Abstract
During April, 2004, 40 sick and dead southern sea otters (Enhydra lutris nereis) were recovered over 18 km of coastline near Morro Bay, California. This event represented the single largest monthly spike in mortality ever recorded during 30 years of southern sea otter stranding data collection. Because of the point-source nature of the event and clinical signs consistent with severe, acute neurological disease, exposure to a chemical or marine toxin was initially considered. However, detailed postmortem examinations revealed lesions consistent with an infectious etiology, and further investigation confirmed the protozoan parasite Sarcocystis neurona as the underlying cause. Tissues from 94% of examined otters were PCR-positive for S. neurona, based on DNA amplification and sequencing at the ITS-1 locus, and 100% of tested animals (n = 14) had elevated IgM and IgG titers to S. neurona. Evidence to support the point-source character of this event include the striking spatial and temporal clustering of cases and detection of high concentrations of anti-S. neurona IgM in serum of stranded animals. Concurrent exposure to the marine biotoxin domoic acid may have enhanced susceptibility of affected otters to S. neurona and exacerbated the neurological signs exhibited by stranded animals. Other factors that may have contributed to the severity of this epizootic include a large rainstorm that preceded the event and an abundance of razor clams near local beaches, attracting numerous otters close to shore within the affected area. This is the first report of a localized epizootic in marine wildlife caused by apicomplexan protozoa.
Index Keywords: sea otter, Enhydra lutris, Sarcocystis neu rona, 18S rDNA, ITS-1, epizootic
Introduction
In April, 2004, biologists and veterinarians responded to an epizootic involving southern sea otters (Enhydra lutris nereis), a federally listed threatened marine mammal. Numerous sick and dead otters were collected over a small geographical area during the first days of the event, suggestive of point-source exposure to a significant and possibly novel toxicant or pathogen. Most live-stranded otters exhibited severe neurological disease and died or were euthanized within 2 days of stranding. Because of the localized case distribution and expression of severe neurological disease in live-stranded animals, mass poisoning resulting from exposure to a chemical pollutant or marine biotoxin was strongly considered. Numerous sea otters had died the year before in the same region and the marine biotoxin domoic acid (DA) was ultimately identified as the cause (Jessup et al., 2004). Other differentials included introduction of a novel virus or point-source exposure to pathogenic parasites or bacteria through plumes of freshwater runoff or sewage.
Results from detailed investigation revealed that nearly all of the stranded sea otters died due to infection by the apicomplexan protozoan parasite Sarcocystis neurona, the causative agent of equine protozoal myeloencephalitis (EPM) (Dubey et al., 2001a). Infectious S. neurona sporocysts are shed only in the feces of Virginia opossums (Didelphis virginiana), an introduced terrestrial omnivore that is now widespread throughout California (Dubey et al., 2001a; 2001b; Grinnell, 1915). Our findings illustrate that introduced terrestrial animals and their indigenous parasite flora can cause substantial mortality of protected marine species. Climactic, biological and environmental factors that may have contributed to the severity of this epizootic are also discussed.
Materials and Methods
2.1 Postmortem examinations and histopathology
Numerous carcasses were recovered within a short time period, so detailed evaluations were completed on the 16 freshest animals available for necropsy. All major tissues were fixed in 10% neutral buffered formalin, trimmed, paraffin-embedded and 5µm-thick, hematoxylin and eosin (H&E)-stained sections prepared. Tissues were examined microscopically and scored for the presence and severity of lesions by a pathologist (MM) who was blinded to results of other diagnostic tests. Then primary and contributing cause(s) of death were determined based on gross and microscopic lesions and results of additional assays, outlined below.
2.2 Sample testing: Bacteria, fungi, viruses and biotoxins
Bacterial and fungal culture was performed at the University of California, Davis (UCD). Tissues and serum were screened for exposure to canine and phocine morbilliviruses (CDV and PMV, respectively), phocine herpesvirus (PHV) I and II, canine parvovirus (CPV), West Nile virus (WNV) and encephalomyocarditis virus (EMCV) using standard procedures at various commercial laboratories. Urine, serum and gastrointestinal contents were submitted to UCD or the California Department of Public Health (CDPH) to screen for the biotoxin domoic acid (DA) using previously published methods (Quilliam et al., 1995; Torr et al., 2003). Test sensitivity, specificity and minimum detection li mits (MDL) were determined using samples spiked with purified DA.
Marine invertebrates consumed by southern sea otters, including razor clams (Siliqua patula), mussels (Mytilus californianus) and Pismo clams (Tivela stultorum) were collected from the affected area, pooled by species and location and submitted to CDPH for DA testing. CDPH provided data on the occurrence of potentially toxic algae in the region and local precipitation. Observations regarding local sea otter abundance, foraging ecology and prey selection were collected by state and federal biologists.
2.3 Sample testing: Protozoa
Cerebrum and cerebellum were processed for parasite isolation in cell culture, as described (Miller et al., 2001). Serological testing for Toxoplasma gondii, S. neurona and Neospora caninum-reactive IgG was performed using indirect immunofluorescent antibody tests (IFAT), as previously described (Miller et al., 2002a). Serum was also tested for the presence and concentration of S. neurona-specific IgM by substituting a 1:100 dilution of goat anti-ferret IgM (Rockland Immunochemicals, Gilbertsville, PA) for anti-IgG antiserum in the IFAT procedure. Formalin-fixed brain was processed for immunohistochemistry for T. gondii and S. neurona, as described (Miller et al., 2002a).
DNA was extracted from sea otter brain and skeletal muscle using the DNeasy Tissue Kit (Qiagen) and genomic DNA preparations were screened for the presence of T. gondii, S. neurona, and/or N. caninum using 18S rDNA pan-specific primers (Miller et al., 2004) and B1 Toxoplasma gondii-specific primers (Grigg and Boothroyd, 2001), followed by sequencing. We also used ITS1 primers that amplify a 500 nucleotide fragment from S. neurona and S. falcatula, but not other Sarcocystis spp, as described (Miller et al., in press) DNA sequencing of the ITS1 amplicons will differentiate between S. neurona and S. falcatula. Positive controls consisted of genomic DNA preparations from well characterized T. gondii isolates RH (Type I), 76K (Type II) and CEP (Type III), S. neurona isolates SN1 and SN3 (Marsh et al., 1996; 1999) and N. caninum isolate NC-1 (ATCC No. 50843). Negative controls consisted of deionised water and genomic DNA from non-infected otters. Amplification products were visualized using ethidium bromide staining in 1% agarose gels. Sequencing was carried out by the Rocky Mountain Lab Genomics Unit DNA Sequencing Center, Division of Intramural Research, Hamilton, Montana.
Results
3.1 Mortality event timing and clinical presentation
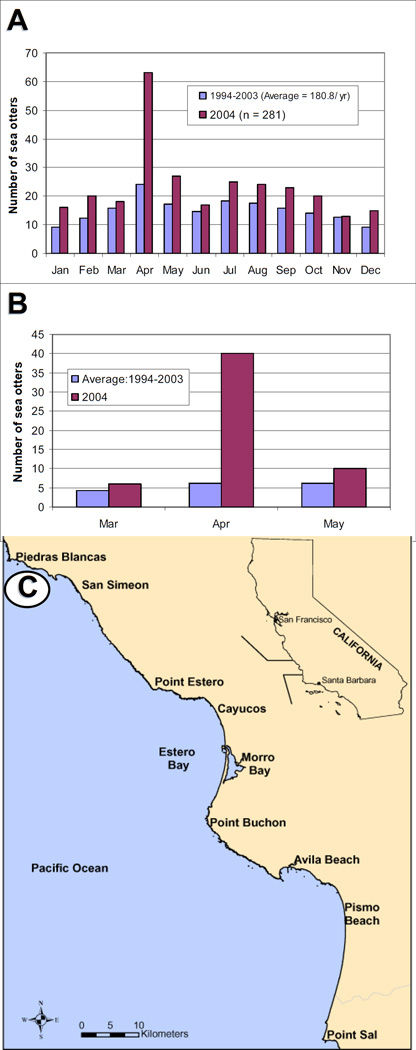
Sea otter strandings typically peak in the spring each year in California, with the highest shoreline deposition of live and dead otters occurring during April (Figure 1A). Between 1994 and 2003, the mean number of otters recovered during April was 24 animals. However, during April, 2004, 63 sick and dead sea otters were found, or >2.6 times the average over the preceding decade. This was the highest monthly deposition of live and dead otters ever recorded during 30 years of stranding data collection in California (Figure 1B). Over half (52.4 %) of the stranded animals were recovered within a small section (1/25th) of the 490 km southern sea otter range, suggestive of point-source exposure to a toxin or pathogen.
