Abstract
Introduction:
We aimed to assess contemporary rates of neoadjuvant chemotherapy (NC) use.
Methods:
We relied on the Surveillance, Epidemiology and End Results (SEER)-Medicare database for non-metastatic, muscle-invasive (T2–T4a) urothelial carcinoma of the urinary bladder (UCUB) patients who underwent radical cystectomy (RC) between 1991 and 2009. Multivariable logistic regression analyses tested predictors of NC use, such as: T-stage, N-stage, year of diagnosis, age at diagnosis, gender, race, use of radiotherapy (RT), marital status, urban status, socioeconomic status, tumour grade, and Charlson comorbidity index (CCI).
Results:
Overall, 5207 patients treated with RC were identified. Of those, 332 (6.4%) received NC. The rate of NC increased over time from 6.1% (1991) to 15.0% (2009) (p<0.001). In multivariable analyses, year of diagnosis (odds ratio [OR]: 4.7; p<0.001), lower T-stage (T3 vs. T2: OR: 0.7; p=0.003), married status (OR: 1.5; p=0.006), and younger age at diagnosis (≥80 vs. 66–69: OR: 0.6; p=0.006) were associated with a higher odds of NC; all represented independent predictors of NC use. Neither race nor CCI demonstrated statistical significance.
Conclusions:
We reported lower than anticipated overall (6.4%) use of NC. Nonetheless, the rate increased from 6.1% (1991) to 15.0% (2009). Older and unmarried individuals were less likely to receive NC. NC rates were higher in T2 UCUB patients. Some of the observed discrepancies, such as lower use in unmarried individuals, may require correction. Better adherence to guidelines should be encouraged and implemented, especially based on the confirmed benefits of NC according to randomized, controlled trials. The study is limited by a retrospective design and limited variables.
Introduction
American Cancer Society estimates 72 570 new cases of bladder cancer (BC) in the U.S. in 2013 and about 15 210 deaths from BC within the same year.1 Muscle-invasive urothelial carcinoma of the urinary bladder (UCUB) presents 25% of incident cases.2 Radical cystectomy (RC) is the standard of care in non-metastatic, muscle-invasive UCUB patients with adequate performance status.3,4 Since improved survival with neoadjuvant chemotherapy (NC) was recorded starting in 2003,5–7 its administration is recommended by guidelines for patients with clinical stage T2–4a N0M0 UCUB.3,4 Despite this broad recommendation for use of NC, population-based reports indicate low rates of NC administration in the context of RC, with 5.9% between 1992 and 2002 and 20.9% in 2010 in the U.S.8,9 Based on this apparent non-adherence to guidelines, we examined the NC rates prior to RC within the Surveillance, Epidemiology and End Results (SEER)-Medicare database focusing on individuals treated between 1991 and 2009.
Methods
Study source
The current study relied on the SEER-Medicare-linked database. This database is 98% complete for case ascertainment. The SEER registries identify 28% of all incident cancer cases in the U.S. Medicare insures approximately 97% of all Americans aged ≥65 years. Linkage to the SEER database is complete for approximately 93% of cases.10
Study population
Overall 15 080 patients with a primary, non-metastatic, muscle-invasive (T2–T4) UCUB (International Classification of disease for Oncology [ICD–O] site code 67.0, histologic code 8120 or 8130), with or without lymph node metastases, diagnosed between January 1991 and December 2009 were abstracted. Patients not enrolled in Medicare parts A or B for a minimum of 12 months prior to their first recorded diagnosis and for six months after diagnosis were not considered. Patients who had health maintenance organization (HMO) enrolment in the year prior to diagnosis or for any period following diagnosis were also excluded. To ensure that all subjects had at least one year of claims from which comorbidities are derived, only those aged ≥66 years old were considered. This resulted in 7337 assessable patients. Additional exclusions comprised those with unknown race (n=36) and unknown marital status (n=432). Furthermore, patients treated with surgery ≥6 months after diagnosis were also not considered in the current study (n=1185). Finally, patients with stages T4b or T4 not otherwise specified were omitted from our analyses, as the current guidelines suggest a different management approach for such individuals (n=477). This resulted in 5207 assessable individuals with T2-T4a, N0 or Nx and N+ patients, who represent potential candidates for NC.
