Abstract
Infection with Sarcocystis species is common in many species of animals, but it has not yet been reported in wolverines (Gulo gulo). Histological sections of tongues from 41 wolverines in the Kitikmeot Region, Nunavut, Canada, were examined for sarcocysts. Sarcocysts were found in 33 (80.4%) wolverines. Two structurally distinct types of sarcocysts were found. Type A sarcocysts were thin (<1 μm thick) walled. Ultrastructurally, the parasitophorous vacuolar membrane (Pvm) had minute undulations, but it lacked villar protrusions and was not invaginated into the granular layer. The bradyzoites were slender, about 5 × 1 μm in size. Structurally, these sarcocysts were distinct from known species of Sarcocystis and possessed a novel 18S and ITS-1 sequence, sharing 98% and 78% sequence similarity with Sarcocystis canis. A new species name, Sarcocystis kalvikus, is proposed for type A sarcocysts. In contrast, type B sarcocysts had relatively thicker (about 2 μm) cyst walls and larger bradyzoites, each about 10 × 2–3 μm. Ultrastructurally, the Pvm on the sarcocyst wall had villar protrusions that were either mushroom-like or sloping. Molecular analysis identified a unique 18S and ITS-1 sequence that placed them in a clade within the Sarcocystidae. Based on histology, TEM, and genetic data, the new name, Sarcocystis kitikmeotensis, is proposed. Sarcocystis kalvikus was found in 14 (34.1%), S. kitikmeotensis was found in 7 (17%), and both species were found in 12 (29.2%) of 41 wolverines.
Parasites belonging to the genus Sarcocystis have a 2-host life cycle (Dubey et al., 1989). The definitive host becomes infected by ingesting the asexual stage (sarcocyst) encysted in the intermediate host’s tissues, whereupon the sexual cycle may commence in the lamina propria of the small intestine of the definitive host. Typically, species of Sarcocystis exclusively parasitize a single intermediate host species. Nothing is known regarding the species of Sarcocystis in wolverines.
The wolverine (Gulo gulo) is the largest member of the weasel family and is an apex animal found in circumpolar regions throughout North America and Eurasia. Here, we describe the sarcocysts of 2 new species, as well as report the prevalence of infections in wolverines for the first time from this host.
MATERIALS AND METHODS
Carcasses of 41 wolverines (Table I) were collected from hunters and trappers in Kugluktuk, Nunavut, Canada, from November 2006 to April 2007. All carcasses were kept frozen until May 2007, when a partial necropsy was performed in Canada. Blood and tissue exudates and samples of several tissues were collected as part of an ongoing Trichinella spp. research project. Samples were frozen again and shipped to Oklahoma State University (OSU; Stillwater, Oklahoma) in August 2007. The samples were then shipped to the Animal Parasitic Diseases Laboratory (APDL) in Beltsville, Maryland, for protozoan diagnosis. At APDL, sera extracted from frozen blood were examined for antibodies to Toxoplasma gondii (Reichard, Torretti, Garvon, and Dubey, 2008), and tongues were examined for Sarcocystis sp. infection in the present study. The results from the previous research on T. gondii and Trichinella spp., as well as the harvest location, have been published elsewhere (Reichard, Torretti, Garvon, and Dubey, 2008; Reichard, Torretti, Snider et al., 2008); the same wolverines were examined for Sarcocystis sp. infection in the present study.
Table I.
Occurrence and prevalence of Sarcocystis kalvikus and S. kitikmeotensis in wolverines from Nunavut, Canada.
| Sex | Age | Number of wolverines | S. kalvikus | S. kitikmeotensis | Co-infection of S. kalvikus and S. kitikmeotensis | Total infected (%) |
|---|---|---|---|---|---|---|
| Male | Juvenile | 16 | 7 | 3 | 3 | 13 (31.7) |
| Adult | 11 | 3 | 2 | 5 | 10 (24.3) | |
| Female | Juvenile | 7 | 3 | 1 | 1 | 5 (12.1) |
| Adult | 7 | 1 | 1 | 3 | 5 (12.1) | |
| Total infected (%) | 14 (34.1) | 7 (17.0) | 12 (29.2) | 33 (80.4) |
At APDL, tissues were thawed, and samples of tongue were fixed in 10% buffered formalin. Routine histological examination was performed on paraffin-embedded sections (5 μm) stained with hematoxylin and eosin (H&E) and 1-μm sections stained with toluidine blue. Three cross sections of tongue that fit in a 35 × 20 mm paraffin block were examined microscopically.
