Abstract
OBJECTIVE
To investigate compositional cartilage changes measured with 3T MRI-based T2 values over 48 months in overweight and obese individuals with different degrees of weight loss and to study whether weight loss slows knee cartilage degeneration and symptom worsening.
DESIGN
We studied participants from the Osteoarthritis Initiative with risk factors or radiographic evidence of mild to moderate knee OA with a baseline BMI ≥25kg/m2. We selected subjects who over 48 months lost a, moderate (BMI change, 5-10%, n=180) or large amount of weight (≥10%, n=78) and frequency-matched these to individuals with stable weight relative to their baseline BMI (<3%, n=258). T2 maps of the cartilage compartments of the right knee, grey-level co-occurrence matrix (GLCM) texture and laminar analyses were evaluated and associations with weight loss and clinical symptoms (WOMAC subscales for pain, stiffness and disability) were assessed using multivariable regression models adjusting for age, sex, baseline BMI and KL.
RESULTS
The amount of weight change was significantly associated with change in cartilage T2 of the medial tibia (β 0.9ms, 95%CI 0.4 to 1.1, P=0.001). An increase of T2 in the medial tibia was significantly associated with an increase in WOMAC pain (β 0.5ms, 95%CI 0.2 to 0.6, P=0.02) and disability (β 0.03ms, 95%CI 0.003 to 0.05, P=0.03. GLCM contrast and variance over all compartments showed significantly less progression in the >10% weight loss group compared to the stable weight group (both comparisons, P=0.04).
CONCLUSIONS
Weight loss over 48 months is associated with slowed knee cartilage degeneration and improved knee symptoms.
Keywords: Osteoarthritis, cartilage imaging, weight loss, magnetic resonance imaging, T2 relaxation time
Introduction
Obesity is a highly prevalent condition and is a major risk factor for knee OA1,2. Biomechanical factors such as increased joint loads and systemic metabolic factors have a negative impact on cartilage degradation3-5. Co-occurrence of these factors with obesity accelerates the cartilage degeneration process and worsens clinical symptoms6,7. Cartilage degeneration is associated with altered content of proteoglycans and water and degradation of the fibrillar collagen network8. Ideally, these findings should be diagnosed at early stages, before irreversible hyaline cartilage damage occurs. MRI-based T2 relaxation time has been identified as an imaging biomarker that provides information on early changes in collagen integrity9. Moreover, previous studies have shown that T2 measurements are able to predict changes in cartilage morphology and radiographic OA10-12.
Studies demonstrated improvement of clinical performance through weight loss13,14. A previous study has shown that T2 relaxation times progress less in subjects with >10% weight loss of their baseline BMI over four years indicating decreased degenerative changes in comparison to controls without weight loss15. However, associations between T2 values in patients loosing weight over time and clinical parameters are not well understood. Another previous study found that percentage weight change was significantly associated with change in medial tibial cartilage volume16, yet this study was limited to investigating cartilage volume only, without compositional imaging, which provides information on early changes in cartilage, even before cartilage volume loss may have taken place9.
Previous studies have shown that quantitative analysis of cartilage texture using grey-level co-occurrence matrix (GLCM) texture parameters contrast, entropy and variance, allows early detection of compositional and structural changes within the cartilage matrix in subjects with risk for osteoarthritis, before radiographic evidence for OA is present, by providing information on the distribution of T2 pixel values17,18. Imaging of homogeneity may visualize early laminar disruption within cartilage, as does laminar analysis, which analyzes the bone and surface layer of cartilage separately19.
Therefore, in this longitudinal study over 48 months in overweight and obese subjects we analyzed the association of different degrees of weight loss with changes in symptoms and change in cartilage T2, laminar and GLCM texture analysis parameters as measures of progression of knee cartilage degeneration.
Method
Subjects
The Osteoarthritis Initiative (OAI; http://www.oai.ucsf.edu) is an ongoing, longitudinal, prospective, multi-center cohort study with 4796 participants of which subjects for this study were selected. The initiative is sponsored by the U.S. National Institutes of Health (NIH) for investigation of diagnosis, treatment and prevention of OA. Subjects between 45 to 79 years of age with (progression cohort) or are at risk for (incidence cohort) symptomatic knee OA were included into this study. Knee imaging and clinical data were been obtained annually. Informed consent was obtained from all subjects and the study was HIPAA compliant. Study protocols, amendments and informed consent documentations were approved by the local institutional review boards. The following OAI datasets were used: baseline imaging dataset 0.E.1, 48 month follow-up imaging dataset 6.E.1, clinical datasets at baseline 0.2.2, 12 month follow-up 1.2.1, 24 month follow-up 3.2.1 and 48 month follow-up 6.2.2.
For our study, subjects with BMI for baseline, 12, 24, 36 and 48-month follow up were selected from the OAI database (progression and incidence cohort). Subsequently, subjects with missing BMI data at any of the four time points, a baseline Kellgren-Lawrence (KL) score > 3 in the right knee, or rheumatoid arthritis diagnosed during follow-up were excluded from these subjects. Of those, patients with an initial BMI of less than 25 kg/m2 and a weight loss of 3–5% were excluded.