Figure 1.
A: Number of southern sea otters stranding rangewide by month. 1B: Number of sea otters stranding between Cayucos and Point Sal, California from March through May. 1C: Locations of Estero Bay and Morro Bay along the central coast of California.
The mortality spike was centered along 18 km of coastline within Estero Bay, near the town of Morro Bay (Figure 1C). Morro Bay is a suburban coastal community that is situated adjacent to a small, enclosed embayment of the same name. The number of sea otters utilizing this region varies by season. During winter, otters (mostly males) migrate through Estero Bay toward the southern range periphery (Jameson, 1989). In spring, this pattern reverses, with otters traveling northward through Estero Bay to the range center. Animals are observed within Estero Bay year-round, reflecting both local and transient sea otter populations. Much of Estero Bay is characterized by sandy beaches and an enclosed, mud bottom harbor. North and south of Estero Bay, the shoreline is comprised of rocky cliffs and poorly accessible beaches where stranded otters are less likely to be recovered.
The epizootic began around April 4th, and 20 live and dead otters recovered over the first 9 days. Live-stranded animals exhibited severe seizures, fine muscle tremors, paresis, somnolence and coma. Less common clinical signs included ptyalism, dyspnea and tachycardia. Throughout April, animals continued to strand over a progressively broader geographical area and by the end of the month, 40 otters had been collected, including 10 sick animals and 30 that were found dead. Of the 30 dead otters, 11 were fresh and 19 were significantly autolyzed. Detailed postmortem examinations were performed for 7 otters that died post-stranding and 9 that were recovered soon after death; summaries were compiled from those 16 cases.
3.2 Postmortem examinations
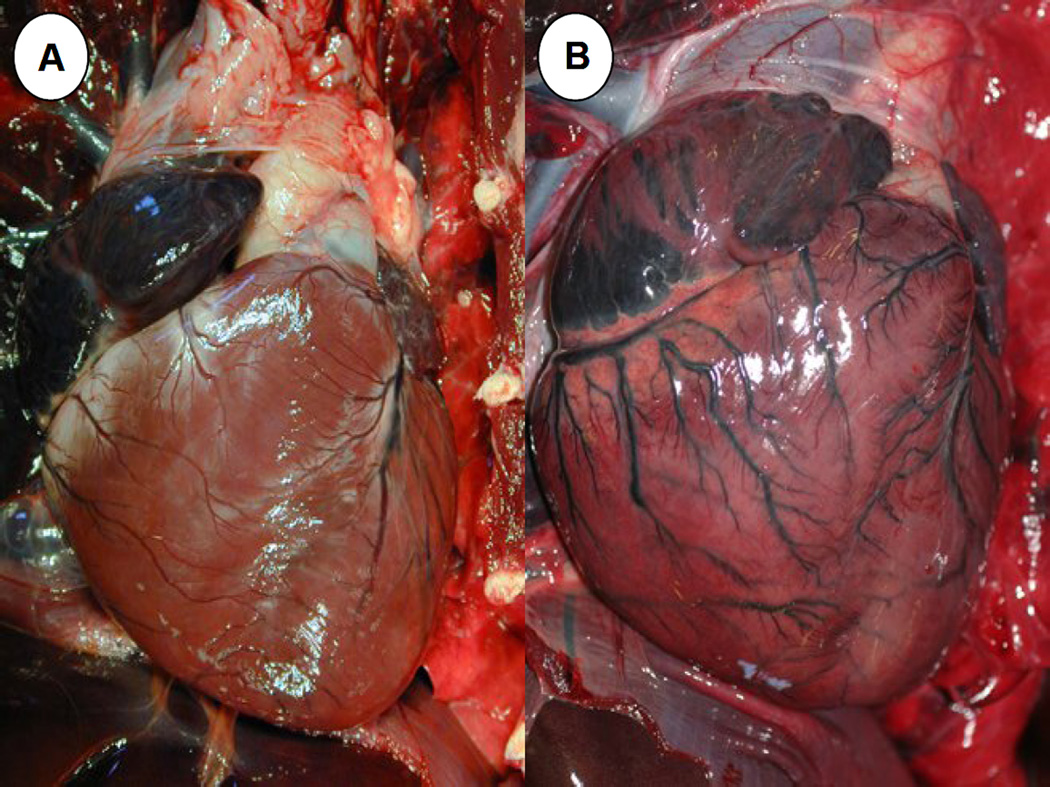
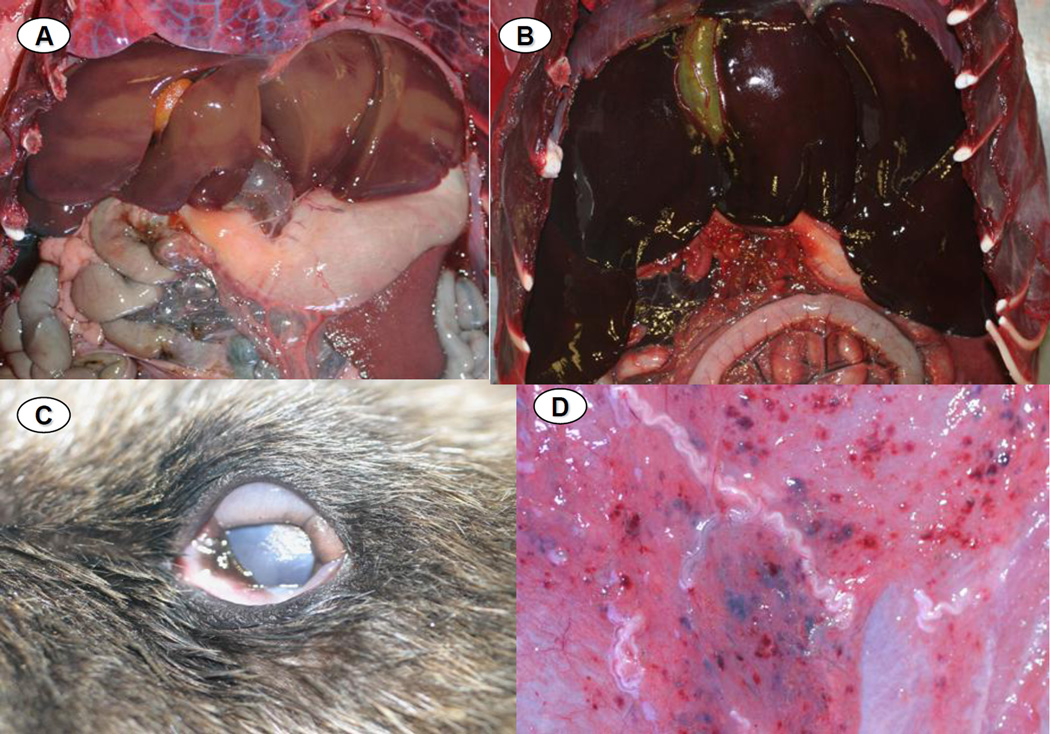
Of 16 otters examined gr ossly and microscopically, one died due to a disseminated fungal infection; the dimorphic fungus Coccidiodes immitis was identified on impression smears, histopathology and fungal culture. This animal was excluded from subsequent analyses as an example of “background” mortality and serology, PCR and parasite isolation were not performed due to human health concerns. Of the remaining 15 animals, 80% exhibited systemic lymphadenopathy, mottled and discolored ventricular myocardium (Figure 2), multi-organ congestion (Figure 3) and emaciation. Less common were pericardial effusion (71.4%), hepatomegaly (66.7%), enlarged, urine-distended bladders (51.1%), splenomegaly with hyperplasia of splenic white pulp (46.7%) and meningeal vascular congestion (42.9%). Pale tan discoloration and swelling of the neuropil was noted in a few cases. Less common were chemosis and subcutaneous petechiation (13.3% for each) (Figure 3). Some of these lesions have been described for individual S. neurona-infected animals in prior reports (Dubey et al., 2001b; Lapointe et al., 1998; Lindsay et al., 2000; Krueder et al., 2003; 2005; Miller et al., 2001a; 2001b; 2008; 2009; Rosonke et al., 1999; Thomas et al., 2007), but this is the first report to summarize gross lesions across a large cohort of otters with PCR-confirmed, S. neurona-associated meningoencephalitis.