For transmission electron microscopy, paraffin-embedded sections from 4 wolverines were post-fixed in 1% osmium tetroxide in Millonig’s phosphate buffer, rinsed in the same buffer, dehydrated in ethanol, and embedded in epoxy resin. Semi-thin sections were stained with toluidine blue in 1% sodium tetraborate. The ultra-thin sections were contrasted with uranyl acetate and lead citrate before examination in a transmission electron microscope. Sections of 10 sarcocysts from 4 wolverines were examined ultrastructurally.
DNA was extracted from frozen tongue according to the manufacturer’s instructions, (DNeasy Tissue kit; Qiagen, Valencia, California). Genomic DNA was screened for the presence of coccidian DNA by polymerase chain reaction (PCR)–amplification using the 18S and internal transcribed spacer 1 (ITS-1) loci within the nuclear ribosomal gene complex. To amplify a portion of the 18S nuclear ribosomal gene complex, 18S rDNA sequences were aligned from all coccidian parasites deposited in GenBank, and primers in conserved sequences among the coccidia were selected in order to amplify approximately 350 nucleotides of 18S sequence for any coccidian DNA present in the wolverine sample. The primer sequences were 18S F: GCAAGGAAGTTTGAGGCA and 18S R: TCCTTCCTCTAAGTGTTAAGGTTC. Primers were also selected within highly conserved portions of the 18S and 5.8S rDNA loci that amplify across the ITS-1 region (R. Miller and M. Grigg, pers. comm.). PCR was conducted using 3 μl of each DNA extraction with 5 μl of PCR buffer (10× containing MgCl2; Sigma, St. Louis, Missouri), 5 μl of 2 mM dNTP mix (Fermentas), 20 pmol of each primer, and 2U of Taq polymerase (Sigma), in a total reaction volume of 50 μl. PCR amplification was carried out for 35 cycles as follows: 94 C for 40 sec, 58 C for 40 sec, 72 C for 90 sec, followed by one 10-min extension at 72 C. Amplicons were visualized in gel-red-stained 1% agarose gels and purified using Exo SAP-IT (USB, Cleveland, Ohio) according to manufacturer’s instructions. Sanger sequencing was performed using both forward and reverse primers on the PCR population at the Rocky Mountain Genomics Unit DNA Sequencing Facility, NIAID, NIH, Hamilton, Montana.
For the phylogenetic analysis, apicomplexan DNA sequences encoding the small subunit 18S rDNA locus within the nuclear ribosomal gene complex were downloaded from the SILVA rDNA database (Pruesse et al., 2007). Perkinsus chesapeake and Perkinsus marinus (Alveolata, Perkinsida) were selected as outgroups. The 18S rDNA sequences from the wolverine type A and type B sarcocysts were aligned using the SINA Aligner tool (Pruesse et al., 2007). Phylogenetic tree reconstruction was carried out using the criterion of minimum evolution method as implemented by MEGA4 after deletion of gapped positions (Tamura et al., 2007).
Age of wolverines was calculated based on cementum annuli (Matson’s Laboratory LLC, Milltown, Montana). Since female wolverines do not become sexually mature until at least 15 or 16 mo and some males do not produce sperm until 26 to 27 mo (Pasitschniak-Arts and Lariviere, 1995; Mulders, 2001), juvenile wolverines were classified as those <2 yr and adults as those ≥2 yr. Chi-square tests (Sokal and Rohlf, 1997) were used to compare the prevalence of infections among species of Sarcocystis as well as age class and sex of wolverines. Statistical analyses were performed with SigmaStat 3.1 statistical software package (Systat Software, Point Richmond, California).