Since the trajectory of weight loss may affect longitudinal changes in joint structures and clinical symptoms, a linear regression model was implemented to assess the annual rate of change in BMI over 4 years. Weight change of the subjects was categorized into “steady” weight and “uneven” weight change, based on the root mean square error (RMSE) of the individual’s regression line. In this study, we excluded subjects (n=84) with uneven weight loss from the overall cohort, which was defined as an RMSE for weight change above the 95th percentile of the RMSE. Reason for this selection criteria was to select patients that follow the linear and steady weight loss trajectory in order to avoid a bias through subjects that “cycle” through weight gain and weight loss periods over 48 months and therefore to isolate the effects of continuous steady weight loss on knee cartilage as good as possible. Also subjects with development of cardiac failure, cancer and/or other severe diseases over the course of the 48-month study period, which could have been responsible for the weight loss, were excluded using the Katz comorbidity questionnaire20. From these subjects only those with right knee MRI T2 mapping sequences available at baseline and 48 months were selected. All subjects that were left after the previous mentioned exclusion criteria (n=1981) were categorized into groups based on their weight loss over 48 months: moderate (BMI decrease of 5-10%), large amount (BMI decrease of >10%) of weight loss (WL) and stable weight (BMI changes <3%). We randomly selected subjects from among those in the 5-10% weight loss group (5-10%WLG; n=180) and the >10% weight loss group (>10%WLG; n=78) and frequency matched these on sex (m/f), age (10 year strata from 45 to 65 and one 14 year stratum from 65 to 79), baseline BMI (BMI in 2.5 kg/m2 strata) and KL (KL in strata of 0/1 and 2/3). Subjects with stable weight (n=258) were randomly selected from each stratum in the frequency matching process and matched to the respective weight loss subjects. This study design was chosen in order to minimize the impact of these covariates (age, sex, baseline BMI and KL) since their impact on the rate of T2 progression is known from previous studies21-25.
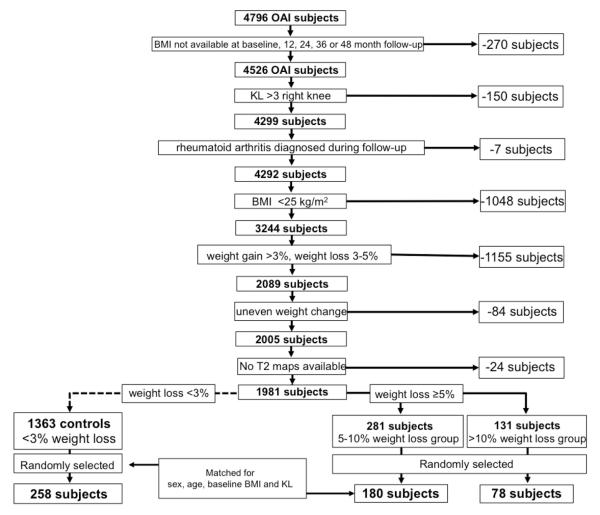
The subject selection process is illustrated in Figure 1 and subject characteristics are shown in Table 1.
Figure 1.
Patient selection from OAI database.
Table 1.
Subject characteristics and differences between stable weight group and weight loss groups. Subjects were divided into groups based on BMI changes over 48 months.
| All | stable weight 1 |
weight loss 5-10%1 |
weight loss > 10%1 |
P-value 5-10% weight loss vs. stable weight group |
P-value >10% weight loss group vs. stable weight group |
|
|---|---|---|---|---|---|---|
| n | 516 (100%) | 258 (50.0%) | 180 (34.9%) | 78 (15.1%) | ||
| Age (years; mean ± SD) |
62.4 ± 9.2 | 62.3 ± 8.9 | 62.5 ± 9.4 | 62.4 ± 9.7 | 1.0 3 | 1.0 3 |
| Sex (females; n (%)) |
314 (60.9%) | 156 (60.5%) | 109 (60.6%) | 49 (62.8%) | 1.0 2 | 0.7 2 |
| Baseline BMI (kg/m2; mean±SD) |
30.3 ± 3.5 | 30.2 ± 3.5 | 30.2 ± 3.5 | 30.7 ± 3.6 | 1.0 3 | 0.9 3 |
| WOMAC pain (mean ± SD) |
2.0 ± 2.6 | 1.8 ± 2.7 | 1.9 ± 2.7 | 2.1 ± 2.5 | 0.6 3 | 0.3 3 |
| WOMAC disability (mean ± SD) |
1.5 ± 1.2 | 1.4 ± 1.1 | 1.5 ± 0.9 | 1.7 ± 1.4 | 0.6 3 | 0.2 3 |
| WOMAC stiffness (mean ± SD) |
6.8 ± 9.6 | 6.7 ± 9.3 | 6.9 ± 9.3 | 7.4 ± 9.8 | 0.8 3 | 0.1 3 |
| Baseline KL Score |
||||||
| KL = 0 (n (%)) |
204 (39.5%) | 107 (41.5%) | 70 (38.9%) | 27 (34.6%) | 0.7 | 0.5 |
| KL = 1 (n (%)) |
213 (41.3%) | 102 (39.5%) | 77 (42.8%) | 34 (43.6%) | 0.8 | 0.6 |
| KL = 2 (n (%)) |
61 (11.8%) | 30 (11.6%) | 22 (12.2%) | 9 (11.5%) | 0.3 | 0.4 |
| Kl = 3 (n (%)) |
38 (7.4%) | 19 (7.4%) | 11 (6.1%) | 8 (10.2%) | 0.8 | 0.3 |
Subjects in the three different groups are matched in terms of age, sex, baseline BMI and baseline KL Score
Pearson's chi-squared test
ANOVA
An a priori power analysis was performed to calculate the appropriate size of each subgroup in order to analyze differences between the groups. Using preliminary data of our previous study in controls (n=65) and subjects with >10%WL (n=62) over 48 months, the average change of T2 in the medial femur was 1.89±1.98ms in the control group and 0.67±2.05ms in the >10%WLG. With these data a comparison between two different WLGs was simulated and we determined a sample size of at least 70 subjects per group would achieve a power >0.9. Therefore we included 78 subjects in the >10%WLG and 180 subjects in the 5-10%WLG in order to ensure adequate group sizes for the comparison.
MR Imaging
MR images were acquired using four identical 3.0T scanners (Siemens Magnetom Trio; Siemens Healthcare, Erlangen, Germany) and quadrature transmit-receive coils (USA Instruments, Aurora, OH, USA) at four sites (University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; Memorial Hospital of Rhode Island, Pawtucket, RI and The Ohio State University, Columbus, OH). T2 values were obtained using sagittal two-dimensional multislice, multiecho sequences with seven echo times (TEs 10ms, 20ms, 30ms, 40ms, 50ms, 60ms, and 70ms). Further details are available in the OAI MR protocol26.
