Abstract
We report a 55-year-old, right-handed patient with intractable left temporal lobe epilepsy, who previously had a partial left temporal lobectomy. The patient could talk during seizures, suggesting that he might have language dominance in the right hemisphere. Presurgical fMRI localization of language processing including reading of exception and regular words, pseudohomophones, and dual meaning words confirmed the clinical hypothesis of right language dominance, with only small amounts of activation near the planned surgical resection and, thus, minimal eloquent cortex to avoid during surgery. Postoperatively, the patient was rendered seizure-free without speech deficits.
Keywords: Language, Temporal lobe epilepsy, fMRI, Neurosurgery, Reading
1. Introduction
In patients with epilepsy, it is especially important to delineate language areas prior to surgery, as functional anatomy may be reorganized with transfer of functions to other areas in the ipsilateral or contralateral hemisphere ([1], [2], see also [3] for evidence following hemispherectomy). For example, functional imaging studies of language processing in patients with chronic epilepsy with a left hemisphere focus have provided evidence for a preoperative right hemispheric activation shift [4], [5], [6], [7], [8], [9]. Surgical resection of epileptogenic regions requires knowledge of any shift of language function, as it may serve as a prognostic indicator and/or impact the surgical approach with the potential to minimize neurologic deficits postsurgery.
The present study describes a patient in whom the surgical resection involved cortical regions that are known in the cognitive neuroscience literature to be a part of the language network (i.e., the left anterior-to-posterior temporal lobe; [10], [11], [12], [13], [14], [15], [16]). The aim was to determine whether language functioning had shifted to the right hemisphere in this patient due to long-standing left temporal lobe epilepsy and a previous partial left temporal lobectomy. By including assessment of reading different types of letter strings in presurgical fMRI (see also [17]), the assessment provided evidence that this type of language processing was localized primarily in the right hemisphere in this patient.
The study examined the activation for reading aloud familiar exception words (words that do not follow regular spelling-to-sound mappings; e.g., one) and regular words (e.g., won), which have been shown to primarily activate brain regions in the temporal lobe, particularly the exception words which rely on ventral stream processing [13], [14], [15], [16]. The study also examined the activation for pseudohomophones (letter strings that when phonetically decoded sound like real words, e.g., wun), which have been shown to activate regions of the dorsal visual processing stream [14]. Lastly, dual-meaning words (e.g., bank) were used to maximally activate the language network [18] by asking the patient to read the word aloud and to think about another word that was associated with it.
2. Case report
The patient is a 55-year-old, right-handed male, presenting with intractable left temporal lobe epilepsy. The surgical resection involved planned access via the left anterior temporal pole. The patient had a previous partial left temporal lobe resection in conjunction with an evacuation of a traumatic intracranial hematoma in 1979. Two years after the trauma, he began to experience complex partial seizures with and without secondary generalization. Speech was not arrested during the seizures. Scalp EEG revealed an epileptogenic focus in the left temporal lobe. On the MRI, the left amygdala and hippocampus were noted to still be present, with evidence of posttraumatic hippocampal encephalomalacia/gliosis. The neurosurgeon and neurologist requested clinical fMRI to localize speech and better delineate the area of planned resection (typically a coronal plane 60 mm posterior to the anterior temporal pole on the lateral surface, and 40 mm posterior on the medial surface). As the patient could speak during seizures, the neurologist suspected that the patient might be right hemisphere language dominant. The patient's consent was obtained, and the experiment was performed in compliance with the Declaration of Helsinki (2008) and the relevant laws and institutional guidelines, and was approved by the University of Saskatchewan Research Ethics Board.
3. Materials and methods
3.1. fMRI protocol
All imaging was conducted using a 3 Tesla Siemens Skyra scanner. Whole-brain anatomical scans were acquired using a high resolution magnetization-prepared rapid acquisition gradient echo sequence consisting of 192 T1-weighted echo-planar image (EPI) slices of 1-mm thickness (no gap) with an in-plane resolution of 1 × 1 mm (field of view 256 × 256, TR = 1900, TE = 2.08). For each of the functional tasks, T2*-weighted single shot gradient echo EPI scans were acquired using an interleaved ascending sequence, consisting of 55 volumes of 25 axial slices of 4-mm thickness (1-mm gap) with an in-plane resolution of 2.65 × 2.65 mm (field of view = 250) using a flip angle of 90°. The top 2 coil sets (16 channels) of a 20-channel Siemens head coil were used. In order to acquire verbal behavioral responses, a blocked (task vs relax) sparse-sampling method was used that allows the participant to respond during a gap in image acquisition (TR = 3300 ms, with a 1650 ms gap of no image acquisition; TE = 30 ms; flip angle = 90°; e.g., 18–21; 25]). The patient responded vocally during the regular, periodic 1650-ms gap in the image acquisition that followed the offset of each volume of image acquisition, which allowed the patient to respond with no noise interference from the MRI and task compliance to be monitored.
