Abstract
The development of inhibitors, neutralizing alloantibodies to Factor VIII (FVIII) or Factor IX (FIX), is the most significant complication of protein replacement therapy for patients with hemophilia and is associated with increased mortality as well as substantial physical, psychosocial, and financial morbidity. Current management, including bypassing agents to treat and prevent bleeding and immune tolerance induction (ITI) to attempt inhibitor eradication, is suboptimal for many patients. Fortunately, there are several emerging gene therapy approaches that aim to address these unmet clinical needs of patients with hemophilia and inhibitors. Herein, we review the mounting evidence from preclinical hemophilia models that the continuous uninterrupted expression of FVIII or FIX delivered as gene therapy can bias the immune system towards tolerance induction and even promote the eradication of pre-existing inhibitors. We also discuss several gene transfer approaches that directly target immune cells in order to promote immune tolerance. These preclinical findings also shed light on the immunological mechanisms that underlie tolerance induction.
Keywords: Hemophilia, Gene Therapy, Factor VIII, Factor IX, Immune Tolerance, Isoantibodies
1. Introduction
Neutralizing alloantibodies against FVIII or FIX, termed inhibitors, are the most significant complication of factor protein replacement therapy for hemophilia A (HA) or hemophilia B (HB) [1–3]. Though inhibitors do not increase the frequency of bleeding, they do decrease the efficacy of replacement factor and at high-titers, render replacement therapy completely ineffective. As such, the development of inhibitors significantly increases the mortality [4–6] and physical morbidity, including higher rates of hemarthropathy and catastrophic bleeds, as well as delaying physical maturation [7–9]. Inhibitor formation also results in substantial financial and psychosocial morbidity, with inhibitors being associated with a 5-fold increase in healthcare costs as well as significantly increasing the burden on families [10, 11].
Inhibitors develop in about 20–30% of patients with severe HA, in about 10% in patients with non-severe HA, and 1–5% of patients with HB, mostly those with severe disease [3, 12]. In HB, more than half of patients who develop inhibitors will also manifest an allergic reaction towards the FIX protein, including anaphylaxis. Both genetic and environmental factors influence the risk of inhibitor formation [13–15]. Genetic factors include the underlying hemophilia causing mutation as well as the genetic background of the patient; environmental factors include the manufacturing process of the product, timing of first factor exposure, factor dosage, and clinical situations that result in immunological “danger signals.” The risk of inhibitor development for patients with severe HA is highest during their initial FVIII exposure [15], while the risk is relatively constant for patients with non-severe HA [12]. Patients with hemophilia-causing mutations resulting in plasma cross-reactive material (CRM), such as missense or small in-frame deletions or insertions, are less likely to develop inhibitors, probably because CRM is present during the negative selection of autoreactive T-cells, which results in central tolerance. Recently, intracellular CRM was similarly identified as a likely contributor to immune tolerance in HA patients with the intron-22 inversion mutation [16]. In preclinical models, CRM-negative animals are similarly more likely to develop inhibitors than models that are CRM-positive [17–19]. Currently, there are no reliable predictors to anticipate the development of inhibitors nor is there therapy that decreases the risk of inhibitor development for a specific patient with severe hemophilia, though prophylactic factor is likely associated with a lower risk than on-demand therapy [20].
In this review, we first discuss current regimens to induce immune tolerance in patients with hemophilia and inhibitors, which are often suboptimal. Encouragingly, there are several emerging gene therapy approaches currently being studied that aim to address these unmet clinical needs. The recent demonstrations of sustained disease ameliorating FIX levels in ongoing AAV-based liver-directed gene therapy clinical trials for HB [21–23], highlight the possibility of future widespread adoption of this therapy for HB and potentially for HA [24]. Herein, we review the mounting evidence from preclinical hemophilia models that the continuous uninterrupted expression of FVIII or FIX by gene therapy can bias the immune system towards immune tolerance induction and potentially promote the eradication of pre-existing inhibitors. We also address additional gene therapy strategies to directly manipulate the immune system to achieve tolerance. These preclinical studies have helped clarify the immunological mechanisms that underlie tolerance induction in hemophilia. Moreover, these promising developments in hemophilia research reflect an emerging scientific understanding that gene therapy for inherited protein deficiencies can be specifically designed to promote immune tolerance [25].
2. Current ITI Regimens and Immunological Mechanisms
The medical management of patients with hemophilia and inhibitors is challenging (Figure 1). The two major treatment goals for these patients are 1) to control and prevent bleeding and 2) to eradicate the alloantibodies and induce immune tolerance. To date, the only effective therapy for inhibitor eradication is the ITI regimen [3, 26–28]. ITI is the regular exposure of the deficient factor to induce peripheral immune tolerance (Figure 2). Though the optimal ITI regimen remains an open scientific question, salient variables include the type of factor product, factor dose, administration frequency, timing of ITI initiation, and the role of adjuvant immunomodulation [2]. On the basis of retrospective registry data [29], “good-risk” HA patients can be stratified from “poor-risk” patients based on 4 factors: 1) historical peak titer <200 BU; 2) inhibitor titer at initiation of ITI <10 BU; 3) less than 2 years between inhibitor identification and initiation of ITI; and 4) age <8 years [27]. Lower peak titer on ITI is also a significant prognostic predictor of success [29]. Combined, these empiric observations suggest that the general strength of the immune response against the exogenous factor is a critical prognostic parameter for ITI success.
Figure 1. Streamlined schematic of current and potential gene therapy based management of patients with inhibitors.
Gene transfer of FVIII or FIX offers the potential of simplifying current management with a single therapeutic administration to provide both ITI and factor prophylaxis.
Figure 2. Gene therapy potentially offers several advantages over current protein ITI.
A single administration (arrow) of a FVIII or FIX gene therapeutic (top panel) provides a steady exposure of antigen (blue) and increasing factor activity (red) with decreasing inhibitor titer (black). Once the inhibitor is eradicated, gene therapy provides ongoing factor prophylaxis. In contrast, protein ITI (bottom panel) requires myriad infusions during inhibitor eradication as well as ongoing administrations for maintenance of tolerance.
Recently, a high-dose (200 U/kg/day) and a low-dose (50 U/kg/3-times per week) ITI regimen were compared in a randomized controlled prospective trial (I-ITI) for “good-risk” HA patients [27]. The I-ITI study concluded that though the overall success of inhibitor eradication at the end of the 3 year study was similar in both cohorts, the high-dose cohort achieved a negative inhibitor titer twice as fast as the low-dose cohort (4.6 months vs 9.2 months, p = .017), which resulted in significantly less total bleeds in the high-dose cohort. However, it is not known whether these advantageous kinetics of inhibitor eradication observed in the high-dose cohort are because of the higher dose and/or the more frequent administration.
In general, ITI is only successful for about 60% of patients with HA and about 30% of HB [3, 27], though ITI in the latter patients is often complicated by non-hematologic problems such as nephrotic syndrome [3]. Furthermore, “poor-risk” HA patients are much less likely to be tolerized [30], and better therapeutic options are needed for these patients. Disparate rates of inhibitor recurrence after ITI have also been reported ranging from 5–35% [2] with the prospective I-ITI study reporting a relapse rate of 13% at 1 year [27]. The importance of continued prophylactic factor replacement is not clear [31], but is widely recommended. The immunological mechanisms that allow the exogenous exposure of factor to successfully induce immune tolerance in some patients is also only incompletely understood [32–36].
The antibody response against exogenous factor consists of both polyclonal neutralizing (i.e. inhibitors) and non-neutralizing antibodies, with the former associated with high affinity IgG4 alloantibodies [37–39]. FVIII inhibitors are primarily directed against either the A2 or C2 domain [40, 41]. Their development and persistence requires the effective cooperation between Factor-specific CD4+ T-cells and Factor-specific memory B-cells, in order for the memory B-cells to differentiate into inhibitor-producing plasma cells, as diagrammed in Figure 3. Experimental disruption of the immunological signaling pathways between these T-cells and B-cells reduce or prevent inhibitor titers in HA mice models [32] and in one clinical pilot study [42]. Furthermore, in HA patients with HIV, the inhibitor-titer is directly correlated with the CD4+ T-cell count [43] and patients with HIV have a lower incidence of inhibitor development [44]. Polymorphisms in cell-signaling molecules, as well as inflammatory and anti-inflammatory cytokines, have all been associated with inhibitor development, though associations between cytokine expression levels and inhibitor development are lacking [13]. Several immunological mechanisms have been suggested to underlie the disappearance of inhibitors after ITI, including direct depletion of the Factor-specific T- and B- cells as well as an increase in regulatory immune pathways [32–36]. In a HA mouse model, very high doses of FVIII have been found to inhibit memory B-cell differentiation into inhibitor producing plasma cells in a T-cell independent manner [45]. Indeed, the proportion of FVIII-specific B-cells was observed to be lower, but not zero, in a small study of HA patients successfully tolerized compared to HA patients with inhibitors [46]. Additionally, Treg-cell induction and expansion likely contributes to immune tolerance during current conventional ITI-protocols as well as during experimental approaches, including oral tolerance regimens (reviewed in [25]) and the gene transfer strategies discussed below. The relative importance of each of these mechanisms for successful immune tolerance induction remains unclear.