Image Analysis
Semi-automatic cartilage segmentation of lateral femur, lateral tibia, medial femur, medial tibia and patella compartments was performed as previously described, using an in-house, spline-based software based on MATLAB (MathWorks, Natick, Massachusetts). This algorithm also calculated the mean cartilage thickness of all ROIs for each compartment as previously described12. Cartilage was segmented and graded by two trained researchers (M.S. and G.F.), applying this semi-automatic cartilage segmentation tool using the first echo of the MSME sequence and manually correcting the position of control points if needed, in consensus, and under supervision of an experienced radiologist (T.M.L.).
Only artifact-free slices with well-defined boundaries of cartilage were segmented. The trochlea was not segmented because of interfering flow artifacts from the popliteal artery. For each compartment, T2 maps were created and T2 relaxation times were estimated by a mono-exponential decay model as a fitting function for the signal intensity at the echo times 20–70ms. The first echo (10ms) was not used in order to improve signal-to-noise ratio27. Laminar analysis was automatically performed, subdividing the segmented compartments into a superficial and deeper cartilage layer of equal thickness17. Superficial layers were orientated to the articular surfaces, deeper layers adjacent to the cartilage–bone interface. The analysis of these cartilage layers separately may provide a more sensitive assessment of T2 measurements and better characterizes cartilage degenerative changes, as previously shown. For correct interpretation of longitudinal data of laminar analysis, cartilage thickness may not show significant cartilage thickness changes over time19. Therefore cartilage thickness of each region was calculated based on distance between the cartilage-bone interface and the closest point on the articular surface. The average thickness of each slice was calculated, and averages were summed to determine overall cartilage thickness28.
Since previous studies have shown that T2 is limited in assessing more advanced degenerative disease21, we performed an additional sub-analysis in which we excluded all KL=3 patients and re-analyzed the data (n=478; number of subjects with KL=3 excluded for analysis (% of cohort): overall cohort, n=38 (7.4%); stable weight group (SWG), n=19 (7.4%); 5-10%WLG, n=11 (6.1%); >10%WLG, n=8 (10.2%); Table 1). This step was undertaken to confirm the results were not caused by a bias in the analysis created by subjects with more severe cartilage defects, since T2 values tend to be less useful in these subjects21.
In addition to standard T2, we also performed an exploratory analysis of cartilage texture using GLCM texture analysis to evaluate the spatial distribution of T2 values, as described previously17,18,29,30. We included two GLCM parameters from the contrast group (contrast and homogeneity), one parameter each from the orderliness group (entropy) and the statistics group (variance), as published previously17,18. GLCM contrast expresses the differences of values of neighboring pixels, consequently high GLCM contrast indicates a high probability of neighboring pixels with large differences in T231. GLCM homogeneity decreases exponentially inversely from the contrast value and expresses the similarity of neighboring pixels, indicating that higher GLCM values reflect high similarity between neighboring pixels17,29. GLCM variance is a measure of the dispersion of pixel values around the mean T2, meaning that high GLCM variance reflects a high number of pixels with T2 co-occurrences dispersed from the mean T218,32. GLCM entropy is a measure of orderliness regarding the distribution of pixel value co-occurrences, therefore high GLCM entropy indicates that pixel pairs with same T2 values are less likely to be found17,18,29,30.
WOMAC questionnaires
Knee pain and function were assessed with Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)33. WOMAC subscales for pain, stiffness and disability (5 point scale) at baseline and 48 months, as described previously34, were investigated in this analysis.
Statistical Analysis
Statistical analysis was performed with Stata/IC Version 13.1 software (StataCorp, College Station, TX) using a two-sided 0.05 level of significance.
One-way analysis of variance (ANOVA; for parametric testing) and chi-square tests (for categorical variables) were used to evaluate differences in subject characteristics between subjects with stable weight, 5-10% weight loss and >10% weight loss.
All models were checked for the following assumptions that were met (values are provided for the dependent variable baseline overall T2): linearity between the predictor variables and the dependent variables (Lack of Fit F=1.42, p=0.33), normality of the dependent variables and the residuals (Shapiro-Wilk test p>0.05), homogeneity of variances (Levene’s test statistic=1.61, p=0.20), absence of influential outliers in the data (max observed: Cook's distance is 0.042 and leverage is 0.049), and absence of multicollinearity (none of predictor variable pairs have correlation above 0.2).
In order to fulfill the assumption of independence, a random data sample was selected. Additionally, the Durbin-Watson statistic (1.98) indicated that the assumption of independence of the errors was satisfied.
The associations between changes in T2 relaxation times and the assignment to different weight loss groups (SW, 5-10%WL, >10%WL) over 48 months were assessed using multivariable regression models adjusting for age, sex, baseline BMI and baseline KL score (independent variables: groups; dependent variable: ΔT2 parameters). The entire T2 analyses were repeated after excluding subjects with KL=3, using the same statistical approach. Due to the large number of parameters, the analyses were split into the following categories (based on previously published data15-18): primary data (compartments: overall, medial tibia, patella; imaging parameter: mean T2, bone layer texture parameters contrast and variance); exploratory data (compartments: lateral femur, lateral tibia, medial femur; imaging parameters: articular layer, texture parameters entropy, homogeneity).
Differences in changes of GLCM texture parameters over 48 months between the SWG and the two weight loss groups were calculated (independent variables: groups; dependent variable: ΔGLCM texture parameters).
Cartilage thickness at baseline and cartilage thickness changes over 48 months and the association with the amount of weight loss was analyzed by using linear regression models, adjusting for age, sex, baseline BMI and baseline KL score (independent variables: groups; dependent variable: baseline cartilage thickness and Δcartilage thickness, respectively).
Associations of changes in WOMAC subscales (pain, stiffness and disability) or changes in cartilage T2 with weight change over 48 months were assessed using multivariable regression models adjusting for age, sex, baseline BMI and baseline KL score (independent variables: Δ BMI; dependent variable: Δ WOMAC subscales or ΔT2 parameters).
