Abstract
Background/aims
To determine if patients with inactive chorioretinitis lesions who experience chronic toxoplasmic uveitis test PCR positive for Toxoplasma in their ocular fluids.
Methods
Two patients undergoing long-term anti-toxoplasmic treatment developed chronic uveitis and vitritis. They underwent therapeutic and diagnostic pars plana vitrectomy. Patient specimens were tested for toxoplasmosis by real-time PCR and nested PCR. Patient specimens were also tested for the presence of Toxoplasma antibodies that recognise allelic peptide motifs to determine parasite serotype.
Results
Patients tested positive for Toxoplasma by real-time PCR at the B1 gene in the vitreous and aqueous humours of patient 1, but only the vitreous of patient 2. Patients were not parasitemic by real-time PCR in plasma and blood. During surgery, only old hyperpigmented toxoplasmic scars were observed; there was no sign of active retinitis. Multilocus PCR–DNA sequence genotyping at B1, NTS2 and SAG1 loci established that two different non-archetypal Toxoplasma strains had infected patients 1 and 2. A peptide-based serotyping ELISA confirmed the molecular findings.
Conclusions
No active lesions were observed, but both patients possessed sufficient parasite DNA in their vitreous to permit genotyping. Several hypotheses to explain the persistence of the vitritis and anterior uveitis in the absence of active retinitis are discussed.
INTRODUCTION
Ocular toxoplasmosis (OT) is the most common ocular infection worldwide and has a profound impact on vision in many countries. OT is frequently recurrent and is caused by congenital and postnatally acquired infection; it affects immunocompetent as well as immunocompromised patients.1–4 The disease classically presents as a unilateral posterior granulomatous uveitis with retinochoroidal lesions that can be single, multiple or satellite to an atrophic-pigmented scar.4 Findings that confirm active disease are intense vitritis over necrotic grey-whitish retinitis associated with choroiditis, vasculitis and haemorrhages.4
The diagnosis of OT is mainly established by its clinical presentation along with the exclusion of a differential diagnosis and evidence for infection by Toxoplasma, such as circulating specific antibody. The diagnosis is often presumptive and the differential diagnosis includes syphilis, herpes and other infections.5,6 The PCR is a molecular biologic technique used for the detection of pathogen DNA and has been used to identify infection in samples extracted from peripheral blood, aqueous and vitreous humours7,8 to confirm active disease.
The purpose of this report is to document two patients with chronic uveitis and vitritis, previously diagnosed as toxoplasmic chorioretinitis and submitted to long-term anti-toxoplasmic treatment without improvement of their active vitritis and anterior chamber reaction.
MATERIALS AND METHODS Clinical information
Case 1
Patient 1 was referred because of redness and loss of vision in his left eye (LE) secondary to a necrotising retinitis, which worsened over the previous year in spite of treatment with Sulfadiazine, Pyrimethamine, Folinic Acid and Prednisone. The patient had been treated also for 6 months with Sulfamethoxazole and Trimethoprim and Prednisone, without decrease of the vitritis and anterior uveitis severity. Workups for tuberculosis, toxoplasmosis, herpes simplex, herpes zoster, cytomegalovirus and HIV were negative except for the presence of positive circulating IgG antibodies for toxoplasmosis.