3.2. Stimuli & language tasks
The stimuli were presented using a PC running EPrime software (Psychology Software Tools, Inc., http://www.pstnet.com) through MRI compatible goggles (Cinemavision Inc., http://www.cinemavision.biz). The leading edge (10 μs) of the fiber-optic signal that is emitted by the MRI at the beginning of each acquisition volume was detected by a Siemens fMRI trigger converter and passed to the EPrime PC via the serial port. As such, perfect continuous synchronization between the MRI and the experimental paradigm computer was obtained at each volume.
The patient was presented 100 monosyllabic letter strings, which consisted of 25 regular words, 25 exception words, 25 pseudohomophones, and 25 dual meaning words in separate runs, in blocks of 5, interspaced with blocks of relaxation. These stimuli were matched on several of the characteristics available from the E-Lexicon Database (http://elexicon.wustl.edu/, [19]). The patient was asked to read the stimulus aloud during the gap in image acquisition. For the dual meaning word task, the patient was also asked to think about another word that is associated with it.
3.3. fMRI analyses
All preprocessing and statistical analyses for functional images were performed using Brain Voyager QX Version 2.6.1 (www.brainvoyager.com). Functional images were preprocessed and corrected for slice scan time acquisition (cubic spline interpolation), 3D motion correction (trilinear/sinc interpolation), and temporal filtering with a high-pass filter to remove frequencies less than two cycles/time course. The first five image volumes were used to achieve steady state of image contrast and were discarded prior to analysis.
4. Neuroimaging results
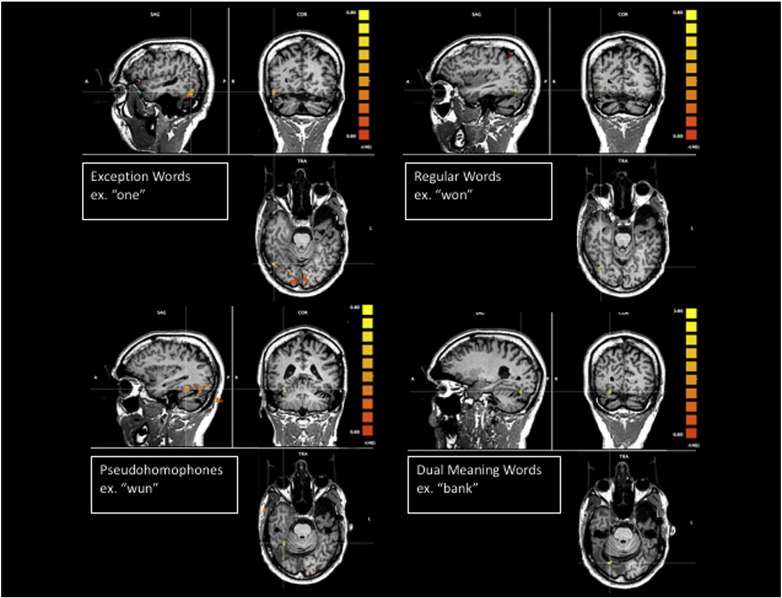
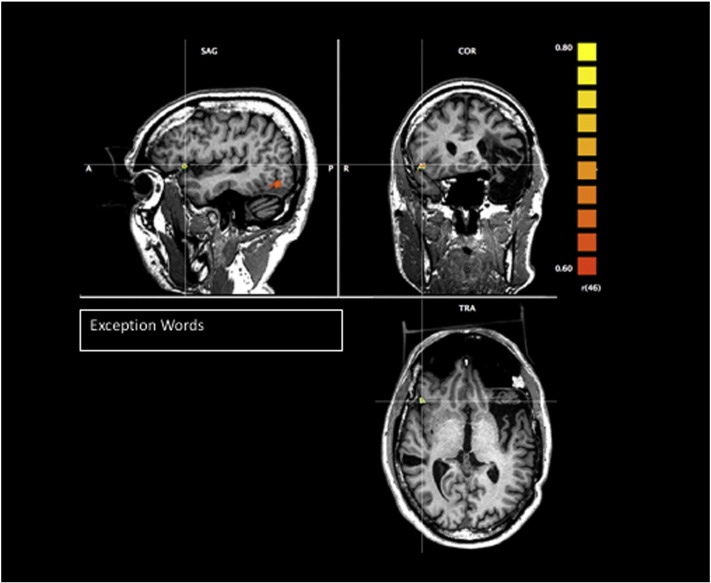
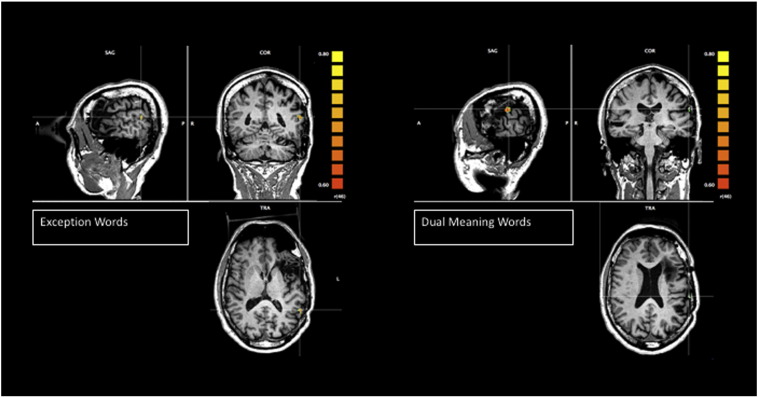
All four reading tasks revealed clear activation of the right posterior occipitotemporal region in the ventral visual stream (see cross hairs in Fig. 1) using a linear correlation threshold of r(46) = 0.60, p < .0001, with the exception words and pseudohomophones showing the greatest area of activation. The pseudohomophones activated a region more anterior and medial in the right temporal lobe, and the dual meaning words activated a region in the right posterior occipital area. Moreover, the exception words also activated a region of the right frontal lobe, which is likely the homologue region of Broca's area (Fig. 2). The exception words and dual meaning words also activated temporal regions of the left hemisphere, which were close to, but posterior to, the planned resection line (Fig. 3).
Fig. 1.
Anatomical T1 images as underlays, and functional T2* images as functional overlays for regions of BOLD activation for task > rest for exception words, regular words, pseudohomophones, and dual meaning words, r(46) = .60, p < .0001.
Fig. 2.
T1 images as underlays, and T2* images as functional overlays for regions of BOLD activation for task > rest for exception words, r(46) = .60, p < .0001.