Reproducibility
Both for the intra- and inter-reader reproducibility, T2 measurements were calculated on a percentage basis as the root mean square average of single coefficients of variation (CV) to assess the reproducibility error, as previously described35.
Inter-reader reproducibility was assessed between the two readers (M.S. and G.F.) in 10 patients and was 1.66% over all compartments. CVs for single compartments were as follows: 1.28% for the lateral femur, 1.11% for the lateral tibia, 1.29% for the medial femur, 2.01% for the medial tibia, and 2.42% for the patella.
For intra-reader reproducibility analysis, the same reader performed repeated T2 measurements in 10 randomly selected patients with readings separated by at least 14 days. Intra-reader CVs were calculated for each compartment using these repeated measurements and compartment specific and overall CVs were as follows: 0.92% for the lateral femur, 1.14% for the lateral tibia, 1.07% for the medial femur, 1.63% for the medial tibia, 2.33% for the patella, and 1.42% over all compartments.
Results
Subject Characteristics
Characteristics of all subjects are described in Table 1. There were no significant differences in mean age, baseline BMI or gender frequencies between the groups. Distribution of KL score, baseline WOMAC subscales, imaging site and race did not differ significantly between any of the groups (P>0.05, respectively). After 48 months, subjects in the >10%WLG lost 4.22±1.97kg/m2 on average while the 5-10%WLG had lost 2.03±1.29kg/m2 on average. BMI change of the SWG was 0.08±0.97kg/m2. The association between change in BMI and change in WOMAC subscales pain, stiffness and disability is shown in Table 4. The more weight was lost, the more WOMAC subscales improved (P<0.05, respectively).
Table 4.
Associations between weight change and change in cartilage T2 and clinical symptoms
| Parameter | Compartment | Adjusted* regression β (95% CI) |
P-value |
|---|---|---|---|
|
Change
cartilage T2 (ms) |
all
compartments |
0.06 (−0.04, 0.1) | 0.2 |
| MT | 0.9 (0.4, 1.1) | 0.001 | |
| MF | 0.02 (−0.1, 0.2) | 0.8 | |
| PAT | 0.09 (−0.07, 0.3) | 0.3 | |
|
Change in
WOMAC |
Pain | 0.4 (0.2, 0.7) | 0.001 |
| Stiffness | 0.2 (0.01, 0.4) | 0.03 | |
| Disability | 0.1 (0.02, 0.2) | <0.001 |
Linear regression analysis adjusting for age, sex, baseline BMI and baseline KL Score
Change of T2 relaxation time and weight loss
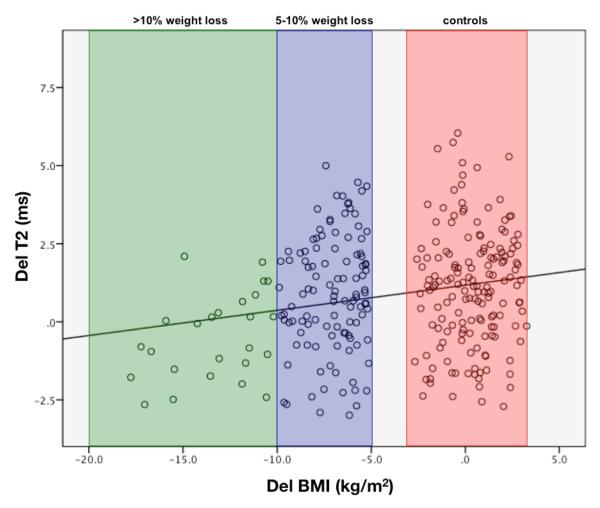
T2 changes for different groups and compartments are shown in Table 2. Increase of global T2 between baseline and 48 month follow-up (ΔT2) was significantly smaller in the medial tibia in the >10%WLG compared to the SWG (P<0.001), as demonstrated in Figure 2, suggesting less progression of cartilage degeneration over time for the >10%WLG in the medial tibia. Yet, there was no significant change of T2 in the 5-10%WLG compared to the SWG (P>0.05). The relationships between percentage of body weight loss and the change of T2 over 48 months are presented in Table 4 (Figure 3). After adjusting for age, sex, baseline BMI and KL, weight change was significantly associated with change in T2 of the medial tibia (β 0.9ms, 95% CI 0.4 to 1.1, P=0.001), but not in the medial femur (β 0.2ms, 95% CI −0.1 to 0.2, P=0.8) or patella (β 0.09ms, 95% CI −0.07 to 0.3, P=0.3). These results demonstrate that for every 1% of weight loss there was 0.9ms less increase of cartilage T2 over 48 months, suggestion less progression of cartilage degeneration the more weight was lost.
Table 2.
Comparison of change in global, articular and bone layer T2 relaxation times (mean [95% confidence interval]) over 48 months of the cartilage compartments of the knees between the group with stable weight and weight loss groups. An increase in T2 values indicates a worsening of cartilage degradation1.