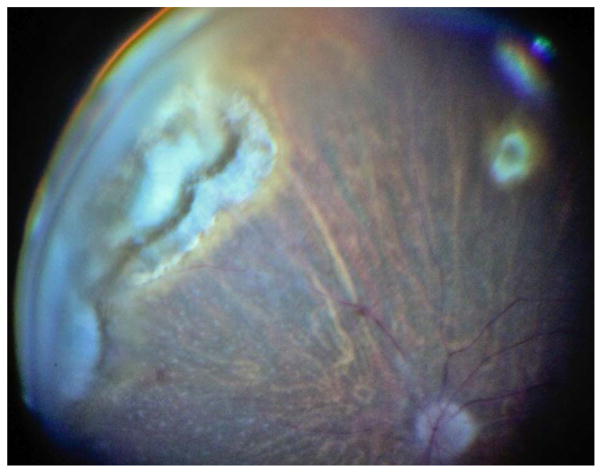
Ophthalmological examination disclosed a normal right eye (RE) and counting fingers in the LE with a severe hyperaemia (+3) an intense anterior chamber reaction (+3 cells) with large granulomatous keratic precipitates and a dense cataract without synechiae. The intraocular pressure (IOP) measurement was 30 mm Hg. There was a +3 cells reaction in the anterior vitreous and the funduscopy was impossible. An ultrasound revealed dense vitritis and a retinal granuloma in the nasal superior quadrant. Clindamycin was added to treatment without clinical improvement. Pars plana vitrectomy with phacoemulsification was performed and an acrylic hydrophobic intraocular lens (IOL) was implanted. During the procedure, peripheral retinal hyperpigmented scars with no exudation or active retinitis were noticed (figure 1). After surgery, the anterior uveitis remained active for 3 months despite continued use of Sulfamethoxazole/Trimethoprim, systemic and local steroids that were slowly tapered.
Figure 1.
Peripheral retinal hyperpigmented scars with no exudation or active retinitis.
Case 2
Patient 2 reported a progressively worsening impaired vision in the LE for 3 months. The ocular history showed a trabeculectomy surgery with phacoemulsification and IOL implantation in both eyes. The referring ophthalmologist had seen a retinal lesion compatible with OT and the patient had been treated with Sulfamethoxazole and Trimethoprim for 45 days and Ganciclovir for 14 days, without improvement of the uveitis.
A routine workup for uveitis was carried out with negative results for syphilis, HIV and with positive results for circulating IgG Toxoplasma gondii antibodies.
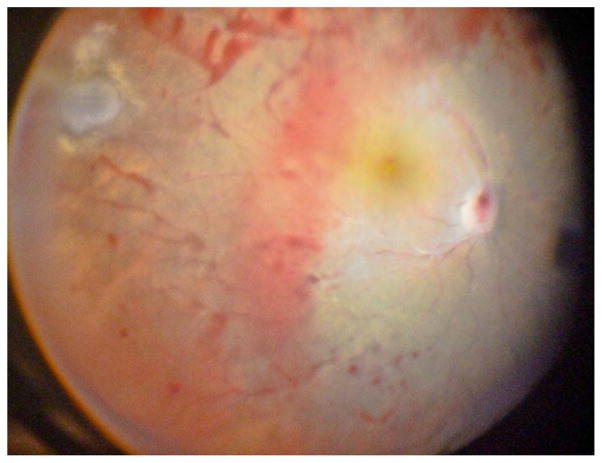
Ophthalmologic evaluation showed a BCVA of 20/20 in RE and hand motion in LE. The RE was normal. The LE showed a moderate conjunctival hyperaemia (+2), a flat superior conjunctival bleb, an oedematous cornea with keratic granulomatous precipitates, +3 anterior chamber cells, a centred IOL placed in the capsular bag and +4 anterior vitreous cells. Funduscopy was impossible. Ocular ultrasound demonstrated vitreous inflammation and macular irregularity without retinal detachment. A vitrectomy was performed and a peripheral toxoplasmic scar without inflammatory activity was noticed (figure 2). After 2 months of treatment with systemic Sulfamethoxazole, Trimethoprim and Prednisone, the BCVA improved to 20/60 and the clinical inflammation disappeared.
Figure 2.
Peripheral non-active lesion, diffuse haemorrhage, a severe vasculopathy and optic nerve involvement.
Serotyping assay
A peptide-based serological ELISA assay was performed using serum, aqueous humour and vitreous to detect allele-specific antibodies. GRA6 and GRA7 strain-specific polymorphic peptides were coupled to keyhole limpet haemocyanin (KLH; Biosource, Camarillo, California, USA), as described previously.9 Peptide names were abbreviated accordingly: ‘6’ denoting peptides from GRA6, ‘7’ from GRA7; ‘I/III’ or ‘II’ indicates the allelic peptide epitope with ‘d’ indicating a truncated version of the diagnostic peptide. Coupled peptides were diluted to 2 μg/mL in 0.1 M carbonate buffer, pH 8.5. The assay was performed as described previously.9 ELISA data were presented as an optical density (OD) index by dividing the OD value obtained at 405 nm for each of the five serotyping peptides (6-I/III, d6-I/III, 6-II, d6-II and 7-II) by the mean of OD readings for two control peptides with results expressed as arbitrary units. Sera from unrelated T gondii-infected individuals for whom the genotype of the infecting parasite was known were included in each experiment as positive controls.