RESULTS
Sarcocysts were found in 33 (80.4%) of 41 wolverines (Table I). Based on structure, 2 types of sarcocysts were identified (Figs. 1–3). Type A sarcocysts were found in 14 (34.1%, wolverine nos. 2, 4, 6, 7, 12, 20, 24, 25, 27, 29, 32, 37, 38, 40), type B sarcocysts were found in 7 (17%, wolverine nos. 5, 14, 19, 21, 31, 34, 41), and both species were found in 12 (29.2%, wolverine nos. 8, 15, 16, 17, 22, 23, 28, 30, 33, 35, 36, 39) of 41 wolverines. Even though wolverine tissues were poorly preserved (freeze artifacts), the sarcocyst walls were fairly well preserved. By light microscopic examination, distinction of the 2 types of sarcocysts was based primarily on bradyzoite morphology: Type A had tiny, 5-μm-long slender bradyzoites, whereas type B sarcocysts had bradyzoites that were twice that size. Although the sarcocyst wall in type A was thinner than the sarcocyst wall in type B, this distinction was not always clear by light microscopy (Fig. 1). Only sarcocysts and Trichinella sp. were found in sections of tongue, and sarcocysts had not provoked an inflammatory response.
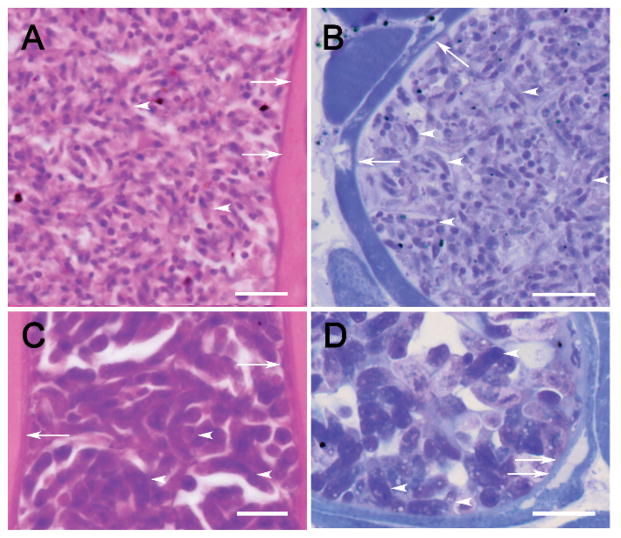
Figure 1.
(A–D) Sarcocysts of Sarcocystis kalvikus and Sarcocystis kitikmeotensis in sections of tongue of wolverine no. 7 (A–B) and wolverine no. 19 (C–D). (A, C) Stained with and H&E, (B, D) stained with toluidine blue. Note cyst wall (arrows) and longitudinally cut bradyzoites (arrowheads). The sarcocyst wall is closely applied to the muscle but has peeled away (double arrows) in (D). Scale bar is 10 μm.
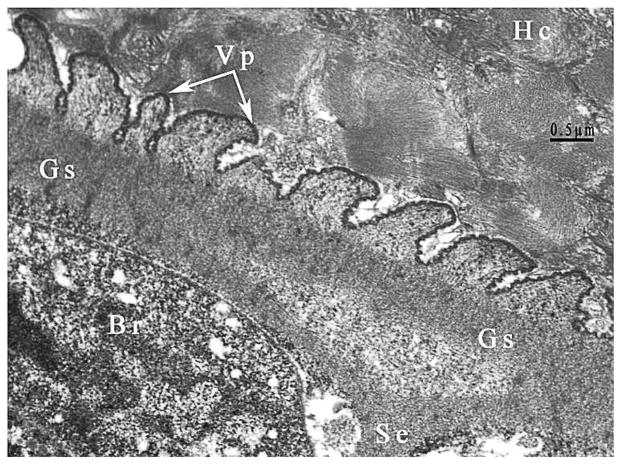
Figure 3.