Figure 2.
A: Grossly normal (control) sea otter heart with moderate coronary groove fat. 2B: Heart from a sea otter that stranded during the epizootic. There is mild, diffuse cardiomegaly, mottling and discoloration of the ventricular myocardium and dilation and hyperemia of the epicardial vasculature.
Figure 3.
A: Normal (control) sea otter liver, characterized by diffuse, pale tan-brown color and sharp lobular margins. 3B: Liver from an otter that died during the epizootic, showing severe, diffuse passive vascular congestion and rounded lobular margins, presumably due to cardiac insufficiency as a result of the myocardial lesions depicted in Figure 2 above. 3C: Diffuse chemosis affecting the ocular conjunctiva of an otter that stranded during the epizootic. 3D: Multifocal petechiation and mild serous atrophy of adipose of the ventral abdominal subcutis of an otter that died during the epizootic.
3.3 Sample testing: Bacteria, fungi, viruses and biotoxins
A few opportunistic bacterial pathogens were isolated from blood, tissues or feces of 13 otters sampled during necropsy, including hemolytic E. coli, Campylobacter, Actinobacillus spp., B-hemolytic Streptococci and Streptococcus phocae. Bacteria contributed to illness and deaths of some otters, but no bacterial or fungal pathogens were shared by a majority of stranded otters. No gross or microscopic lesions consistent with morbilliviral infection were apparent. Although some otters had low antibody titers for CDV, CPV and EMCV, lesions consistent with death due to these pathogens were not observed microscopically and submitted tissues were PCR-negative (Table 1).
Table 1.
Results of viral screening
| Virusa | Sample tested |
Number of otters tested |
Virus isolaton |
Overview of serology resultsb |
Proportion seropositive |
HPa | PCRa | IHCa | TEMa | Laboratories completing tests a |
|---|---|---|---|---|---|---|---|---|---|---|
| CDV | Brain, lymph node, serum |
12 | Neg | Weak pos (1:16, 1:24) |
3/12 | Neg | Neg | Neg | ND | OK, UCD, CU |
| PDV | Brain, lymph node, serum |
12 | Neg | Neg (<1:8) |
0/6 | Neg | Neg | ND | ND | OK, UCD |
| CPV | Feces, serum |
11 | Neg | Weak pos (1:20) |
4/11 | Neg | Neg | ND | Neg | OK, UCD, CU |
| WNV | Feces, serum |
2 | Neg | ND | ND | Neg | Neg | ND | ND | OK, UCD |
| PHV1 | Serum | 6 | Neg | Neg (<1:8) |
0/6 | Neg | ND | ND | ND | OK |
| PVH2 | Serum | 6 | Neg | Neg (<1:8) |
0/6 | Neg | ND | ND | ND | OK |
| EMCV | Serum | 12 | Neg | Weak pos (1:16) |
3/12 | Neg | ND | ND | ND | CU |
CDV = canine distemper virus, PDV = phocine distemper virus, CPV = canine parvovirus, WNV = west nile virus, PHV1, 2 = phocine herpesviruses 1 & 2, EMCV = encephalomyocarditis virus, HP = histopathology, PCR = polymerase chain reaction, IHC = immunohistochemistry, TEM = transmission electron microscopy, OK = Oklahoma Veterinary Diagnostic Laboratory, UCD = UC Davis School of Veterinary Medicine, CU = Cornell School of Veterinary Medicine:
Tests completed using serum neutralization (SN) or hemagglutanin inhibition (HI) is also confirmed
Analysis of wild marine invertebrates and urine or stomach contents from stranded otters established that DA was present locally during the mortality event (Table 2). Of 21 otters tested, 9 (42.9%) had urine DA concentrations above the minimum detection limit (≥ 0.005 ppm), ranging from 0.009 to 2.06 ppm. Minimally digested marine invertebrates were recovered from the stomachs of 2 otters: sand and spiny mole crabs (Emerita analoga and Blepharipoda occidentalis respectively) recovered from the stomach of 1 otter tested negative for DA. However, postmortem urine and razor clams from the stomach of a second otter tested strongly positive for DA. Local wild razor clams tested PSP-negative, but DA-positive, and some mussels also contained DA (Table 2), while locally collected Pismo clams were DA-negative. Based on prior testing of necropsied southern sea otters and their prey, DA detection at levels near or just above the MDL is a common incidental finding due to the pervasiveness of this biotoxin in the marine environment. However, detection of high levels of DA (≥10 ppm) in urine, serum or digesta of acutely dead animals is suggestive of death due to DA intoxication (M. Miller, unpub. data). Possible indirect effects of DA exposure may occur at lower levels of exposure.
Table 2.
Test results for domoic acid (DA) from stranded sea otters and marine invertebrates from the affected area
| Otter number |
Animal status |
Sample collection or stranding date |
Locationb | DA test results in ppm (µg/G)a | Diagnostic testb |
|
|---|---|---|---|---|---|---|
| Stomach contents/ invertebrates |
Urine | |||||
| 1 | Fresh dead |
4/4/2004 | Morro Bay | Sand/ mole crabs: neg |
Neg | LC/MS |
| 2 | Fresh dead |
4/8/2004 | Morro Bay | N/A | 0.381 | LC/MS |
| 3 | Fresh dead |
4/8/2004 | Morro Bay | N/A | Neg | LC/MS |
| 4 | Fresh dead |
4/9/2004 | Morro Bay | N/A | 0.25 | LC/MS |
| 5 | Fresh dead |
4/9/2004 | Morro Bay | N/A | Neg | LC/MS |
| 6 | Fresh dead |
4/9/2004 | Morro Bay | N/A | Neg | LC/MS |
| 7 | Fresh dead |
4/10/2004 | Morro Bay | N/A | Neg | LC/MS |
| 8 | Fresh dead |
4/10/2004 | Morro Bay | N/A | Neg | LC/MS |
| 9 | Fresh dead |
4/10/2004 | Morro Bay | N/A | Neg | LC/MS |
| 10 | Fresh dead |
4/12/2004 | Morro Bay | N/A | 0.038 | LC/MS |
| 11 | Fresh dead |
4/13/2004 | Pismo Beach | N/A | 0.009 | LC/MS |
| 12 | Fresh dead |
4/19/2004 | Pismo Beach | N/A | Neg | LC/MS |
| 13 | Fresh dead |
4/20/2004 | Morro Bay | N/A | 0.725 | LC/MS |
| 14 | Fresh dead |
4/22/2004 | Morro Bay | N/A | Neg | LC/MS |
| 16 | Fresh dead |
4/25/2004 | Oceano Dunes | N/A | 0.018 | LC/MS |
| 18 | Live | 4/12/2004 | Morro Bay | N/A | Neg | LC/MS |
| 19 | Autolyzed | 4/11/2004 | Morro Bay | Razor clams: 1.860 (CAHFS), 1.200 (CDPH) |
2.060 (CAHFS) | HPLC-UV & LC/MS |
| 21 | Autolyzed | 4/27/2004 | Morro Bay | N/A | 0.029 | LC/MS |
| Wild invertebratesc | ||||||
| Razor clams |
Live | 4/12/2004 | Morro Bay | 3.1 | N/A | HPLC-UV |
| Razor clams |
Live | 4/12/2004 | Morro Bay | 4 | N/A | HPLC-UV |
| Razor clams |
Live | 4/13/2004 | Morro Bay | <1 | N/A | HPLC-UV |
| Razor clams |
Live | 4/14/2004 | Morro Bay | 41 | N/A | HPLC-UV |
| Mussels | Live | 4/12/2004 | Morro Bay | 1.4 | N/A | HPLC-UV |
| Mussels | Live | 4/13/2004 | Morro Bay | <1 | N/A | HPLC-UV |
| Mussels | Live | 4/14/2004 | Morro Bay | <1 | N/A | HPLC-UV |
| Mussels | Live | 4/14/2004 | Morro Bay | <1 | N/A | HPLC-UV |
| Mussels | Live | 4/14/2004 | Morro Bay | <1 | N/A | HPLC-UV |
| Pismo Clam |
Live | 4/12/2004 | Morro Bay | <1 | N/A | HPLC-UV |
| Pismo Clam |
Live | 4/13/2004 | Morro Bay | <1 | N/A | HPLC-UV |
ppm = parts per million, Neg. = test result is below minimum detection limits, N/A = test not performed, LC/MS = liquid chromatography/ mass spectrophotography, HPLC-UV = high performance liquid chromatography with ultraviolet detection
Animals denoted as “Morro Bay” were recovered near the towns of Morro Bay, Los Osos or Cayucos
Samples of 5 to 30 invertebrates collected at various locations within the affected area & pooled by location, collection date & species
3.4 Sample testing: Protozoal culture and serology
Results for IgG serology and pa rasite isolation for S. neurona, T. gondii and N. caninum are summarized in Table 3. Sera and/or tissues were evaluated from 2 live and 15 necropsied otters; most (n= 14) had high IFAT titers against S. neurona (≥ 10,240 serum dilution). Twelve otters were also seropositive for T. gondii (≥ 320). IFAT testing for N. caninum identified 4 otters with titers ≥ 320, but no otters had positive titers to N. caninum in the absence of higher titers to T. gondii.
