Abstract
Tuberous sclerosis is associated with epilepsy in up to 85% of cases, and in 2/3, the onset is within the first year of life. An early antiepileptic treatment is crucial to minimize the consequences of epilepsy on cognition and behavior. We present a case report of a child with tuberous sclerosis who presented with infantile spasms at the age of 6 months, immediately treated with vigabatrin. Because of the presence of a subependymal giant cell astrocytoma, he also received everolimus since 18 months of age. We might wonder if an earlier treatment could have produced a better outcome; in fact, despite a targeted combined treatment, he continues to suffer from sporadic focal motor seizures, and at the age of 40 months, he presents severe developmental delay with autism-like behavior.
Keywords: Tuberous sclerosis, Everolimus, Vigabatrin, Early treatment, Epilepsy, Neurodevelopmental disorders
1. Introduction
Tuberous sclerosis complex (TSC) is a genetic multisystem disorder in which benign hamartomas develop in multiple organ systems [1]. The disorder has an estimated birth incidence of 1:5800 [2]. Epilepsy is the most frequent neurological manifestation in patients affected by TSC, involving up to 80% of subjects [3]. Epileptic seizures begin in the first year of life in about one-third of TSC patients, and sometimes in the very first weeks of life [4]. Focal seizures may be the first seizure type and may coexist or evolve into infantile spasms [5]. Children with TSC presenting an early seizure onset are at high risk of developing refractory epilepsy and/or an epileptic encephalopathy; by the age of 2 years, 42% of children with TSC will manifest drug-resistant seizures [4]. Early life seizures are also associated with a greater incidence of severe/profound cognitive impairment and autism-like behavior [4], [6]. However, cognitive–behavioral disturbances might be present independently of seizures. Manifestations related to TSC are the consequence of the hyperactivation of the so-called mTOR (mammalian target of rapamycin) pathway, resulting from the loss of TSC1 or TSC2 gene, and leading to abnormalities in cell growth and differentiation, synaptogenesis, and impaired protein synthesis [7].
A definite clinical diagnosis of TSC can be made in the presence of two major features, but identification of either a TSC1 or TSC2 pathogenic mutation is sufficient to make a definite diagnosis too [8]. Cardiac rhabdomyomas, cortical tubers, subependymal nodules, and renal angiomyolipomas may be detected by prenatal imaging allowing an early diagnosis [9], [10]. Today, TSC can be diagnosed prenatally, or early postnatally in a growing number of patients [11], and all the diagnosed infants should be considered at a high risk of developing early onset seizures [12]. Therefore, clinicians should educate parents to recognize subtle focal seizures or epileptic spasms as early as possible, thus, trying to reduce the gap between seizure onset and treatment initiation [13], [14], [15].
Similarly, all children should be considered at high risk for neurocognitive and behavioral impairments, and should be regularly evaluated in order to highlight early signs of deviation from the typical developmental trajectory so that an early intensive behavioral treatment program could be applied. In particular, there is still little evidence whether early treatment with everolimus or combined treatment with vigabatrin and everolimus is able to reduce the risk of epilepsy and neurodevelopmental disability.
In this paper, we report the case of a child with TSC and early onset epilepsy, treated with a combined targeted therapy of vigabatrin and everolimus, and followed up until the age of 40 months.