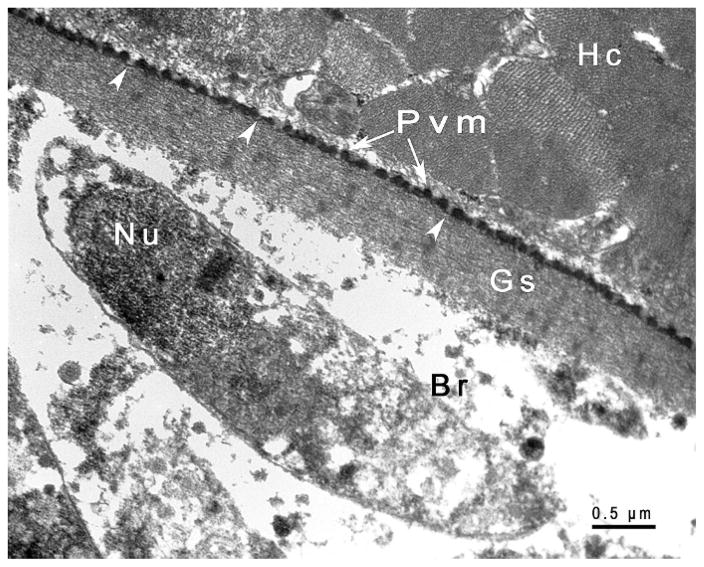
TEM of the sarcocyst of Sarcocystis kitikmeotensis. Note relatively thicker cyst wall with villar protrusions (Vp) that are up to 1 μm long. Faint microtubules are apparent in the Vp and do not extend into the ground substance (Gs) layer. The Gs is continued into the interior of the cyst as septa. Note host cells (Hc) without any inflammation.
The DNA extracted from infected tissues was used as a template for genetic characterization of the 2 different sarcocyst types. PCR amplification readily distinguished between the 2 types based on size polymorphisms using the more variable internal transcribed spacer ITS-1 marker within the nuclear ribosomal gene complex. Type A and B sarcocysts yielded an ~900- and ~600-bp band, respectively (Fig. 4). DNA sequencing identified a single homogeneous novel sequence at the ITS-1 locus that was either divergent or had no sequence similarity to any ribosomal DNA sequences deposited in GenBank for the 2 sarcocyst types, respectively. Type A sarcocysts possessed 78% nucleotide sequence similarity over ungapped positions with Sarcocystis canis (see GenBank GU200661), whereas the type B sarcocyst sequence (GenBank GU200662) had no significant similarity.
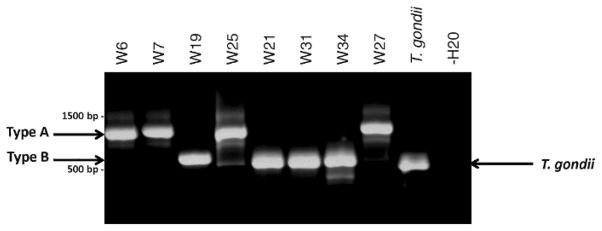
Figure 4.
Differentiation of type A (S. kalvikus) from type B (S. kitikmeotensis) sarcocysts by PCR and DNA sequencing. DNA extracts from wolverine tongue were tested using primers designed to PCR amplify across the ITS-1 rDNA, and products were separated in a 1% gel-red-stained agarose gel. Two bands were resolved: Tongues with type A sarcocyst morphologies yielded an ~900-bp band (GenBank GU200661), whereas those with type B sarcocyst morphologies gave an ~600-bp band (GenBank GU200662). An ~550-bp T. gondii band was detected using type I RH strain genomic DNA and served as the positive control; water only served as the no-template negative control.
