Abstract
Background
Finding compatible feline blood donors can be challenging. Canine blood has been occasionally used when compatible feline blood was not available in emergency situations.
Objectives
The study goals were to describe the effects of xenotransfusion in two anemic cats receiving canine blood because of discordant blood types and acute transfusion reaction, respectively, and to report in vitro heterotyping and – crossmatching results between canine and feline blood samples.
Material and Methods
Blood samples from patients and other cats and dogs were typed, crossmatched, and assessed for alloantibodies using gel, card, and immunochromatographic strip techniques.
Results
Cat 1 was found to have type AB blood. Cat 2, which experienced an acute transfusion reaction, had type A blood. Neither had detectable alloantibodies against feline RBC. Both cats transiently improved after transfusion with canine blood, however, acute intravascular hemolysis occurred and the PCV rapidly declined. Blood typing post xenotransfusion with DEA 1 strips revealed a positive control band that was absent in feline blood, thus allowing for the identification of transfused canine RBC. Longitudinal assessment revealed that canine RBC could no longer be detected 4 days after xenotransfusion.
Major crossmatching (feline plasma with canine RBC) resulted in both positive and negative reactions, depending on the cat. Minor crossmatching results showed mostly incompatibility.
Conclusion
While both cats survived xenotransfusion, the positive control band on the DEA 1 strip revealed that transfused canine RBC were short-lived, and intravascular hemolysis occurred. Crossmatch results between cats and dogs showed varied incompatibilities, and may not predict transfusion reactions.
Keywords: Crossmatch, Feline, Hemolysis, Transfusion reaction, Blood typing
Introduction
Because of the natural occurrence of strong anti-A and varied anti-B alloantibodies, feline patients and blood donors are blood typed for the AB blood group system and/or crossmatched in order to assure blood type compatibility. Several AB blood typing kits are available for in-clinic and laboratory use, although occasionally typing difficulties occur. Additional blood type (eg, Mik) incompatibilities and acute hemolytic and other transfusion reactions have been observed, particularly in previously transfused cats.1 Crossmatching is particularly indicated after prior transfusions, but is not generally performed for a first transfusion.2–7
Transfusions are far less frequently performed in cats than in dogs for various reasons, including fewer clinical signs associated with anemia, fewer bleeding problems, the need to sedate donor cats, and difficulties in collecting and processing blood. When AB-matched feline blood is unavailable and conditions are dire, xenotransfusion (ie transfusion of blood from one species to another species) with canine blood has been attempted, particularly in Australia and France.8–11 A recent review of canine blood transfused to cats12, mostly based upon 1960’s experimental studies8,9,13, and a few anecdotal clinical reports14,15, claimed compatible crossmatch results between dogs and cats, and clinical improvement of anemic cats receiving canine blood for the first time. Clinical improvement was seen despite the very short life span of transfused cells. However, fatal reactions occurred when a second transfusion was given after 7–8 days. Those authors concluded that canine blood could be given for a first transfusion when feline blood was not available.12 Alternatively, a highly purified polymerized bovine hemoglobin solution may be considered under such circumstances, although this product is currently only available in Europe.16
Here we report on 2 anemic cats receiving canine RBCs because of discordant AB blood typing results and an acute transfusion reaction to feline blood, respectively. The mixing effects of canine and feline blood were assessed in typing and crossmatch tests.
Animals and Methods
Blood Samples
Small (0.5–1 ml) EDTA anticoagulated blood samples were available from 2 anemic cats that received one therapeutic canine packed red blood cell (pRBC) transfusion. One fresh whole blood transfusion from a canine blood donor at the ASPCA Animal Hospital, New York, another at the Animal Health Center, College of Veterinary Medicine, Mississippi State University Starkville, MS, respectively, and several other blood donors were studied at PennGen Laboratories, School of Veterinary Medicine of the University of Pennsylvania, Philadelphia, PA (PennGen). Medical records and laboratory test results from both feline patients were reviewed.
Additionally, left-over EDTA anticoagulated blood samples from ill and healthy cats and dogs, submitted to the Clinical Pathology Laboratory at the Ryan Veterinary Hospital, University of Pennsylvania, were evaluated at PennGen. All samples were obtained and analyzed with approval of the University of Pennsylvania institutional animal care and use committee.
Blood Typing Techniques
Samples were typed at PennGen using 3 methods according to the manufacturers’ instructions and published methods with minor modifications briefly described below.
Immunochromatographic Typing Strips (“Strip”; Alvedia, Lab Test A+B and DEA 1, Limonest, France)4,17
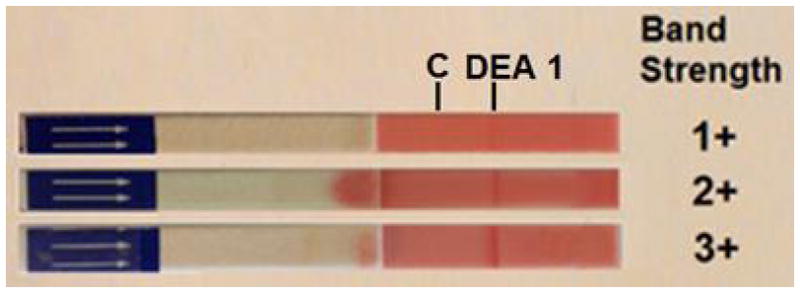
Briefly, 3 drops of diluent (Buffer, canine or feline) were placed into wells of a 96-well plate. Ten μl of washed RBC suspension (PCV 20%) was mixed with the diluent. An immunochromatographic strip (impregnated with monoclonal antibodies) was then dipped into the RBC suspension for approximately 2–3 min until the RBC had migrated to the other end of the strip. The results were interpreted as follows: a red band at the position marked C (control) must appear for result interpretation (note exception when performing heterotyping, because control reagent reacts species-specifically [see below]), and the presence of a visible red band at the position marked DEA 1 (for canine typing) or A and/or B (for feline typing) indicated the expression of the antigen on RBC membranes, respectively. The band intensity was then further measured as follows: 0, no visible band; 1+ very faint band; 2+ light band; 3+ bright red band; 4+ intense red band. Any visible bands of 1+ or stronger were considered as positive.17
Agglutination Typing Card (“Card”; DMS Laboratories Inc., RapidVet-H, Flemington, NJ).3,4
Briefly, 1 drop of diluent was placed into the 3 wells marked autocontrol, A, or B on a feline blood typing card and 3 wells marked autocontrol, positive control and DEA 1 on a canine blood typing card, respectively. The reagents were rubbed off the well surface into solution with a wooden stick. Then, 1 drop of EDTA anticoagulated blood was added to each well, and the card was carefully agitated. The results were read after 1 min as follows: 0, no agglutination; 1+, many small agglutinates; 2+, some larger agglutinates and many small ones; 3+, a few large agglutinates; and 4+, 1 large agglutinate. Visible agglutination reactions of 2+ or stronger were considered as positive.