1.1. Case presentation
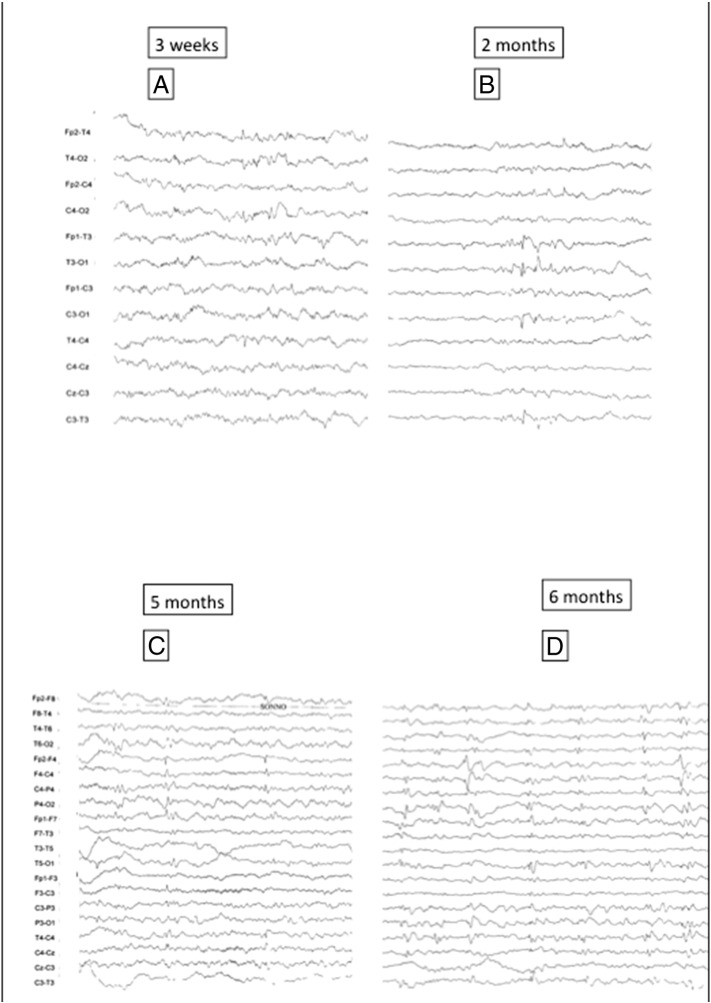
A 3-week-old male newborn came to our attention after the detection of cardiac rhabdomyomas at 28 weeks of gestational age, and the subsequent identification of cortical tubers and a subependymal nodule by fetal brain MRI. He was born at 32 gestation weeks after an uneventful pregnancy by healthy, nonconsanguineous parents. An early postnatal MRI confirmed the TSC diagnosis. Genetic molecular analysis revealed a de novo mutation on the TSC2 gene (c.687_690del CTGC). Electroencephalogram monitoring was started at 3 weeks of life and was continued at 4-week intervals, for the first 6 months of life, and every 6–8 weeks, or as clinically needed thereafter, according to the recommendations from the European Consensus Conference on TSC-related epilepsy [14]. During the first evaluations, his neurologic examination and the wakefulness and sleep video-EEG were normal for his age (Fig. 1A). At the age of 2 months, some isolated sharp waves and spikes appeared on the left temporal region during sleep (Fig. 1B); this epileptic focus appeared to be in topographic concordance with a large left temporal tuber seen by MRI. At the age of 5 months, the epileptiform abnormalities became multifocal, and bilateral temporoparietal slow waves were also recorded (Fig. 1C). However, the background EEG activity showed a good organization, with regional differentiation and sleep spindles and K complex during NREM sleep. At the age of 6 months, the EEG was more active, showing recurrent multifocal slowing and epileptiform abnormalities, with a pseudo-periodism (Fig. 1D). Epileptiform abnormalities became more frequent and often generalized, with epileptic foci mainly localized in the right hemisphere. Starting from the age of 6 months, serial neuropsychological evaluations were performed every 6 months using the Griffiths Mental Developmental Scale (GMDS). All the single items have been analyzed in full detail to identify a pattern of gain or loss of specific abilities over time. The first structured neuropsychological evaluation revealed a normal developmental quotient (DQ) of 99.
Fig. 1.
Sleep EEGs of the patient at different timepoints.
A. The first EEG at 3 weeks of life showed a good background organization with no clear epileptiform abnormalities.
B. At the age of 2 months, sleep and awake EEG continued to show a good background organization. Very rare and isolated spikes appeared only during sleep on the left temporal region (T3).
C. At the age of 5 months, the EEG pattern begins to change. Epileptiform abnormalities now become sustained and multifocal, with an initial tendency to spread, at least in the same hemisphere. This kind of EEG pattern might be considered as a “point of no return”, after which, a progressive EEG deterioration with a significant increase of epileptiform abnormalities is likely to occur in a few weeks.
D. Significant evolution of the EEG pattern, which now shows recurrent and sustained epileptiform abnormalities with spikes and spike and waves with a tendency to spread with a pseudo-periodism.