Table 3.
Results of protozoal serology and in vitro parasite cultivation for otters stranding during the 2004 epizootic
| Otter number |
Age | Sex | Sarcocystis neurona | Toxoplasma gondii |
Neospora caninum |
||
|---|---|---|---|---|---|---|---|
| IFATa | Isolationa | IFA T | Isolation | IFAT | |||
| Necropsied otters | |||||||
| 1 | subadult | F | 10,240 | SNa | >40,960 | TGa | 160 |
| 2 | adult | F | 2,560 | npsa | 1,280 | nps | 160 |
| 3 | adult | M | 20,480 | SN | <40 | nda | <40 |
| 4 | pup | F | 10,240 | SN | <40 | nd | <40 |
| 5 | adult | M | 20,480 | SN | 40,960 | TG | 160 |
| 6 | adult | M | 10,240 | SN | 10,240 | TG | <40 |
| 7 | subadult | M | 81,920 | SN | 320 | nps | 80 |
| 8 | adult | M | 81,920 | SN | 40,960 | TG | 320 |
| 9 | subadult | F | 10,240 | SN | 160 | nd | 80 |
| 10 | pup | F | <40 | nd | <40 | nd | <40 |
| 11 | aged adult | M | 40,960 | nps | 10,240 | nps | 1,280 |
| 12 | adult | M | 10,240 | nps | >81,920 | TG | 640 |
| 13 | adult | F | 10,240 | nps | 81,920 | TG | <40 |
| 14 | adult | M | 10,240 | zoites early, died out | 81,920 | nps | 320 |
| 16 | aged adult | M | 10,240 | nps | 320 | nps | 160 |
| Live, rehabilitated otters | |||||||
| 17b | pup | F | <40 | n/d | <40 | nd | nd |
| 18 | subadult | M | 40,960 | n/d | 20,480 | nd | nd |
IFAT = indirect fluorescent antibody test; isolation = parasite isolation from brain tissue in cell culture; TG = Toxoplasma gondii; SN = Sarcocystis neurona; nps = no parasites seen; nd = not done
Clinically normal, presumably orphaned sea otter pup
Parasite isolation was attempted from the brains of 14 otters and an S. neurona-like parasite was isolated from 8 animals (57.1%)(Table 3). Parasites exhibiting morphology consistent with S. neurona were observed transiently after cell culture inoculation with brain from a ninth otter, but then disappeared. Toxoplasma gondii was isolated from 6 otters (42.9%), consistent with prior reports of the prevalence of T. gondii infection in the central nervous system of sea otters (Miller et al., 2002b; 2004). Comparison of IgM and IgG antibody titers directed against S. neurona are summarized in Table 4. Nearly all of the otters stranding during the epizootic had elevated IgM and IgG, supportive of subacute S. neurona infection. For comparison, archived sera from 2 otters with acute, fatal S. neurona-associated interstitial pneumonia, but no brain lesions exhibited high IgM, but low IgG titers; 3 otters with PCR-confirmed, S. neurona-associated meningoencephalitis had elevations of both IgM and IgG, supportive of subacute infection; and 2 otters with chronic, incidental S. neurona infection, characterized by the presence of PCR-positive, mature S. neurona sarcocysts in muscle and PCR-negative, lesion-negative brains had low IgM and IgG titers (Table 4). Sera from 2 otters that were negative for S. neurona on parasite isolation, DNA PCR, histopathology and immunohistochemistry served as negative controls. Serological data may have been impacted to some extent by gender and age bias that is inherent to sea otter populations from the Estero Bay region, where males and older age class animals are more prevalent.
Table 4.
Indirect fluorescent antibody titers to Sarcocystis neurona for sea otters from the epizootic: Comparison with control otters of known S. neurona infection status
| Sarcocystis neurona titers | ||
|---|---|---|
| Serum sample typea | IgM | IgG |
| 2004 epizootic | 81,920 | 81,920 |
| 2004 epizootic | 81,920 | 40,960 |
| 2004 epizootic | 81,920 | 20,480 |
| 2004 epizootic | 40,960 | 20,480 |
| 2004 epizootic | 40,960 | 2,560 |
| 2004 epizootic | 20,480 | 10,240 |
| 2004 epizootic | 10,240 | 10,240 |
| 2004 epizootic | 1,280 | 10,240 |
| 2004 epizootic | 1,280 | 10,240 |
| 2004 epizootic | 640 | 10,240 |
| Positive control: Acute S. neurona infection | 81,920 | <80 |
| Positive control: Acute S. neurona infection | 40,960 | 640 |
| Positive control: Subacute S. neurona infection | 81,920 | 81,920 |
| Positive control: Subacute S. neurona infection | 40,960 | 10,240 |
| Positive control: Subacute S. neurona infection | 1,280 | 10,240 |
| Positive control: Chronic/ incidental S. neurona infection | 1,280 | 80 |
| Positive control: Chronic/ incidental S. neurona infection | 80 | 640 |
| Negative control: S. neurona-negative otter | 80 | <80 |
| Negative control: S. neurona-negative otter | 80 | 160 |
Case definitions: Acute infection = PCR-confirmed, S. neurona-associated interstitial pneumonia with mild/ no brain disease Subacute infection = PCR-confirmed, S. neurona-associated meningoencephalitis as the main finding on necropsy & histopathology Chronic infection = Muscle PCR + for S. neurona with histologically mature-appearing sarcocysts, but no brain lesions & brain is PCR-negative Negative control = negative for S. neurona or Sarcocystis spp. on histopathology, immunohistochemistry and PCR