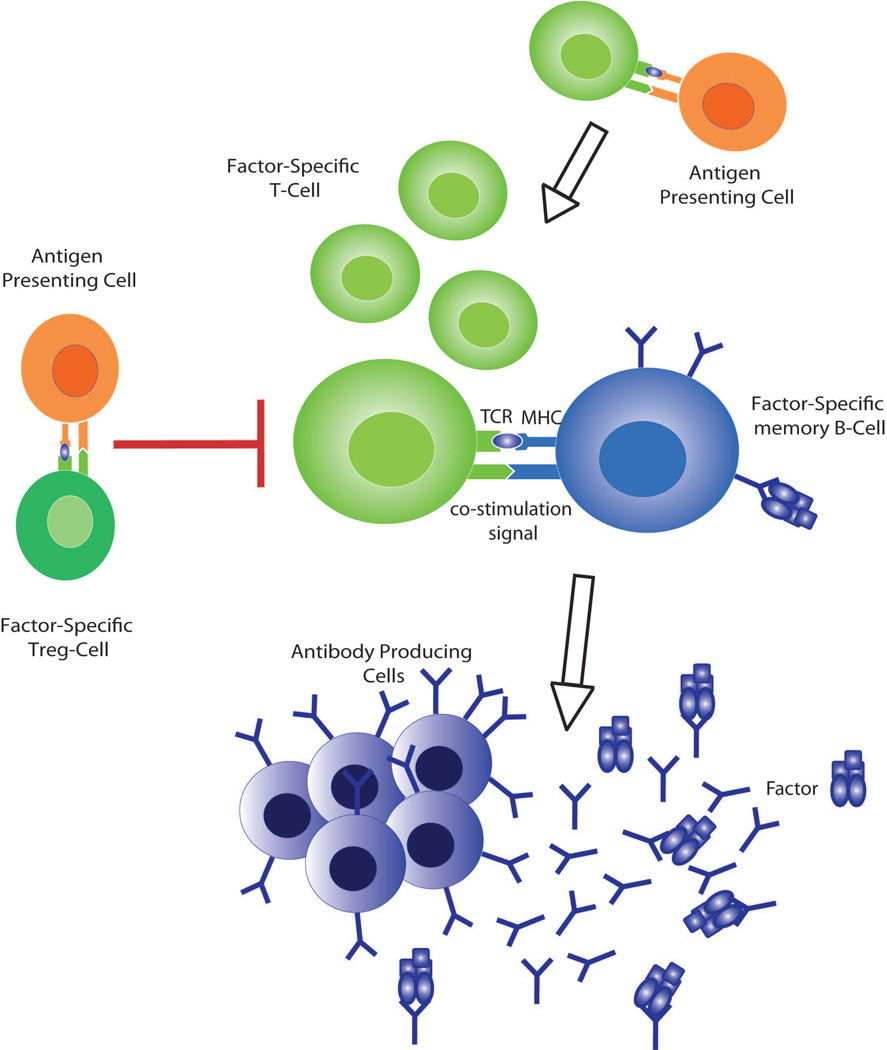
Figure 3. A simplified illustration of the cellular compartments responsible for the persistence of inhibitors.
Effective cooperation between Factor-specific CD4+ T-cells and Factor-specific memory B-cells leads to the differentiation and proliferation of the latter to form inhibitor-producing plasma cells. This cooperation requires the expression of T-cell receptors (TCR) that recognize Factor-peptides presented by MHC class II as well as effective co-stimulatory interactions, such as B7 (CD80/86) binding with CTLA-4. The presentation of factor by professional Antigen Presenting Cells (APC) facilitates that activation of these T-cells, which in turn, activates additional B-cells increasing inhibitor secretion; conversely, the activation of factor-specific Treg-cells impedes this process through the production of inhibitory cytokines including IL-10. As detailed in the text, reductions in Factor-specific B- and T-cells and increased Factor-specific Treg-cells contribute to inhibitor eradication and tolerance induction.
Currently, ITI is the only long-term strategy for patients with inhibitors, but it remains a very intensive and difficult therapy. Indeed, almost 20% of randomized subjects were withdrawn from I-ITI [27], highlighting that even in a research setting, ITI is challenging for patients and their families. Furthermore, ITI itself can cost over a million dollars a year, though this price has been found to be cost-effective when compared to the price of a life-time of bypassing therapy and associated morbidities [47]. Finally, the need for frequent, usually daily, intravenous administration of factors often necessitates placement of a central venous catheter in pediatric patients, which in turn leads to high rates of thrombotic and infectious complications [27, 48]. Thus, the single therapeutic administration proffered by gene transfer (see Figure 2) directly addresses these obstacles of compliance and central venous access. The physical, financial, and social morbidity of inhibitors in hemophilia patients also help justify the expense and intrinsic uncertainty of novel therapies.
3. ITI with FVIII or FIX Gene Transfer
There are currently few alternative therapeutic options for patients with hemophilia and inhibitors recalcitrant to standard ITI regimens, especially HA patients with “poor-risk” features. Encouragingly, there is accumulating data from preclinical animal models of hemophilia that the constitutive exposure of factor after gene transfer both predisposes the immune system towards transgene-specific tolerance and can reverse an already established anti-factor immune response, including eradicating pre-existing inhibitors.
Several animal models are used in the study of hemophilia[17, 18, 33]. A major limitation of all animal models, however, is that most immunocompetent animals will develop antibodies against a xenoprotein, such as human (h) FVIII or FIX, which complicates the assessment of immunogenicity. The best assessment of immunogenicity, therefore, is the use of species-specific protein or transgene, which also best models the clinical situation. Severe HA and HB naturally occur in dogs. Similar to patients, these animals have frequent spontaneous bleeds. The canine models of hemophilia have also reliably predicted the clinical experience of novel replacement factors, bypassing agents, and gene therapy [17, 33]. Most importantly, the canine HA and HB models are outbred with a diverse genetic, and thus immunogenic backgrounds, which better mirrors the patient population than rodent models. Indeed, HA dogs from the Queens University (QU) colony and the University of North Carolina (UNC) colony share a plasma CRM-negative FVIII gene mutation similar to the human intron-22 inversion [17]; however, these models historically exhibited distinct risks of inhibitor formation, with the QU animals being considered “inhibitor-prone” since about 25% develop inhibitors after exposure to canine cryoprecipitate [49], while the UNC animals had a lower rate of inhibitor formation. Intriguingly, there is now a subset of animals within the UNC colony, the progeny of an outside male breeder, that also develop inhibitors with a frequency similar to the QU dogs. These observations highlight that genetic factors, other than the hemophilia causing mutation, help define the risk of inhibitor development. The University of Alabama (UAB) HB dogs, due to a complex null mutation with no FIX transcript (plasma and intracellular CRM-negative), are similarly “inhibitor-prone” [50]. In contrast, the UNC HB dogs, due to a missense mutation, have normal levels of FIX transcript and are likely intracellular CRM-positive, and have not routinely developed inhibitors to canine FIX protein to-date [17].
Similarly, the propensity of murine hemophilia models to develop inhibitors is highly dependent on the strain background [51, 52]; however, the genetic and immunogenic homogeneity of the murine models allows for more precise mechanistic studies. Furthermore, though they display increased bleeding and decreased clot formations during hemostatic challenges, the murine hemophilia models do not spontaneously bleed unlike the canine models.