To identify the clade and related taxa for type A and B sarcocyst sequences, a partial region of the small subunit 18S rDNA sequence within the nuclear ribosomal gene complex was successfully amplified using primers designed to amplify coccidian DNA (see Materials and Methods). PCR amplification produced ~350-bp amplicons. DNA sequencing of the PCR products yielded a single, homogeneous sequence for each sarcocyst type. The type A sequence was 331 bp in length and possessed 99% and 98% sequence identity to Sarcocystis muris and Sarcocystis neurona, respectively. The type B sequence was 339 bp and possessed 98% sequence identity to Sarcocystis rangiferi and Sarcocystis tarandi. A phylogenetic tree was constructed using the criterion of minimum evolution method as implemented by the MEGA4 program, and both sarcocyst types resolved as discrete taxa within the Sarcocystidae (Fig. 5). In summary, the histology, electron microscopy, and genetic data support the conclusion that the two sarcocyst types are as yet undescribed, new Sarcocystis species.
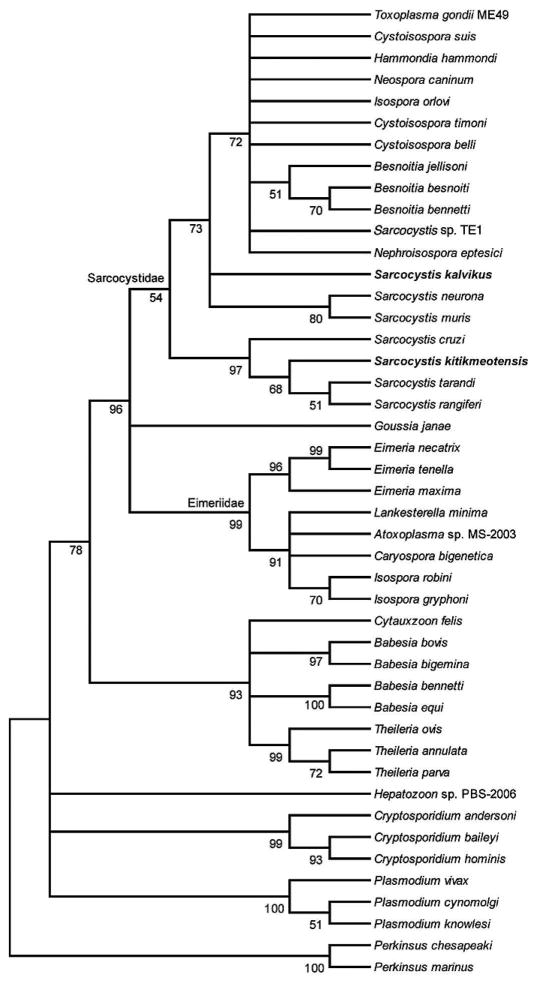
Figure 5.
Minimum evolution tree reconstructed using partial 18S rDNA sequences within the nuclear ribosomal gene complex. The percentage of replicate trees in which the associated taxa clustered together in the 1,000 bootstrap test is shown next to the branches. The evolutionary distances were computed using the Tamura 3-parameter method (Tamura, 1992). All positions containing alignment gaps and missing data were eliminated only in pairwise sequence comparisons (pairwise deletion option). There were a total of 248 positions in the final data set. Phylogenetic analyses were conducted in MEGA4 (Tamura et al., 2007). Sarcocystis kalvikus (GenBank ID HM053963) and S. kitikmeotensis (GenBank ID HM053964) group within the Sarcocystidae.
DESCRIPTIONS
Sarcocystis kalvikus n. sp. (Figs. 1, 2)
Figure 2.
TEM of the sarcocyst of Sarcocystis kalvikus. The parasitophorous vacuolar membrane (Pvm) is wavy in outline, has small blebs, and is lined by electron dense layer that has thinned out at irregular distances (arrowheads). The ground substance is smooth. One bradyzoite (Br) lies beneath the Gs. Although other organelles are washed out, the sub-terminal nucleus (Nu) is visible.