Covariates
Covariates comprised age at diagnosis, comorbidities (derived using a validated algorithm based on the Charlson comorbidity index [CCI]11), tumour extent (T2, T3, T4a) represented as a consensus state (highest of either clinical or pathological), tumour grade (low-, high-grade), nodal stage (N0/Nx, N+), gender, race (White, other), marital status (married, unmarried), socioeconomic status (SES) (composite variable of income, education, and poverty levels),12 year of diagnosis, urban residence status (non-, metropolitan), and radiotherapy (RT).
Statistical analyses
We compared baseline characteristics between RC patients, who were either administered to NC or not (Table 1). Subsequently, univariable and multivariable logistic regression analyses were performed to identify the clinical and demographic characteristics associated with the use of NC. Means, medians, and interquartile ranges were reported for continuous variables. Frequencies and proportions were reported for categorical variables. The t-test, the Mann-Whitney test, and chi-square tests were used to compare the statistical significance of differences in means, medians, and proportions, respectively. All statistical tests were performed using R software environment for statistical computing and graphics (Vienna, Austria, version 3.0.1). All tests were two-sided, with a significance level set at p<0.05.
Table 1.
Baseline descriptives of 5207 patients treated with RC for non-metastatic, muscle-invasive UCUB (T2-T4a) between 1991 and 2009 within the SEER-Medicare-linked database
| Variables | RC without NC n=4875 (93.6%) | RC with NC n=332 (6.4 %) | p value |
|---|---|---|---|
| Age | |||
| Mean (median) | 75.6 (75.0) | 74.3 (74.0) | <0.001 |
| IQR | 71.0–80.0 | 70.0–78.0 | |
| Age categories, n (%) | |||
| 66–69 | 952 (19.5) | 81 (24.4) | |
| 70–74 | 1292 (26.5) | 101 (30.4) | 0.02 |
| 75–79 | 1319 (27.1) | 80 (24.1) | |
| ≥80 | 1312 (26.9) | 70 (21.1) | |
| Charlson comorbidity index, n (%) | |||
| 0 | 1899 (39.0) | 136 (41.0) | |
| 1 | 747 (15.3) | 51 (15.4) | 0.6 |
| 2 | 1006 (20.6) | 72 (21.7) | |
| ≥3 | 1223 (25.1) | 73 (22.0) | |
| Gender, n (%) | |||
| Male | 3487 (71.5) | 246 (74.1) | 0.3 |
| Female | 1388 (28.5) | 86 (25.9) | |
| Race, n (%) | |||
| White | 4388 (90.0) | 309 (93.1) | 0.1 |
| Other | 487 (10.0) | 23 (6.9) | |
| Marital status, n (%) | |||
| Unmarried | 1713 (35.1) | 87 (26.2) | 0.001 |
| Married | 3162 (64.9) | 245 (73.8) | |
| Socioeconomic status, n (%) | |||
| High | 2478 (50.8) | 143 (43.1) | 0.006 |
| Low | 2397 (49.2) | 189 (56.9) | |
| Tumour stage, n (%) | |||
| T2 | 2365 (48.5) | 188 (56.6) | |
| T3 | 1732 (35.5) | 93 (28.0) | 0.01 |
| T4a | 778 (16.0) | 51 (15.4) | |
| Organ-confined, n (%) | |||
| Yes (T2 Nx/0) | 2194 (45.0) | 177 (53.3) | 0.003 |
| No (T2 N1-3,T3, or T4a) | 2681 (55.0) | 155 (46.7) | |
| Nodal stage, n (%) | |||
| Nx/N0 | 4024 (82.5) | 278 (83.7) | 0.6 |
| N+ | 851(17.5) | 54 (16.3) | |
| Tumour grade, n (%) | |||
| High | 4580 (93.9) | 322 (97.0) | 0.02 |
| Metropolitan, n (%) | 4418 (90.6) | 296 (89.2) | 0.4 |
| Radiotherapy, n (%) | 706 (14.5) | 68 (20.5) | 0.003 |
NC: neoadjuvant chemotherapy; RC: radical cystectomy; SEER: Surveillance, Epidemiology and End Results; UCUB: urothelial carcinoma of the urinary bladder.