Gel Column (“Gel”; BioRad ID-Cards, NaCl, Enzyme Test and Cold Agglutinins DiaMed GmbH, Cressier, Switzerland) 3,4,18
Ten μl of RBC pellets (washed 3 times with phosphate buffered saline [PBS], each time discarding the supernatant) were added to 1000 μl of low-ionic strength solution (LISS) to prepare a 1% RBC solution. A volume of 25 μl of one of the following reagents: anti-A, anti-Mik, or anti-DAL serum (PennGen), Triticum vulgaris lectin (anti-B; Sigma Co. LLC, St Louis, MO), anti-DEA 4 serum (Animal Blood Resources International, Dixon, CA) or recipient serum (for backtyping and crossmatching) were pipetted on top of the gel columns. Then 50 μl of the RBC solution was loaded on top of the gel. Gel columns were incubated for 15 min at 37°C and then centrifuged for 15 min at 85 g in the manufacturer’s incubator and centrifuge, respectively. The distribution of RBC in the gel column was graded as follows: 0, all RBC at the bottom of the gel; 1+ a few RBC agglutinates in the lower half of the gel, but the large majority of RBC at the bottom of the gel; 2+, RBC agglutinates throughout the gel, 3+, RBC throughout the gel as well as on top of the gel; and 4+, all RBC on top of the gel column. A grading of ≥2+ was considered a positive test result.
Crossmatch Tests
For major and minor crossmatch and backtyping assays, the gel column method was applied as described above for typing, but using plasma instead of typing reagent.18
Results
Case Reports
Cat 1
Following skin debridement and neutering, a young adult, male domestic shorthair cat, weighing 3.3 kg and testing negative for feline leukemia virus (FeLV) antigen and feline immunodeficiency virus (FIV) antibody, started oozing immediately from the surgical sites and developed marked anemia by the next day at the ASPCA Animal Hospital, New York, NY. A CBC revealed a severe non-regenerative normocytic and normochromic anemia and mild thrombocytopenia, without evidence of intravascular hemolysis. The PT and APTT were infinitely prolonged. An acute blood loss anemia due to severe coagulopathy, presumably due to ingestion of an anticoagulant rodenticide, was diagnosed.
The cat was treated with vitamin K1 (10 mg SC, SID) and wound compression. In-clinic blood typing tests indicated type B blood with the Card, while the Strip revealed type AB blood. The apparent discordant blood typing results and lack of readily accessible type-compatible feline packed RBC (pRBC) led to xenotransfusion with 64 ml of canine pRBC (DEA 1−) given in divided doses as 3 4-hour infusions, with the initial portion of the first transfusion given as a rapid bolus due to the cat’s critical condition.
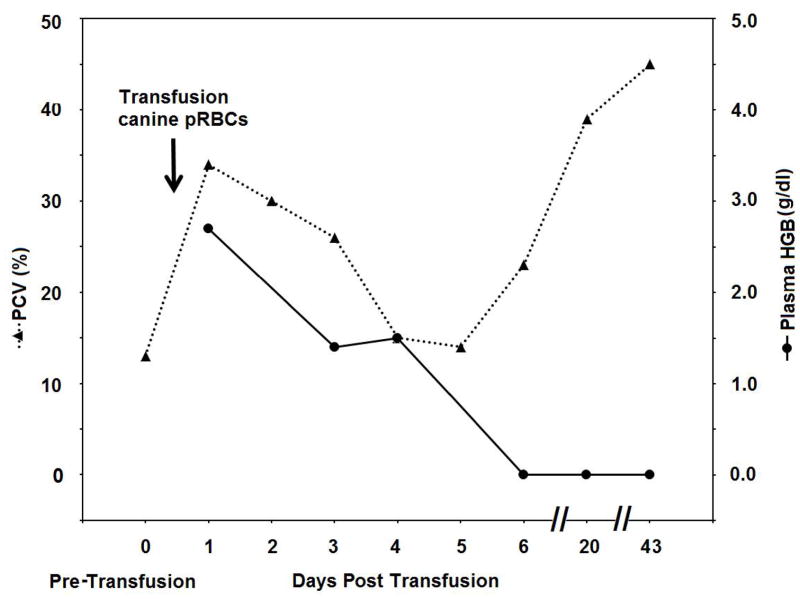
Within 1 hour of initiating transfusion, the cat started eating and grooming. The mucous membranes turned slightly pink after 4 hours, the oozing stopped, and no overt adverse reactions were observed any time after the transfusion. The next day (Day 1), the PCV was 21%, but the plasma appeared hemolyzed, with a plasma hemoglobin level of 2.7 g/dl (Figure 1A, B). Repeat PT and APTT test results on Day 1 were within the normal range. While the cat’s PCV declined close to pre-transfusion levels (14%) by Day 4, the cat did clinically well and did not show any further bleeding. The wounds healed well over the next 3 weeks. Furthermore, the PCV returned to the normal range and the coagulation tests remained normal (Figure 1A).
Figure 1.