3.5 Histopathology, immunohistochemistry and PCR
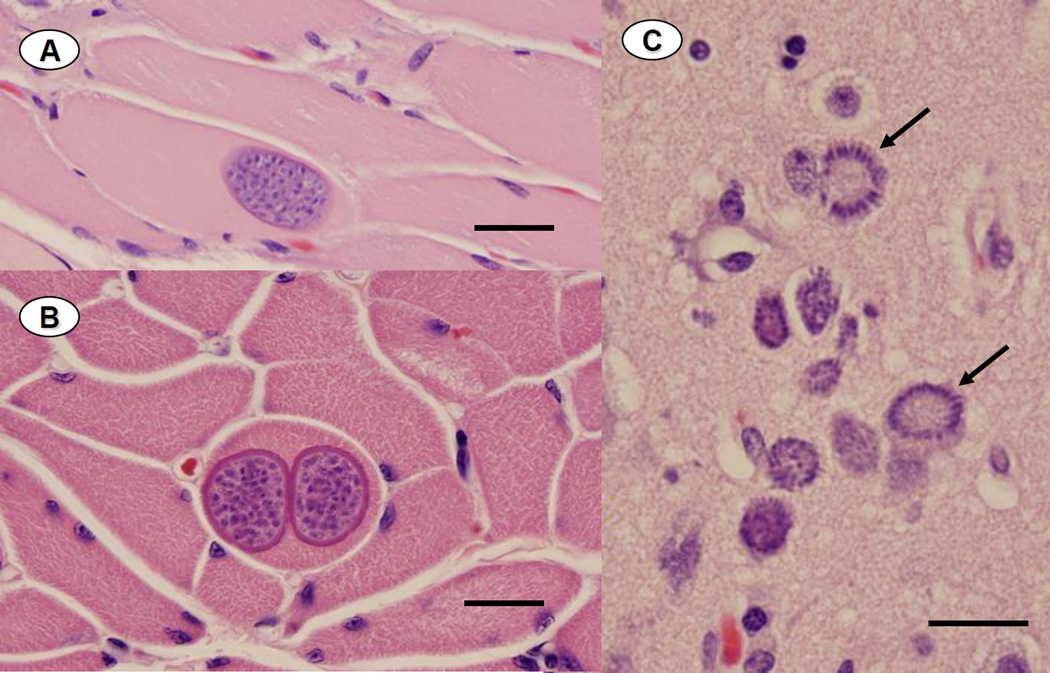
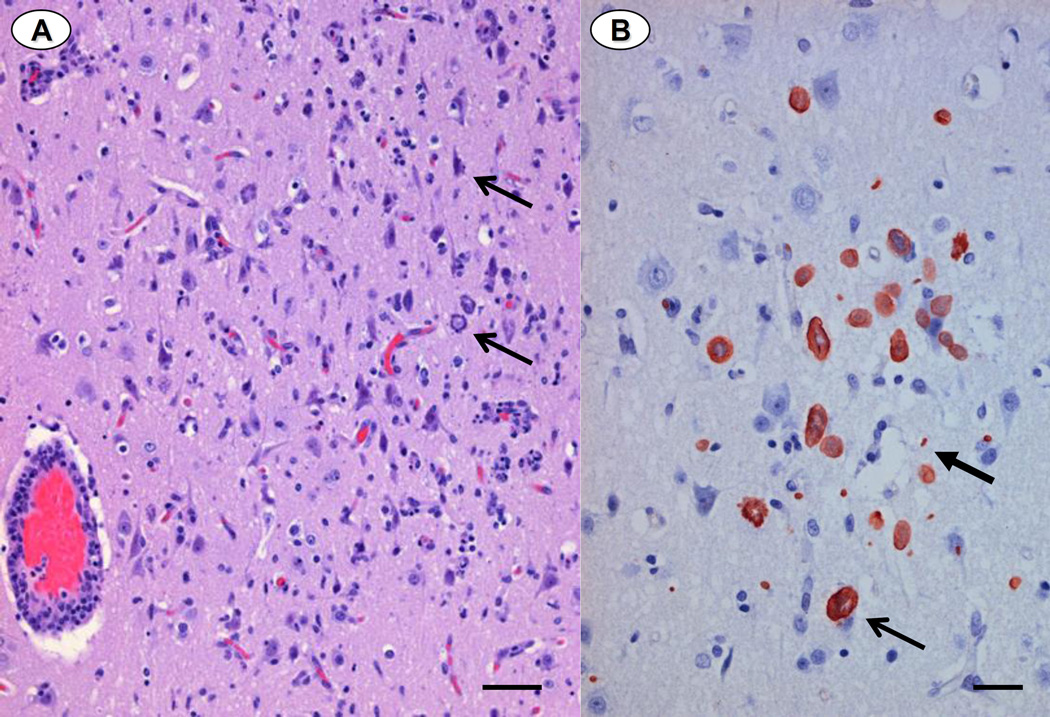
Findings from necropsy, histopathological examination and PCR analysis of brain and skeletal muscle are summarized in Table 5. Sarcocysts with microscopic features consistent with immature (Figure 4A) or mature (Figure 4B) S. neurona tissue cysts were observed within skeletal and/ or cardiac muscle fibers of most animals, along with patchy lymphoplasmacytic myositis and myocarditis. Nearly all otters had moderate to severe lymphoplasmacytic, histiocytic and mildly neutrophilic meningoencephalitis, often associated with significant tissue necrosis and white matter rarefaction (Figure 5A). Protozoal schizonts and merozoites ranged from rare to numerous on brain histopathology and immunohistochemistry (Figures 4C and 5B), (Table 5). DNA sequences consistent with S. neurona were identified from ITS-1500 amplicons from brain and/or muscle of all 15 otters (Table 5). Based on overall assessment of lesions and diagnostic data, S. neurona was considered a primary or major contributing factor in the death or euthanasia for 15 of the 16 otters that received full necropsies.
Table 5.
Findings from microscopic examination, immunohistochemistry and DNA PCR of tissues from 16 freshly dead sea otters recovered during the epizootic
| Otter # |
Sarcocystis neurona | Toxoplasma gondii | Primary cause of death |
Protozoal parasites detecteda |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Muscle-HPa | Muscle-PCR | Brain-HP | Brain-IHCa | Brain- PCR |
Muscle & Brain HP |
Brain- IHC |
Brain-PCR | |||
| 1 | Yesb | Yes | Yes | Yes | Yes | Nob | No | Yes | Protozoal meningoencephalitis |
S. neurona |
| 2 | No | Yes | No | ndb | No | No | nd | No | Acanthocephalan peritonitis |
S. neurona |
| 3 | Yes | Yes | Yes | Yes | Yes | No | No | No | Protozoal meningoencephalitis |
S. neurona |
| 4 | No | No | No | No | Yes | No | nd | No | Protozoal meningoencephalitis |
S. neurona |
| 5 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Protozoal meningoencephalitis |
S. neurona (T. gondii) |
| 6 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Protozoal meningoencephalitis |
S. neurona (T. gondii) |
| 7 | Yes | Yes | Yes | Yes | Yes | No | No | No | Protozoal meningoencephalitis |
S. neurona |
| 8 | Yes | Yes | Yes | Yes | Yes | No | No | No | Protozoal meningoencephalitis |
S. neurona (T. gondii) |
| 9 | Yes | Yes | Yes | Yes | Yes | No | No | No | Protozoal meningoencephalitis |
S. neurona |
| 10 | No | Yes | No | nd | Yes | No | nd | Yes | Protozoal meningoencephalitis |
S. neurona (T. gondii) |
| 11 | Yes | Yes | Yes | Yes | Yes | No | No | No | Protozoal meningoencephalitis |
S. neurona |
| 12 | Yes | Yes | Yes | Yes | Yes | No | No | No | Protozoal meningoencephalitis |
S. neurona (T. gondii) |
| 13 | Yes | Yes | Yes | Yes | Yes | No | No | No | Protozoal meningoencephalitis |
S. neurona |
| 14 | Yes | Yes | Yes | nd | Yes | No | nd | Yes | Protozoal meningoencephalitis |
S. neurona |
| 15c | No | nd | No | nd | nd | Yes | nd | nd | Coccidiomycosis | T. gondii |
| 16 | Yes | Yes | Yes | Yes | Yes | No | nd | No | Protozoal meningoencephalitis |
S. neurona |
HP= histopathology, IHC= immunohistochemistry
“Yes” = test positive; “No” = test negative; nd= not done; S. neurona (T. gondii) = S. neurona implicated as main cause of brain disease and death, but concurrent T. gondii infection was confirmed
Death due to disseminated coccidiomycosis; considered incidental mortality for the affected region & not part of the epizootic
Figure 4.
Photomicrographs of paraffin-embedded, H&E-stained tissues from otters dying during the epizootic. 4A: Skeletal muscle containing a single smooth-walled, immature-appearing sarcocyst encompassing numerous zoites (Bar = 30 µm). 4B: Two intramuscular sarcocysts that are larger and more fully developed, with a thick cyst wall and prominent surface villi (Bar = 25 µm). 4C: Neuropil containing numerous S. neurona schizonts, including 2 rosette-form schizonts, characterized by a distinctive radial arrangement of budding merozoites (arrows) (Bar = 20µm).
Figure 5.
Paraffin-embedded brain from a sea otter dying from disseminated Sarcocystis neurona infection during the epizootic. 5A: Hematoxylin and eosin-stained cerebrum, demonstrating a marked diffuse and perivascular infiltrate of lymphocytes and plasma cells, with fewer macrophages and neutrophils. Protozoal schizonts of varying stages of development are apparent in the cytoplasm of neurons and glial cells arrows) and there is moderate rarefaction of adjacent neuropil (Bar = 40µm). 5B: Paraffin-embedded cerebrum from the same otter labeled with antibodies to S. neurona using an immunoperoxidase method. Numerous protozoal schizonts (slender arrow) and both free and intracytoplasmic merozoites are apparent (thicker arrow) (Bar = 20µm).