Results
Baseline characteristics
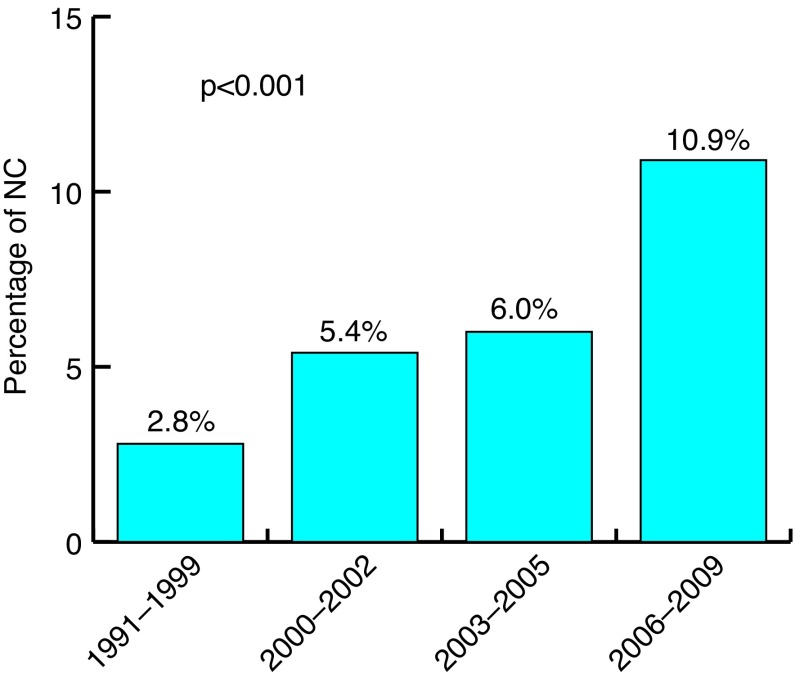
Overall, 5207 patients with non-metastatic, muscle-invasive UCUB who underwent RC were included in the study cohort (Table 1). Mean (median) age at diagnosis was 75.5 (75.0) years. Overall, 332 (6.4%) RC patients were treated with NC vs. 4875 (93.6%) who were not. Within these groups, statistically significant differences were recorded according to age at diagnosis, marital status, socioeconomic status, tumour stage, rate of organ-confined tumours, tumour grade, and administration of RT (Table 1; all p<0.05). Specifically, NC patients were younger (median age: 74.0 vs. 75.5 years) and were more frequently married (73.8 vs. 64.9%). Additionally, a larger proportion of NC patients was included in the low SES group (56.9 vs. 49.2%). NC patients more often had organ-confined disease (53.3 vs. 45.0%). RT was also more frequently delivered in NC patients (20.5 vs. 14.5%). The rate of NC increased over time from 2.8% to 10.9% (Fig. 1; p<0.001), where the highest rate was recorded in 2009 (15.0%).
Fig. 1.
Rate of neoadjuvant chemotherapy according to year of diagnosis.
Logistic regression models
In univariable logistic regression analyses predicting the administration of NC, six variables emerged as statistically significant predictors of NC prior to RC. The strongest predictor was the year of diagnosis. Individuals treated and diagnosed between 2006 and 2009 had a higher rate of NC administration than their counterparts treated and diagnosed between 1991 and 1999 (odds ratio [OR]: 4.2 (95% confidence interval [CI]: 2.9–6.0; p<0.001). Married individuals were more likely to receive NC compared to their unmarried counterparts (OR: 1.5; 95% CI: 1.2–2.0; p=0.001). Lower socioeconomic status was associated with a higher probability of receiving NC (OR: 1.4; 95% CI: 1.1–1.7; p=0.006). Higher tumour grade indicated higher odds of NC administration (OR: 2.1; 95% CI: 1.1–3.9; p=0.03). Additionally, patients with tumour stage T3 had a significantly lower probability of receiving NC (OR: 0.7; 95% CI: 0.5–0.9; p=0.003). Similarly, patients ≥80 years had a lower probability of being exposed to NC compared to their counterparts between 66 and 69 years (OR: 0.6; 95% CI: 0.5–0.9; p=0.006). Importantly, nodal stage, CCI, and race were not associated to NC administration (Table 2).