In the research setting, the anti-factor immune response can be quantified both by anti-factor immunoglobin levels as well as neutralizing assays such as the Bethesda assay, with the latter being used clinically. However, these two measurements do not always completely correspond, with factor-specific immunoglobin levels (non-neutralizing antibodies) often persisting after the disappearance of neutralizing antibodies. Clinically, this is probably responsible for the continued poor recovery or decreased half-life after factor infusion in patients with negative inhibitor titers [27]. Interestingly, decreased recovery is a risk factor for inhibitor recurrence [31], which may suggest that the persistence of anti-factor antibodies after the disappearance of neutralizing antibodies, is evidence of a continued anti-factor immune response. True immune tolerance, therefore, may require the disappearance of all anti-factor antibodies.
It is also worth emphasizing that decreased anti-factor antibodies, lack of inhibitor development (immune hyporesponsiveness), or even the disappearance of Bethesda titers in animal models are of interest, but do not equate with immune tolerance. Indeed, Bethesda titers typically wane in inhibitor patients who are no longer exposed to factor. Immune tolerance is best demonstrated when subsequent challenges with factor protein do not elicit immune responses, as evidenced by continued negative neutralizing assays and typical pharmacokinetics of infused factor.
Liver-Directed Gene Therapy
Emerging evidence demonstrates that liver-directed gene therapy for hemophilia is tolerogenic even towards a primed immune system with pre-existing inhibitors. The liver is the main source of coagulation factors [53, 54]. Furthermore, not only is the liver the primary metabolic organ responsible for the production of most blood proteins, it is continuously exposed to foreign antigens from the gut. As such, there exists in the liver a unique balance between immunosuppressive and inflammatory responses to antigens, which has been termed the “liver tolerance effect” [55, 56].
Liver-directed gene therapy for naive HA dogs using canine (c)FVIII transgene has consistently demonstrated very low relative rates of inhibitor formation (summarized in Table 1). These experiments, using the large outbred canine models from different colonies combined with a therapeutic species-specific FVIII transgene, are likely the most informative preclinical assessment of immunogenicity. Sabatino et al. reported that only 1 out of 8 naive HA dogs from UNC developed a transient low-titer inhibitor (2.5 BU), that resolved within 7 weeks; furthermore, this tolerance was maintained despite subsequent protein challenges in this animal and six other dogs [57]. Of note, this animal was subsequently identified as part of the “inhibitor-prone” subset of the UNC colony. Combined, the results of these 23 naive HA animals demonstrate the low-risk of inhibitor formation after AAV-based liver-directed FVIII gene transfer and, moreover, the ability for the same gene therapy to induce immune tolerance if inhibitors develop. However, this lack of an anti-FVIII immune response after cFVIII liver-directed gene transfer in HA dogs is not absolute; earlier work utilizing cFVIII adenoviral vector gene therapy showed that 4 out of 4 HA dogs from QU developed inhibitors in the setting of liver toxicity [58], which is consistent with the clinical observation that there is an increased risk of inhibitor development in the setting of “danger signals” [20].
Table 1.
Rate of Inhibitor Development After FVIII or FIX Liver-Directed Gene Transfer in Large Animal Models
| Model | Vector | Rate of Inhibitor |
Peak Inhibiter Titer (BU) |
Disappearance Kinetics (weeks) |
Challenged (#) |
Comments | Reference |
|---|---|---|---|---|---|---|---|
| FVIII | |||||||
| Canine- QU |
Adeno- Viral |
4/4 | 1.4 – 1210 | sustained | 0 | Adenoviral-associated liver toxicity |
[58] |
| Canine- QU |
Adeno- Viral |
0/1 | NA | NA | 0 | [94] | |
| Canine- QU |
Adeno- Viral |
1/4 | > 100 | sustained | 0 | [95] | |
| Canine- QU |
AAV | 1/9 | 9 | 9 | 0 | [49, 96] | |
| Canine- UNC |
Adeno- Viral |
0/3 | NA | NA | 0 | [97] | |
| Canine- UNC |
AAV | 0/6 | NA | NA | 0 | [93] | |
| Canine- UNC |
AAV | 1/8 | 2.5 | 7 | 7 | [57] | |
| NHP | AAV | 3/4 | 3 – 15 | 30 – 35 | 0 | Rituximab and Cytoxan Immuno- suppression |
[98] |
| FIX | |||||||
| Canine- UAB |
AAV | 1/3 | 100 | sustained | 2 | Underlying liver disease |
[63, 64] |
| Canine- UNC |
AAV | 0/1 | NA | NA | 0 | [63] | |
| Canine- UNC |
AAV | 0/4 | NA | NA | 0 | [65] | |
| Canine- UNC |
AAV | 0/2 | NA | NA | 2 | FIX-Padua | [67] |
| Canine- UNC |
AAV | 0/2 | NA | NA | 1 | [66] | |
| Canine- UNC |
Lenti- viral |
0/3 | NA | NA | 0 | FIX-Padua and wild type |
[99] |
| NHP | AAV | 3/3 | 1 – 215 | sustained | 0 | Immuno-suppression with anti-CD25 |
[69] |
| NHP | AAV | 0/12 | NA | NA | 0 | [69] | |
| NHP | AAV | 1/16 | 12 | sustained | NA | Nephrotic Syndrome | [21] |
Note: In utero and neonatal gene transfer studies not included
NA, not applicable; NHP, non-human primates
Our group has recently demonstrated that liver-directed FVIII gene transfer not only promotes immune tolerance but can also reverse an already established anti-factor immune response and eradicate pre-existing inhibitors [59], as summarized in Table 2. Three HA dogs with persistent high-titer inhibitors from the “inhibitor-prone” subset of the UNC colony received AAV liver-directed cFVIII gene transfer. In all of these animals, the inhibitor was undetectable 5 weeks post-therapy and the animals demonstrated typical pharmacokinetics of infused protein; this immune tolerance was maintained despite subsequent factor infusion. A fourth dog from the QU colony received the same gene therapy. He had only a historical titer of 4 BU, but had initially developed an inhibitor against hFVIII, after a therapeutic exposure that cross-reacted with cFVIII. This animal had an amnestic response after gene therapy with a peak inhibitor titer of 216 BU that become undetectable after 18 months; this immune tolerance was maintained even after subsequent protein challenges. Clinically, inhibitor titers >100 BU during ITI are a predictor for ITI failure [60]; this dog’s tolerization is, therefore, relatively rapid compared to the several years ITI is typically attempted. Immune tolerance in all 4 dogs remains intact more than five years after initial publication (VRA, unpublished observation). Mechanistically, we observe that there is a spike in the number of CD4+CD25+FOXP3+ cells that presages the loss of inhibitors in these dogs (Figure 4), which supports the role Treg-cells in inhibitor eradication and immune tolerance. The vector used in this study consisted of dual-chain system [61], in which there is a >5-fold excess of FVIII antigen to activity. As such, our results support that the uninterrupted endogenous expression of the antigen and/or an antigen threshold level is the principle mechanism of immune tolerance induction. To help distinguish between these two possibilities, we are currently testing a novel single-chain cFVIII variant [62], in which there is equivalent antigen and activity ratio.
Table 2.
Inhibitor Eradication After FVIII or FIX Liver-Directed Gene Transfer in Large Animal Models
| Model | Vector | Age | Number | Inhibitor Duration (months) |
Historical- Peak Titer (BU) |
Pre-Gene Transfer Inhibiter Titer (BU) |
Peak Inhibiter Titer Post-Gene Therapy (BU) |
Eradication Kinetics (weeks) |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| FVIII | |||||||||
| Canine- UNC |
AAV | 1 – 2 | 3 | 4 – 8 | 4–12 | 2 – 3 | 2 – 7 | 4 – 5 | [59] |
| Canine- QU |
AAV | 5 | 1 | 24 | 4 | 4 | 216 | 100 | [59] |
| FIX | |||||||||
| Canine- UAB |
AAV | 3 | 1 | ND | 0.6* | 5 | 4 | [67] |
Note: Immune tolerance maintained in all animals despite protein challenge
Initial inhibitor against human FIX
Figure 4. Inhibitor eradication after FVIII gene therapy is associated with an increase in Treg-cells.
(A) As previously reported [59], liver-directed cFVIII gene therapy for a HA dog (K01) with a pre-existing inhibitor resulted in the disappearance of both the inhibitor-titer and FVIII-specific immunoglobins; this eradication coincided with an increase in the percentage of CD25+FoxP3+, CD4+ positive cells, assumed to be Treg-cells, from baseline. Gene therapy also tolerized K01, as demonstrated by the unchanged inhibitor-titer and FVIII-specific immunoglobin levels after protein challenge (dotted vertical line). This exposure of exogenous FVIII protein was, however, associated with a rebound of Treg-cells. (B) A similar spike in Treg-cells was seen in two other HA dogs (K03, L44) with pre-existing inhibitors, but not in HA dogs without inhibitors (M06, Linus, L51) [57, 93].