One day, after the child presented subtle spasms, vigabatrin (VGB) was immediately started and titrated up to 128 mg/kg/day to obtain a complete cessation of the epileptic spasms and a progressive disappearance of secondary bilateral synchrony on the EEG. After 3 weeks of VGB treatment, the EEG showed only the persistence of isolated multifocal sharp waves. The child never presented a hypsarrhythmic EEG pattern.
After spasm cessation, the baby presented good clinical conditions and continued to be interactive and participative. However, the EEG showed the persistence of focal slow abnormalities, and the representation of NREM physiologic elements began to be insufficient. After a seizure-free period of 5 weeks, focal seizures characterized by psychomotor arrest and left eye deviation appeared. Clobazam was introduced, initially producing a seizure-free period, but seizures soon appeared again, and he also began to have daily focal tonic seizures, sometimes followed by myoclonic jerks. At the age of 18 months, after the detection of a growth in the size of a subependymal giant cell astrocytoma, everolimus was introduced at an initial dosage of 2.5 mg every other day, subsequently increased at 2.5 mg/day due to low blood levels. About one month after everolimus introduction, the child ceased to have convulsive seizures, only showing brief duration psychomotor arrests, with a global decrease in daily seizure frequency. At the age of 26 months, he began again have focal motor seizures, and because of the high myoclonic component, clobazam was replaced with clonazepam with good clinical results. He subsequently had three months of seizure freedom, followed by the reappearance of brief sporadic seizures characterized by psychomotor arrest.
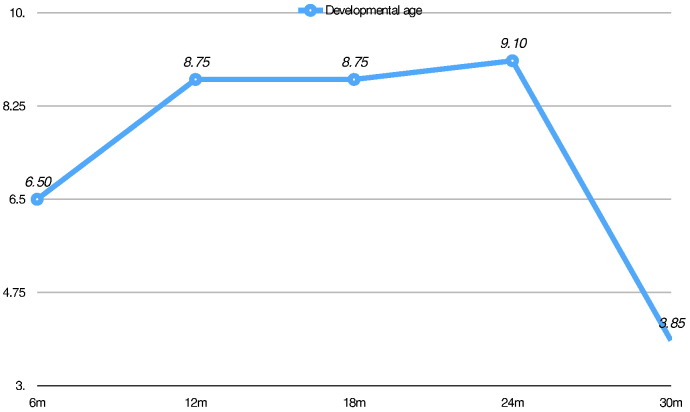
After the age of 8 months, a gradual deceleration of the developmental trajectory was observed, confirmed by a decrease in DQ values (99 at 6 months, 71 at 12 months, and below 50 at 18, 24, and 30 months of age) reflecting a lack of acquisitions with a minimal increase of developmental age over time up to the age of 24 months followed by a severe regression (Fig. 2). After the age of 12 months, the child also began to show some autistic traits including alterations in sociocommunication areas, such as poor eye contact, failure to respond to name, and deficit of visual and joint attention, associated with patterns of stereotyped and repetitive behaviors. After the second year of life, a progressive decrease in the functional usage of his hands appeared, worsening the general performances of the child, and severely influencing the last neuropsychological evaluation performed at the age of 30 months. Autism Diagnostic Observation Schedule (ADOS), a semistructured, interactive schedule designed to assess social and communicative functioning, could not be administered to better investigate autism symptoms, since a minimal mental age of 12 months is required.
Fig. 2.
This chart shows the developmental age of the child over time. After the first evaluation at the age of 6 months, he never appeared to develop according to his chronological age, and after 1 year without significant acquisitions, he significantly lost many abilities.
By analyzing in detail the different evolution of the various developmental areas as assessed through GMDS, we detected a slow but continuous gain of acquisition of motor abilities, associated with stagnation in performance and oculomanual coordination. On the other hand, a clear regression of developmental age was observed in the areas of hearing and language, but above all, in the personal–social abilities. In particular, he lost the social smiling with the mirror from the 12th month of age, and later, he also ceased to show interest for other persons, to electively recognize his parents, and to interactively play. Expressive language was never achieved, and after some months of babbling, he completely stopped producing sounds.