Table 2.
Univariable and multivariable logistic regression analyses evaluating the predictors of administration of NC in 5207 patients treated with RC for non-metastatic, muscle-invasive UCUC between 1991 and 2009 within SEER-Medicare-linked database
| Variables | Univariable analyses | Multivariable analyses | ||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age at diagnosis | ||||
| 66–69 | 1 (ref.) | 1 (ref.) | ||
| 70–74 | 0.9 (0.7–1.3) | 0.6 | 1.0 (0.7–1.3) | 0.8 |
| 75–79 | 0.7 (0.5–1.0) | 0.04 | 0.7 (0.5–1.0) | 0.07 |
| ≥80 | 0.6 (0.5–0.9) | 0.006 | 0.6 (0.4–0.9) | 0.006 |
| Year of diagnosis | ||||
| 1991–1999 | 1 (ref.) | 1 (ref.) | ||
| 2000–2002 | 2.0 (1.3–3.0) | 0.001 | 2.2 (1.4–3.4) | 0.001 |
| 2003–2005 | 2.2 (1.5–3.3) | <0.001 | 2.4 (1.6–3.7) | <0.001 |
| 2006–2009 | 4.2 (2.9–6.0) | <0.001 | 4.7 (3.2–7.0) | <0.001 |
| Gender | ||||
| Male | 1 (ref.) | 1 (ref.) | ||
| Female | 0.9 (0.7–1.1) | 0.3 | 1.1 (0.8–1.4) | 0.6 |
| Race | ||||
| White | 1 (ref.) | 1 (ref.) | ||
| Other | 0.7 (0.4–1.0) | 0.07 | 0.7 (0.5–1.1) | 0.1 |
| Marital status | ||||
| Unmarried | 1 (ref.) | 1 (ref.) | ||
| Married | 1.5 (1.2–2.0) | 0.001 | 1.5 (1.1–1.9) | 0.006 |
| Population density | ||||
| Metropolitan | 1 (ref.) | 1 (ref.) | ||
| Non-metropolitan | 1.2 (0.8–1.7) | 0.4 | 1.1 (0.7–1.6) | 0.7 |
| Socioeconomic status | ||||
| High | 1 (ref.) | 1 (ref.) | ||
| Low | 1.4 (1.1–1.7) | 0.006 | 1.0 (0.7–1.2) | 0.7 |
| Charlson comorbidity index | ||||
| 0 | 1 (ref.) | 1 (ref.) | ||
| 1 | 1.0 (0.7–1.3) | 0.8 | 0.9 (0.7–1.3) | 0.6 |
| 2 | 1.0 (0.7–1.3) | 1.0 | 1.0 (0.7–1.3) | 0.9 |
| ≥3 | 0.8 (0.6–1.1) | 0.2 | 0.8 (0.6–1.0) | 0.07 |
| Tumour grade | ||||
| Low | 1 (ref.) | 1 (ref.) | ||
| High | 2.1 (1.1–3.9) | 0.03 | 1.9 (0.97–3.5) | 0.06 |
| Tumour stage | ||||
| T2 | 1 (ref.) | 1 (ref.) | ||
| T3 | 0.7 (0.5–0.9) | 0.003 | 0.7(0.5–0.9) | 0.003 |
| T4a | 0.8 (0.6–1.1) | 0.2 | 0.9 (0.7–1.3) | 0.6 |
| Nodal stage | ||||
| N0/Nx | 1 (ref.) | 1 (ref.) | ||
| N+ | 0.9 (0.7–1.2) | 0.6 | 0.9 (0.6–1.2) | 0.4 |
CI: confidence interval; NC: neoadjuvant chemotherapy; OR: odds ratio;
RC: radical cystectomy; ref: reference; SEER: Surveillance, Epidemiology and End Results; UCUB: urothelial carcinoma of the urinary bladder.