In murine HA models, Herzog and coworkers have demonstrated that liver-directed gene transfer of hFVIII can induce immune tolerance that is resistant to subsequent protein challenges, where control mice that did not receive gene therapy uniformly develop inhibitors [51]. Adoptive CD4+CD25+ cell transfer from these tolerized mice conferred a statistically significant blunted FVIII immune response to subsequent protein challenges, supporting the role of Treg-cells in FVIII tolerance after gene therapy. CD20+ B-cells, however, also contribute to FVIII tolerance, as subsequent treatment with anti-CD20 antibodies helps promote tolerance to succeeding protein challenges. Lastly, there is likely a minimal FVIII threshold that shifts the balance from immunogenicity towards tolerance after AAV liver-directed gene therapy, as an increase of circulating FVIII from <1% to 4% decreased the anti-FVIII antibody response by 10-fold in mice [51].
Inhibitors in patients with HB are rare, though more difficult to treat [3]. None of the reported 22 subjects who have received AAV liver-directed FIX gene therapy have developed inhibitors, though the eligibility criteria for these clinical trials were prudently constructed to minimize the risk of inhibitor formation [21–23]. Liver-directed cFIX gene transfer successfully tolerized 2 out of 3 “inhibitor-prone” HB dogs from the UAB colony, which was maintained despite subsequent protein challenges (a single FIX concentrate protein infusion is generally sufficient to provoke inhibitor development in these animals) [63, 64].) The animal that developed the inhibitor also had evidence of inflammatory liver disease, which may have contributed to inhibitor development either through propagation of a “danger signal” or by disrupting the unique immunological environment of the liver. Seven other HB dogs from the UNC colony (Table 1) have received liver-directed gene therapy without inhibitor formation, though only 1 animal was subsequently challenged with plasma [63, 65, 66].
To assess the potential of gene therapy for eradication of FIX inhibitors, our group has recently demonstrated the successful eradication of a preexisting inhibitor (5 BU) in 1 HB dog as well as successful stringent tolerization, maintained despite protein challenges, in 2 additional dogs from the “inhibitor-prone” UAB colony after cFIX Padua gene transfer [67], where FIX Padua is a naturally occurring hyperactive FIX variant currently being used in several gene therapy clinical trials [23, 68]. Inhibitory FIX antibodies became undetectable by 10 weeks, but the circulating FIX levels only stabilized after 800 days, suggesting the persistence of anti-FIX antibodies that we were unable to detect. Prior to his gene therapy, this dog had a pro-inflammatory cytokine profile (characterized by elevated GM-CSF, IL-15, IL-6, and IL-18), that slowly diminished as his FIX levels increased, suggesting a waning of his anti-FIX immune response. His loss of inhibitor was associated with a rise in his IL-10 levels, which then decreased as his FIX levels increased, but then rebounded upon protein challenge (Figure 5). Combined, these observations support the role of Treg-cells in FIX inhibitor eradication and immune tolerance. The data from this single animal need to be taken carefully, but it does support the concept that lack of inhibitory antibodies does not necessarily indicate the absence of an immune response. The lack of non-neutralizing antibodies and/or no evidence of pro-inflammatory cytokines may be useful to assess the status of immune tolerance during attempts to eradicate inhibitors. Additional studies in both HA and HB models are needed to support these initial findings. The contribution of Treg-cells in FIX tolerance is further supported by the observation that immunosuppressive regimens leading to CD4+CD25+FOXP3+ depletion (anti-CD25 antibody), concomitantly with liver-directed hFIX gene transfer, resulted in 3 out of 3 rhesus macaques developing antibodies to hFIX. In contrast, 0 out of 12 animals that received similar gene therapy, but whose immunosuppression did not change their CD4+CD25+FOXP3+ frequency, developed antibodies [69].
Figure 5. Inhibitor eradication after FIX gene therapy is associated with an increase in anti-inflammatory cytokines.
As previously reported [67], liver-directed FIX gene therapy results in inhibitor eradication and immune tolerance induction for a HB dog (Wiley). The amnestic rise of the inhibitor is associated with a reactive increase in IL-10 levels, which slowly decreases with increasing FIX activity levels. IL-10 is an inhibitory cytokine produced by Treg-cells. This tolerance was maintained despite protein challenge (dotted vertical line), though it was associated with a rebound in IL-10 levels.
Herzog and coworkers were the first to demonstrate, in mouse models, that liver-directed gene therapy could induce immune tolerance to hFIX that is resistant to subsequent FIX challenges [70] and eradicate pre-existing high-titer inhibitors (15 BU) [71]. Both of these phenomena are dependent on Treg activity, as was demonstrated by adoptive transfer. Similar to their results in HA mice, they conclude that there is also a threshold FIX level that moves the balance of the immune system towards tolerance and away from immunogenicity [70, 71]. Inhibitor eradication after liver-directed gene therapy in HB mice is also associated with a decrease in FIX-specific plasma and memory B cells [72].
In summary, studies in large outbred animal models have demonstrated that liver-directed gene therapy for HA or HB biases the immune system towards tolerance. Liver-directed gene therapy has consistently demonstrated lower rates of new inhibitor development compared to other therapeutic modalities. Moreover, new inhibitors were transient giving way to stringent immune tolerance, which was maintained through subsequent antigen challenges. Notable exceptions occurred in the setting of liver damage, which is likely an example of how “danger signals” are associated with inhibitor development. Lastly, liver-directed gene therapy has also been able to induce robust immune tolerance despite pre-existing inhibitors in HA and HB models, including in situations that would be clinically considered poor-risk. These studies suggest that successful ITI is: 1) dependent on Treg-cell induction; 2) promoted by B-cell depletion; and 3) there is a threshold that tilts the immune system towards tolerance, but above this threshold, constitutive factor exposure provided by gene therapy may be more important than antigen level. Collectively, after inhibitor eradication, endogenous transgene expressions levels were associated with sustained improvement of the bleeding phenotypes, as observed by a greater than 90% reduction in the bleeding episodes in these fragile dogs with pre-existing inhibitors. Liver-directed gene therapy may, therefore, be an idealized ITI-regimen as it delivers continuous uninterrupted factor after a single therapeutic administration, as illustrated in Figure 2. A single administration of FVIII or FIX liver-directed gene therapy, therefore, was able to achieve multiple goals of care for hemophilia with inhibitors: 1) inhibitor eradication; 2) prevention of amnestic responses upon subsequent protein challenges (stringent immune tolerance); and 3) ongoing disease phenotype improvement.
Hematopoietic Stem Cell (HSC) Directed Gene Therapy
HSCs are also an attractive target for gene therapy because of their proven ability to provide life-long protein expression in the clinical setting [73]. Promoters and enhancers can be chosen to be either lineage-specific, such as megakaryocytes [74–76], or non-lineage specific. HSC-directed gene therapy generally involves ex vivo transduction and subsequent bone marrow conditioning and transplant. There is accruing data from murine studies that HSC-directed gene therapy combined with the appropriate bone marrow conditioning can also bias the immune system towards tolerance of FVIII or FIX. This is consistent with the conclusions discussed above that the continuous uninterrupted factor exposure above a certain threshold promotes tolerance; however, the bone-marrow conditioning and the use of immunosuppressive drugs are confounders in assessing the immune tolerance induction of the HSC-based therapy. Furthermore, detargeting antigen presenting cells with hematopoietic-specific microRNA actually enhances tolerance induction after liver-directed gene transfer with lentiviral vectors [77].
Spencer and coworkers have been able to induce immune tolerance in HA mice with pre-existing anti-FVIII antibodies after ex vivo transduction of HSC with porcine FVIII, which is maintained despite protein challenges with hFVIII [78], though this tolerance is dependent on the bone marrow conditioning regimen with anti-T-cell immunosuppression also contributing [79]. Clinically, however, bone marrow transplant by itself has not been a successful strategy to eradicate inhibitors [80]. HSC-directed hFIX gene transfer also robustly tolerized HB mice gene that was maintained despite protein and adjuvant challenges that resulted in high levels of anti-FIX antibodies in control mice [81].