To establish a specific cut-off, 14 sera samples from volunteers in São Paulo—Brazil were evaluated (IgG and IgM negative to T gondii). These sera samples were tested against the panel of seven polymorphic allelic peptide epitopes and the mean and SD for all sera samples were defined for each peptide. The cut-off value was equal to the mean plus 2 SD.
Multilocus PCR genotyping
DNA was extracted from the peripheral blood, aqueous and vitreous humours using QIAamp DNA Blood Midi Kit (Qiagen, Valencia, California, USA) following the manufacturer’s instructions. After the DNA extraction, real-time PCR was performed using primers and probe designed for the B1 gene as described by Fekkar and collaborators.10 Assays were performed using Taqman Universal Master Mix 2x (Applied Biosystems, USA) to a 25 μL final reaction volume containing 0.3 μM forward primer, 0.5 μM reverse primer, 0.15 μM flouorescent probe and 5 μL of DNA. PCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR System. As a negative control, DNA was replaced with deionised water, and as positive control we used 4 μL of strain type I (RH).
For the PCR genotyping, a multilocus PCR–DNA was performed on genomic DNA at B1, SAG-1 and NTS2 loci as described previously.11 PCR products were incubated for 15 min with ExoSAP-IT (USB Corp, Cleveland, Ohio, USA) prior to DNA sequencing. DNA sequencing was performed by the NIAID RML, Genomics Unit, Hamilton, Montana, USA.
RESULTS
The cytology of the vitreous was performed and revealed lymphocytes, atypical plasmocytes and rare macrophages in both cases (data not shown). The real-time PCR at B1 gene was positive in the vitreous and aqueous humours in patient 1. Patient 2 was positive at B1 in the vitreous, but not the aqueous humour (table 1) indicating that the both patients were positive for T gondii. Patients were not parasitemic; real-time PCR was negative for plasma and blood.
Table 1.
Real-time PCR (B1 marker)
| Samples | Plasma | Blood | Aqueous | Vitreous |
|---|---|---|---|---|
| Patient 1 | − | − | + | + |
| Patient 2 | − | − | − | + |
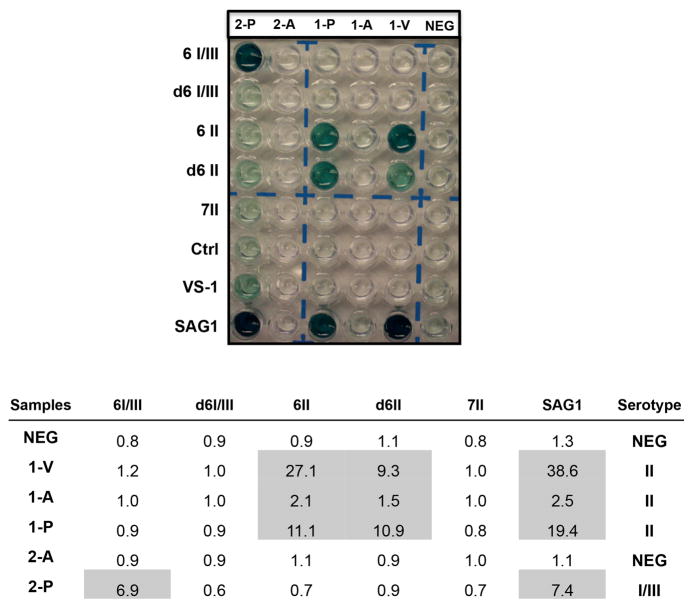
A T gondii ELISA strain-typing serologic assay was next performed. Both patients reacted with recombinant SAG1, confirming the presence of infection antibodies against T gondii. Patient 1 also possessed antibodies in their vitreous, aqueous humour and plasma that specifically reacted with type II allele-specific peptide motifs, whereas patient 2 reacted with type I/III allele-specific peptide motifs, but only in their plasma (figure 3).