| Parameter |
Compart
ment |
stable weight |
5-10% weight
loss |
P- value 5-10% weight loss vs. stable weight group |
>10% weight loss |
P-value >10% weight loss group vs. stable weight group |
|
|---|---|---|---|---|---|---|---|
|
Global
ΔT2 (in ms) |
all
compart ments |
Baseline mean T2 (ms) | 32.4 [32.1, 32.7] | 32.5 [32.0, 32.7] | 0.8 | 32.7 [32.2, 33.2] | 0.3 |
| Change in mean T2 (ms) | 1.0 [0.8, 1.1] | 0.8 [0.6, 1.1] | 0.6 | 0.6 [0.4, 1.0] | 0.3 | ||
| LF | Baseline mean T2 (ms) | 36.3 [35.9, 36.7] | 35.8 [35.3, 36.3] | 0.3 | 36.4 [35.8, 37.1] | 0.7 | |
| Change in mean T2 (ms) | 1.5 [1.2, 1.8] | 1.3 [0.8, 1.7] | 0.7 | 1.2 [0.7, 1.6] | 0.7 | ||
| LT | Baseline mean T2 (ms) | 27.1 [26.8, 27.3] | 27.3 [27.0, 27.7] | 0.3 | 27.4 [26.7, 28.0] | 0.4 | |
| Change in mean T2 (ms) | 1.2 [1.0, 1.6] | 1.0 [0.7, 1.3] | 0.2 | 1.0 [0.8, 1.2] | 0.3 | ||
| MF | Baseline mean T2 (ms) | 39.7 [39.3, 40.1] | 39.2 [38.7, 39.7] | 0.2 | 39.0 [38.6, 39.5] | 0.8 | |
| Change in mean T2 (ms) | 0.6 [0.2, 1.0] | 0.8 [0.4, 1.1] | 0.6 | 0.4 [0.0, 0.9] | 0.5 | ||
| MT | Baseline mean T2 (ms) | 29.1 [28.9, 29.4] | 29.3 [28.8, 29.6] | 0.6 | 29.3 [28.7, 29.9] | 0.6 | |
| Change in mean T2 (ms) | 1.1 [0.8, 1.4] | 0.8 [0.4, 1.0] | 0.2 | −0.3 [−0.9, 0.4] | <0.001 | ||
| PAT | Baseline mean T2 (ms) | 30.5 [30.1, 30.9] | 30.7 [30.1, 31.1] | 0.7 | 31.3 [30.5, 32.1] | 0.08 | |
| Change in mean T2 (ms) | 0.9 [0.5, 1.3] | 0.5 [0.3, 1.1] | 0.2 | 0.4 [0.02, 1.0] | 0.3 | ||
|
Articular
Layer ΔT2 (in ms) |
all
compart ments |
Baseline mean T2 (ms) | 35.3 [35.0, 35.6] | 35.2 [34.8, 35.6] | 0.9 | 35.6 [35.0, 36.2] | 0.5 |
| Change in mean T2 (ms) | 1.1 [0.8, 1.3] | 1.0 [0.6, 1.3] | 0.6 | 0.9 [0.3, 1.5] | 0.3 | ||
| MT | Baseline mean T2 (ms) | 32.4 [32.1, 32.7] | 32.4 [32.1, 32.7] | 0.1 | 32.4 [32.1, 32.7] | 0.7 | |
| Change in mean T2 (ms) | 1.3 [0.9, 1.6] | 0.9 [0.4, 1.3] | 0.1 | 0.0 [−0.2, 0.7] | <0.001 | ||
| PAT | Baseline mean T2 (ms) | 33.1 [32.7, 33.6] | 33.4 [32.8, 33.9] | 0.5 | 34.0 [33.1, 34.9] | 0.1 | |
| Change in mean T2 (ms) | 0.9 [0.4, 1.3] | 0.5 [0.0, 1.0] | 0.3 | 0.5 [0.0, 1.1] | 0.8 | ||
|
Bone Layer
ΔT2 (in ms) |
all
compart ments |
Baseline mean T2 (ms) | 29.6 [29.3, 29.8] | 29.5 [29.2, 29.8] | 0.9 | 29.4 [29.1, 30.2] | 0.3 |
| Change in mean T2 (ms) | 1.0 [0.7, 1.1] | 0.9 [0.6, 1.1] | 0.7 | 0.4 [0.0, 0.8] | 0.03 | ||
| MT | Baseline mean T2 (ms) | 26.4 [26.1, 26.7] | 26.5 [26.1, 26.8] | 0.9 | 26.5 [26.0, 27.0] | 0.7 | |
| Change in mean T2 (ms) | 1.0 [0.6, 1.2] | 0.8 [0.4, 1.2] | 0.06 | −0.2 [−0.5, 0.3] | <0.001 | ||
| PAT | Baseline mean T2 (ms) | 27.9 [27.5, 28.2] | 27.9 [27.4, 28.2] | 0.9 | 28.6 [27.9, 29.4] | 0.2 | |
| Change in mean T2 (ms) | 1.0 [0.6, 1.3] | 0.5 [0.3, 0.8] | 0.07 | 0.2 [−0.5, 0.5] | 0.04 |
The associations between ΔT2 relaxation times and weight loss (5-10% weight loss and >10% weight loss) over 48 months were assessed using linear regression models adjusting for age, sex, baseline BMI and baseline KL score.
BMI, body mass index; LF, lateral femur; LT, lateral tibia; MF, medial femur; MT, medial tibia; PAT, patella.
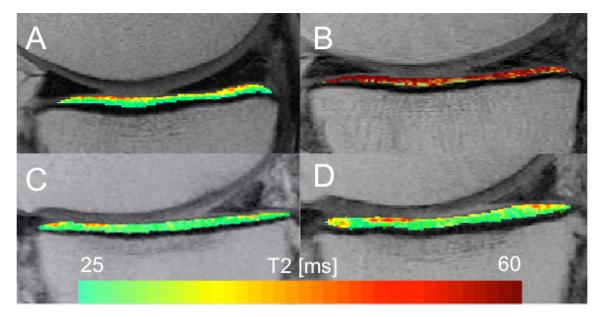
Figure 2.
Representative T2 maps from a subject with stable weight (A, B) and a > 10% weight loss subject (C, D) at baseline (left) and 48 months follow up (right). Stable weight subject (A, B): In this subject mean T2 relaxation times increase (red) in the medial tibia. > 10% weight loss subject (C, D): Only slight progression of cartilage T2 relaxation times. T2 increase is a measure for progression of cartilage degeneration.
Figure 3.
Scatterplot of change in mean T2 relaxation times versus change in BMI over 48 months in the medial tibial compartment (r=0.37, P < 0.001). Group with more than 10% weight loss is marked green; group with 5-10% weight loss is marked blue; control group is marked red.
In laminar subanalyses, changes of global T2 over 48 months were significantly lower in the >10%WLG compared to the SWG, showing globally slowed cartilage degeneration after >10%WL in the bone layer (P=0.03). Moreover, changes of T2 over 48 months of the bone layer of the patella showed significantly lower values in the >10%WLG (P=0.04) compared to the SWG, suggesting less progression of cartilage degeneration in the patella in the >10%WLG over 48 months.