Discussion and Conclusions
Here we summarize findings from investigation of an epizootic affecting southern sea otters in 2004. The point-source character of this event, the striking consistency of lesions and detection of high concentrations of anti-S. neurona IgM in affected animals is unprecedented and suggests point-source exposure to S. neurona, most likely as infective sporocysts from feces of the terrestrial definitive hosts; opossums (Dubey et al., 2001a; 2001b). Outbreaks of gastrointestinal illness in humans and animals resulting from infection by enteric protozoa such as Cryptosporidium and Giardia have strong associations with fecally polluted water (Fayer et al., 2004; Craun et al., 2005). Reports of mass-exposure to apicomplexan protozoa such as T. gondii are also numerous and the importance of aqueous dissemination of these systemic pathogens is increasingly recognized (Benenson et al., 1982; Bowie et al., 1997; Aramini et al., 1999; Eng et al., 1999; Arkush et al., 2003; Bahia-Olivera et al., 2003; Fayer et al., 2004; Craun et al., 2005; de Moura et al., 2006). These events are often first identified as localized outbreaks that are ultimately traced back to a shared food or water resource. No prior reports of marine epizootics caused by S. neurona exist in the scientific literature and epizootics caused by tissue cyst-forming protozoa have not been reported previously for any wildlife species. Documentation of the deaths of numerous adult animals during this epizootic is also novel and emphasizes the significant pathogenicity of S. neurona for sea otters. Based on carcass collection, necropsy, histopathology and PCR of tissues from this same region during the preceding decade, the maximum number of S. neurona-related sea otter strandings during the month of April would be ≥ 4 otters (M. Miller, unpub. data).
Conversely, some sea otters infected with S. neurona strains not associated with the epizootic survived and became chronically infected, based on comparison of IgM and IgG titers with results from DNA PCR and histopathology (Table 4). These comparison otters that were not associated with the epizootic had numerous mature sarcocysts in skeletal muscle on histopathology and minimal lesions associated with protozoal infection in the brain or other tissues. Similar to T. gondii infections of marine species (Miller et al, 2004), strain-specific variations in parasite prevalence, infectivity and pathogenicity may prove to be important in the ecology of S. neurona infections of marine mammals.
One important distinction in this epizootic is the means of exposure: unlike other pathogens such as viruses that cause epizootics in marine species, S. neurona and related protozoa are unlikely to be transmitted horizontally. Instead, the primary means of exposure is probably through direct contact with infective sporocysts or oocysts, or by ingestion of filter-feeding invertebrates that concentrate these pathogens from fecally-polluted water (Lindsay et al, 2001; Arkush et al., 2003; Miller et al., 2008).
Prior studies on the environmental dissemination of bacterial and parasitic pollutants along the central California coast demonstrate enhanced pathogen detection in marine invertebrates after storm events (Miller et al., 2005a; 2005b; 2006). A wild mussel (M. californianus) collected in central California during the rainy season was PCR-positive for T. gondii, and the genotype detected was the same as the dominant T. gondii genotype observed in sea otters and coastal-dwelling terrestrial felids from the same region (Miller et al., 2008). The ability of marine bivalves to remove and concentrate T. gondii oocysts during filter-feeding has been demonstrated experimentally (Lindsay et al., 2001; Arkush et al., 2003). Sarcocystis neurona sporocysts are comparable in size to T. gondii oocysts (10–12 µm), are passed in high numbers in the feces of definitive hosts and are environmentally persistent (Dubey et al., 2001a).
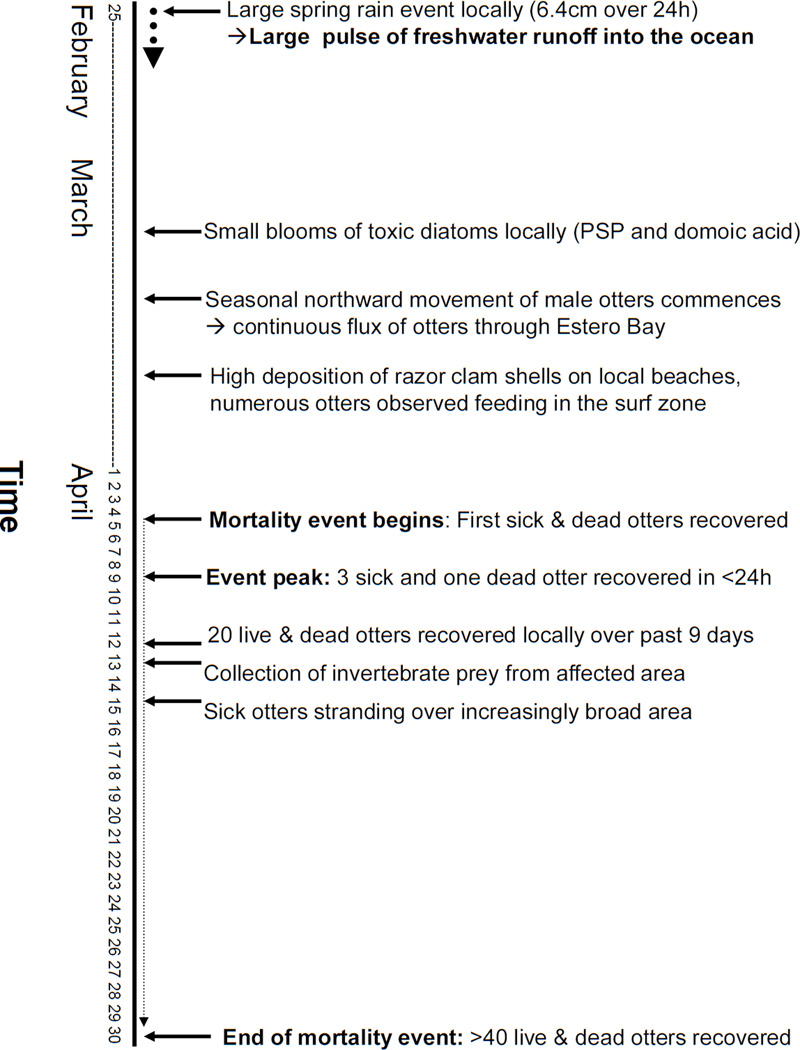
Mortality of marine wildlife due to S. neurona often peaks during spring (Kreuder et al., 2003), but large-scale, localized epizootics have not previously been recognized. Factors that may have contributed to the scope of the epizootic include a large rainstorm that occurred prior to the onset of sea otter deaths and concentration of numerous otters along the shoreline, exploiting invertebrate prey (razor clams) that may be capable of concentrating protozoal sporocysts (Figure 6). Although opossums are common in California, the terrestrial-to-marine flow of fecal waste from these animals is probably patchy and episodic, entering the ocean through multiple point-source discharges from rivers and stormwater drainages interspersed along the shore. Rivers that enter the ocean at sandy beaches are often blocked intermittently by sand berms produced by wave action. Freshwater becomes impounded, forming large, shallow coastal lagoons that provide habitat for fish, birds, insects and carnivores and omnivores (like opossums) that prey upon them. Over time, this impounded water may become enriched with nutrients and pathogens that are hazardous to marine wildlife when rapidly discharged. The mouths of several streams at the site of this epizootic are intermittently blocked by sand. If the late rain event in February, 2004 caused local berm disruption, it could have triggered a rapid release of sporocyst-laden water to the ocean.
Figure 6.
Timeline of major local events preceding and including the epizootic that could have enhanced exposure of susceptible sea otters to Sarcocystis neurona.
Post-discharge concentration of sporocysts by marine bivalves could also favor S. neurona exposure. Pathogens and toxins present in wastewater plumes can be concentrated by filter-feeding invertebrates (Goldberg, 2003; Fayer et al., 2004; O’Connor and Lauenstine, 2006) and concurrent exposure to anthropogenic pollutants, biotoxins, or viruses may enhance marine mammal susceptibility to infectious agents like S. neurona (Ross, 2002; Kanaan et al., 2006; 2007).
During the 2004 epizootic, concurrent sea otter exposure to domoic acid (DA) and S. neurona was confirmed through diagnostic testing. Uptake and slow depuration of DA by razor clams has been demonstrated (Wekell et al., 2002) and these nearshore-dwelling bivalves could serve as an efficient source for both DA and S. neurona exposure. Although DA concentrations in local bivalves were below regulatory levels for human consumption (≥ 20 ppm, http://www.cfsan.fda.gov/~comm/haccp4f.html), sea otters consume far greater masses of food per kg body weight than do humans (Reidman and Estes, 1990) and could ingest cumulative levels of DA sufficient to cause clinical or subclinical disease, even when concentrations in shellfish are below levels considered safe for humans.
