Abstract
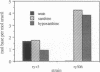
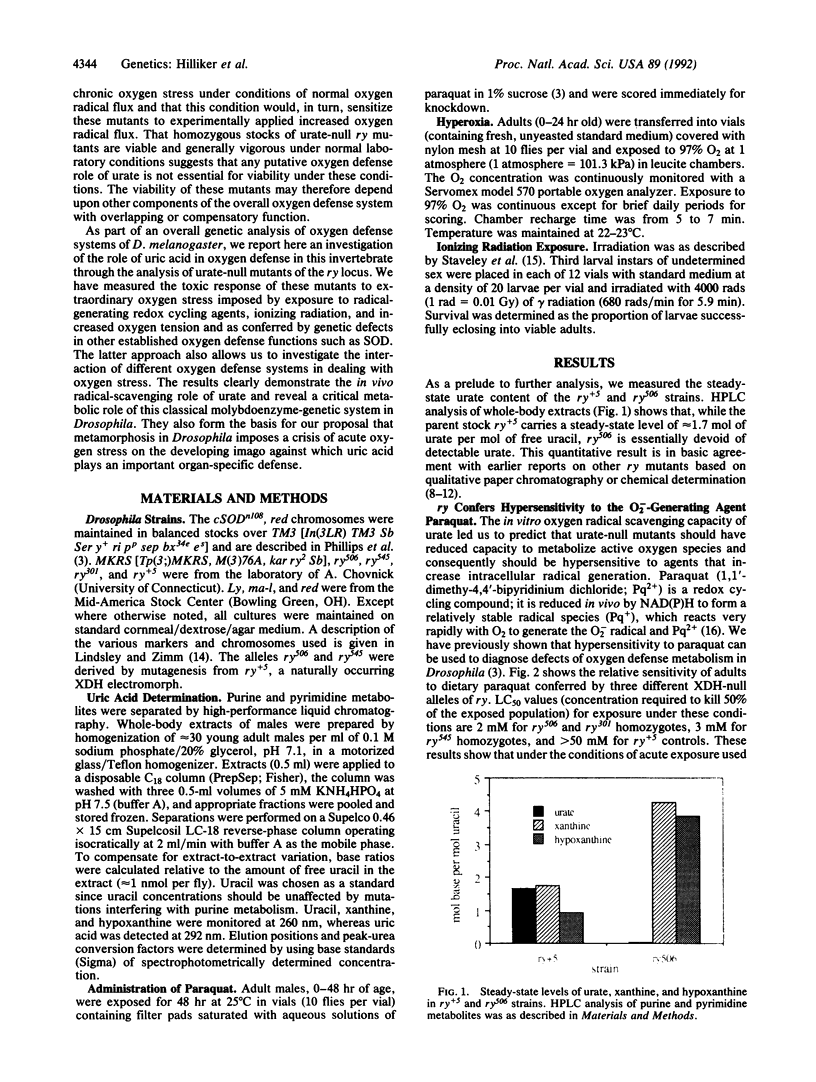
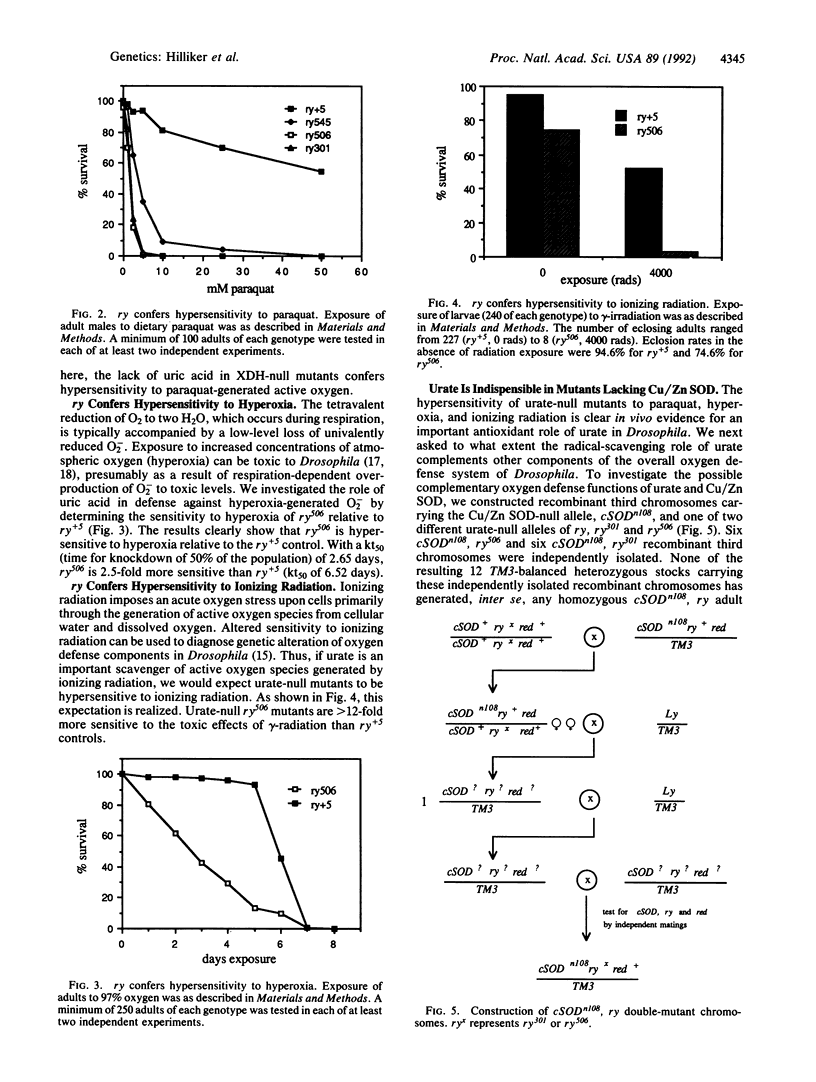
It has been proposed that uric acid is an important scavenger of deleterious oxygen radicals in biological systems [Ames, B. N., Cathcart, R., Schwiers, E. & Hochstein, P. (1981) Proc. Natl. Acad. Sci. USA 78, 6858-6852]. We report here an in vivo investigation of the oxygen defense role of uric acid through an analysis of mutants of the rosy (ry) gene of Drosophila melanogaster. The ry gene is the structural gene for the molybdoenzyme, xanthine dehydrogenase; xanthine dehydrogenase-null ry mutants are therefore unable to synthesize urate. The rationale of our approach was to measure the response of urate-null ry mutants to extraordinary oxygen stress as imposed by exposure to radical-generating agents and as conferred by a genetic defect in superoxide dismutase, an established oxygen defense function. We show that urate-null mutants of the ry locus are hypersensitive to paraquat, ionizing radiation, and hyperoxia. Furthermore, compound mutants doubly deficient for uric acid and Cu/Zn-containing superoxide dismutase are synthetic lethals, which are unable to complete metamorphosis under normal growth conditions. These experiments demonstrate unambiguously the importance of urate in oxygen defense in vivo and support our earlier proposal that the molybdoenzyme genetic system plays a critical role in oxygen defense in Drosophila. They also form the basis for our proposal that metamorphosis in Drosophila imposes a crisis of oxygen stress on the developing imago against which uric acid plays an important organ-specific defense. Finally, the results provide a basis for understanding the syndrome of phenotypes, including the hallmark dull brown eye color, which characterizes mutants of this classic genetic system of Drosophila.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonse A. Untersuchungen über die chemische Natur und die Bildung der Harnkonglomerate in den Malpighischen Gefässen der Mutante rosy von Drosophila melanogaster. Z Naturforsch B. 1967 Oct;22(10):1027–1029. [PubMed] [Google Scholar]