After excluding subjects with KL = 3 and re-analyzing the dataset, mean T2 of all compartments combined was significantly lower in the >10%WLG compared the SWG (P=0.02) and significance levels of the differences between these two groups in the other compartments improved (supplemental data).
Changes in GLCM, cartilage thickness and weight loss
In the >10%WLG, changes in GLCM contrast and variance over all compartments (P=0.04 and P=0.04, respectively) as well as in the patella (P=0.01 and P=0.04, respectively) over 48 months were significantly lower than in the SWG, suggesting less progression of cartilage inhomogeneity and increased orderliness in the >10%WLG.
Decrease of GLCM homogeneity over 48 months was significantly lower in the medial tibia in the >10%WLG compared to the SWG (P=0.004; Table 3). We re-analyzed the data with Bonferoni correction, to verify that our significant findings were not caused by the number of hypotheses tested, yet this did not change the results.
Table 3.
Comparison of texture parameters over 48 months (mean difference (95% confidence interval)) between groups1
| stable weight vs. 5- 10% weight loss |
P-value | stable weight vs. >10% weight loss |
P-value | |
|---|---|---|---|---|
|
Mean over all
compartments |
||||
| Δ contrast | 10.7 (−9.1, 30.4) | 0.4 | 29.4 (1.5, 58.3) | 0.04 |
| Δ entropy | 0.1 (−0.2, 0.4) | 0.7 | 0.4 (−0.1, 0.9) | 0.2 |
| Δ variance | 7.0 (−3.9, 17.6) | 0.3 | 14.9 (0.4, 30.2) | 0.04 |
| Δ homogeneity | −0.02 (−0.06, 0.02) | 0.4 | −0.04 (−0.1, 0.01) | 0.1 |
| Medial tibia | ||||
| Δ contrast | 1.6 (−28.7, 31.9) | 0.8 | 38.2 (−4.7, 81.2) | 0.08 |
| Δ entropy | 0.1 (−0.5, 0.4) | 0.5 | 0.5 (−0.2, 1.2) | 0.2 |
| Δ variance | 4.3 (−16.2, 15.4) | 0.8 | 19.4 (2.1, 42.0) | 0.07 |
| Δ homogeneity | −0.006 (−0.08, 0.06) | 0.8 | −0.1 (−0.2, −0.01) | 0.004 |
| Patella | ||||
| Δ contrast | 14.0 (1.5, 58.3) | 0.3 | 42.2 (10.0, 74.4) | 0.01 |
| Δ entropy | 0.4 (−0.1, 0.9) | 0.1 | 0.3 (−0.5, 1.2) | 0.5 |
| Δ variance | 11.4 (0.4, 30.2) | 0.4 | 23.7 (12.3, 50.1) | 0.04 |
| Δ homogeneity | −0.09 (−0.1, 0.01) | 0.02 | −0.04 (−0.1, 0.06) | 0.4 |
Multivariable regression analysis adjusting for age, sex, baseline BMI and baseline KL Score
Over all compartments, there were no significant differences in baseline cartilage thickness and in cartilage thickness changes over 48 months between the SWG and both WLGs (P>0.05) assessed.
Associations of changes in clinical symptoms and cartilage T2
Increase of T2 in the medial tibia was significantly associated with an increase in the WOMAC subscales for pain (β 0.5ms, 95% CI 0.2 to 0.6, P=0.02) and disability (β 0.03ms, 95% CI 0.003 to 0.05, P=0.03). These results demonstrate 0.5ms increase in cartilage T2 in the medial tibia per point increased on the WOMAC subscore pain scale.
Over all compartments, increase in T2 was significantly associated with increase in the WOMAC subscale for stiffness (β 0.1ms, 95% CI 0.003 to 0.2, P=0.04). Similarly to the findings in the medial tibia, WOMAC subscale for disability showed a statistical trend (β 0.02ms, 95% CI −0.002 to 0.4, P=0.07), again, suggesting progression of cartilage degeneration over all compartments being associated with clinical worsening.
Discussion
In this study, the effect of weight loss on the biochemical composition, texture and thickness of cartilage in individuals with risk factors for OA was analyzed using MR-based T2 relaxation time measurements. We found significantly less increase of cartilage T2 in overweight and obese subjects in the medial tibia over 48 months. Less clinical worsening was associated with both, lower T2 increase over time and a substantial amount of weight loss. Moreover, especially in the >10%WLG, slower cartilage matrix degeneration through weight loss over all compartments was found with texture analysis. The highest association of weight loss and reduced cartilage degeneration was found in the medial tibia, which adds to the hypothesis that weight loss is most protective in the medial weight-bearing compartments36,37.
These findings are supported by the GCLM homogeneity parameter measured, which suggests less progression of cartilage degeneration after a large amount of weight loss, especially within the medial tibia36. Also, a previous study has demonstrated that percentage of weight change was significantly associated with change in cartilage volume of the medial tibia, yet although our range of weight change was smaller our overall study cohort was larger16.
This study is the first to examine the longitudinal association between weight loss over 48 months, and change in both, cartilage T2 and symptoms. The association found between changes in cartilage T2 in the medial tibia with change in clinical symptoms pain and disability emphasizes weight loss being an important primary management strategy in obese individuals in order to avoid or slow progression of OA.
Previous studies suggested a link between obesity and progression of OA38,39 and demonstrated that weight gain was strongly associated with increased31 and >10%WL was associated with slower progression of cartilage degeneration15. Neither of the mentioned studies have investigated a group with 5-10%WL, nor have they evaluated cartilage texture analysis, laminar analysis or clinical data15. To our knowledge, this is the first study to quantify cartilage composition with texture and laminar analysis in obese subjects after stratifying these in two groups with different amounts of weight loss.
Nevertheless, similar to the previous study, the >10%WLG had significantly less increase of T2 in the medial tibia. With laminar analysis, less increase of T2 was found in the bone layer over all compartments combined in the >10%WLG compared to the SWG17, which emphasizes the superiority of laminar analysis in detecting changes in cartilage composition and differences over all compartments in between the groups, compared to using solely mean T2 values as used in previous studies15. These results derived from laminar analysis suggest a slowed progression of cartilage degeneration in subjects with >10%WL effects all compartments.