HSC-directed, megakaryocyte-restricted, FVIII or FIX gene transfer is an alternative strategy to promote hemostasis despite the presence of inhibitors since the FVIII or FIX expressed in megakaryocytes is subsequently packaged into platelet alpha-granules [82, 83]. This may shield FVIII and FIX from circulating inhibitors until delivered to the site of injury upon platelet activation. However, the in vivo hemostatic efficacy of platelet FVIII or FIX in the presence of inhibitors remains controversial, with different laboratories reporting different levels of efficacy based on different bleeding models [84, 85].
Platelet FVIII or FIX generated by ex vivo lentiviral-based gene transfer followed by bone marrow conditioning and transplant has resulted in hemostatic improvement in both HA [74] and HB mice models, and has also induced immune tolerance in HB mice [76]. The obvious safety concern of bleeding due to hemophilia combined with the thrombocytopenia associated with the conditioning regimen was partially addressed by the demonstration of the feasibility of this strategy in HA dogs, though both the conditioning regimen and the hemostatic support were empirically adjusted during the study [86]. Notably, platelet FVIII after gene therapy completely ameliorated the bleeding phenotype in 2 out of the 3 animals. An alternative in vivo lentiviral-based approach to generate platelet FVIII, which avoids the concerns associated with bone marrow conditioning and transplant, also decreased bleeding after a tail-clip challenge in HA mice with inhibitors [87].
The platelet factor level necessary to overcome clinically-relevant inhibitor titers and, in turn, the vector dose required to produce this threshold, remain open scientific questions. Additional preclinical work regarding HSC-directed gene therapy for hemophilia will need to address: 1) the efficacy of this approach in large animal models of hemophilia with inhibitors; 2) the safety concerns of genotoxicity and insertional mutagenesis intrinsic to integrative gene transfer techniques; and 3) the safety concerns associated with bone marrow conditioning regimens, including the risk of bleeding in the setting of hemophilia with inhibitors and chemotherapy-induced-thrombocytopenia. Since murine models of hemophilia have relatively short lifespans and do not exhibit spontaneous bleeding, these latter safety concerns likely require additional large-animal studies.
4. ITI with Immune-Cell Targeted Gene Therapy
Rather than mimic conventional ITI with constitutive expression of FVIII or FIX from the liver or HSCs, an alternative gene therapy strategy to induce immune tolerance is to directly manipulate immune cells to promote tolerance. Scott and coworkers pioneered this approach in developing tolerogenic antigen-presenting cells by transducing murine B-cells with a retroviral construct that fused either the A2 or C2 domains of hFVIII in-frame with murine IgG. In HA mice, these B-cells both significantly attenuated the anti-FVIII immune response upon subsequent protein challenge and significantly decreased an already established anti-FVIII immune response [88]. Herzog and coworkers recently demonstrated similar results with an analogous full-length hFIX construct [89]. Treg activity was implicated in the immune hyporesponsiveness in both these studies. More recently, Scott and coworkers have reported another strategy utilizing ex vivo gene transfer of a FVIII-specific TCR into FOXP3+ Treg-cells. This manipulation resulted in FVIII-specific human Tregs that significantly suppressed both humoral and cellular pro-inflammatory responses compared to mock controls in vitro upon FVIII exposure [90]. Their in vivo potency, however, has not been reported.
These initial studies suggest a possible role for the direct manipulation of the immune system by gene therapy to promote tolerance to FVIII or FIX. The most salient safety concern is insertional mutagenesis and risk of neoplastic transformation associated with integrative gene transfer into the lymphoid lineage. There are also a number of obstacles currently impeding the therapeutic use of Tregs, as discussed in [91]. However, since the goal of this approach is the stimulation of immune tolerance and not long-term factor expression, suicide switches have been suggested to address the oncological safety concerns [89]. Moreover, these approaches could be combined with either the liver-directed or HSC-directed gene therapeutics discussed in Section 3.
5. Conclusions
Inhibitor development is the most significant complication of factor replacement for patients with hemophilia [1–3] and is associated with increased morbidity and mortality [4–11]. Current medical management (diagrammed in Figure 1) includes bypassing agents to treat and prevent bleeding and ITI to attempt inhibitor eradication. However, bypassing agents remain hemostatically inferior to factor replacement without inhibitors and ITI is unsuccessful in about 40% of patients with HA and 70% of patients with HB [3, 27]. Furthermore, there are few published protocols for “poor-risk” HA patients or HB patients with FIX allergic reactions. Gene therapy approaches are beginning to address these unmet clinical needs.
Preclinical studies have demonstrated that several gene therapy strategies can promote immune tolerance and eradicate pre-existing antibodies in both murine and canine models. Multiple studies in large outbred hemophilia dogs have demonstrated that AAV-based liver-directed gene transfer of both FVIII and FIX—including similar constructs used in ongoing clinical trials for HB [21–23]—can shift the immune system towards tolerance, including eradicating pre-existing inhibitors in clinical situations that would be considered poor prognosis. The translational implication of these results is that liver-directed gene therapeutics for hemophilia, though originally designed to deliver therapeutic levels of factor, may be beneficial for patients with inhibitors resistant to conventional ITI. This proposed AAV-based liver-directed gene therapy ITI can capitalize on the accumulating safety data from ongoing and future clinical trials for HB and HA as well as AAV-based gene therapies for other monogenic diseases [92], including studies enrolling pediatric subjects. To date, gene therapy trials for HA and HB have only included adult subjects, but pediatric patients are the most likely to benefit from gene therapy given the epidemiology of inhibitors and the complications of central venous access associated with current ITI protocols [27, 48]. As there are few current therapeutic options for patients with refractory inhibitors, the potential benefits of liver-directed gene therapy in this situation may outweigh the risks of a clinically unproven approach. Future studies addressing the underlying immunologic mechanisms for successful immune tolerance induction, including determining the contributions of antigen threshold levels and the uninterrupted expression of the missing clotting factor, will help refine this novel strategy. Importantly, this gene-based strategy has the dual benefits of not only eradicating alloantibodies, but also providing continuous disease ameliorating factor expression. Future gene therapy clinical trials for hemophilia should consider this rationale in their design.
Acknowledgments
Disclosure
B. J. Samelson-Jones reports Bayer Fellowship Project Award 2015 and a HTRS/Novo Nordisk 2015 Mentored Research Award in Hemophilia or Rare Bleeding Disorders from the Hemostasis and Thrombosis Research Society, Inc., which was supported by Novo Nordisk Inc.