Figure 3.
An ELISA plate identified two distinct serotype patterns. Both patients reacted to SAG1 confirming the presence of infection. Patient 1 reacted with type II allele-specific peptide motifs. Patient 2 reacted with type I/III allele-specific peptide motifs. V, vitreous humour; A, aqueous humour; P, plasma; NEG, negative.
To confirm that both patients were infected with different strains of T gondii as determined serologically, DNA extraction was performed in the peripheral blood from patients 1 and 2, and vitreous humour from patient 1. The samples were processed for multilocus PCR–DNA sequencing at the B1, SAG1 and NTS2 markers to identify T gondii genotypes. Highly sensitive nested PCR analysis established that both patients were in fact parasitemic; peripheral blood tested PCR positive at the NTS2 marker (table 2), whereas vitreous from patient 1 was positive at the B1 and SAG1 markers, but not at NTS2 (table 2). DNA sequencing at the NTS2 locus identified a type I allele in patient 1, and II or III allele in patient 2. DNA sequencing at the B1 locus identified a non-archetypal allele (designated U) and a II or III allele at SAG1 in the vitreous of patient 1 (table 2). Multilocus genotyping established that patients 1 and 2 were each infected with different, non-archetypal strains of T gondii in support of the serological findings.
Table 2.
PCR—DNA sequencing
| 225 | 360 | 366 | 378 | 504 | 533 | Allele | 211 | 246 | 263 | 356 | 444 | Allele | 723 | 761 | 765 | 843 | 848 | 856 | 991 | Allele | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Consensus | C | C | t/c | G | g/c | A | T | C | G | G | C | T | G | T | A | T | G | T | |||
| Type I | . | . | T | . | G | . | I | C | T | C | A | T | I | C | T | A | G | . | A | . | I |
| Type II | . | . | . | . | . | . | II | . | . | . | . | . | II | . | . | . | . | . | . | . | II |
| Type III | . | . | . | . | . | . | III | . | . | . | . | . | III | . | . | . | . | . | . | . | III |
| 1-V | . | . | T | a/g | . | . | u3 | . | . | . | . | . | II/III | ||||||||
| 1-B | C | T | A | G | . | A | . | I | |||||||||||||
| 2-B | . | . | . | . | . | . | . | II/III | |||||||||||||
| B1 | SAG1 | NTS2 | |||||||||||||||||||
DISCUSSION
It is known that there are patients with OT in whom the vitreous reaction fails to clear over time despite evidence of healing of their retinal lesions. These patients probably do not have indication for long-term treatment since there is no active inflammation.
Patients with large necrotic toxoplasmic lesions typically require treatment for many months with anti-toxoplasma drugs associated with systemic steroids to mitigate disease. The anterior chamber and vitreous inflammation is usually considered secondary to the retinal lesion with the intensity of the anterior chamber reaction considered as a parameter to evaluate inflammatory activity.12 However, these two patients experienced a chronic iridocyclitis and vitritis that remained severe and progressively worsened despite continued anti-toxoplasma prophylaxis and prednisone treatment for many months. Vitrectomy was performed also for diagnostic purposes.
During vitrectomy, aqueous and vitreous humours were taken to confirm the suspected diagnosis of OT and to determine the genotype(s) of the strain(s) associated with disease. Plasma was also obtained to determine the parasite strain serotype(s). Other differential diagnoses were negative and could be excluded.
In both patients, there was a chronic inflammation in the vitreous expressed by the presence of the monomorphonuclear inflammatory cells. Although the chronic inflammation remained active despite long-term therapy with anti-toxoplasma drugs, the clinical examination during and after surgery showed, in both cases, that there were no active foci of chorioretinitis and only pre-existing inactive toxoplasmic scars. However, parasite DNA, although not detected by real-time PCR, tested positive in the peripheral blood by nested PCR indicating parasitaemia or the presence of circulating parasites. In 2011, Silveira and collaborators showed circulating parasites in the peripheral blood from patients with acute and chronic OT.13 It is important to mention that real-time PCR on blood may have a high false-negative rate and miss systemic parasitaemia. When the more sensitive nested PCR approach was used, it was capable of detecting parasitaemia in the blood of these patients.