Fig. 3.
T1 images as underlays, and T2* images as functional overlays for regions of BOLD activation for task > rest for exception words and dual meaning words, r(46) = .60, p < .0001.
5. Surgical procedure
A left temporal lobectomy was performed using neuronavigation with the STEALTH system, with intraoperative EEG and awake speech mapping using the same reading stimuli as used during fMRI. After exposure of the temporal lobe surface, speech was mapped using an Ojemann stimulator, and the absence of speech arrest was confirmed over the entirety of the planned resection area. Surface grids were placed over the left temporal lobe and intraoperative EEG used to identify areas of abnormal seizure activity. The anterior left temporal lobe was excised, opening the temporal horn of the lateral ventricle and exposing the hippocampal head, which was in turn removed. Removal of the seizure focus was confirmed by intraoperative EEG after completion of the resection, followed by hemostasis and closure.
6. Discussion
The results of the present study demonstrate that fMRI of reading processing, as used here, can be helpful in identifying the language-dominant hemisphere in an individual patient with epilepsy. In addition to aiding global localization, our fMRI protocol lateralized particular language regions, such as the homologue region of Broca's area. As the surgery involved the resection of the hippocampus, amygdala, and remaining neocortical tissue of the left temporal lobe, which can be critical in language processing, the tasks included reading words, decoding pseudohomophones, and semantic retrieval (by asking the patient to also think about another word that is associated with the dual meaning words). It was found that BOLD fMRI signal consistently activated the right temporal lobe across four reading-aloud tasks (see Fig. 1).
In this case, the neurologist had suspected right language dominance because of the patient's intact speech during seizures. The fMRI supported right language dominance, and that there was minimal activation near the planned resection and, thus, minimal eloquent cortex to avoid during surgery. These findings suggest a redistribution of language function in the brain that may have occurred because of recurrent ictal activity in the left hemisphere language areas. An alternative explanation is that reorganization may have occurred following the earlier remote partial left temporal lobectomy with a shift of language areas to the right hemisphere.
7. Limitations
Great caution is always required when trying to interpret the absence of activation in fMRI, and especially so in presurgical localization of relevant language processing. However, there was some reassurance in that both fMRI and direct cortical stimulation were consistent in this null finding, and moreover, the patient has no speech deficits and remains seizure-free thus far (one year) following the surgery.