- Burcombe J. V., Hollingsworth M. J. The total nitrogen, protein, amino acid and uric acid ntent of ageing Drosophila. Exp Gerontol. 1970 Sep;5(3):247–255. doi: 10.1016/0531-5565(70)90045-8. [DOI] [PubMed] [Google Scholar]
- Campbell S. D., Hilliker A. J., Phillips J. P. Cytogenetic analysis of the cSOD microregion in Drosophila melanogaster. Genetics. 1986 Feb;112(2):205–215. doi: 10.1093/genetics/112.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. G. Urate and ascorbate: their possible roles as antioxidants in determining longevity of mammalian species. Arch Gerontol Geriatr. 1984 Dec;3(4):321–348. doi: 10.1016/0167-4943(84)90033-5. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Sevanian A., Muakkassah-Kelly S. F., Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986 May 1;235(3):747–754. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORREST H. S., GLASSMAN E., MITCHELL H. K. Conversion of 2-amino-4-hydroxypteridine to isoxanthopterin in D. Melanogaster. Science. 1956 Oct 19;124(3225):725–726. doi: 10.1126/science.124.3225.725. [DOI] [PubMed] [Google Scholar]
- Farrington J. A., Ebert M., Land E. J., Fletcher K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta. 1973 Sep 26;314(3):372–381. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- Fenn W. O., Henning M., Philpott M. Oxygen poisoning in Drosophila. J Gen Physiol. 1967 Jul;50(6):1693–1707. doi: 10.1085/jgp.50.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman E, Mitchell H K. Mutants of Drosophila Melanogaster Deficient in Xanthine Dehydrogenase. Genetics. 1959 Mar;44(2):153–162. doi: 10.1093/genetics/44.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADORN E., SCHWINCK I. Fehlen von Isoxanthopterin und Nicht-Autonomie in der Bildung der roten Augenpigmente bei einer Mutante (rosy2) von Drosophila melanogaster. Z Indukt Abstamm Vererbungsl. 1956;87(4):528–553. [PubMed] [Google Scholar]