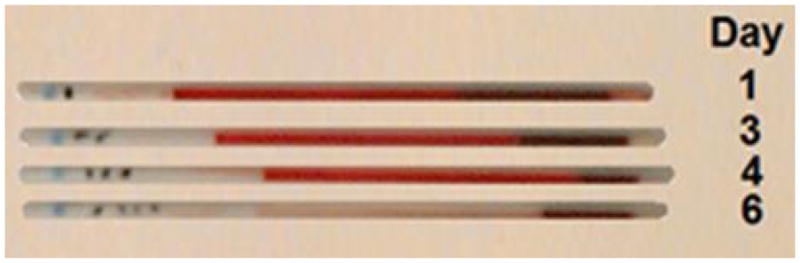
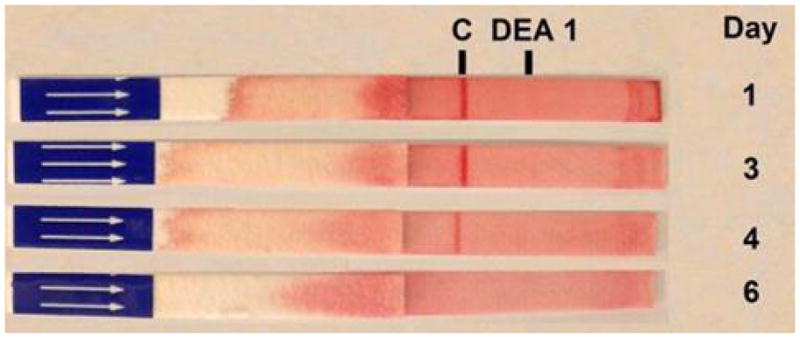
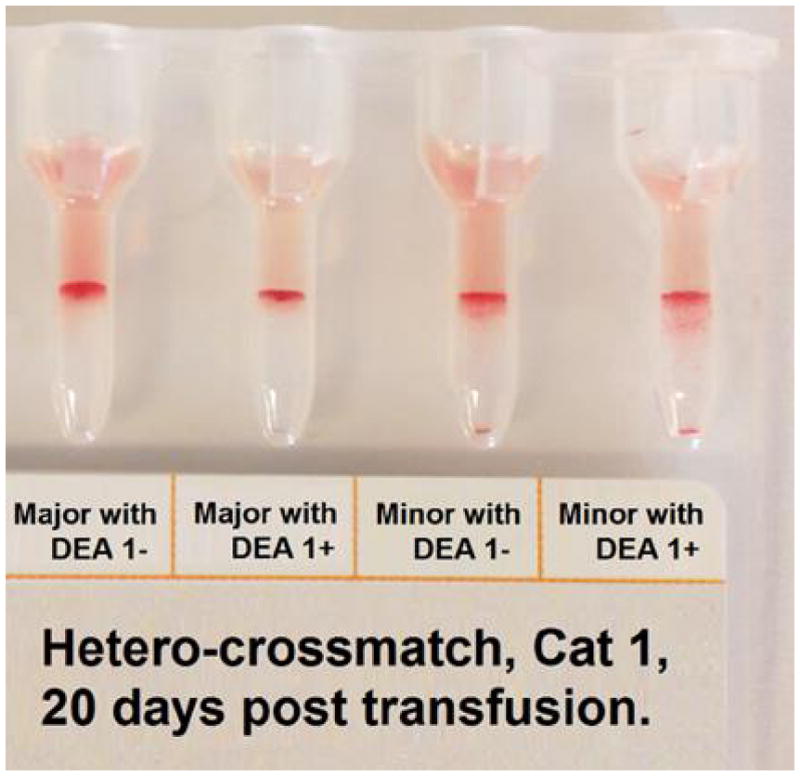
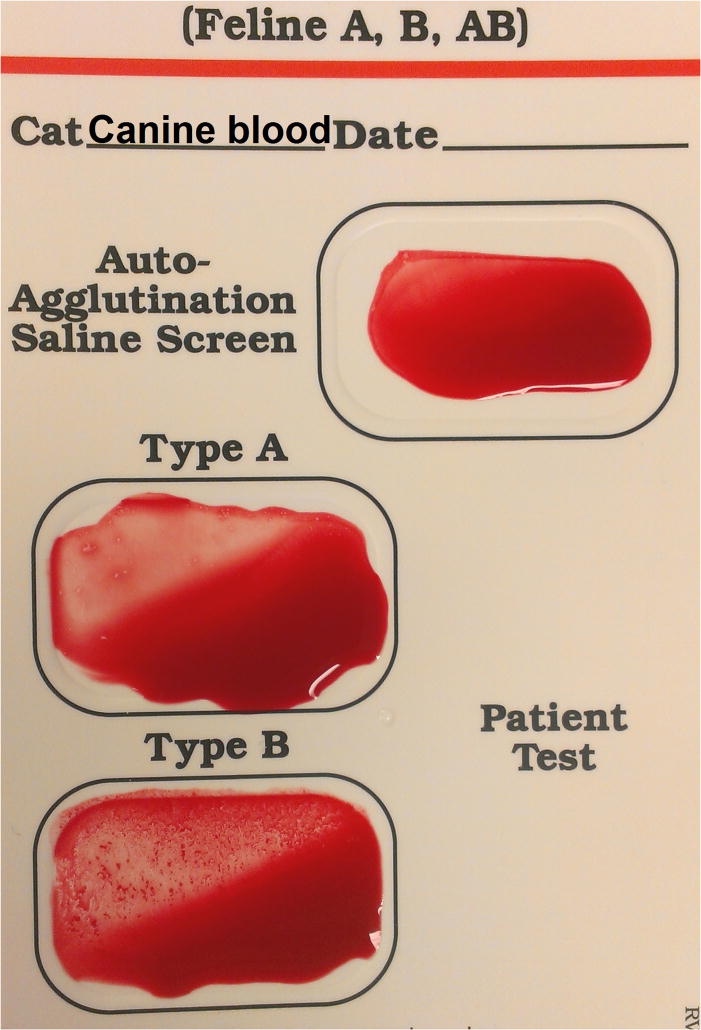
Illustrative results from Cat 1, which received a xenotransfusion with canine pRBC (A). PCV and plasma HGB values (On day 0 the plasma appeared non-hemolyzed). (B) Microcapillary tubes showing PCV decline and hemolyzed plasma. (C) DEA 1 Strip showing a fading positive control band. (D) Crossmatch with canine blood, showing incompatibility for the major and minor crossmatch with DEA 1+ and - blood
Cat 2
A 10 month old, FeLV- and FIV-, male domestic shorthair cat weighing 4.1 kg, with no history of prior transfusion, was presented with a severe non-regenerative normocytic-normochromic anemia (PCV of 6% [Figure 2A] and 0% reticulocytes) to the Animal Health Center, College of Veterinary Medicine, Mississippi State University, Starkville, MS. The in-clinic Card indicated type A blood. After transfusing ~1 ml of feline blood from a known type A donor, the recipient cat exhibited acute bradycardia, open mouth breathing, hypothermia, and collapse, and the transfusion was immediately stopped. Subsequent major crossmatch test results between patient plasma and the original donor blood used and 2 other feline blood donors, both also typed as type A, were strongly incompatible using a commercial gel tube crossmatch kit (Companion Animal Crossmatch Major, DMS Laboratories Inc., RapidVet-H, Flemington, NJ). Repeated blood typing, using the Card, confirmed that the recipient and all donors tested had type A blood.
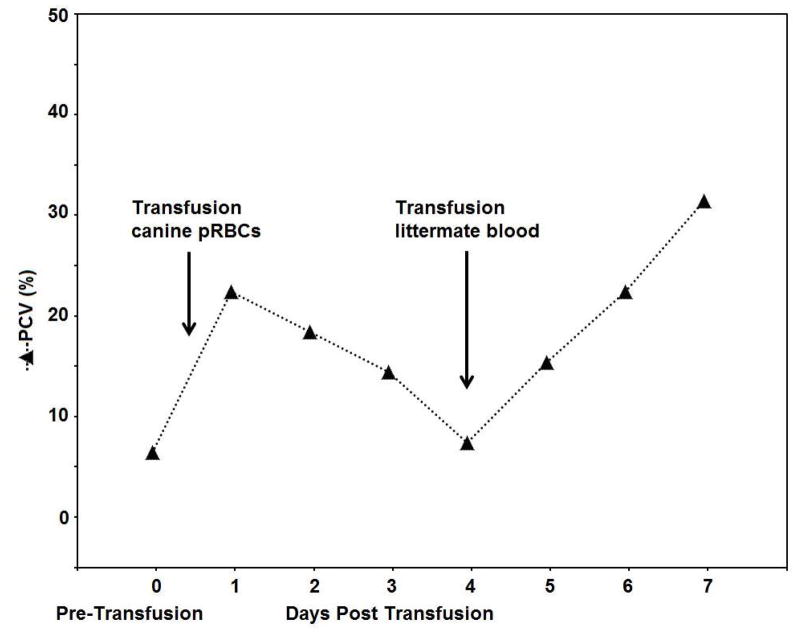
Figure 2.