Diagnosis
In 5-μm sections stained with H&E, sarcocyst wall thin (<1 μm) and smooth (Fig. 1 A). Sarcocysts up to 900 μm long and up to 100 μm wide. Sarcocyst interior contained slender 5 × 1 μm bradyzoites. Ultrastructurally, outer layer of the sarcocyst (parasitophorous vacuolar membrane [Pvm]), wavy in outline with minute undulations or blebs that had no stalk (Fig. 2). Undulations appeared to be at irregular intervals. Interior of undulations lined by electron-dense layer (Edl) that did not invaginate further in ground substance. Edl approximately 65 nm thick and thinned out at irregular distances. Beneath Pvm was ground substance (Gs) layer. Gs approximately 600 nm thick; thickest at origin of septa, and smooth in character (Fig. 2). Gs continued into interior of sarcocyst as septa (Fig. 2). All sarcocysts mature, containing fully formed bradyzoites. Groups of bradyzoites separated by septa. Longitudinally cut bradyzoites measured 4–5 × 1.0–1.2 μm. Organelles poorly preserved (Fig. 2).
Taxonomic summary
Type host
Wolverine (Gulo gulo), wolverine no. 7, an adult female.
Other hosts
Unknown.
Type locality
Wolverine no. 7 trapped 22 November 2006, lat/long 67.41667°N, 115.0667°W, Kitikmeot Region, Nunavut, Canada.
Etymology
The species is named after the host species, i.e., kalvik, local language, Inuinnaqtun name for wolverine, and the description is from wolverine no. 7, with only this type of sarcocyst found.
Specimens deposited
Histological sections stained with H&E and toluidine blue from wolverine no. 7 (syntype specimens for S. kalvikus USNPC 102435) and voucher specimens of H&E-stained sections from wolverine nos. 2, 4, 5, 6, 8, 12, 20, 24, 25, 27, 32, 37, 38, 39, and 40, are deposited in the U.S. National Parasite Collection (USNPC nos. 102437–102449), U.S. Department of Agriculture, Beltsville, Maryland.
Sarcocystis kitikmeotensis n. sp. (Figs. 1, 3)
Diagnosis
In 5-μm sections stained with H&E and 1-μm sections stained with toluidine blue, sarcocyst wall relatively thick (<2 μm) and smooth (Fig. 1C). Sarcocysts up to 1,100 μm long and up to 120 μm wide. Sarcocyst interior contained relatively large bradyzoites. Ultrastructurally, outer layer of sarcocyst, Pvm, wavy in outline and thrown in to straight to sloping up to 1-μm-long villar protrusions (Vp) (Fig. 3B). Interior of the Pvm lined by approximately 40-nm-thick Edl that thinned out at irregular distances. Gs 600–1000 nm thick; thickest at origin of septa (Fig. 3). Gs continued into interior of sarcocyst as septa (Fig. 3). All sarcocysts mature, containing fully formed bradyzoites. Groups of bradyzoites separated by septa, longitudinally cut bradyzoites measured 9–13 × 2–3 μm.
Taxonomic summary
Type host
Wolverine (Gulo gulo), no. 19, adult, male.
Other hosts
Unknown.
Type locality
Wolverine no. 19 trapped 17 January 2007: lat/long 67.3333°N, 114.03333°W, Kitikmeot Region, Nunavut, Canada.
Etymology
The species is named after the geographic area, i.e., Kitikmeot, and the description is from wolverine no. 19; only this type of sarcocyst was found. Kitikmeot refers to the westernmost region of Nunavut.
Specimens deposited
Histological sections stained with H&E and toluidine blue from wolverine no. 19 (syntype specimens for S. kitikmeotensis USNPC no. 102436), and voucher specimens of H&E-stained sections from wolverine nos. 5, 14, 21, 31, 34, and 41 are deposited in the U.S. National Parasite Collection (USNPC nos. 102450–102455), U.S. Department of Agriculture, Beltsville, Maryland. Additional vouchers of both species from wolverine nos. 8,15,16,17, 22, 23, 28, 30, 33, 35, 36, 39 are also deposited at the USNPC, nos. 102456–102467.