- HARMAN D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956 Jul;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Kaur H., Halliwell B. Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem Biol Interact. 1990;73(2-3):235–247. doi: 10.1016/0009-2797(90)90006-9. [DOI] [PubMed] [Google Scholar]
- MITCHELL H. K., GLASSMAN E. Hypoxanthine in rosy and maroon-like mutants of Drosophila melanogaster. Science. 1959 Jan 30;129(3344):268–268. doi: 10.1126/science.129.3344.268. [DOI] [PubMed] [Google Scholar]
- MORITA T. Purine catabolism in Drosophila melanogaster. Science. 1958 Nov 7;128(3332):1135–1135. doi: 10.1126/science.128.3332.1135. [DOI] [PubMed] [Google Scholar]
- Mackay W. J., Bewley G. C. The genetics of catalase in Drosophila melanogaster: isolation and characterization of acatalasemic mutants. Genetics. 1989 Jul;122(3):643–652. doi: 10.1093/genetics/122.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. P., Hilliker A. J. Genetic analysis of oxygen defense mechanisms in Drosophila melanogaster. Adv Genet. 1990;28:43–71. doi: 10.1016/s0065-2660(08)60523-4. [DOI] [PubMed] [Google Scholar]
- Reaume A. G., Clark S. H., Chovnick A. Xanthine dehydrogenase is transported to the Drosophila eye. Genetics. 1989 Nov;123(3):503–509. doi: 10.1093/genetics/123.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J. C., Walldorf U., Hug P., Gehring W. J. Fruit flies with additional expression of the elongation factor EF-1 alpha live longer. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7520–7521. doi: 10.1073/pnas.86.19.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley B. E., Phillips J. P., Hilliker A. J. Phenotypic consequences of copper-zinc superoxide dismutase overexpression in Drosophila melanogaster. Genome. 1990 Dec;33(6):867–872. doi: 10.1139/g90-130. [DOI] [PubMed] [Google Scholar]
- Wallrath L. L., Burnett J. B., Friedman T. B. Molecular characterization of the Drosophila melanogaster urate oxidase gene, an ecdysone-repressible gene expressed only in the malpighian tubules. Mol Cell Biol. 1990 Oct;10(10):5114–5127. doi: 10.1128/mcb.10.10.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]