In 2012, McLeod et al14 presented data to establish that specific serotypes associate with severe disease in patients with congenital toxoplasmosis in North America. We have showed previously that serotyping, which detects strain-specific antibodies in circulation, is a powerful tool for the identification of specific strains associated with disease in Brazil.15 However, the two patients reported here who were experiencing a recalcitrant, severe vitritis and active uveitis without necrotising retinitis did not possess a single serotype or genotype, indicating that parasite strain type was not likely a determining risk factor in the development of their OT disease.
The absence of any clear foci of chorioretinitis with associated parasite growth is hard to explain. One possible mechanism to explain the chronic uveitis is that parasite antigens in the blood restimulate circulating inflammatory cells, including activated Toxoplasma-restricted T cells, that home back to the choroid to mediate the inflammatory uveitis, even in the absence of visible parasite growth. This could also explain the presence of parasite DNA in the vitreous in the absence of active retinitis and the persistence of the vitritis and the anterior uveitis. Circulating monocytes/macrophages/lymphocytes, harbouring T gondii organisms, could also be recruited to the eye, leading to the positive real-time PCR. It is known that often the vitreous cavity is a reservoir for a long-lasting drug administration.16,17 It has been reported that the vitreous can also work as a stagnant reservoir for resulting substances and debris from previous choroid, retina, optic nerve or ciliary body infection and inflammation.18 That would explain the chronic inflammation, which improved after diagnostic and therapeutic vitrectomy. Alternatively, latent T gondii organisms or their antigens, present in inactive retinal cysts, could be released into the vitreous, causing the uveitis, without any associated foci of active infection or retinal necrosis. Some authors believe that proteins from T gondii, such as SAG119,20 or others21, are strong immunogens that attract immunologic responses that could conceivably manifest as persistent inflammation.
It is also possible that the DNA itself may be inflammatory. Lee et al22 showed that unmodified DNA does not induce inflammation in mice unless the DNA is modified by immunostimulatory motifs such as unmethylated CpG motifs. CpG motifs are recognised by macrophages via toll-like receptor ligation, which induces downstream signalling leading to enhanced secretion of a variety of proinflammatory cytokines typically found in vitreous, including tumour necrosis factor, interleukin (IL)-6 and IL-12. Whether Toxoplasma DNA is capable of exacerbating inflammation remains to be established. This study identified that patients undergoing long-term anti-parasitic prophylactic treatment can develop a severe and persistent vitritis and active uveitis without necrotising retinitis, and that their OTcannot be explained by parasite genetics or proliferation at the foci of disease.
Acknowledgments
Funding This work was supported in part by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (MEG) and CNPq (AGC—Grant number 237252/2012).
Footnotes
Contributors Analysis of the specimens: EAN, AGC and FS. Discussion of the results: EAN, AGC, FS, CM, MEG, LVR and RB. Manuscript preparation: EAN, AGC, MEG and RB. Surgery procedures: AM, HN and CTAM.