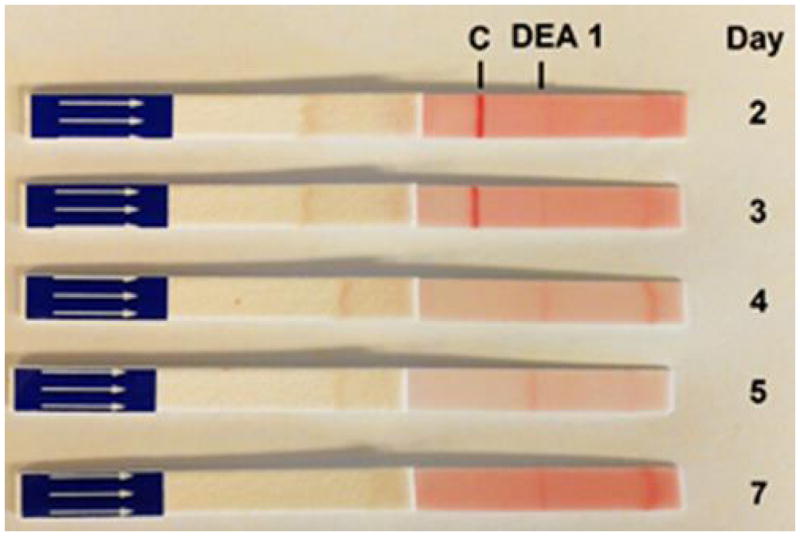
Illustrative results from Cat 2, which received a xenotransfusion with canine whole blood. (A) PCV values. (B) DEA 1 Strip showing a fading positive control band
The cat recovered uneventfully from the acute transfusion reaction with supportive care (intravenous fluids, flow-by oxygen and warming). The inexplicable acute transfusion reaction paired with initially incompatible crossmatch test results led to a xenotransfusion the following morning (12 hours after the transfusion reaction) with 60 ml of fresh whole blood over 4 hours from a canine donor (DEA 1−, DEA 4+, DEA 7+). The cat showed no overt clinical reaction. The PCV was 22% shortly after the transfusion, but the plasma appeared hemolyzed over the following days. Four days after the initial transfusion, the PCV fell to 7%, and the cat developed open mouth breathing, bradycardia and collapse, again necessitating emergency supportive care as described previously. A littermate was located, found to have type A blood and be compatible to the patient on major and minor crossmatch using the commercial gel tube crossmatch kit. Sixty ml of fresh whole blood from the FeLV− and FIV− littermate was transfused over 4 hours without any transfusion reaction. The recipient cat stabilized shortly after starting the transfusion with the littermate’s blood and the PCV rose to 15%. While a cause for the severe non-regenerative anemia could not be identified, the cat was treated twice daily with oral doxycycline (25 mg), prednisolone (7.5 mg) and cyclosporine (25 mg). The cat recovered uneventfully, with a PCV of 22% and 31% at discharge on Day 7 and recheck on Day 13, respectively, on a tapering course of prednisolone and cyclosporine (Figure 2A).
Follow up Blood Typing Studies of Transfused Cats at PennGen
Feline Typing
Pre- and post-transfusion blood samples from Cat 1 consistently typed as AB by Strip and Gel, but typed as B by Card. Furthermore, DNA testing revealed a heterozygosity for the B allele which is consistent with having the other allele for the AB type (Laboklin, Bad Kissing, Germany).19 Retyping Cat 2 and the initial incompatible feline donor, the 2 other feline donors and the littermate donor by applying different techniques consistently showed type A blood. All of these cats also typed as Mik+.
Canine Typing
When using the DEA 1 Strip with post-transfusion blood from both cats, no or only faint bands were detected at the DEA 1 site, which was expected as DEA 1− blood was transfused to both cats. However, a strongly positive control band was noted on Day 1 post-transfusion with samples from both cats, which completely disappeared by Day 3 to 4 (Figure 1C and 2B), respectively.
Feline Major Crossmatch and Alloantibody Studies
In major crossmatch tests, plasma samples from both anemic cats were compatible with RBC from 4 type A cats. Neither cat had any detectable anti-A or anti-B alloantibodies in their plasma by Gel.
Major Crossmatch with Other Canine Red Blood Cells
A pre-transfusion plasma sample from Cat 1 showed no agglutination reaction with canine RBC by Gel; no pre-transfusion sample was available from Cat 2. In contrast, strong agglutination reactions (3+/4+) were noted between plasma from both Cat 1 (Figure 1D) and Cat 2 and RBC from dogs at PennGen at every time point tested post-transfusion.