Further previous studies have shown that T2 values are limited in assessing more advanced degenerative disease21 and interestingly, after excluding subjects with radiographically moderate OA (KL=3), T2 relaxation times in both cartilage layers significantly decreased in the >10%WLG compared to the controls. These findings underline the global protective effect of weight loss for the entire joint cartilage matrix, which confirms and broadens previous findings15. Additionally, in the >10%WLG, slower cartilage degeneration through weight loss over all compartments was found with texture analysis, which supports the importance of more sophisticated analysis to increase accuracy in the investigation of cartilage composition.
Moreover, the assessed GLCM parameters of contrast, variance and entropy reflect heterogeneity of the cartilage extracellular matrix12,18,30. Previous studies have shown that elevated GLCM parameters suggest cartilage degeneration18. Interestingly, significantly less increase of GLCM contrast and variance was found over all compartments as well as at the patella in the >10%WLG compared to controls, suggesting decelerated degenerative changes of cartilage in all compartments18. This may be caused by texture parameters showing earlier and stronger signal changes through weight loss in all compartments. Previous studies confirmed that texture and laminar analysis add valuable information to solely assessed mean T2 values, detecting smaller changes, which may be otherwise masked by overall spatial T2 changes in the collagenous extra-cellular matrix18. This underlines the importance of our findings of texture analysis over all compartments, since in the previous studies no differences in the global T2 changes over all compartments were detected between the >10%WL and the SWG. This study demonstrates that subjects without weight loss showed more elevated and heterogeneous cartilage T2 compared to subjects that lost a substantial amount of weight over all compartments. Therefore texture analysis was able to detect differences in biomechanical cartilage composition over all compartments while simultaneously cartilage T2 alone was not able to detect differences in changes of cartilage quality.
Our study has limitations, which need to be reported: no additional image analysis was performed assessing morphological changes of MRI findings. Nonetheless, further investigations in which structural MR findings are associated with weight loss in obese and overweight subjects, are warranted. Moreover, we performed a retrospective analysis of subjects’ weight loss data. Therefore many confounders can only be controlled in a prospective study, such as activity levels, diet, and comorbidities. These confounders might cause issues regarding the analysis, especially at a 5-10%WL cohort. Also, the analysis of effects of weight gain on cartilage degeneration is of great clinical importance and needs to be investigated in future studies.
In summary, our study demonstrated that weight change was significantly associated with change in cartilage composition in the medial tibia and in WOMAC subscales for pain and disability in obese and overweight individuals. Yet, after creating subgroups, only the >10%WLG was associated with significantly decreased progression of compositional cartilage degeneration compared to stable weight in individuals with risk factors or mild to moderate radiographic evidence for OA. Based on our findings we hypothesize that weight loss has a protective effect on cartilage, which is detected in all compartments, and that a larger amount of weight loss is more beneficial in obese and overweight subjects in order to slow progression of cartilage matrix deterioration and worsening of clinical symptoms.
Supplementary Material
Table 5.
Associations between change in symptoms and change in cartilage T2
| Cartilage Compartments |
Change in WOMAC |
Adjusted* regression β (95% CI) |
P-value |
|---|---|---|---|
|
T2 (ms) over
all compartments |
Pain | 0.04 (−0.02, 1.0) | 0.2 |
| Stiffness | 0.1 (0.003, 0.2) | 0.04 | |
| Disability | 0.02 (−0.002, 0.4) | 0.07 | |
| T2 (ms) of MT | Pain | 0.5 (0.2, 0.6) | 0.02 |
| Stiffness | 0.1 (−0.03, 0.3) | 0.11 | |
| Disability | 0.03 (0.003, 0.05) | 0.03 |
Linear regression analysis adjusting for age, sex, baseline BMI and baseline KL Score
Acknowledgements
We would like to thank the participants and staff of the Coordinating Center of the OAI, as well as the UCSF QUIP-C group, for their invaluable assistance with patient selection, statistical analysis, and technical support.
Role of funding sources
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. The analyses in this study were funded through the NIH (National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01AR064771 and P50-AR060752).
Footnotes
Author contributions
Alexandra Gersing, M.D. and Thomas Link, Ph.D., M.D. (thomas.link@ucsf.edu) take responsibility for the integrity of the work as a whole, from inception to finished article.