V. Arruda reports NIH/NHLBI P01 HL64190.
References
- 1.DiMichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia. 2007;13(Suppl 1):1–22. doi: 10.1111/j.1365-2516.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 2.Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124:3365–3372. doi: 10.1182/blood-2014-05-577643. [DOI] [PubMed] [Google Scholar]
- 3.DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. British Journal of Haematology. 2007;138:305–315. doi: 10.1111/j.1365-2141.2007.06657.x. [DOI] [PubMed] [Google Scholar]
- 4.Walsh CE, Soucie JM, Miller CH United States Hemophilia Treatment Center N. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 2015;90:400–405. doi: 10.1002/ajh.23957. [DOI] [PubMed] [Google Scholar]
- 5.Eckhardt CL, Loomans JI, van Velzen AS, Peters M, Mauser-Bunschoten EP, Schwaab R, Mazzucconi MG, Tagliaferri A, Siegmund B, Reitter-Pfoertner SE, van der Bom JG, Fijnvandraat K Group IS. Inhibitor development and mortality in non-severe hemophilia A. J Thromb Haemost. 2015;13:1217–1225. doi: 10.1111/jth.12990. [DOI] [PubMed] [Google Scholar]
- 6.Darby SC, Keeling DM, Spooner RJ, Wan Kan S, Giangrande PL, Collins PW, Hill FG, Hay CR Organisation UKHCD. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–99. J Thromb Haemost. 2004;2:1047–1054. doi: 10.1046/j.1538-7836.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 7.Donfield SM, Lynn HS, Lail AE, Hoots WK, Berntorp E, Gomperts ED. Delays in maturation among adolescents with hemophilia and a history of inhibitors. Blood. 2007;110:3656–3661. doi: 10.1182/blood-2007-05-088062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witmer C, Presley R, Kulkarni R, Soucie JM, Manno CS, Raffini L. Associations between intracranial haemorrhage and prescribed prophylaxis in a large cohort of haemophilia patients in the United States. Br J Haematol. 2011;152:211–216. doi: 10.1111/j.1365-2141.2010.08469.x. [DOI] [PubMed] [Google Scholar]
- 9.Hoots WK. Arthropathy in inhibitor patients: differences in the joint status. Semin Hematol. 2008;45:S42–S49. doi: 10.1053/j.seminhematol.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Guh S, Grosse SD, McAlister S, Kessler CM, Soucie JM. Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia. 2012;18:268–275. doi: 10.1111/j.1365-2516.2011.02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindvall K, von Mackensen S, Elmstahl S, Khair K, Stain AM, Ljung R, Berntorp E. Increased burden on caregivers of having a child with haemophilia complicated by inhibitors. Pediatr Blood Cancer. 2014;61:706–711. doi: 10.1002/pbc.24856. [DOI] [PubMed] [Google Scholar]
- 12.Eckhardt CL, van Velzen AS, Peters M, Astermark J, Brons PP, Castaman G, Cnossen MH, Dors N, Escuriola-Ettingshausen C, Hamulyak K, Hart DP, Hay CR, Haya S, van Heerde WL, Hermans C, Holmstrom M, Jimenez-Yuste V, Keenan RD, Klamroth R, Laros-van Gorkom BA, et al. Factor VIII gene (F8) mutation and risk of inhibitor development in nonsevere hemophilia A. Blood. 2013;122:1954–1962. doi: 10.1182/blood-2013-02-483263. [DOI] [PubMed] [Google Scholar]
- 13.Astermark J. FVIII inhibitors: pathogenesis and avoidance. Blood. 2015;125:2045–2051. doi: 10.1182/blood-2014-08-535328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gouw SC, van den Berg HM, Oldenburg J, Astermark J, de Groot PG, Margaglione M, Thompson AR, van Heerde W, Boekhorst J, Miller CH, le Cessie S, van der Bom JG. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. 2012;119:2922–2934. doi: 10.1182/blood-2011-09-379453. [DOI] [PubMed] [Google Scholar]
- 15.Gouw SC, van der Bom JG, Marijke van den Berg H. Treatment-related risk factors of inhibitor development in previously untreated patients with hemophilia A: the CANAL cohort study. Blood. 2007;109:4648–4654. doi: 10.1182/blood-2006-11-056291. [DOI] [PubMed] [Google Scholar]
- 16.Pandey GS, Yanover C, Miller-Jenkins LM, Garfield S, Cole SA, Curran JE, Moses EK, Rydz N, Simhadri V, Kimchi-Sarfaty C, Lillicrap D, Viel KR, Przytycka TM, Pierce GF, Howard TE, Sauna ZE Investigators PS. Endogenous factor VIII synthesis from the intron 22-inverted F8 locus may modulate the immunogenicity of replacement therapy for hemophilia A. Nat Med. 2013;19:1318–1324. doi: 10.1038/nm.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols TC, Hough C, Agerso H, Ezban M, Lillicrap D. Canine Models of Inherited Bleeding Disorders in the Development of Coagulation Assays, Novel Protein Replacement and Gene Therapies. J Thromb Haemost. 2016 doi: 10.1111/jth.13301. [DOI] [PubMed] [Google Scholar]
- 18.Reipert B, Arruda V, Lillicrap D. Animal models of inhibitors. Haemophilia. 2010;16(Suppl 5):47–53. doi: 10.1111/j.1365-2516.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 19.Bril WS, van Helden PM, Hausl C, Zuurveld MG, Ahmad RU, Hollestelle MJ, Reitsma PH, Fijnvandraat K, van Lier RA, Schwarz HP, Mertens K, Reipert BM, Voorberg J. Tolerance to factor VIII in a transgenic mouse expressing human factor VIII cDNA carrying an Arg(593) to Cys substitution. Thromb Haemost. 2006;95:341–347. doi: 10.1160/TH05-08-0559. [DOI] [PubMed] [Google Scholar]
- 20.Astermark J, Altisent C, Batorova A, Diniz MJ, Gringeri A, Holme PA, Karafoulidou A, Lopez-Fernandez MF, Reipert BM, Rocino A, Schiavoni M, von Depka M, Windyga J, Fijnvandraat K European Haemophilia Therapy Standardisation B. Non-genetic risk factors and the development of inhibitors in haemophilia: a comprehensive review and consensus report. Haemophilia. 2010;16:747–766. doi: 10.1111/j.1365-2516.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- 21.Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D, Nawathe S, Waddington SN, Bronson R, Jackson S, Donahue RE, High KA, Mingozzi F, Ng CY, Zhou J, Spence Y, McCarville MB, Valentine M, Allay J, Coleman J, et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D, Riddell A, Pie J, Rangarajan S, Bevan D, Recht M, Shen YM, Halka KG, Basner-Tschakarjan E, Mingozzi F, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monahan PE, Walsh CE, Powell JS, Konkle B, Josephson NC, Escobar MA, McPhee SJ, Litchev B, Cecerle M, Ewenstein BM, Rottensteiner H, Rose MDI, Reipert BM, Samulski RJ, Orloff J, Scheiflinger F. Update on phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy for program for phemophilia B. Journal of Thrombosis and Haemostasis. 2015;13:87. [Google Scholar]
- 24.Arruda VR, Samelson-Jones BJ. Obstacles and future of gene therapy for hemophilia. Expert Opinion on Orphan Drugs. 2015;3:997–1010. doi: 10.1517/21678707.2015.1069179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sack BK, Herzog RW, Terhorst C, Markusic DM. Development of Gene Transfer for Induction of Antigen-specific Tolerance. Mol Ther Methods Clin Dev. 2014;1:14013. doi: 10.1038/mtm.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiMichele DM. Immune tolerance in haemophilia: the long journey to the fork in the road. Br J Haematol. 2012;159:123–134. doi: 10.1111/bjh.12028. [DOI] [PubMed] [Google Scholar]
- 27.Hay CR, DiMichele DM International Immune Tolerance S. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119:1335–1344. doi: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 28.Brackmann HH, Gormsen J. Massive factor-VIII infusion in haemophiliac with factor-VIII inhibitor, high responder. Lancet. 1977;2:933. doi: 10.1016/s0140-6736(77)90871-6. [DOI] [PubMed] [Google Scholar]
- 29.Dimichele D. The North American Immune Tolerance Registry: contributions to the thirty-year experience with immune tolerance therapy. Haemophilia. 2009;15:320–328. doi: 10.1111/j.1365-2516.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 30.Mariani G, Kroner B Immune Tolerance Study G. Immune tolerance in hemophilia with factor VIII inhibitors: predictors of success. Haematologica. 2001;86:1186–1193. [PubMed] [Google Scholar]
- 31.Antun A, Monahan PE, Manco-Johnson MJ, Callaghan MU, Kanin M, Knoll C, Carpenter SL, Davis JA, Guerrera MF, Kruse-Jarres R, Ragni MV, Witmer C, McCracken CE, Kempton CL. Inhibitor recurrence after immune tolerance induction: a multicenter retrospective cohort study. J Thromb Haemost. 2015;13:1980–1988. doi: 10.1111/jth.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott DW, Pratt KP, Miao CH. Progress toward inducing immunologic tolerance to factor VIII. Blood. 2013;121:4449–4456. doi: 10.1182/blood-2013-01-478669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saint-Remy JM, Reipert BM, Monroe DM. Models for assessing immunogenicity and efficacy of new therapeutics for the treatment of haemophilia. Haemophilia. 2012;18(Suppl 4):43–47. doi: 10.1111/j.1365-2516.2012.02828.x. [DOI] [PubMed] [Google Scholar]