2. Discussion
To the best of our knowledge, this is the first case report of a long-term follow-up of a child with TSC treated with a targeted combined treatment, including vigabatrin and everolimus, and carefully evaluated both from a neurodevelopmental point of view and an electroclinical point of view. The clinical evolution of this young boy shows that, even if early VGB treatment might minimize the long-term neurocognitive outcome, it is not able to totally revert the neurobehavioral phenotype, which is a multifactorial condition [13, 16]. To better understand this process, it is important to consider that neurocognitive symptoms and epilepsy in TSC share a common neurobiological causative pathway, in which mTOR overactivation is responsible for an imbalance between excitation and inhibition, as well as for synaptic and connectivity abnormalities [17]. In particular, experimental data suggest that mTOR overactivation resulting from loss of TSC1 or TSC2 gene determines abnormal dendritic spines, enhancement of glutamatergic neurotransmission, as well as a contribution to the reduction of GABAergic transmission [17]. Although this is not a rule, it is well known that patients with TSC2 mutations are at higher risk of earlier onset and more refractory seizures when compared with patients with TSC1, and that TSC2 is associated with more severe neurodevelopmental impairment [18]. Furthermore, a higher tuber burden as well as a greater involvement of white matter has been shown to be associated with a more severe neurological phenotype [16], [19], [20]. Our patient presented several risk factors usually associated with a worse prognosis. It is becoming increasingly evident that in children with TSC, the presence of epileptiform discharges before any clinical symptom should be regarded with caution, since the risk of subsequent seizures is to be considered 100% [21], [22]. The patient showed a negative cognitive evolution not apparently linked to epilepsy, consequently not depicting a real epileptic encephalopathy. Indeed, we have to consider an epileptic encephalopathy as a condition in which cognitive, sensory, and/or motor functions deteriorate as a consequence of epileptic activity, which consists of frequent seizures and/or major so-called interictal paroxysmal activity [23]. However, in this child, a progressive deceleration of acquisitions was noticed after spasm cessation and with a lack of major interictal paroxysmal activity, although background organization progressively worsened over time.
This severe phenotype was present in the child despite promptly receiving a targeted combined treatment with a strong rationale for efficacy in TSC. In particular, VGB acts on GABAergic neurotransmission, while both VGB and everolimus act on mTOR inhibition, thus, providing a hypothetical wide spectrum treatment for TSC-related seizures and its comorbidities [12]. Furthermore, clobazam has also been shown to have a potential benefit on TSC-related epilepsy [24].
Despite experiencing some seizure-free periods, our patient never presented adequate psychomotor evolution after the age of 6 months. Neither the early administration of synergic GABAergic antiepileptic drugs, nor the addition of mTOR inhibition was able to prevent the evolution toward an encephalopathy in which epilepsy was present but did not appear to be a causative factor.
The most significant open question in this case remains the timing of treatment. We might wonder if a more precocious treatment with VGB started right at the beginning of multifocal epileptiform abnormalities, and/or an earlier start of everolimus could have been able to lead toward a less severe neurocognitive phenotype. Our clinical experience also suggests that this risk is much higher when multifocal abnormalities and a tendency toward generalization occur.
Acknowledgments
Paolo Curatolo and Romina Moavero received funding from the European Commission Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 602391 (www.epistop.eu).
Conflict of interest
The Authors declare that they have no conflict of interest.