Remarks
Morphologically, sarcocysts from wolverines were distinct from known species of Sarcocystis in different hosts (Dubey et al., 1989). We are not aware of any previous report of sarcocysts in wolverines. The sarcocyst wall of S. kalvikus described here resembled type 1 sarcocyst wall of Dubey et al. (1989), but further comparison was not relevant because type 1 sarcocysts are found in variety of mammals, including various rodents, voles, carnivores, and primates (Dubey et al., 1989). The closest 18S orthologous sequence were those of Sarcocystis spp., and for ITS-1, Sarcocystis canis, which has been described previously infecting marine mammals and 2 polar bears (Garner et al., 1997; Dubey et al., 2006). The closest resemblance of sarcocysts in wolverines is to Sarcocystis spp. from the European badger (Meles meles) (Tadros and Laarman, 1976; Odening, Stolte, Walter, and Bockhardt, 1994; Odening, Stolte, Walter, Bockhardt, and Jakob, 1994); its sarcocysts are macroscopic (up to 9 mm long), and bradyzoites were approximately 7 × 2 μm. Sarcocystis species in wolverines are different from Sarcocystis species in badger and in mink (Ramos-Vara et al., 1997).
The sarcocyst wall in S. kitikmeotensis was highly different from other species of Sarcocystis, a fact further supported by the unique ITS-1 sequence that had no homology with any published GenBank sequence. However, it shared significant sequence identity in the 18S rDNA locus with numerous Sarcocystis spp. (Fig. 5), confirming that the parasite belongs in the Sarcocystidae.
DISCUSSION
Complete life cycles of Sarcocystis spp. are known for only a few species of animals, mostly those in livestock (Dubey et al., 1989). Most Sarcocystis species have been named based on their intermediate host occurrence and their sarcocyst structure. Dubey et al. (1989) and Dubey and Odening (2001) recognized 35 types of sarcocysts based on the structure of the sarcocyst walls. Complete life cycles of only a few species of Sarcocystis in wildlife are known because of the difficulties of rearing wildlife in a parasite-free environment. Thus, it is unlikely that the life cycles of Sarcocystis species of wolverines will be completed with certainty. We have not yet succeeded in obtaining carcasses of unfrozen wolverines from Canada to better describe the parasite structure morphology. Based on the histologic, transmission electron microscopy, and molecular genotyping within the 18S and ITS-1 nuclear ribosomal gene complex, we have proposed names for the novel Sarcocystis species in wolverines.
The 80% prevalence of sarcocysts in wolverines indicates that this host is a true host of these parasites. Carnivores are generally the definitive hosts for Sarcocystis species. There are very few animals that prey on wolverines, but cannibalism among wolverines has been documented (Flook and Rimmer, 1965). Mulders (2001) reported finding a wolverine claw in the stomach of another wolverine that was captured in the same area as samples of the present study. It is possible that wolverines act as both intermediate and definitive hosts for Sarcocystis, and there are other such examples for other species of Sarcocystis (Dubey et al., 1989).
The proportion of wolverines infected with S. kalvikus or S. kitikmeotensis, or co-infected with both parasites, was not statistically different (χ2 = 3.230, df = 2, P = 0.199) than what one would expect at random. Because a significant difference was not found among single or multiple infections of S. kalvikus and S. kitikmeotensis, data were combined for comparisons between sex and age of wolverines. The prevalence of Sarcocystis spp. did not differ significantly between sex (χ2 = 0.693, df = 1, P = 0.405) or age (χ2 = 0.427, df = 1, P = 0.513) of wolverines.