Feline and Canine Typing and Crossmatching Studies with Other Cats and Dogs
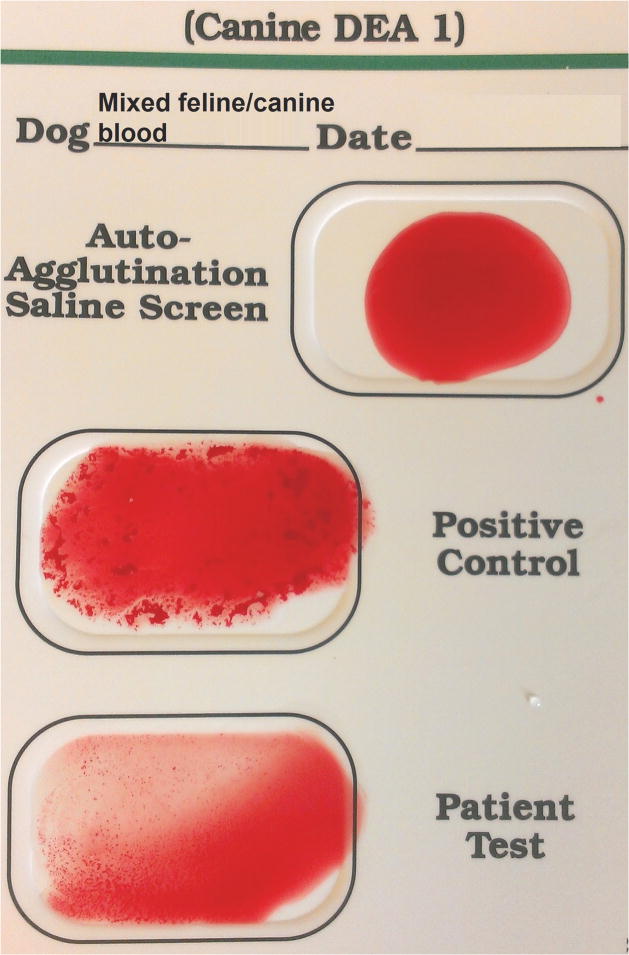
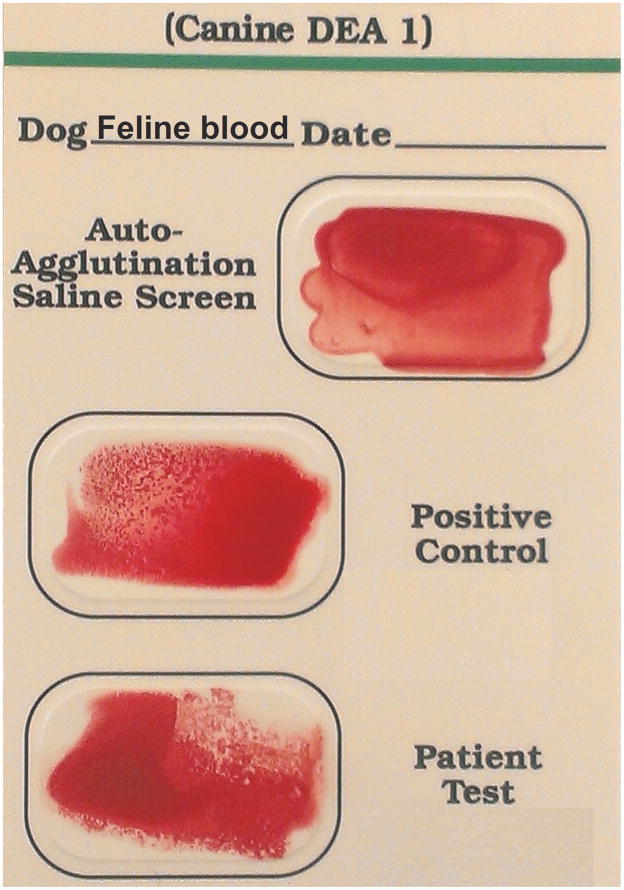
In order to better assess the reactions of feline blood typed by the DEA 1 Strip and Card, and crossmatch test results of feline blood with canine blood by Gel, we performed additional typing and crossmatch/mixing studies (Figure 4A, B). None of the 20 feline blood samples tested showed a positive control band on the DEA 1 Strip, but all of them showed a weak (1+) to moderate (3+) DEA 1 band (Table 1; Figure 3A). Moreover, testing feline blood samples using the DEA 1 Card, produced negative to moderate (3+) agglutination reactions in the positive control as well as in the DEA 1 well (Table 1; Figure 3B).
Figure 4.

Mixture of feline and canine blood tested with canine DEA 1 blood typing Strip (A) and Card (B). Strip and Card showed regular reactions with the positive control band and well, respectively.
Table 1.
Heterotyping of feline and canine blood with Strip and Card. Canine typing with AB Strip was negative, both for the AB Band and Control. All cats tested were blood type A
| Band Strength (0 to 4+) with DEA 1 Strip | ||
|---|---|---|
| # Cats | DEA 1 Band | Control Band |
| 3 | 1+ | 0 |
| 11 | 2+ | 0 |
| 6 | 3+ | 0 |
| Agglutination Strength (0 to 4+) with Card | ||
|---|---|---|
| # Cats | DEA 1 Well | Control Well |
| 7 | 1+ – 3+ | 0 – 1+ |
| 4 | 1+ – 4+ | 2+ |
| 12 | 1+ – 3+ | 3+ – 4+ |
Figure 3.
Heterotyping feline and canine blood with Strip (A), and Card (B). Canine typing with Card showed weakly positive (1+ / 2+) agglutination in the B well but no reaction in the A or Control well (C)
Crossmatching 20 feline control samples (16 type A and 4 type B cats) individually with 5 DEA 1+ and 5 DEA 1− canine blood samples showed both positive and negative results (Table 2, Figure 5). However the major crossmatch of each individual cat was either consistently compatible or consistently incompatible with all canine donor RBC. Further crossmatching of Cat 1 with 2 DEA 1+ and DEA 1− dogs on Day 20 and 43 post-transfusion revealed strongly positive major and minor crossmatch results. The minor crossmatch was incompatible with most feline plasma and canine RBC combinations. Finally, typing of 10 canine blood samples with the feline AB Strip showed no reaction at the A, B, or control band sites. Typing these canine samples with the feline AB Card showed no reaction in the A well but consistently mild agglutination reactions in the B well (Figure 3C).
Table 2.
Hetero-crossmatch by Gel of 20 cats with 5 different DEA 1− and 5 different DEA 1+ dogs (see also Figure 5). Minor hetero-crossmatch tests by Gel showed strongly incompatible results independent of the canine DEA 1 blood type and few crossmatch compatible reactions. (data not shown)
| Major Crossmatch with | |||
|---|---|---|---|
| Cats | DEA1− (n=5) | DEA1+ (n=5) | |
| Type | n | ||
| A | 5 | 0 – 1+ | 0 – 1+ |
| 1 | 1+ – 3+ | 1+ – 3+ | |
| 2 | 0 – 2+ | 0 – 2+ | |
| 1 | 2+ – 4+ | 0 – 4+ | |
| 7 | 2+ – 4+ | 2+ – 4+ | |
|
| |||
| B | 2 | 2+ – 3+ | 1+ – 4+ |
| 1 | 0 – 2+ | 2+ – 3+ | |
| 1 | 0 | 0 | |
Figure 5.
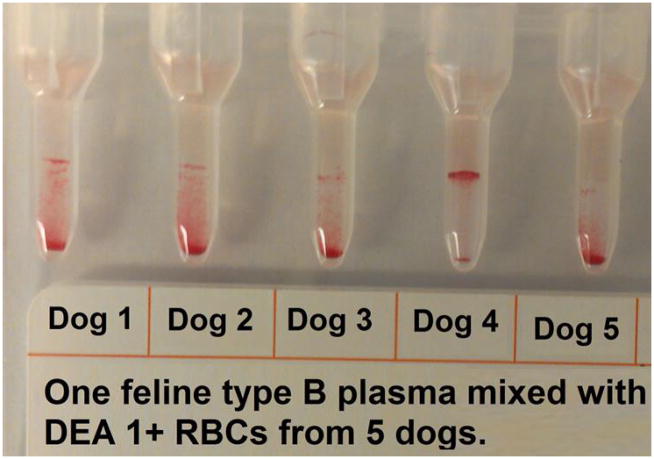
Hetero-crossmatch of feline plasma and canine RBC by Gel. Plasma from 1 feline type B cat mixed with DEA 1+ RBC from 5 dogs showing negative and positive agglutination reactions.