Developing a concise and precise case definition for DA intoxication has proven to be challenging in sea otters, when compared to marine pinnipeds. This is due in part to the short half life of DA in the body, instability of DA in cryoarchived samples and concurrent presence of multiple diseases with potential to cause neurological disease in sea otters (including protozoal disease, domoic acid, other biotoxins, hyperthermia, and hypoglycemia). Also, many otters are found dead with no clinical history and significant postmortem autolysis, which can obscure lesions subscribed to DA intoxication. DA-associated brain lesions are present in some sea otters and there is no doubt that DA intoxication kills otters (Kreuder et al, 2003; 2005), but the true population impact has been difficult to ascertain, given that up to 70% of necropsied otters test DA-positive, often at levels just above minimum detection limits (M. Miller, unpublished data). An epidemiological study is in progress to determine associations between DA concentrations in urine, serum and digesta with results from blind-scoring of specific lesions in the brain and other tissues. Potential immunomodulatory effects for DA have been reported for leukocytes (Jones et al., 1995). If an immunosuppressive or immunopotentiating effect for DA is confirmed, exposure to sublethal concentrations could render exposed wildlife (and humans) more susceptible to opportunistic pathogens like S. neurona and T. gondii. DA exposure was thought to have played a contributory role in the 2004 event, but the magnitude and significance of its overall contribution to sea otter mortality could not be determined given our current state of knowledge.
Given the strong seasonal pattern of detection of S. neurona-associated disease in sea otters (Kreuder et al., 2003), it is likely that sporocyst shedding in opossum populations is seasonally influenced, thus enhancing risk for marine mammals feeding in areas impacted by freshwater drainage during periods of high coastal precipitation. Also, based on past sea otter capture records, Estero Bay is heavily used by immature and subadult otters, forming a large, local population of immunologically naïve animals that are more likely to die due to S. ne urona-associated meningoencephalitis (Kreuder et al., 2003).
The nearshore aggregation of sea otters due to ready availability of razor clams was another possible contributing factor (Figure 6). Prior to and during the epizootic large numbers of otters were observed foraging in shallow water along local beaches and significant quantities of razor clam shell fragments were observed along the adjacent shoreline. Only sand-dwelling invertebrates (razor clams and sand crabs) were recovered from the digestive tracts of affected sea otters. Also, over half of stranded otters had intestinal infections by Profillicolis spp. acanthocephalans whose main intermediate hosts are sand and mole crabs that reside along sandy beaches (Mayer et al., 2003). To summarize, it appears that the otters dying due to S. neurona infection fed on a common food source (razor clams) near the shoreline, thereby enhancing their exposure to S. neurona sporocysts released from coastal lagoons during the large storm event.
Our investigation of the 2004 mortality event has provided important insights regarding the potential of terrestrial pathogens to negatively impact protected marine species. The ability of marine and estuarine invertebrates to bioconcentrate both DA and protozoa is well documented (Goldberg, 2003; Fayer et al, 2004). The recent confirmation of Type X T. gondii uptake by a wild marine mussel from coastal California and demonstration of the same unusual genotype in coastal-dwelling terrestrial felids and sympatric sea otters strongly supports the hypothesis of land-sea transfer of protozoal pathogens (Miller et al., 2008). Here we describe an additional model system; that of S. neurona, opossums, invertebrates and sea otters that demonstrates the importance of land-sea transport as a potential source of human and animal exposure to biological pathogens.
Acknowledgments
We thank J. Ames, E. Berberich, D. Brownstein, E. Dodd, E. Dorfmeier, J. Mazet and W. Miller for assistance. The Microbial Diseases Laboratory and the Food and Drug Laboratory, CDPH, for DA analyses. This research was supported in part by the Intramural Research Program of the NIH and NIAID (MEG), CDFG-OSPR, USGS, Monterey Bay Aquarium and the United States Fish and Wildlife Service. This manuscript is dedicated to Dr Peter Kennedy, an outstanding pathologist, mentor and lifelong student who believed in doing good things for sea otters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aramini JJ, Stephen C, Dubey JP, Engelstoft C, Schwantje H, Ribble CS. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol. Infect. 1999;122:305–315. doi: 10.1017/s0950268899002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkush KD, Miller MA, Leutenegger CM, Gardner IA, Packham AE, Heckeroth AR, Tenter AM, Barr BC, Conrad PA. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis) Int. J. Parasitol. 2003;33:1087–1097. doi: 10.1016/s0020-7519(03)00181-4. [DOI] [PubMed] [Google Scholar]
- Bahia-Oliveira LM, Jone LM, Azevedo-Silva CCF, Alves, Orefice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg. Infect. Dis. 2003;9:55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benenson MW, Takafuji ET, Lemon SM, Greenup RL, Sulzer AJ. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N. Engl. J. Med. 1982;307:666–669. doi: 10.1056/NEJM198209093071107. [DOI] [PubMed] [Google Scholar]
- Bowie WR, King AS, Werker DH, Isaac-Renton JL, Bell A, Eng SB, Marion SA. Outbreak of toxoplasmosis associated with municipal drinking water. Lancet. 1997;350:173–177. doi: 10.1016/s0140-6736(96)11105-3. [DOI] [PubMed] [Google Scholar]
- Craun GF, Calderon RL, Craun MF. Outbreaks associated with recreational water in the United States. Int. J. Environment. Health Res. 2005;15:243–262. doi: 10.1080/09603120500155716. [DOI] [PubMed] [Google Scholar]
- de Moura L, Bahia-Oliveira LMG, Wada MY, Jones JL, Tuboi SH, Carmo EH, Ramalho WM, Camargo NJ, Trevisan R, Graca RMT, da Silva AJ, Moura I, Dubey JP, Garrett DO. Waterborne Toxoplasmosis, Brazil, from Field to Gene. Emerg. Infect. Dis. 2006;12:326–329. doi: 10.3201/eid1202.041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Saville WJ, Reed SM, Granstrom DE, Speer CA. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM) Vet. Parasitol. 2001a;95:89–131. doi: 10.1016/s0304-4017(00)00384-8. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Rosypal AC, Rosenthal BM, Thomas NJ, Lindsay DS, Stanek JF, Reed SM, Saville WJ. Sarcocystis neurona infections in sea otter (Enhydra lutris): Evidence for natural infection with sarcocysts and transmission of infection to opossums. J. Parasitol. 2001b;87:1387–1393. doi: 10.1645/0022-3395(2001)087[1387:SNIISO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Eng SB, Werker DH, King AS, Marion SA, Bell A, Isaac-Renton JL, Irwin GS, Bowie WR. Computer-generated dot maps as an epidemiologic tool: Investigating an outbreak of toxoplasmosis. Emerg. Infect. Dis. 1999;5:815–819. doi: 10.3201/eid0506.990613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R, Dubey JP, Lindsay DS. Zoonotic protozoa: from land to sea. Trends Parasitol. 2004;20:531–536. doi: 10.1016/j.pt.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Goldberg J. MS Thesis. Monterey Bay: California State University; 2003. Domoic acid in the benthic food web of Monterey Bay, California; p. 41. [Google Scholar]
- Grigg ME, Boothroyd JC. Rapid identification of virulent Type I strains of the protozoan pathogen Toxoplasma gondii by PCR-Restriction Fragment Length Polymorphism analysis at the B1 gene. J. Clin. Microbiol. 2001;39:398–400. doi: 10.1128/JCM.39.1.398-400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell J. The Tennessee possum has arrived in California. Calif. Fish and Game. 1915;1:114–116. [14] [Google Scholar]
- Jameson R. Movements, home range and territories of male sea otters off California. Mar. Mam. Sci. 1989;5:159–172. [Google Scholar]