8. Conclusions
In summary, the present case study demonstrates that fMRI of reading processes can be a useful clinical tool for language localization and lateralization presurgically in temporal lobe epilepsy cases. Moreover, the results suggest that protocols similar to the one used here including different stimulus types (e.g., exception and regular words, pseudohomophones, dual meaning words) are valuable for presurgical cortical localization of language function in patients with epilepsy. These findings, once replicated and extended, will inform future presurgical localization of eloquent cortex in temporal lobe resection cases and provide further insights into the functional reorganization in patients with long-standing temporal lobe epilepsy.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
This work was supported through the Natural Sciences and Engineering Research Council (NSERC) of Canada in the form of NSERC Canada Graduate Scholarships to the lead author, L. Gould, and C. Ekstrand, NSERC summer research scholarships to E. Lorentz, and an NSERC Discovery Grant to the senior author, R. Borowsky, under grant 183968-2013-18. It was also supported through a Post-Doctoral Research Fellowship from the Saskatchewan Health Research Foundation (SHRF) to M. Mickleborough (#2843).
References
- 1.Duffau H., Capelle L., Denvil D., Sichez N., Gatignol P., Lopes M. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatry. 2003;74:901–907. doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen T., Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–365. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- 3.Cummine J., Borowsky R., Stockdale Winder F., Crossley M. Basic reading skills and dyslexia: three decades following right versus left hemispherectomy for childhood-onset intractable epilepsy. Epilepsy Behav. 2009;15:470–475. doi: 10.1016/j.yebeh.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Billingsley R., McAndrews M., Crawley A., Mikulis D. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124:1218–1227. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier A., Pugh K.R., Westerveld M., Studholme C., Skrinjar O., Thompson J.R. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42:1241–1252. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- 6.Gaillard W.D., Balsamo L., Xu B., Grandin C.B., Braniecki S.H., Papero P.H. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–265. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- 7.Rutten G.J.M., Ramsey N.F., van Rijen P.C., Alpherts W.C., van Veelen C.W.N. fMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage. 2002;17:447–460. doi: 10.1006/nimg.2002.1196. [DOI] [PubMed] [Google Scholar]
- 8.Adcock J.E., Wise R.G., Oxbury J.M., Oxbury S.M., Matthews P.M. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 9.Sabsevitz D.S., Swanson S.J., Hammeke T.A., Spanaki M.V., Possing E.T., Morris G.L., III Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- 10.Grabowski T.J., Damasio H., Tranel D., Boles Ponto L.L., Hichwa R.D., Damasio A.R. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visser M., Jefferies E., Lambon Ralph M.A. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2010;22:1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- 12.Price C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62:816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borowsky R., Loehr J., Friesen C.K., Kraushaar G., Kingstone A., Sarty G. Modularity and intersection of ‘what’, ‘where’, and ‘how’ processing of visual stimuli: a new method of fMRI localization. Brain Topogr. 2005;18:67–75. doi: 10.1007/s10548-005-0276-8. [DOI] [PubMed] [Google Scholar]
- 14.Borowsky R., Cummine J., Owen W.J., Friesen C.K., Shih F., Sarty G. fMRI of ventral and dorsal processing streams in basic reading processes: insular sensitivity to phonology. Brain Topogr. 2006;18:233–239. doi: 10.1007/s10548-006-0001-2. [DOI] [PubMed] [Google Scholar]
- 15.Borowsky R., Esopenko C., Cummine J., Sarty G.E. Neural representations of visual words and objects: a functional MRI study on the modularity of reading and object processing. Brain Topogr. 2007;20:89–96. doi: 10.1007/s10548-007-0034-1. [DOI] [PubMed] [Google Scholar]
- 16.Cummine J., Gould L., Zhou C., Hrybouski S., Siddiqi Z., Chouinard B. Manipulating instructions strategically affects reliance on the ventral–lexical reading stream: converging evidence from neuroimaging and reaction time. Brain Lang. 2013;125:203–214. doi: 10.1016/j.bandl.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Mickleborough M.J.S., Kelly M.E., Gould L., Ekstrand C., Lorentz E., Ellchuk T. Inclusion of attentional networks in the pre-surgical neuroimaging assessment of a large deep hemispheric cavernous malformation: an fMRI case report. Cerebrovasc Dis. 2015;39:202–208. doi: 10.1159/000376612. [DOI] [PubMed] [Google Scholar]
- 18.Borowsky R., Esopenko C., Gould L., Kuhlmann N., Sarty G., Cummine J. Localization of function for noun and verb reading: converging evidence for shared processing from fMRI activation and reaction time. Lang Cognit Process. 2013;28:789–809. [Google Scholar]
- 19.Balota D.A., Yap M.J., Hutchison K.A., Cortese M.J., Kessler B., Loftis B. The English Lexicon Project. Behav Res Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]