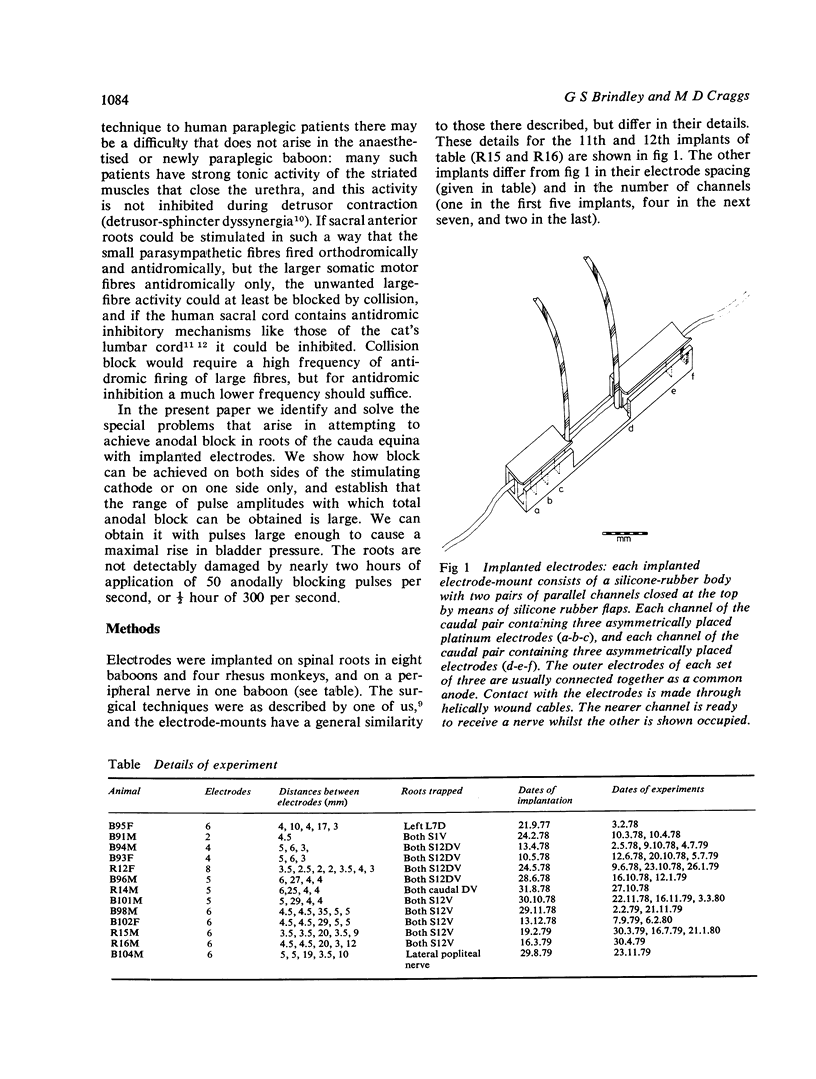
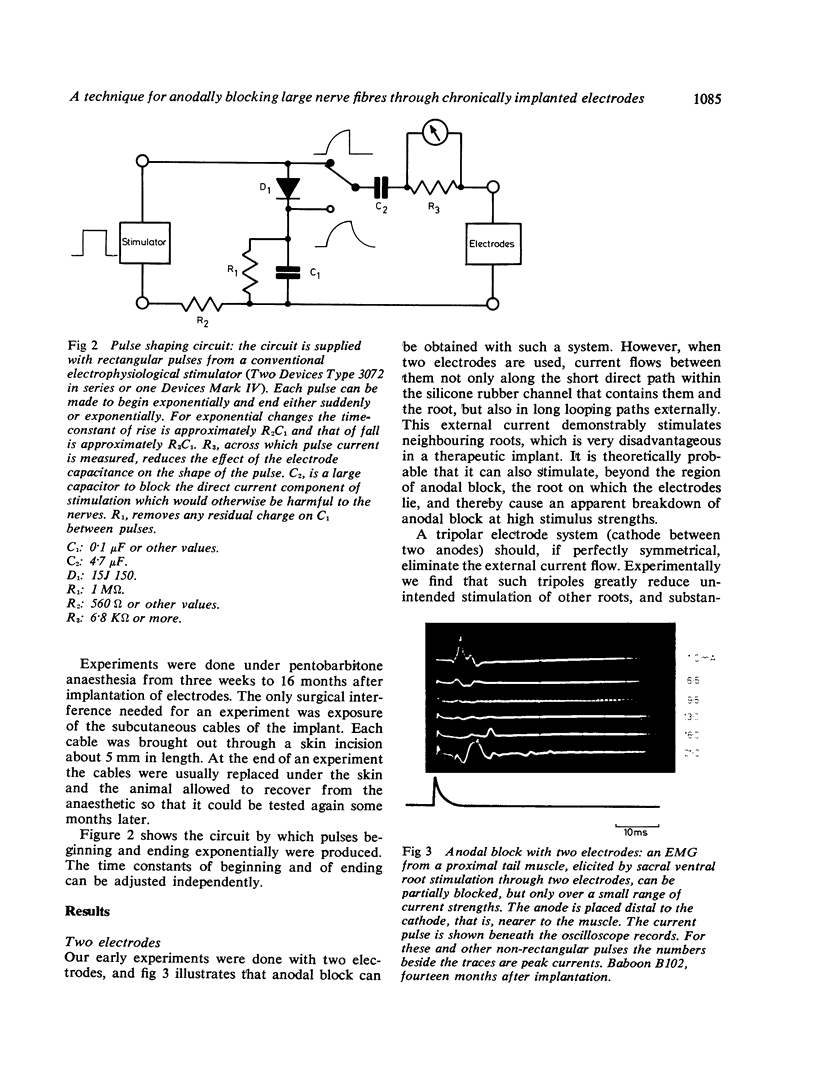
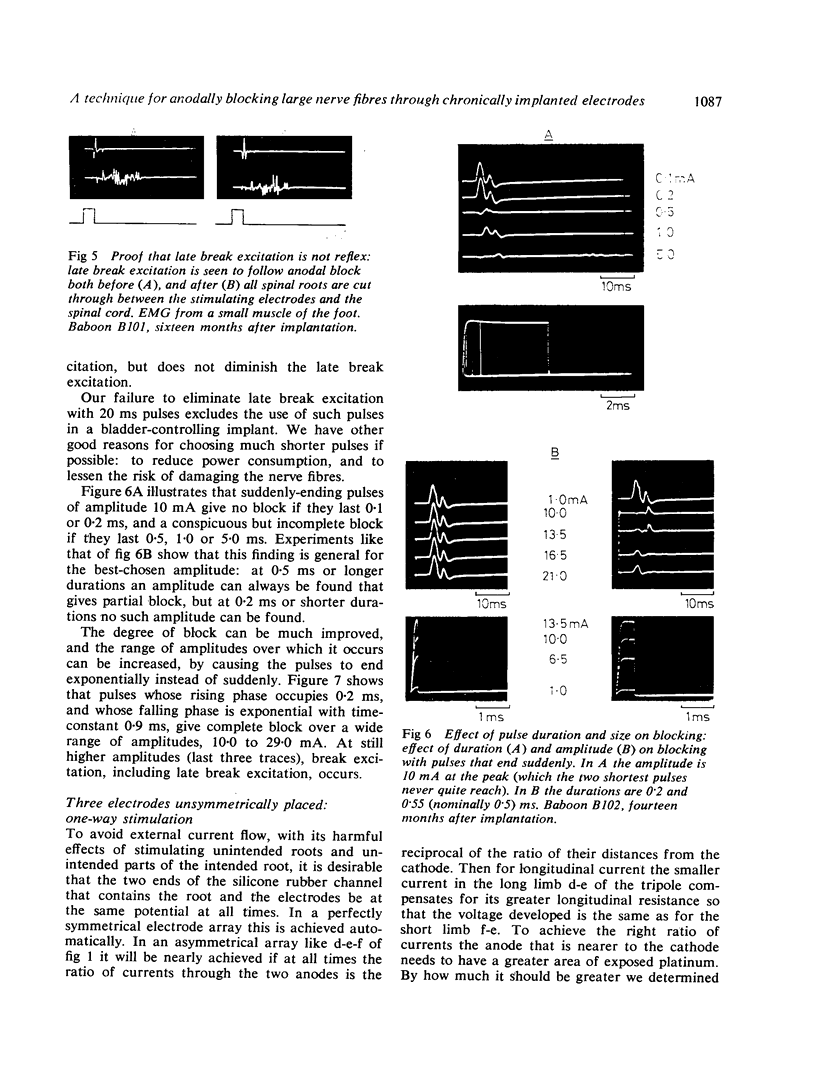
Abstract
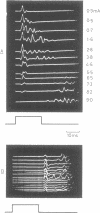
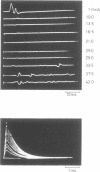
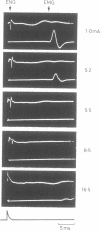
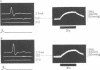
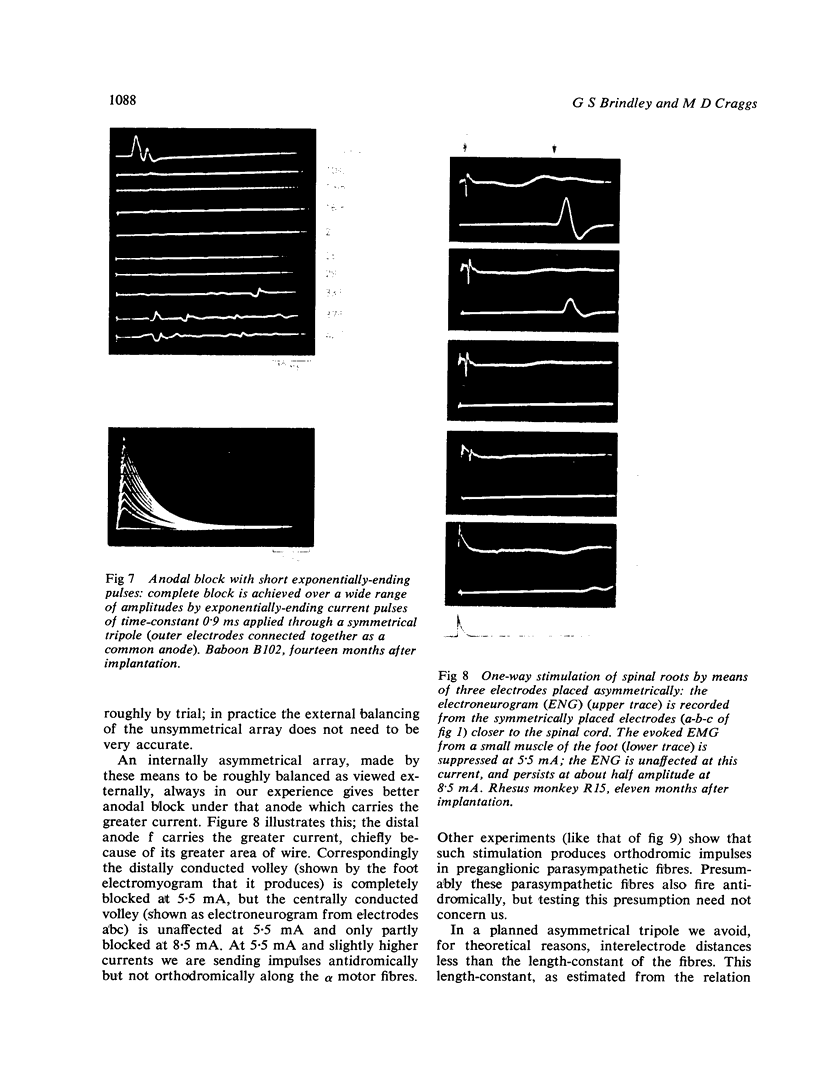
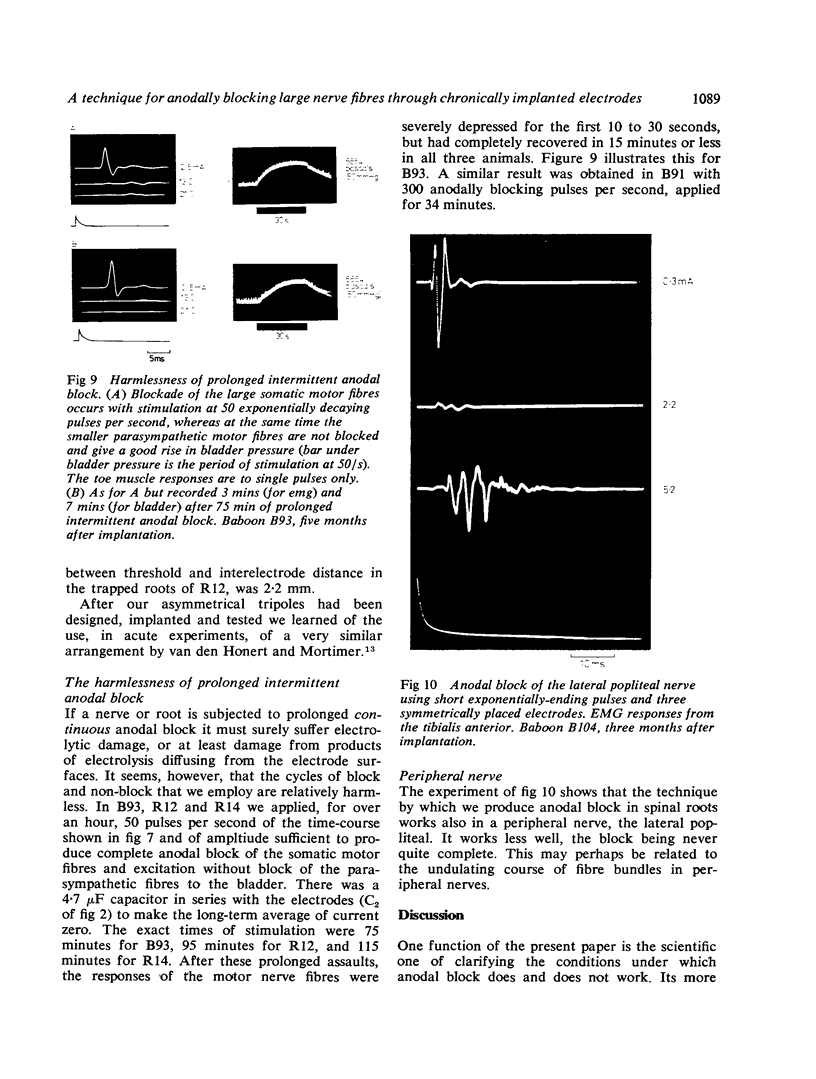
If a spinal root of a baboon or rhesus monkey is trapped in an initially loose-fitting silicone rubber channel containing two or more platinum electrodes, electrical pulses sent through these electrodes can stimulate nerve fibres close to the cathode and block the resulting impulses close to the anode. We show (1) how anodal break excitation and