Discussion
This report describes two anemic cats that received canine RBC as a xenotransfusion on an emergency basis because of issues with feline blood typing and compatibility. The cats eventually recovered clinically despite acute hemolytic transfusion reactions following transfusion with canine blood. Monitoring the control band on DEA 1 typing Strips allowed tracking of transfused canine RBC and revealed that these cells were very short-lived. Testing hetero-crossmatches between feline patients and canine donors revealed both compatible and incompatible major crossmatch results and frequent agglutination reactions in minor crossmatch studies independent of AB and DEA 1 blood type, respectively. As our results indicate that canine DEA 1− RBC are rapidly cleared following transfusion into cats and our hetero-crossmatch studies indicate that compatibility of canine blood cannot be assured in feline patients, xenotransfusions should be avoided except when compatible feline blood and ultra-purified polymerized bovine hemoglobin cannot be located in time. Based upon our hetero-crossmatch studies, results seem to be mostly incompatible and do not assure compatibility of canine blood in feline patients.
While several feline AB typing kits are available for clinical practice, typing of AB and B cats can still pose challenges. Erroneous and discordant blood typing results have been previously reported in cats, and can occur as a result of human error in performing the test and interpreting results, as well as damage to and limitations of test kits used.3,4,20 Detection of the rare type AB is a particular concern as the Card does not recognize all type AB cats and may wrongly type them as type B as noted in Cat 1 of this report (the type A blood of Cat 2 was correctly typed). Furthermore, some FeLV+ anemic cats have reportedly been typed as AB when actually having type A blood.4 Both cats of this report were FeLV−, but other yet to be identified diseases may affect RBC antigen expression. We have previously recommended having type AB results confirmed in a reference laboratory, as was done with Cat 1 in this report. We have also recommended that type B results be confirmed by backtyping, because every type B cat older than 12 weeks has strong anti-A alloantibodies (kittens may also have anti-alloantibodies after colostrum ingestion).21 Neither adult Cat 1 nor 2 in this report had any detectable anti-A or anti-B antibodies.
While AB blood typing of both recipient and donor cats is generally recommended and performed prior to transfusion in clinical settings, crossmatching has been much less likely performed before a first transfusion in cats, although a recent study indicated that ensuring that both typing and crossmatching results are compatible prior to a first transfusion in cats may serve to maximize post-transfusion hematocrits.22 Clearly, if AB blood typing is not available, a crossmatch test as simple as mixing a drop of plasma with whole blood should be used, as the strong anti-A alloantibodies in type B cats readily detects any A–B incompatibility.2 However, the detection of other blood type incompatibilities requires RBC washing (a more cumbersome procedure), technical skills at performing the test, and expertise in interpreting results. An in-practice commercial gel tube crossmatch kit was used for Cat 2, but its reliability has thus far only been evaluated for dogs.5, 23
Beyond the AB blood type, there is little information about other feline blood groups and RBC antigens. The Mik antigen is quite common, as very few Mik− cats have been found, but Mik− cats may have naturally occurring alloantibodies. However, typing for Mik is restricted, because of a lack of typing reagents.1 Incompatible major crossmatches are readily observed following prior and repeat transfusions, supporting the presence of additional unknown RBC antigens.24 Cat 2 had not been transfused previously, but was initially found to have an apparent incompatible major crossmatch result using a commercial gel tube crossmatch kit. Follow-up assessments in the reference laboratory showed that both cats of this report were Mik+, and neither showed any agglutination reactions in crossmatch tests against initial and other donor cats. The discrepancy between the test results at the original hospital and the PennGen laboratory remain unexplained, but may include differences in testing protocols and the potential for non-specific autoagglutination falsely interpreted as incompatibility. The latter, however, is unlikely, since the autoagglutination saline control well on the patient’s original pre-transfusion AB typing Card was negative.
When blood incompatibilities are detected against unknown and high frequency RBC antigens, which can even happen when using the same donor due to prior sensitization, compatible donors can still be found among relatives. Indeed, a littermate of Cat 2 in this report was located and found to be compatible with Cat 2 on both major and minor crossmatch test. After it was recognized that the beneficial effects of the transfused canine RBC had been very transient, a unit from this littermate cat was transfused on an emergency basis and rapidly stabilized this critically ill and anemic patient.
Adverse transfusion reactions can be divided into immunologic and non-immunologic reactions, and both have been reported in cats.25 Reactions may occur with as little as 1 ml of blood 26, as observed with Cat 2 of this report. The AB and other blood group incompatibilities are the most feared immunologic reactions, and can typically be identified by typing and crossmatching. Other immunologic reactions can be due to reactions to white blood cell antigens as well as various plasma components. Other hemolytic but non-immunologic reactions may be caused by bacterial contamination of units during collection, processing and storing of blood products.27,28 Non-immunologic reactions also include citrate toxicity, volume overload, and infectious disease transmission from the donor.5,29,30
Frequently, the precise cause of a transfusion reaction remains unknown, as in the case of Cat 2 of this report. When receiving blood from the original feline donor, Cat 2 exhibited an immediate severe reaction, which was very comparable to the reaction observed in cats following transfusion of incompatible blood (for example, transfusion of type A blood to a type B cat)31, but the reactions could also have been caused by other immunologic reactions. Blood type incompatibility was initially suspected, but follow-up crossmatch studies could not confirm this, as no alloantibodies could be detected. There was no evidence of hemolysis in Cat 2 after transfusion with feline blood, but as only 1 ml of blood was transfused, the amount of released hemoglobin might not have been detectable. A complete follow-up investigation was not performed to determine the cause, but whenever severe acute reactions consistent with blood group incompatibility are observed, transfusion should be immediately discontinued and fluids, antihistamines and glucocorticosteroids must be administered as supportive care.
Xenotransfusions with canine blood have been previously used in anemic cats, when feline blood was not available10–12,15 or not compatible, as illustrated in the case reports described here. Cat 1 had discordant typing results and therefore received canine pRBC. However, this AB cat could have been safely transfused with type A pRBC or even whole blood if AB pRBC were not available, because type A cats rarely have strong alloantibodies 2,30,31 Moreover, a major crossmatch with a type A donor could detect any anti-A alloantibodies. Cat 2 experienced an acute transfusion reaction and initial crossmatches were incompatible, although subsequent testing could not confirm this. Cat 2 could potentially have been safely transfused with type A blood from another donor cat and not just the littermate.