Competing interests RBJ, CM and LVR are researchers of CNPq, Brazil. MEG is a scholar of the Canadian Institute for Advanced Research Integrated Microbial Biodiversity Program.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25:264–96. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey JP, Lago EG, Gennari SM, et al. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. 2012;139:1375–424. doi: 10.1017/S0031182012000765. [DOI] [PubMed] [Google Scholar]
- 3.Montoya JG, Remington JS. Toxoplasmic chorioretinitis in the setting of acute acquired toxoplasmosis. Clin Infect Dis. 1996;23:277–82. doi: 10.1093/clinids/23.2.277. [DOI] [PubMed] [Google Scholar]
- 4.Nussenblatt RB, Belfort R., Jr Ocular toxoplasmosis. An old disease revisited. JAMA. 1994;271:304–7. doi: 10.1001/jama.271.4.304. [DOI] [PubMed] [Google Scholar]
- 5.Belfort R, Jr, Silveira C, Muccioli C. Retina. 5. London: W.B. Saunders; 2013. Chapter 85—ocular toxoplasmosis; pp. 1494–9. [Google Scholar]
- 6.Arévalo JF, Belfort R, Jr, Espinoza JV, et al. Retinal and choroidal manifestations of toxoplasmosis. In: Arévalo JF, editor. Retinal and choroidal manifestations of selected systemic diseases. Springer; New York: 2013. pp. 79–104. [Google Scholar]
- 7.Harper TW, Miller D, Schiffman JC, et al. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol. 2009;147:140–47. e2. doi: 10.1016/j.ajo.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Gelder RN. Applications of the polymerase chain reaction to diagnosis of ophthalmic disease. Surv Ophthalmol. 2001;46:248–58. doi: 10.1016/s0039-6257(01)00274-0. [DOI] [PubMed] [Google Scholar]
- 9.Kong JT, Grigg ME, Uyetake L, et al. Serotyping of Toxoplasma gondii infections in humans using synthetic peptides. J Infect Dis. 2003;187:1484–95. doi: 10.1086/374647. [DOI] [PubMed] [Google Scholar]
- 10.Fekkar A, Bodaghi B, Touafek F, et al. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol. 2008;46:1965–7. doi: 10.1128/JCM.01900-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottos J, Miller RH, Belfort RN, et al. Bilateral retinochoroiditis caused by an atypical strain of Toxoplasma gondii. Br J Ophthalmol. 2009;93:1546–50. doi: 10.1136/bjo.2009.162412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commodaro AG, Belfort RN, Rizzo LV, et al. Ocular toxoplasmosis: an update and review of the literature. Mem Inst Oswaldo Cruz. 2009;104:345–50. doi: 10.1590/s0074-02762009000200030. [DOI] [PubMed] [Google Scholar]
- 13.Silveira C, Vallochi AL, Rodrigues da Silva U, et al. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol. 2011;95:396–400. doi: 10.1136/bjo.2008.148205. [DOI] [PubMed] [Google Scholar]
- 14.McLeod R, Boyer KM, Lee D, et al. Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981–2009) Clin Infect Dis. 2012;54:1595–605. doi: 10.1093/cid/cis258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaudaux JD, Muccioli C, James ER, et al. Identification of an atypical strain of toxoplasma gondii as the cause of a waterborne outbreak of toxoplasmosis in Santa Isabel do Ivai, Brazil. J Infect Dis. 2010;202:1226–33. doi: 10.1086/656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahsuvaryan ML. Therapeutic potential of intravitreal pharmacotherapy in retinal vein occlusion. Int J Ophthalmol. 2012;5:759–70. doi: 10.3980/j.issn.2222-3959.2012.06.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas JB. Intravitreal triamcinolone acetonide: a change in a paradigm. Ophthalmic Res. 2006;38:218–45. doi: 10.1159/000093796. [DOI] [PubMed] [Google Scholar]
- 18.Asencio-Duran M, Vallejo-Garcia JL, Pastora-Salvador N, et al. Vitreous diagnosis in neoplastic diseases. Mediators Inflamm. 2012;2012:930704. doi: 10.1155/2012/930704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang R, Feng H, Hu M, et al. Evaluation of immune responses induced by SAG1 and MIC3 vaccine cocktails against Toxoplasma gondii. Vet Parasitol. 2012;187:140–6. doi: 10.1016/j.vetpar.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Hoseinian Khosroshahi K, Ghaffarifar F, D’Souza S, et al. Evaluation of the immune response induced by DNA vaccine cocktail expressing complete SAG1 and ROP2 genes against toxoplasmosis. Vaccine. 2011;29:778–83. doi: 10.1016/j.vaccine.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, He S, Zhao G, et al. Toxoplasma gondii: bioinformatics analysis, cloning and expression of a novel protein TgIMP1. Exp Parasitol. 2012;132:458–64. doi: 10.1016/j.exppara.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Lytton-Jean AK, Chen Y, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7:389–93. doi: 10.1038/nnano.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]