excitation of fibres outside the silicone rubber channel can be avoided; (2) that an implant 26 months old behaves like a recent one; (3) that in a root containing somatic motor fibres and parasympathetic fibres, all somatic motor fibres can be blocked and most or all parasympathetic fibres excited but not blocked; (4) that provided that the electrodes pass no net direct current, prolonged stimulation with block can be harmless; (5) how block can be achieved in one direction only along a root; (6) that a peripheral nerve can be blocked by the same techniques.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accornero N., Bini G., Lenzi G. L., Manfredi M. Selective Activation of peripheral nerve fibre groups of different diameter by triangular shaped stimulus pulses. J Physiol. 1977 Dec;273(3):539–560. doi: 10.1113/jphysiol.1977.sp012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE W., GINSBORG B. L. The electrical properties of the slow muscle fibre membrane. J Physiol. 1956 Jun 28;132(3):586–598. doi: 10.1113/jphysiol.1956.sp005551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley G. S. An implant to empty the bladder or close the urethra. J Neurol Neurosurg Psychiatry. 1977 Apr;40(4):358–369. doi: 10.1136/jnnp.40.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotch F., Macdonald J. S. Temperature and Excitability. J Physiol. 1896 Oct 19;20(4-5):247–297. doi: 10.1113/jphysiol.1896.sp000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. H. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951 Sep;115(1):101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSHTON W. A. H. The site of excitation in the nerve trunk of the frog. J Physiol. 1949 Sep;109(3-4):314–326. doi: 10.1113/jphysiol.1949.sp004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. G., Smallwood R., Graham D. Urodynamic observations following spinal trauma. Br J Urol. 1975 Apr;47(2):161–175. [PubMed] [Google Scholar]
- van den Honert C., Mortimer J. T. Generation of unidirectionally propagated action potentials in a peripheral nerve by brief stimuli. Science. 1979 Dec 14;206(4424):1311–1312. doi: 10.1126/science.515733. [DOI] [PubMed] [Google Scholar]