As observed in other rare case reports and series 8–11,15, both cats in this report initially recovered uneventfully following canine xenotransfusion. However, as in other studies, both cats also experienced hemolysis and rapid clearance of canine RBC within 4 days.33 A rapid return of the PCV to a pre-transfusion level and evidence of intravascular hemolysis are indicative of an acute hemolytic transfusion reaction. In support, our ability to use the control band on the DEA 1 Strip to follow the canine RBC in the transfused cats revealed that transfused cells disappeared by Day 4. The control band on the DEA 1 strip binds to a glycophorin present on canine but not feline RBC. This observation may be helpful for other cases in order to differentiate between lysis of patient’s RBC caused by an underlying disease or trigger, and lysis of canine RBC following xenotransfusion. Worth noting are the different sequels to intravascular hemolytic transfusion reactions between animals and people. In cats and dogs, intravascular hemolysis neither causes acute kidney injury nor major vascular compromise in A–B and other mismatched transfusions or xenotransfusions.17,34–38 Alternatively, when compatible feline blood is not available for an anemic cat, a highly purified polymerized bovine hemoglobin-based oxygen carrier (eg, Oxyglobin, Dechra Pharmaceuticals PLC, Headquarters, Northwich, UK) may be considered as an alternative to canine xenotransfusion, since it can improve the clinical signs of anemia for at least 24 hours and is not associated with exposure of the patient to harmful membranes and other components from lyzed canine erythrocytes.16
The rapid disappearance of canine RBC following xenotransfusion into cats suggests that naturally occurring alloantibodies against canine RBC are present in cats. In order to investigate this further, we utilized currently available typing and crossmatch tests to detect antigens and alloantibodies. Feline blood samples typed with the DEA 1 Strip revealed a usually faint and rarely stronger band at the DEA 1 location, but never a positive control band. In the 2 anemic cats in this case report, the band at DEA 1 was initially not appreciated, likely due to the relative absence of feline RBC. However, after recovery, when feline RBC had been replenished, both showed a slight DEA 1 band similar to other cats. As the DEA 1 protein recognized by anti-DEA 1 in dogs is not known, it is unclear if cats have this antigen or if the antibody cross-reacts with another protein.
In contrast, canine blood does not react with the A, B and control agent on the AB Strip, but always shows positive Card agglutination reactions in the type B well and no reaction in the type A well. The monoclonal antibodies used in Strip and card test are different. Mixing canine and feline blood produced the expected patterns: feline blood mixed with DEA 1− canine blood, showed a moderate DEA 1 band and a positive control, or a positive control and weak agglutination in the patient well with the Card.
Earlier experimental studies with transfusion of canine blood into feline patients suggested initial compatible major crossmatches with incompatible crossmatch agglutination reactions after ≥8 days.8,9,13, In the crossmatches reported here, however, we found that some cats had compatible major crossmatch results to nearly all dogs, while other cats exhibited incompatible results to nearly all dogs. These patterns seemed independent of the cats’ AB blood type and dogs’ DEA 1 type. Furthermore, most canine plasmas caused agglutination reactions with feline RBC. These studies indicate the presence of alloantibodies in some feline and nearly all canine plasma samples against the other species’ RBC. The observed in vivo acute hemolytic reactions indicate the presence of alloantibodies against canine RBC in at least some cats, even if crossmatch results suggest compatibility. These alloantibodies may be hemolysins (rather than agglutinating antibodies), which were not specifically tested for in this and other studies.
Beside the RBC-related incompatibilities, considerations may also be given to hetero-compatibility of plasma transfusions. Cat 1 of this report had a severe coagulopathy, presumably due to an anticoagulant rodenticide poisoning, and could also have benefited from plasma with coagulation factors, but only received canine pRBC and vitamin K1. The potential benefits of xenotransfusion of cats with canine plasma is unknown, albeit early xenotransfusion studies used canine whole blood, and even pRBC contain some plasma (~10%). In fact, hemophilic human patients who develop inhibitors (antibodies) against human factor VIII can be sometimes successfully treated with heterologous porcine coagulation factors.39,40 It is therefore conceivable that canine coagulation factors could be active in vivo and interact with feline coagulation factors to enhance fibrin formation and hemostasis.
Finally, while there seem to be hemolytic incompatibility reactions seen in every transfused cat receiving canine blood, the acute fatal reactions observed following repeat xenotransfusions in cats may not be explained solely by alloantibodies, but may also be related to plasma components, and may represent serum sickness.41
In conclusion, these 2 cases and our blood typing and compatibility study reiterate that xenotransfusions should only be considered in dire emergency situations, since canine blood can cause incompatibility reactions in cats.
Acknowledgments
This study was supported in part by NIH OD 010939 and a fellowship to C. C. Euler by Laboklin. The authors thank Alvedia and DMS laboratories for providing typing kits, and Jackie King for her assistance with the management of Cat 2.
Footnotes
Disclosure
The authors at the University of Pennsylvania are affiliated with the PennGen laboratory, which offers some of the blood typing and crossmatch studies.
References
- 1.Weinstein NM, Blais MC, Harris K, Oakley DA, Giger U. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen. J Vet Intern Med. 2007;21:287–92. doi: 10.1892/0891-6640(2007)21[287:anrbgi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griot-Wenk ME, Giger U. Feline transfusion medicine. Blood types and their clinical importance. Vet Clin North Am Small Anim Pract. 1995;25:1305–22. doi: 10.1016/s0195-5616(95)50156-1. [DOI] [PubMed] [Google Scholar]
- 3.Stieger K, Palos H, Giger U. Comparison of various blood-typing methods for the feline AB blood group system. Am J Vet Res. 2005;66:1393–9. doi: 10.2460/ajvr.2005.66.1393. [DOI] [PubMed] [Google Scholar]
- 4.Seth M, Jackson KV, Giger U. Comparison of five blood-typing methods for the feline AB blood group system. AJVR. 2011;72:203–209. doi: 10.2460/ajvr.72.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidow B. Transfusion medicine in small animals. Vet Clin Small Anim. 2013;43:735–756. doi: 10.1016/j.cvsm.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Giger U. Blood typing and crossmatching to ensure blood compatibility. In: Bonagura JD, Twedt DC, editors. Kirk’s Current Veterinary Therapy XV ed. Web Chapter 26. WB Saunders; 2014. p. e143. [Google Scholar]