In multivariable logistic regression analyses, four variables emerged as independent predictors of NC administration. Patients diagnosed and treated in most recent years had a higher likelihood of receiving NC. Specifically, individuals treated and diagnosed between 2006 and 2009 had a 4.7-fold higher rate of NC administration than their counterparts treated and diagnosed between 1991 and 1999 (OR: 4.7; 95% CI: 3.2–7.0; p<0.001). It is of interest to note that married individuals had 1.5-fold higher odds of receiving NC, compared to their unmarried counterparts (OR: 1.5; 95% CI: 1.1–1.9, p=0.006). With respect to tumour characteristics, patients with tumour stage T3 had a significantly lower probability of receiving NC (OR: 0.7; 95% CI: 0.5–0.9; p=0.003). It is also of note that advanced age decreased the likelihood of NC administration. Specifically, patients ≥80 years had a 40% lower probability of being exposed to NC (OR: 0.6; 95% CI: 0.4–0.9; p=0.006, Table 2).
Discussion
Our hypothesis stated that the rates of NC administration have increased in most contemporary years. We were able to confirm an overall increase in the rates of NC. However, the absolute rate of NC use was low during the initial, as well as the final time periods of the study. Specifically, NC rates ranged from 2.8–10.9% according to time periods (1991–1999 vs. 2000–2002 vs. 2003–2005 vs. 2006–2009), with a peak of 15.0% in 2009. These low rates sharply contrast with the National Comprehensive Cancer Network (NCCN) and European Association of Urology (EAU) guidelines that recommend routine use of NC in individuals with T2-T4a UCUB.3,4 The increasing trend is nonetheless explained by increasing dissemination of existing guidelines. This, in turn, may indicate that guidelines result in optimization of patient care.
Several important additional observations also warrant mention. For example, our multivariable models results identified several patient characteristics that represented relative barriers to administration of NC. Advanced patient age limited NC administration rate. This finding is in agreement with an acknowledged relative contraindication to NC for patients with advanced age, especially when cisplatin-based chemotherapy is considered.13 However, such limitation is only relative. Age alone should not be used to exclude patients from this potentially highly beneficial treatment.14,15
Other patient characteristics, such as marital status, also represented barriers to NC administration. For example, married individuals were 1.5-fold more likely to receive NC than their unmarried counterparts. This finding is in agreement with several other urologic malignancies, where married status is associated with better access or higher rates of treatment delivery.16,17
Discrepancies related to age and/or marital status are important to note. From a clinical standpoint, physicians may choose to pay particular attention to individuals with characteristics that decrease access or patient interest in NC. Age and marital status represent such characteristics.
Additionally, tumour characteristics also represented a statistically significant variable that influenced the rate of NC administration. For example, patients with tumour stage T3 were 30% less likely to receive NC. The association between tumour stage and NC administration rate, where patients with T3 disease are less likely to receive NC, might be explained by patient and physician concerns related to potential surgical treatment delays if NC is chosen.18 The NC recommendations hinge on the Grossman et al data,5 which included a 60% majority of T3-4a patients.
Our findings are in agreement with previously published reports,8,9 where the rates of NC were also low. Porter et al8 relied on the SEER-Medicare database. In that study, the rate of NC administration within 8719 patients with muscle-invasive UCUB treated by RC between 1992 and 2002 was 5.6%. Relying on the National Cancer Database, Zaid et al9 reported on 5692 patients. Those patients had UCUB stage cT2 or higher and underwent RC between 2006 and 2010. In that patient cohort, the rates of NC increased from 7.6% in 2006 to 20.9% in 2010. Similar to our study, Gotto et al19 recorded increased rates of NC administration over time according to different Canadian centres between 2007 and 2011. Higher rate of NC in the Zaid et al report may relate to age differences between the current populations and theirs. In the latter, the mean age was 66.6 years and the range was 28–90 years. Conversely, the mean age in our study was 75.5 years and ranged from 66–95 years. To the best of our knowledge, no population-based data indicate higher rates than those described in our study and that of Zaid et al. Moreover, we are unaware of population-based European studies reporting on the use of NC.