References
- 1.Curatolo P., Bombardieri R., Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 2.O'Callaghan F.J., Shiell A.W., Osborne J.P., Martyn C.N. Prevalence of tuberous sclerosis estimated by capture–recapture analysis. Lancet. 1998;351:1490. doi: 10.1016/S0140-6736(05)78872-3. [DOI] [PubMed] [Google Scholar]
- 3.Curatolo P., Moavero R., de Vries P.J. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol. 2015;14:733–745. doi: 10.1016/S1474-4422(15)00069-1. [DOI] [PubMed] [Google Scholar]
- 4.Chu-Shore C.J., Major P., Camposano S., Muzykewicz D., Thiele E.A. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusmai R., Chiron C., Curatolo P., Dulac O., Tran-Dinh S. Topographic comparative study of magnetic resonance imaging and electroencephalography in 34 children with tuberous sclerosis. Epilepsia. 1990;31:747–755. doi: 10.1111/j.1528-1157.1990.tb05516.x. [DOI] [PubMed] [Google Scholar]
- 6.Talos D.M., Sun H., Zhou X., Fitzgerald E.C., Jackson M.C., Klein P.M. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crino P.B. Evolving neurobiology of tuberous sclerosis complex. Acta Neuropathol. 2013;125:317–332. doi: 10.1007/s00401-013-1085-x. [DOI] [PubMed] [Google Scholar]
- 8.Northrup H., Krueger D.A., International Tuberous Sclerosis Complex Consensus G. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49:243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gedikbasi A., Oztarhan K., Ulker V., Aslan G., Gul A., Sener-Arslan E. Prenatal sonographic diagnosis of tuberous sclerosis complex. J Clin Ultrasound. 2011;39:427–430. doi: 10.1002/jcu.20857. [DOI] [PubMed] [Google Scholar]
- 10.Gusman M., Servaes S., Feygin T., Degenhardt K., Epelman M. Multimodal imaging in the prenatal diagnosis of tuberous sclerosis complex. Case Rep Pediatr. 2012;2012:925646. doi: 10.1155/2012/925646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yates J.R. Tuberous sclerosis. Eur J Hum Genet. 2006;14:1065–1073. doi: 10.1038/sj.ejhg.5201625. [DOI] [PubMed] [Google Scholar]
- 12.Moavero R., Cerminara C., Curatolo P. Epilepsy secondary to tuberous sclerosis: lessons learned and current challenges. Childs Nerv Syst. 2010;26:1495–1504. doi: 10.1007/s00381-010-1128-8. [DOI] [PubMed] [Google Scholar]
- 13.Cusmai R., Moavero R., Bombardieri R., Vigevano F., Curatolo P. Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis. Epilepsy Behav. 2011;22:735–739. doi: 10.1016/j.yebeh.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Curatolo P., Jozwiak S., Nabbout R., on behalf of the participants of the TSC Consensus Meeting for SEGA and Epilepsy Management Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol. 2012;16:582–586. [Google Scholar]
- 15.Krueger D.A., Northrup H., International Tuberous Sclerosis Complex Consensus G. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:255–265. doi: 10.1016/j.pediatrneurol.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen F.E., Vincken K.L., Algra A., Anbeek P., Braams O., Nellist M. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70:916–923. doi: 10.1212/01.wnl.0000280579.04974.c0. [DOI] [PubMed] [Google Scholar]
- 17.Napolioni V., Moavero R., Curatolo P. Recent advances in neurobiology of tuberous sclerosis complex. Brain Dev. 2009;31:104–113. doi: 10.1016/j.braindev.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Curatolo P., Moavero R., Roberto D., Graziola F. Genotype/phenotype correlations in tuberous sclerosis complex. Seminars in Pediatric Neurology. 2015;22:259–273. doi: 10.1016/j.spen.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Peters J.M., Sahin M., Vogel-Farley V.K., Jeste S.S., Nelson C.A., 3rd, Gregas M.C. Loss of white matter microstructural integrity is associated with adverse neurological outcome in tuberous sclerosis complex. Acad Radiol. 2012;19:17–25. doi: 10.1016/j.acra.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis W.W., Sahin M., Scherrer B., Peters J.M., Suarez R.O., Vogel-Farley V.K. Impaired language pathways in tuberous sclerosis complex patients with autism spectrum disorders. Cereb Cortex. 2013;23:1526–1532. doi: 10.1093/cercor/bhs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domanska-Pakiela D., Kaczorowska M., Jurkiewicz E., Kotulska K., Dunin-Wasowicz D., Jozwiak S. EEG abnormalities preceding the epilepsy onset in tuberous sclerosis complex patients — a prospective study of 5 patients. Eur J Paediatr Neurol. 2014;18:458–468. doi: 10.1016/j.ejpn.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Wu J.Y., Peters J.M., Goyal M., Krueger D., Sahin M., Northrup H. Clinical electroencephalographic biomarker for impending epilepsy in asymptomatic tuberous sclerosis complex infants. Pediatr Neurol. 2016;54:29–34. doi: 10.1016/j.pediatrneurol.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg A.T., Berkovic S.F., Brodie M.J., Buchhalter J., Cross J.H., van Emde B.W. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 24.Jennesson M., van Eeghen A.M., Caruso P.A., Paolini J.L., Thiele E.A. Clobazam therapy of refractory epilepsy in tuberous sclerosis complex. Epilepsy Res. 2013;104:269–274. doi: 10.1016/j.eplepsyres.2012.10.010. [DOI] [PubMed] [Google Scholar]