- 34.Lacroix-Desmazes S, Navarrete AM, Andre S, Bayry J, Kaveri SV, Dasgupta S. Dynamics of factor VIII interactions determine its immunologic fate in hemophilia A. Blood. 2008;112:240–249. doi: 10.1182/blood-2008-02-124941. [DOI] [PubMed] [Google Scholar]
- 35.Waters B, Lillicrap D. The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 2009;7:1446–1456. doi: 10.1111/j.1538-7836.2009.03538.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Terhorst C, Herzog RW. In vivo induction of regulatory T cells for immune tolerance in hemophilia. Cell Immunol. 2015 doi: 10.1016/j.cellimm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whelan SF, Hofbauer CJ, Horling FM, Allacher P, Wolfsegger MJ, Oldenburg J, Male C, Windyga J, Tiede A, Schwarz HP, Scheiflinger F, Reipert BM. Distinct characteristics of antibody responses against factor VIII in healthy individuals and in different cohorts of hemophilia A patients. Blood. 2013;121:1039–1048. doi: 10.1182/blood-2012-07-444877. [DOI] [PubMed] [Google Scholar]
- 38.Hofbauer CJ, Whelan SF, Hirschler M, Allacher P, Horling FM, Lawo JP, Oldenburg J, Tiede A, Male C, Windyga J, Greinacher A, Knobl PN, Schrenk G, Koehn J, Scheiflinger F, Reipert BM. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood. 2015;125:1180–1188. doi: 10.1182/blood-2014-09-598268. [DOI] [PubMed] [Google Scholar]
- 39.Lollar P. Pathogenic antibodies to coagulation factors. Part one: factor VIII and factor IX. J Thromb Haemost. 2004;2:1082–1095. doi: 10.1111/j.1538-7836.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 40.Prescott R, Nakai H, Saenko EL, Scharrer I, Nilsson IM, Humphries JE, Hurst D, Bray G, Scandella D. The inhibitor antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with factor VIII autoantibodies. Recombinate and Kogenate Study Groups. Blood. 1997;89:3663–3671. [PubMed] [Google Scholar]
- 41.Lollar P. Pathogenic antibodies to coagulation factors. Part one: factor VIII and factor IX. J Thromb Haemost. 2004;2:1082–1095. doi: 10.1111/j.1538-7836.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 42.Ewenstein BM, Hoots WK, Lusher JM, DiMichele D, White GC, 2nd, Adelman B, Nadeau K. Inhibition of CD40 ligand (CD154) in the treatment of factor VIII inhibitors. Haematologica. 2000;85:35–39. [PubMed] [Google Scholar]
- 43.Bray GL, Kroner BL, Arkin S, Aledort LW, Hilgartner MW, Eyster ME, Ragni MV, Goedert JJ. Loss of high-responder inhibitors in patients with severe hemophilia A and human immunodeficiency virus type 1 infection: a report from the Multi-Center Hemophilia Cohort Study. Am J Hematol. 1993;42:375–379. doi: 10.1002/ajh.2830420408. [DOI] [PubMed] [Google Scholar]
- 44.Hay CR, Palmer B, Chalmers E, Liesner R, Maclean R, Rangarajan S, Williams M, Collins PW United Kingdom Haemophilia Centre Doctors O. Incidence of factor VIII inhibitors throughout life in severe hemophilia A in the United Kingdom. Blood. 2011;117:6367–6370. doi: 10.1182/blood-2010-09-308668. [DOI] [PubMed] [Google Scholar]
- 45.Hausl C, Ahmad RU, Sasgary M, Doering CB, Lollar P, Richter G, Schwarz HP, Turecek PL, Reipert BM. High-dose factor VIII inhibits factor VIII-specific memory B cells in hemophilia A with factor VIII inhibitors. Blood. 2005;106:3415–3422. doi: 10.1182/blood-2005-03-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Helden PM, Van Haren SD, Fijnvandraat K, van den Berg HM, Voorberg J. Factor VIII-specific B cell responses in haemophilia A patients with inhibitors. Haemophilia. 2010;16:35–43. doi: 10.1111/j.1365-2516.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- 47.Colowick AB, Bohn RL, Avorn J, Ewenstein BM. Immune tolerance induction in hemophilia patients with inhibitors: costly can be cheaper. Blood. 2000;96:1698–1702. [PubMed] [Google Scholar]
- 48.Van Dijk K, Van Der Bom JG, Bax KN, Van Der Zee DC, Van Den Berg MH. Use of implantable venous access devices in children with severe hemophilia: benefits and burden. Haematologica. 2004;89:189–194. [PubMed] [Google Scholar]
- 49.Scallan CD, Lillicrap D, Jiang H, Qian X, Patarroyo-White SL, Parker AE, Liu T, Vargas J, Nagy D, Powell SK, Wright JF, Turner PV, Tinlin SJ, Webster SE, McClelland A, Couto LB. Sustained phenotypic correction of canine hemophilia A using an adeno-associated viral vector. Blood. 2003;102:2031–2037. doi: 10.1182/blood-2003-01-0292. [DOI] [PubMed] [Google Scholar]
- 50.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 51.Sack BK, Merchant S, Markusic DM, Nathwani AC, Davidoff AM, Byrne BJ, Herzog RW. Transient B cell depletion or improved transgene expression by codon optimization promote tolerance to factor VIII in gene therapy. PLoS One. 2012;7:e37671. doi: 10.1371/journal.pone.0037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qadura M, Waters B, Burnett E, Chegeni R, Hough C, Othman M, Lillicrap D. Immunoglobulin isotypes and functional anti-FVIII antibodies in response to FVIII treatment in Balb/c and C57BL/6 haemophilia A mice. Haemophilia. 2011;17:288–295. doi: 10.1111/j.1365-2516.2010.02397.x. [DOI] [PubMed] [Google Scholar]
- 53.Fahs SA, Hille MT, Shi Q, Weiler H, Montgomery RR. A conditional knockout mouse model reveals endothelial cells as the principal and possibly exclusive source of plasma factor VIII. Blood. 2014;123:3706–3713. doi: 10.1182/blood-2014-02-555151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Everett LA, Cleuren AC, Khoriaty RN, Ginsburg D. Murine coagulation factor VIII is synthesized in endothelial cells. Blood. 2014;123:3697–3705. doi: 10.1182/blood-2014-02-554501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 57.Sabatino DE, Lange AM, Altynova ES, Sarkar R, Zhou S, Merricks EP, Franck HG, Nichols TC, Arruda VR, Kazazian HH., Jr Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol Ther. 2011;19:442–449. doi: 10.1038/mt.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallo-Penn AM, Shirley PS, Andrews JL, Tinlin S, Webster S, Cameron C, Hough C, Notley C, Lillicrap D, Kaleko M, Connelly S. Systemic delivery of an adenoviral vector encoding canine factor VIII results in short-term phenotypic correction, inhibitor development, and biphasic liver toxicity in hemophilia A dogs. Blood. 2001;97:107–113. doi: 10.1182/blood.v97.1.107. [DOI] [PubMed] [Google Scholar]
- 59.Finn JD, Ozelo MC, Sabatino DE, Franck HW, Merricks EP, Crudele JM, Zhou S, Kazazian HH, Lillicrap D, Nichols TC, Arruda VR. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116:5842–5848. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coppola A, Margaglione M, Santagostino E, Rocino A, Grandone E, Mannucci PM, Di Minno G Group APS. Factor VIII gene (F8) mutations as predictors of outcome in immune tolerance induction of hemophilia A patients with high-responding inhibitors. J Thromb Haemost. 2009;7:1809–1815. doi: 10.1111/j.1538-7836.2009.03615.x. [DOI] [PubMed] [Google Scholar]
- 61.Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, Wilson JM, Kazazian HH., Jr Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–1260. doi: 10.1182/blood-2003-08-2954. [DOI] [PubMed] [Google Scholar]
- 62.Siner JI, Samelson-Jones BJ, Crudele JM, Zhou S, Merricks E, Nichols T, Camire R, Arruda V. Enhanced factor VIII gene therapy for hemophilia a dogs with a novel Furin-evading factor VIII variant. Journal of Thrombosis and Haemostasis. 2015;13:43. [Google Scholar]