- Jessup DA, Miller MA, Harris M, Hatfield B, Estes J. The 2003 southern sea otter (Enhydra lutris nereis) unusual mortality event. Official report to the National Oceanic and Atmospheric Association and United States Fish and Wildlife Service. 2004:31. [Google Scholar]
- Jones TO, Whyte JNC, Ginther NG, Townsend LD, Iwama GK. Haemocyte changes in the pacific oyster, crassostrea gigas, caused by exposure to domoic acid in the diatom pseudonitzschia pungens f. multiseries. Toxicon. 1995;3:347–353. doi: 10.1016/0041-0101(94)00170-d. [DOI] [PubMed] [Google Scholar]
- Kannan K, Perrotta E, Thomas NJ. Association between perfluorinated compounds and pathological conditions in southern sea otters. Environ. Sci. Technol. 2006;40:4943–4948. doi: 10.1021/es060932o. [DOI] [PubMed] [Google Scholar]
- Kannan K, Perrotta E, Thomas NJ, Aldus KM. A comparative study of polybrominated diphenyl ethers and polychlorinated biphenyls in southern sea otters that died of infectious diseases and noninfectious causes. Arch. Envir. Contam. Toxicol. 2007;53:293–302. doi: 10.1007/s00244-006-0251-8. [DOI] [PubMed] [Google Scholar]
- Kreuder C, Miller MA, Jessup DA, Lowenstine LJ, Harris MD, Ames JA, Carpenter TE, Conrad PA, Mazet JA. Patterns of mortality in southern sea otters (Enhydra lutris nereis) from 1998–2001. J. Wildl. Dis. 2003;39:495–509. doi: 10.7589/0090-3558-39.3.495. [DOI] [PubMed] [Google Scholar]
- Kreuder C, Miller MA, Lowenstine LJ, Conrad PA, Carpenter TE, Jessup DA, Mazet JK. Evaluation of cardiac lesions and risk factors associated with myocarditis and dilated cardiomyopathy in southern sea otters (Enhydra lutris nereis) Am. J. Vet. Res. 2005;66:289–299. doi: 10.2460/ajvr.2005.66.289. [DOI] [PubMed] [Google Scholar]
- Lapointe JM, Duignan PJ, Marsh AE, Gulland FM, Barr BC, Naydan DK, King DP, Farman CA, Burek-Huntingdon KA, Lowenstine LJ. Meningoencephalitis due to a Sarcocystis neurona-like protozoan in Pacific Harbor Seals (Phoca vitulina richardsi) J. Parasitol. 1998;84:1184–1189. [PubMed] [Google Scholar]
- Lindsay DS, Thomas NJ, Dubey JP. Biological characterization of Sarcocystis neurona isolated from a southern sea otter (Enhydra lutris nereis) Int. J. Parasitol. 2000;30:617–624. doi: 10.1016/s0020-7519(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Phelps KK, Smith SA, Flick G, Dubey JP. Removal of Toxoplasma gondii oocysts from seawater by Eastern Oysters. (Crassostrea virginica) J. Eukaryot. Microbiol. 2001:197S–198S. doi: 10.1111/j.1550-7408.2001.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Marsh AE, Barr BC, Madigan J, Lakritz J, Conrad PA. Sequence analysis and polymerase chain reaction amplification of small subunit ribosomal DNA from Sarcocystis neurona. Am J Vet Res. 1996;57:975–981. [PubMed] [Google Scholar]
- Marsh AE, Barr BC, Tell L, Bowman DD, Conrad PA, Ketcherside C, Green T. Comparison of the internal transcribed spacer, ITS-1, from Sarcocystis falcatula isolates and Sarcocystis neurona. J. Parasitol. 1999;85:750–757. [PubMed] [Google Scholar]
- Mayer K, Dailey M, Miller MA. Helminth parasites of the southern sea otter (Enhydra lutris nereis) from central California: abundance, distribution and pathology. Dis. Aquat. Org. 2003;53:77–82. doi: 10.3354/dao053077. [DOI] [PubMed] [Google Scholar]
- Miller MA, Sverlow K, Crosbie PR, Barr BC, Lowenstine LJ, Gulland FM, Packham A, Conrad PA. Isolation and characterization of two parasitic protozoa from a Pacific harbor seal (Phoca vitulina richardsi) with meningoencephalomyelitis. J. Parasitol. 2001;87:816–822. doi: 10.1645/0022-3395(2001)087[0816:IACOTP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Miller MA, Gardner IA, Packham AE, Maz et JK, Hanni KD, Jessup DA, Jameson R, Dodd E, Barr BC, Lowenstine LJ, Gulland FM, Conrad PA. Evaluation of an indirect fluorescent antibody test (IFAT) for demonstration of antibodies to Toxoplasma gondii in the sea otter (Enhydra lutris) J. Parasitol. 2002a;88:594–599. doi: 10.1645/0022-3395(2002)088[0594:EOAIFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Miller MA, Gardner IA, Kreuder C, Paradies D, Worcester K, Jessup D, Dodd E, Harris M, Ames J, Packham A, Conrad PA. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int. J. Parasitol. 2002b;32:997–1006. doi: 10.1016/s0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Miller MA, Grigg ME, Kreuder C, James ER, Melli AC, Crosbie PR, Jessup DA, Boothroyd JC, Brownstein D, Conrad PA. An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. Int. J. Parasitol. 2004;34:275–284. doi: 10.1016/j.ijpara.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Miller MA, Miller WAPA, Conrad PA, James ER, Melli AC, Leutenegger CM, Dabritz HA, Packham AE, Paradies D, Harris M, Ames J, Jessup DA, Worcester K, Grigg ME. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: new linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters, Int. J. Parasitol. 2008;38:1319–1328. doi: 10.1016/j.ijpara.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Miller MA, Barr BC, Nordhausen R, James ER, Magargal SJ, Conrad PA, Murray M, Packham AE, Melli AC, Toy-Choutka S, Jessup D, Grigg ME. Ultrastructural and molecular confirmation of the development of Sarcocystis neurona tissue cysts in the central nervous system of southern sea otters (Enhydra lutris nereis) Int. J. Parasitol. doi: 10.1016/j.ijpara.2009.04.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WA, Atwill ER, Gardner IA, Miller MA, Fritz H, Hedrick RP, Melli AC, Barnes NC, Conrad PA. Clams (Corbicula fluminea) as bioindicators of fecal contamination with Cryptosporidium and Giardia spp. in freshwater ecosystems in California. Int. J. Parasitol. 2005a;l35:673–684. doi: 10.1016/j.ijpara.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Miller WA, Miller MA, Gardner IA, Atwill ER, Harris M, Ames J, Jessup DA, Melli AC, Paradies D, Worcester K, Olin P, Barnes NM, Conrad PA. New genotypes and factors associated with Cryptosporidium detection in mussels (Mytilus spp.) along the California coast. Int. J. Parasitol. 2005b;35:1103–1113. doi: 10.1016/j.ijpara.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Miller WA, Miller MA, Gardner IA, Atwill ER, Byrne BA, Jang S, Harris M, Ames J, Jessup D, Paradies D, Worcester K, Melli A, Conrad PA. Salmonella spp., Vibrio spp., Clostridium perfringens and Plesiomonas shigelloides in freshwater and marine invertebrates from coastal California ecosystems. Microb. Ecol. 2006;52:198–206. doi: 10.1007/s00248-006-9080-6. [DOI] [PubMed] [Google Scholar]
- O’Connor TP, Lauenstine GG. Trends in chemical concentrations in mussels and oysters collected along the U.S. Coast: Update to 2003. Mar. Envir. Res. 2006;62:261–285. doi: 10.1016/j.marenvres.2006.04.067. [DOI] [PubMed] [Google Scholar]
- Quilliam MA, Xhie M, Hardstaff WR. J. Assoc. Offic. An. Chem. Int. 1995;78:543–555. [Google Scholar]
- Riedman ML, Estes JA. The sea otter (Enhydra lutris): Behavior, ecology and natural history. USFWS Biol. Rep. 1990;90:126. [Google Scholar]
- Rosonke BJ, Brown SR, Tornquist SJ, Snyder SP, Garner MM, Blythe LL. Encephalomyelitis associated with a Sarcocystis neurona-like organism in a sea otter. J. Am. Vet. Med. Assoc. 1999;215:1839–1842. [PubMed] [Google Scholar]
- Ross PS. The role of immunotoxic environmental contaminants in facilitating the emergence of infectious diseases in marine mammals. Human and Ecological Risk Assessment. 2002;8:277–292. [Google Scholar]
- Thomas NJ, Dubey JP, Lindsay DS, Cole RA, Meteyer CU. Protozoal meningoencephalitis in sea otters (Enhydra lutris): a histopathological and immunohistochemical study of naturally occurring cases. J. Comp. Path. 2007;137:102–121. doi: 10.1016/j.jcpa.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Torr ER, Puschner B, Whitehead WE. Rapid determination of domoic acid in serum and urine by liquid chromatography-electrospray tandem mass spectrometry. J. Agric. Food. Chem. 2003;51:1791–1796. doi: 10.1021/jf020947f. [DOI] [PubMed] [Google Scholar]
- Wekell JC, Trainer VL, Ayres D, Simons D. A study of spatial variability of domoic acid in razor clams: Recommendations for resource management on the Washington coast. Harmful Algae. 2002;1:35–43. [Google Scholar]