Acknowledgments
We sincerely thank Dr. Eric Hoberg for nomenclature advice. We would like to thank John Jenkins, Armed Institute of Pathology, Washington, DC 20306, for electron microscopy, Leandra Ferreira for illustrations, James Wasmuth for help with the phylogenetic analyses, and Patricia Pilitt for cataloging the specimens. This study was funded in part by Nunavut Wildlife Management Board and GN-Department of Environment, the Center for Veterinary Health Sciences at Oklahoma State University, and by the Intramural Research Program of the NIH and NIAID. M.E.G. is a scholar of the Canadian Institute for Advanced Research (CIFAR) Integrated Microbial Biodiversity Program.
LITERATURE CITED
- Dubey JP, Chapman JL, Rosenthal BM, Mense M, Schueler RL. Clinical Sarcocystis neurona, Sarcocystis canis, Toxoplasma gondii, and Neospora caninum infections in dogs. Veterinary Parasitology. 2006;137:36–49. doi: 10.1016/j.vetpar.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Odening K. Toxoplasmosis and related infections. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic diseases of wild mammals. Iowa State University Press; Ames, Iowa: 2001. pp. 478–519. [Google Scholar]
- Dubey JP, Speer CA, Fayer R. Sarcocystosis of animals and man. CRC Press; Boca Raton, Florida: 1989. p. 215. [Google Scholar]
- Flook DR, Rimmer J. Cannibalism in starving wolverines and sex identification from skulls. Canadian Field Naturalist. 1965;79:171–173. [Google Scholar]
- Garner MM, Barr BC, Packham AE, Marsh AE, Burek-Huntington KA, Wilson RK, Dubey JP. Fatal hepatic sarcocystosis in two polar bears (Ursus maritimus) Journal of Parasitology. 1997;83:523–526. [PubMed] [Google Scholar]
- Mulders R. Final Report to the West Kitikmeot/Slave Study Society. Yellowknife; Northwest Territories, Canada: 2001. Wolverine ecology, distribution, and productivity in the Slave Geological Province; p. 92. [Google Scholar]
- Odening K, Stolte M, Walter G, Bockhardt I. The European badger (Carnivora: Mustelidae) as intermediate host of further three Sarcocystis species (Sporozoa) Parasite. 1994;1:23–30. doi: 10.1051/parasite/1994011023. [DOI] [PubMed] [Google Scholar]
- Odening K, Stolte M, Walter G, Bockhardt I, Jakob W. Sarcocysts (Sarcocystis sp.: Sporozoa) in the European badger, Meles meles. Parasitology. 1994;108:421–424. doi: 10.1017/s0031182000075971. [DOI] [PubMed] [Google Scholar]
- Pasitschniak-Arts M, Lariviere S. Gulo gulo. Mammalian Species. 1995;499:1–10. [Google Scholar]
- Pruesse E, Quast C, Knittel K, Fuchs B, Ludwig W, Peplies J, Glöckner FO. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Research. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Vara JA, Dubey JP, Watson GL, Winn-Elliot M, Patterson JS, Yamini B. Sarcocystosis in mink (Mustela vison) Journal of Parasitology. 1997;83:1198–1201. [PubMed] [Google Scholar]
- Reichard MV, Torretti L, Garvon JM, Dubey JP. Prevalence of antibodies to Toxoplasma gondii in wolverines from Nunavut, Canada. Journal of Parasitology. 2008;94:764–765. doi: 10.1645/GE-1497.1. [DOI] [PubMed] [Google Scholar]
- Reichard MV, Torretti L, Snider TA, Garvon JM, Marucci G, Pozio E. Trichinella T6 and Trichinella nativa in wolverines (Gulo gulo) from Nunavut, Canada. Parasitology Research. 2008;103:657–661. doi: 10.1007/s00436-008-1028-y. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3. W. H. Freeman and Company; New York, New York: 1997. p. 887. [Google Scholar]
- Tadros W, Laarman JJ. Sarcocystis and related coccidian parasites: A brief general review, together with a discussion on some biological aspects of their life cycles and a new proposal for their classification. Acta Leidensia. 1976;44:1–107. [PubMed] [Google Scholar]
- Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Molecular Biology and Evolution. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software, version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]