- 63.Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, Bellinger D, Nichols TC, Arruda VR, Lothrop CD, Jr, High KA. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 64.Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD., Jr Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, Wilson JM. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- 66.Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, Stafford DW, Patel S, Thompson AR, Nichols T, Read MS, Bellinger DA, Brinkhous KM, Kay MA. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 67.Crudele JM, Finn JD, Siner JI, Martin NB, Niemeyer GP, Zhou S, Mingozzi F, Lothrop CD, Jr, Arruda VR. AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood. 2015;125:1553–1561. doi: 10.1182/blood-2014-07-588194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simioni P, Tormene D, Tognin G, Gavasso S, Bulato C, Iacobelli NP, Finn JD, Spiezia L, Radu C, Arruda VR. X-linked thrombophilia with a mutant factor IX (factor IX Padua) N Engl J Med. 2009;361:1671–1675. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 69.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, Zhou S, Wright JF, Jiang H, Pierce GF, Arruda VR, High KA. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, Arruda VR, High KA, Herzog RW. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Markusic DM, Hoffman BE, Perrin GQ, Nayak S, Wang X, LoDuca PA, High KA, Herzog RW. Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med. 2013;5:1698–1709. doi: 10.1002/emmm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Annoni A, Cantore A, Della Valle P, Goudy K, Akbarpour M, Russo F, Bartolaccini S, D'Angelo A, Roncarolo MG, Naldini L. Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol Med. 2013;5:1684–1697. doi: 10.1002/emmm.201302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doering CB, Spencer HT. Replacing bad (F)actors: hemophilia. Hematology Am Soc Hematol Educ Program. 2014;2014:461–467. doi: 10.1182/asheducation-2014.1.461. [DOI] [PubMed] [Google Scholar]
- 74.Kuether EL, Schroeder JA, Fahs SA, Cooley BC, Chen Y, Montgomery RR, Wilcox DA, Shi Q. Lentivirus-mediated platelet gene therapy of murine hemophilia A with pre-existing anti-factor VIII immunity. J Thromb Haemost. 2012;10:1570–1580. doi: 10.1111/j.1538-7836.2012.04791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Greene TK, Wang C, Hirsch JD, Zhai L, Gewirtz J, Thornton MA, Miao HZ, Pipe SW, Kaufman RJ, Camire RM, Arruda VR, Kowalska MA, Poncz M. In vivo efficacy of platelet-delivered, high specific activity factor VIII variants. Blood. 2010;116:6114–6122. doi: 10.1182/blood-2010-06-293308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y, Schroeder JA, Kuether EL, Zhang G, Shi Q. Platelet gene therapy by lentiviral gene delivery to hematopoietic stem cells restores hemostasis and induces humoral immune tolerance in FIX(null) mice. Mol Ther. 2014;22:169–177. doi: 10.1038/mt.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Annoni A, Brown BD, Cantore A, Sergi LS, Naldini L, Roncarolo MG. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ide LM, Iwakoshi NN, Gangadharan B, Jobe S, Moot R, McCarty D, Doering CB, Spencer HT. Functional aspects of factor VIII expression after transplantation of genetically-modified hematopoietic stem cells for hemophilia A. J Gene Med. 2010;12:333–344. doi: 10.1002/jgm.1442. [DOI] [PubMed] [Google Scholar]
- 79.Ide LM, Gangadharan B, Chiang KY, Doering CB, Spencer HT. Hematopoietic stem-cell gene therapy of hemophilia A incorporating a porcine factor VIII transgene and nonmyeloablative conditioning regimens. Blood. 2007;110:2855–2863. doi: 10.1182/blood-2007-04-082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uprichard J, Dazzi F, Apperley JF, Laffan MA. Haemopoietic stem cell transplantation induces tolerance to donor antigens but not to foreign FVIII peptides. Haemophilia. 2010;16:143–147. doi: 10.1111/j.1365-2516.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 81.Bigger BW, Siapati EK, Mistry A, Waddington SN, Nivsarkar MS, Jacobs L, Perrett R, Holder MV, Ridler C, Kemball-Cook G, Ali RR, Forbes SJ, Coutelle C, Wright N, Alison M, Thrasher AJ, Bonnet D, Themis M. Permanent partial phenotypic correction and tolerance in a mouse model of hemophilia B by stem cell gene delivery of human factor IX. Gene Ther. 2006;13:117–126. doi: 10.1038/sj.gt.3302638. [DOI] [PubMed] [Google Scholar]
- 82.Yarovoi HV, Kufrin D, Eslin DE, Thornton MA, Haberichter SL, Shi Q, Zhu H, Camire R, Fakharzadeh SS, Kowalska MA, Wilcox DA, Sachais BS, Montgomery RR, Poncz M. Factor VIII ectopically expressed in platelets: efficacy in hemophilia A treatment. Blood. 2003;102:4006–4013. doi: 10.1182/blood-2003-05-1519. [DOI] [PubMed] [Google Scholar]
- 83.Zhang G, Shi Q, Fahs SA, Kuether EL, Walsh CE, Montgomery RR. Factor IX ectopically expressed in platelets can be stored in alpha-granules and corrects the phenotype of hemophilia B mice. Blood. 2010;116:1235–1243. doi: 10.1182/blood-2009-11-255612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC, Desai D, Morateck PA, Gorski J, Montgomery RR. Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies. J Clin Invest. 2006;116:1974–1982. doi: 10.1172/JCI28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gewirtz J, Thornton MA, Rauova L, Poncz M. Platelet-delivered factor VIII provides limited resistance to anti-factor VIII inhibitors. J Thromb Haemost. 2008;6:1160–1166. doi: 10.1111/j.1538-7836.2008.02992.x. [DOI] [PubMed] [Google Scholar]
- 86.Du LM, Nurden P, Nurden AT, Nichols TC, Bellinger DA, Jensen ES, Haberichter SL, Merricks E, Raymer RA, Fang J, Koukouritaki SB, Jacobi PM, Hawkins TB, Cornetta K, Shi Q, Wilcox DA. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat Commun. 2013;4:2773. doi: 10.1038/ncomms3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Shin SC, Chiang AF, Khan I, Pan D, Rawlings DJ, Miao CH. Intraosseous delivery of lentiviral vectors targeting factor VIII expression in platelets corrects murine hemophilia A. Mol Ther. 2015;23:617–626. doi: 10.1038/mt.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105:4865–4870. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang X, Moghimi B, Zolotukhin I, Morel LM, Cao O, Herzog RW. Immune tolerance induction to factor IX through B cell gene transfer: TLR9 signaling delineates between tolerogenic and immunogenic B cells. Mol Ther. 2014;22:1139–1150. doi: 10.1038/mt.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim YC, Zhang AH, Su Y, Rieder SA, Rossi RJ, Ettinger RA, Pratt KP, Shevach EM, Scott DW. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood. 2015;125:1107–1115. doi: 10.1182/blood-2014-04-566786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trzonkowski P, Bacchetta R, Battaglia M, Berglund D, Bohnenkamp HR, ten Brinke A, Bushell A, Cools N, Geissler EK, Gregori S, Marieke van Ham S, Hilkens C, Hutchinson JA, Lombardi G, Madrigal JA, Marek-Trzonkowska N, Martinez-Caceres EM, Roncarolo MG, Sanchez-Ramon S, Saudemont A, Sawitzki B. Hurdles in therapy with regulatory T cells. Sci Transl Med. 2015;7:304ps18. doi: 10.1126/scitranslmed.aaa7721. [DOI] [PubMed] [Google Scholar]
- 92.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 93.Sarkar R, Mucci M, Addya S, Tetreault R, Bellinger DA, Nichols TC, Kazazian HH., Jr Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum Gene Ther. 2006;17:427–439. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- 94.Chuah MK, Schiedner G, Thorrez L, Brown B, Johnston M, Gillijns V, Hertel S, Van Rooijen N, Lillicrap D, Collen D, VandenDriessche T, Kochanek S. Therapeutic factor VIII levels and negligible toxicity in mouse and dog models of hemophilia A following gene therapy with high-capacity adenoviral vectors. Blood. 2003;101:1734–1743. doi: 10.1182/blood-2002-03-0823. [DOI] [PubMed] [Google Scholar]
- 95.Brown BD, Shi CX, Powell S, Hurlbut D, Graham FL, Lillicrap D. Helper-dependent adenoviral vectors mediate therapeutic factor VIII expression for several months with minimal accompanying toxicity in a canine model of severe hemophilia A. Blood. 2004;103:804–810. doi: 10.1182/blood-2003-05-1426. [DOI] [PubMed] [Google Scholar]
- 96.Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, Powell S, Keller T, McMurray M, Labelle A, Nagy D, Vargas JA, Zhou S, Couto LB, Pierce GF. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 97.McCormack WM, Jr, Seiler MP, Bertin TK, Ubhayakar K, Palmer DJ, Ng P, Nichols TC, Lee B. Helper-dependent adenoviral gene therapy mediates long-term correction of the clotting defect in the canine hemophilia A model. J Thromb Haemost. 2006;4:1218–1225. doi: 10.1111/j.1538-7836.2006.01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D, Patel N, Tuddenham EG, Christophe OD, McVey JH, Waddington S, Nienhuis AW, Gray JT, Fagone P, Mingozzi F, Zhou SZ, High KA, Cancio M, Ng CY, Zhou J. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cantore A, Ranzani M, Bartholomae CC, Volpin M, Valle PD, Sanvito F, Sergi LS, Gallina P, Benedicenti F, Bellinger D, Raymer R, Merricks E, Bellintani F, Martin S, Doglioni C, D'Angelo A, VandenDriessche T, Chuah MK, Schmidt M, Nichols T, et al. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci Transl Med. 2015;7:277ra28. doi: 10.1126/scitranslmed.aaa1405. [DOI] [PMC free article] [PubMed] [Google Scholar]