Despite its strengths, our study is not devoid of limitations. First, its retrospective design limits the quality of the data. Second, population-based studies contain limited numbers of variables. Moreover, the pathological T and N stages represent consensus stages, where the highest of either clinical or pathological is included. Third, several important characteristics that may represent exclusion criteria from NC, such as performance status, renal function, patients willingness to undergo NC, or physician interest in recommending NC prior to RC were not available. Fourth, it is of note that the exact timing of RT administration was not established. It might be postulated that the majority of such patients received this treatment modality after RC. Last, but not least, our study relies on patients older than 65 years. Consequently, our findings might not be generalizable to younger individuals.
In conclusion, we reported lower than anticipated overall (6.4%) use of NC during the study period. Nonetheless, the rate increased from 6.1% (1991) to 15.0% (2009). Older and unmarried individuals were less likely to receive NC. Finally, NC rates were higher in T2 UCUB patients. Some of the observed discrepancies, such as lower use in unmarried individuals, may require correction. Better adherence to guidelines should be encouraged and implemented, especially based on the confirmed benefits of NC according to randomized, controlled trials.
Footnotes
Competing interests: The authors declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Ca Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Howlader NA, Krapcho M, Garshell J, et al., editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. Accessed February 1, 2016. [Google Scholar]
- 3.Stenzl A, Cowan NC, De Santis M, et al. Treatment of muscle-invasive and metastatic bladder cancer: Update of the EAU guidelines. Eur Urol. 2011;59:1009–18. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–75. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 5.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Bellmunt J, Sonpavde G, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol. 2013;63:58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–5. doi: 10.1016/j.eururo.2005.04.006. discussion 205–6. [DOI] [PubMed] [Google Scholar]
- 8.Porter MP, Kerrigan MC, Donato BM, et al. Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urol Oncol. 2011;29:252–8. doi: 10.1016/j.urolonc.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Zaid HB, Patel SG, Stimson CJ, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: Results from the National Cancer Database. Urology. 2014;83(1):75–80. doi: 10.1016/j.urology.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Medical Care. 2002;40:IV3–18. doi: 10.1097/00005650-200208001-00002. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/S0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 12.Singh GK, National Cancer Institute (U.S.) Area socioeconomic variations in U.S. cancer incidence, mortality, stage, treatment, and survival, 1975–1999. Bethesda, MD: U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute; 2003. Available at http://open-dev.umms.med.umich.edu/sites/default/files/1232/reading-resources-1/Singh-fullarticle.pdf. Accessed February 8, 2016. [Google Scholar]
- 13.Thompson RH, Boorjian SA, Kim SP, et al. Eligibility for neoadjuvant/adjuvant cisplatin-based chemotherapy among radical cystectomy patients. BJU Int. 2014;113:E17–21. doi: 10.1111/bju.12274. [DOI] [PubMed] [Google Scholar]
- 14.Hollenbeck BK, Miller DC, Taub D, et al. Aggressive treatment for bladder cancer is associated with improved overall survival among patients 80 years old or older. Urology. 2004;64:292–7. doi: 10.1016/j.urology.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Bamias A, Efstathiou E, Moulopoulos LA, et al. The outcome of elderly patients with advanced urothelial carcinoma after platinum-based combination chemotherapy. Ann Oncol. 2005;16:307–13. doi: 10.1093/annonc/mdi039. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi M, Becker A, Abdollah F, et al. Rates of open versus laparoscopic and partial versus radical nephrectomy for T1a renal cell carcinoma: A population-based evaluation. Int J Urol. 2013;20:1064–71. doi: 10.1111/iju.12110. [DOI] [PubMed] [Google Scholar]
- 17.Denberg TD, Beaty BL, Kim FJ, et al. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer. 2005;103:1819–25. doi: 10.1002/cncr.20982. [DOI] [PubMed] [Google Scholar]
- 18.Chang SS, Hassan JM, Cookson MS, et al. Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stage. J Urol. 2003;170:1085–7. doi: 10.1097/01.ju.0000086828.26001.ca. [DOI] [PubMed] [Google Scholar]
- 19.Gotto GT, Shea-Budgell MA, Rose MS, et al. Predictors of referral for neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer and changes in practice over time. Can Urol Assoc J. 2015;9:236–41. doi: 10.5489/cuaj.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]