Conception and design of the study: Gersing, Solka, Heilmeier, Joseph, Schwaiger, McCulloch, Nevitt, Link
Acquisition of data: Solka, Feuerriegel, Gersing, Schwaiger, Heilmeier, Joseph, Link
Analysis and interpretation of data: Gersing, Solka, Schwaiger, Nevitt, McCulloch, Link
Drafting of article or revising it critically for important intellectual content: Gersing, Solka, Schwaiger, Joseph, Nevitt, McCulloch, Link
Final approval of the version of the article to be published: Gersing, Solka, Joseph, Schwaiger, Heilmeier, Feuerriegel, McCulloch, Nevitt, Link
Competing interest statement
None of the authors have any financial or other interests related to the manuscript submitted to Osteoarthritis and Cartilage that might constitute a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzgerald KR. Review of article: Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010 by Katherine M. Flegal, PhD; Margaret D. Carroll, MSPH; Brian K. Kit, MD; Cynthia L. Ogden, PhD (JAMA 2012;307:491-7) J Vasc Nurs. 2013;31(3):131–132. doi: 10.1016/j.jvn.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis and Cartilage. 2010;18(1):24–33. doi: 10.1016/j.joca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi T, Favre J. The Nature of In Vivo Mechanical Signals That Influence Cartilage Health and Progression to Knee Osteoarthritis. Current Rheumatology Reports. 2014;16(11):1–8. doi: 10.1007/s11926-014-0463-2. [DOI] [PubMed] [Google Scholar]
- 4.Koonce RC, Bravman JT. Obesity and Osteoarthritis: More Than Just Wear and Tear. Journal of the American Academy of Orthopaedic Surgeons. 2013;21(3):161–169. doi: 10.5435/JAAOS-21-03-161. [DOI] [PubMed] [Google Scholar]
- 5.Jungmann PM, Kraus MS, Alizai H, et al. Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: data from the osteoarthritis initiative. Arthritis care & research. 2013;65(12):1942–1950. doi: 10.1002/acr.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apold H, Meyer HE, Nordsletten L, Furnes O, Baste V, Flugsrud GB. Weight gain and the risk of knee replacement due to primary osteoarthritis: a population based, prospective cohort study of 225,908 individuals. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2014;22(5):652–658. doi: 10.1016/j.joca.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman IN, Osborne RH. Obesity and increased burden of hip and knee joint disease in Australia: results from a national survey. BMC musculoskeletal disorders. 2012;13:254. doi: 10.1186/1471-2474-13-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 9.Choi J-A, Gold G. MR Imaging of Articular Cartilage Physiology. Magnetic resonance imaging clinics of North America. 2011;19(2):249–282. doi: 10.1016/j.mric.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum T, Joseph G, Karampinos D, Jungmann P, Link T, Bauer J. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21(10):1474–1484. doi: 10.1016/j.joca.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Majumdar S. Quantitative MRI of articular cartilage and its clinical applications. Journal of magnetic resonance imaging : JMRI. 2013;38(5):991–1008. doi: 10.1002/jmri.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebl H, Joseph G, Nevitt MC, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Annals of the rheumatic diseases. 2015;74(7):1353–1359. doi: 10.1136/annrheumdis-2013-204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent HK, Heywood K, Connelly J, Hurley RW. Obesity and Weight Loss in the Treatment and Prevention of Osteoarthritis. Pm&R. 2012;4(5):S59–S67. doi: 10.1016/j.pmrj.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis. Nature reviews. Rheumatology. 2013;9(4):225–235. doi: 10.1038/nrrheum.2012.224. [DOI] [PubMed] [Google Scholar]
- 15.Serebrakian AT, Poulos T, Liebl H, et al. Weight loss over 48 months is associated with reduced progression of cartilage T2 relaxation time values: data from the osteoarthritis initiative. Journal of magnetic resonance imaging : JMRI. 2015;41(5):1272–1280. doi: 10.1002/jmri.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teichtahl AJ, Wluka AE, Tanamas SK, et al. Weight change and change in tibial cartilage volume and symptoms in obese adults. Ann Rheum Dis. 2015;74(6):1024–1029. doi: 10.1136/annrheumdis-2013-204488. [DOI] [PubMed] [Google Scholar]
- 17.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011;65(4):1184–1194. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 18.Joseph GB, Baum T, Carballido-Gamio J, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls--data from the osteoarthritis initiative. Arthritis research & therapy. 2011;13(5):R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carballido-Gamio J, Blumenkrantz G, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative. Magn Reson Med. 2010;63(2):465–472. doi: 10.1002/mrm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Jungmann PM, Kraus MS, Nardo L, et al. T(2) relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: longitudinal data from the osteoarthritis initiative. Journal of magnetic resonance imaging : JMRI. 2013;38(6):1415–1424. doi: 10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baum T, Joseph GB, Nardo L, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six-month followup data from a longitudinal, observational multicenter study. Arthritis care & research. 2013;65(1):23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan J, Pialat JB, Joseph T, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011;261(2):507–515. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laberge MA, Baum T, Virayavanich W, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects--data from the Osteoarthritis Initiative. Skeletal radiology. 2012;41(6):633–641. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai PH, Chou MC, Lee HS, et al. MR T2 values of the knee menisci in the healthy young population: zonal and sex differences. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2009;17(8):988–994. doi: 10.1016/j.joca.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 measurement in articular cartilage: Impact of the fitting method on accuracy and precision at low SNR. Magnetic Resonance in Medicine. 2010;63(1):181–193. doi: 10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 28.Carballido-Gamio J, Bauer JS, Stahl R, et al. Inter-subject comparison of MRI knee cartilage thickness. Medical image analysis. 2008;12(2):120–135. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haralick R, Shanmugam K, Dinstein I. Textural Features for Image Classification. IEEE Transactions on Systems, Man, and Cybernetics. 1973:610–618. SMC-1. [Google Scholar]
- 30.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2--preliminary findings at 3 T. Radiology. 2000;214(1):259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 31.Bucknor MD, Nardo L, Joseph GB, et al. Association of cartilage degeneration with four year weight gain--3T MRI data from the Osteoarthritis Initiative. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(4):525–531. doi: 10.1016/j.joca.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu A, Heilmeier U, Kretzschmar M, et al. Racial differences in biochemical knee cartilage composition between African-American and Caucasian-American women with 3 T MR-based T2 relaxation time measurements - data from the Osteoarthritis Initiative. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(9):1595–1604. doi: 10.1016/j.joca.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of rheumatology. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 34.Jungmann PM, Nevitt MC, Baum T, et al. Relationship of unilateral total hip arthroplasty (THA) to contralateral and ipsilateral knee joint degeneration - a longitudinal 3T MRI study from the Osteoarthritis Initiative (OAI) Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(7):1144–1153. doi: 10.1016/j.joca.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1995;5(4):262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 36.Anandacoomarasamy A, Leibman S, Smith G, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Ann Rheum Dis. 2012;71(1):26–32. doi: 10.1136/ard.2010.144725. [DOI] [PubMed] [Google Scholar]
- 37.Teichtahl AJ, Wluka AE, Tanamas SK, et al. Weight change and change in tibial cartilage volume and symptoms in obese adults. Annals of the rheumatic diseases. 2014;74(6):1024–1029. doi: 10.1136/annrheumdis-2013-204488. [DOI] [PubMed] [Google Scholar]
- 38.Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis and rheumatism. 2000;43(5):995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Losina E, Walensky RP, Reichmann WM, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Annals of internal medicine. 2011;154(4):217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.