- 7.Giger U. Transfusion therapy. In: Silverstein DC, Hopper K, editors. Small Animal Critical Care Medicine. 2. WB Saunders; 2015. pp. 327–332. [Google Scholar]
- 8.Lautie R, Coulon J, Geral MF, Cazieux A, Griess F. L’hétéro-transfusion sanguine chez le chat, Etude immunologique-Etude clinique. [Blood transfusion from dogs to cats. Immunological and clinical study] Rev Med Vet. 1969;120:311–323. In French. [Google Scholar]
- 9.René JGF. École Nationale Vétérinaire de Toulouse: Thèse pour le Doctorat Vétérinaire, Diplôme d’État. Vol. 50. Toulouse, F. Édouard Privat impr; 1968. L’hétéro-transfusion sanguine chez le chat [Hetero-transfusion in the cat] In French. [Google Scholar]
- 10.Gowan R. Canine blood transfusion in a cat with erythroid leukemia. Proceedings of the Australian College of Veterinary Scientists Week; Surfers’s Paradise, QLD, Australia. 2004; pp. 29–30. [Google Scholar]
- 11.Burke N. C&T Control and Therapy Series, Centre for Veterinary Education. Vol. 266. The University of Sydney; 2012. Flea-related anaemic crisis in a young kitten; pp. 24–25. [Google Scholar]
- 12.Bovens C, Gruffydd-Jones T. Xenotransfusion with canine blood in the feline species: review of the literature. J Feline Med Surg. 2012;15:62–67. doi: 10.1177/1098612X12460530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessler J, Davis LE, Dale HE. Effect of repeated transfusions of dog blood to cats. Small Anim Clin. 1962;2:684–687. [Google Scholar]
- 14.Malik R, et al. The prevalence of feline A/B blood types in the Sydney region. Aust Vet J. 2005;83:38–44. doi: 10.1111/j.1751-0813.2005.tb12190.x. [DOI] [PubMed] [Google Scholar]
- 15.Weingram T. Xenotransfusion of canine Bblood to a cat. Israel J of Vet Medicine. 2014;69:50–52. [Google Scholar]
- 16.Gibson GR, Callan MB, Hoffman V, Giger U. Use of a hemoglobin-based oxygencarrying solution in cats: 72 cases (1998–2000) J Am Vet Med Assoc. 2002;221:96–102. doi: 10.2460/javma.2002.221.96. [DOI] [PubMed] [Google Scholar]
- 17.Acierno MM, Raj K, Giger U. DEA 1 expression on canine erythrocytes analyzed by immunochromatographic and flow cytometric techniques. J Vet Intern Med. 2014;28:592–598. doi: 10.1111/jvim.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler RJ, Reese J, Chang D, Seth M, Hale AS, Giger U. Dog erythrocyte antigens 1.1, 1.2, 3, 4, 7 and Dal blood typing and cross-matching by gel column technique. Vet Clin Pathol. 2010;39:306–16. doi: 10.1111/j.1939-165X.2010.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bighignoli B, et al. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genetics. 2007;8:27. doi: 10.1186/1471-2156-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hourani L, Weingart C, Kohn B. Evaluation of a novel feline AB blood typing device. J Feline Med Surg. 2014;16:826–831. doi: 10.1177/1098612X14522052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bücheler J, Giger U. Alloantibodies against A and B blood types in cats. Veterinary Immunology and Immunopathology. 1993;38:283–295. doi: 10.1016/0165-2427(93)90088-l. [DOI] [PubMed] [Google Scholar]
- 22.Weltman J, Rogers C, Fletcher D. Influence of cross-match on posttransfusion packed cell volume in feline packed red blood cell transfusion. J Vet Emerg Crit Care. 2014;24:429–436. doi: 10.1111/vec.12204. [DOI] [PubMed] [Google Scholar]
- 23.Villarnovo D, Burton S, Horney B, MacKenzie A, Vanderstichel R. Evaluation of commercial gel column agglutination cross-match kit in dogs. Vet Clin Path. doi: 10.1111/vcp.12374. in progress for submission. [DOI] [PubMed]
- 24.Weingart C, Giger U, Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation. J Feline Med Surg. 2004;6:139–148. doi: 10.1016/j.jfms.2004.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael M. Prevention and treatment of transfusion reactions. In: Silverstein DC, Hopper K, editors. Small Animal Critical Care Medicine. 2. WB Saunders; 2015. pp. 333–341. [Google Scholar]
- 26.Auer L, Bell K, Coates S. Blood transfusion reactions in the cat. J Am Vet Med Assoc. 1982;180:729–30. [PubMed] [Google Scholar]
- 27.Hohenhaus AS, Drusin LM, Garvey MS. Serratia marcescens contamination of feline whole blood in a hospital blood bank. J Am Vet Med Assoc. 1997;210:794–798. [PubMed] [Google Scholar]
- 28.Kessler R, et al. Pseudomonas fluorescens contamination of a feline packed red blood cell unit and studies of canine units. Vet Clin Pathol. 2010;39:29–38. doi: 10.1111/j.1939-165X.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klaser DA, Reine NJ, Hohenhaus AE. Red blood cell transfusion in cats: 126 cases (1999) JAVMA. 2005;226:920–923. doi: 10.2460/javma.2005.226.920. [DOI] [PubMed] [Google Scholar]
- 30.Patterson J, Rousseau A, Kessler RJ, Giger U. In vitro lysis and acute transfusion reactions with hemolysis caused by inappropriate storage of canine red blood cell products. J Vet Intern Med. 2011;25:927–933. doi: 10.1111/j.1939-1676.2011.0737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giger U, Bücheler J. Transfusion of type-A and type-B blood to cats. J Am Vet Med Assoc. 1991;198:411–87. [PubMed] [Google Scholar]
- 32.Niggemeier A, Haberstroh AF, Nelson VE, Giger U. An Accidental Transfusion of Type A kitten with type B blood causes a transient switch from blood type A to B. J Vet Intern Med. 2000;14:214–216. [PubMed] [Google Scholar]
- 33.Clark CH, Kiesel GK. Longevity of red blood cells in interspecies transfusion. J Am Vet Med Assoc. 1963;143:400–401. [PubMed] [Google Scholar]
- 34.Klein HG, Anstee DJ. Haemolytic transfusion reactions: Intravascular and extravascular destruction. In: Klein HG, editor. Mollison’s Blood Transfusion in Clinical Medicine. 12. New York, NY: John Wiley and Sons; 2014. pp. 458–459. [Google Scholar]
- 35.Yuile CL, Van Zandt TF, Ervin DM, Young LE. Hemolytic reactions produced In dogs by transfusion of incompatible dog blood and plasma: II. Renal aspects following whole blood transfusions. Blood. 1949;4:1232–1239. [PubMed] [Google Scholar]
- 36.Christian RM, Stewart WB, Yuile CL, Ervin DM, Young LE. Limitation of hemolysis in experimental transfusion reactions related to depletion of complement and isoantibody in the recipient: Observations on dogs given successive transfusions of incompatible red cells tagged with radioactive iron. Blood. 1951;6:142–150. [PubMed] [Google Scholar]
- 37.Andersen MN, Mouritzen CV, Gabrielli ER. Mechanism of plasma hemoglobin clearance after acute hemolysis in dogs: Serum haptoglobin levels and selective deposition in liver and kidney. Ann Surg. 1966;164:905–912. doi: 10.1097/00000658-196611000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd JV, Street AM, Berry E, et al. Cross-reactivity to porcine factor VIII of factor VIII inhibitors in patients with haemophilia in Australia and New Zealand. Aust N Z J Med. 1997;27:658–64. doi: 10.1111/j.1445-5994.1997.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 40.Giangrande PLF. Porcine factor VIII (Commemorative Article) Haemophilia. 2012;18:305–309. doi: 10.1111/j.1365-2516.2012.02803.x. [DOI] [PubMed] [Google Scholar]
- 41.Tizard IR. Generalized type III hypersensitivity reactions: Serum sickness. In: Tizard IR, editor. Veterinary Immunology. 9. St. Louis, MO: WB Saunders; 2013. pp. 359–360. [Google Scholar]