2.15376. [DOI] [PubMed] [Google Scholar]
- Zhou S, Cui Z, Urban JPG. Nutrient gradients in engineered cartilage: Metabolic kinetics measurement and mass transfer modeling. Biotechnology and Bioengineering. 2008;101:408–421. doi: 10.1002/bit.21887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.