Abstract
Neuroactive steroids (NAS) are allosteric modulators of the γ-aminobutyric acid (GABA) system. NAS and GABA are implicated in depression. The peripartum period involves physiologic changes in NAS which may be associated with peripartum depression and anxiety. We measured peripartum plasma NAS and GABA in healthy comparison subjects (HCS) and those at-risk for postpartum depression (AR-PPD) due to current mild depressive or anxiety symptoms or a history of depression. We evaluated 56 peripartum medication-free subjects. We measured symptoms with the Hamilton Depression Rating Scale (HAM-D17), Hamilton Anxiety Rating Scale (HAM-A) and Spielberger State-Trait Anxiety Inventory-State (STAI-S). Plasma NAS and GABA were quantified by liquid chromatography-mass spectrometry. We examined the associations between longitudinal changes in NAS, GABA and depressive and anxiety symptoms using generalized estimating equation methods. Peripartum GABA concentration was 1.9 ± 0.7 ng/mL (p=0.004) lower and progesterone and pregnanolone were 15.8 ± 7.5 (p=0.04) and 1.5 ± 0.7 ng/mL (p=0.03) higher in AR-PPD versus HCS, respectively. HAM-D17 was negatively associated with GABA (β=−0.14 ± 0.05, p=0.01) and positively associated with pregnanolone (β=0.16 ± 0.06, p=0.01). STAI-S was positively associated with pregnanolone (β=0.11 ± 0.04, p=0.004), allopregnanolone (β=0.13 ± 0.05, p=0.006) and pregnenolone (β=0.02 ± 0.01, p=0.04). HAM-A was negatively associated with GABA (β=−0.12 ± 0.04, p=0.004) and positively associated with pregnanolone (β=0.11 ± 0.05, p=0.05). Altered peripartum NAS and GABA profiles in AR-PPD women suggest that their interaction may play an important role in the pathophysiology of peripartum depression and anxiety.
Keywords: neuroactive steroid, γ-aminobutyric acid, pregnancy, postpartum, depression, anxiety
1. INTRODUCTION
Postpartum depression (PPD) affects 1 in 8 women (Gavin et al., 2005) and negatively impacts child development. Antepartum depressive or anxiety symptoms are a risk factor for PPD (Milgrom et al., 2008) and may represent an early manifestation of the disorder. Recently, the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) revised the “postpartum onset” specifier for major depression to “peripartum onset” in recognition that 50% of “postpartum” major depressive episodes begin antepartum (American Psychiatric Association, 2013). Additional risk factors associated with PPD include: prior depressive episode (Meltzer-Brody et al., 2013) or history of PPD (Wisner et al., 2001), premenstrual syndrome or premenstrual dysphoric disorder (PMDD) (Buttner et al., 2013) and history of childhood trauma (Meltzer-Brody et al., 2013).
While the pathogenesis of PPD is likely multifactorial, research has focused on neuroendocrine mechanisms given the physiologic changes occurring within the endocrine system across the peripartum period. Hypothalamic-pituitary-adrenal (HPA) (Bloch et al., 2005; Skrundz et al., 2011) functioning has been investigated in the context of prolonged and elevated antepartum exposure of the neurocircuit to the effects of neuroactive steroids (NAS) followed by their rapid withdrawal after delivery. Thus a proposed model is a “hormone-sensitive” PPD phenotype in which a subgroup of women develops affective symptomatology when the neurocircuit fails to adapt to the normal fluctuating peripartum hormonal milieu (Bloch et al., 2000; Deligiannidis et al., 2013).
Γ-aminobutyric acid (GABA) is the dominant inhibitory neurotransmitter within the hypothalamic paraventricular nucleus, a region important in the initiation of the neuroendocrine and autonomic response to stress (Herman and Cullinan, 1997), and exerts inhibitory tone upon the HPA axis function (Makara and Stark, 1974). Derivatives of cholesterol or steroidal precursors, NAS are among the most rapid and potent allosteric modulators of GABAA receptor (GABAAR) function and, as such, alter the excitability of the neurocircuit (Majewska et al., 1986). NAS, especially allopregnanolone, modulate the extent and duration of stress-induced inhibition of GABAergic transmission ((Barbaccia et al., 1998; Barbaccia et al., 1996; Barbaccia et al., 1997)). The peripartum period involves significant changes in NAS levels which are associated with GABAAR neuroplasticity (Follesa et al., 1998; Gilbert Evans et al., 2005). In healthy peripartum women, NAS levels increase across pregnancy and then fall precipitously at delivery (Hill et al., 2000; Hill et al., 2002; Parizek et al., 2005). Animal models suggest that differential metabolism of NAS during late pregnancy and the postpartum period is associated with the development of depressive-like symptoms through their interaction with the GABAergic system (Mostallino et al., 2009). NAS abnormalities and abnormalities in the GABAergic system response to normal NAS levels have additionally been implicated in clinical reproductive (Bloch et al., 2000; Martinez et al., 2016) and non-reproductive related affective disorders (Eser et al., 2006). Elevated plasma progesterone has been associated with poorer mood in healthy pregnant women (N=19) (Buckwalter et al., 1999) and 5α-dihydroprogesterone (DHP), the precursor to 3α,5α-tetrahydroprogesterone (THP) [i.e. allopregnanolone] and 3α,5β–THP [i.e. pregnanolone], was elevated in a sample of women (N=9) with antepartum major depressive disorder (MDD) (Pearson Murphy et al., 2001). In one study, low allopregnanolone levels were associated with the development of postpartum blues (N=18) (Nappi et al., 2001), however other studies reported no difference in plasma progesterone, pregnenolone or allopregnanolone concentrations between euthymic postpartum women and those with PPD (Deligiannidis et al., 2013; Epperson et al., 2006).
The GABAergic system, evidenced by abnormal plasma and brain MRS concentrations, has been implicated in the pathogenesis of MDD (Sanacora et al., 1999) and the hormonally-modulated PMDD (Epperson et al., 2002; Halbreich et al., 1996). Studies in the occipital cortex suggest that reductions in cortical GABA in the postpartum may be a risk factor for PPD development (Epperson et al., 2006). GABAAR plasticity correlates with the marked fluctuations in NAS throughout pregnancy and after delivery (Mostallino et al., 2009). GABAAR subunit knockout mice exhibit abnormal GABA conductance with depression-like and abnormal maternal behaviors that result in reduced pup survival (Maguire and Mody, 2008). Rodent models of postpartum syndrome demonstrate allopregnanolone-mediated changes in gene transcription of GABAAR (Smith et al., 1998). GABA plasma levels have been studied in relationship to cerebral spinal fluid (CSF) levels in several species (Bohlen et al., 1979; Ferkany et al., 1979; Ferkany et al., 1978; Loscher, 1979; Loscher and Frey, 1982; Petty, 1994; Petty et al., 1987). GABA plasma and CSF levels are correlated in some but not all studies (Berrettini et al., 1982; Bohlen et al., 1979; Loscher and Frey, 1982; Loscher et al., 1981; Schmidt and Loscher, 1982; Uhlhaas et al., 1986). GABA concentration is reduced in many brain areas during pregnancy in rats (Smolen et al., 1993) and in the CSF of healthy women (Altemus et al., 2004) but the role of GABA has not been otherwise investigated in peripartum women.
Given preliminary evidence for alterations in plasma NAS in PMDD and antepartum depression and the evidence for lower plasma GABA concentrations in unipolar depression, the primary aim of this study was to examine plasma NAS and GABA in peripartum women at-risk for PPD (AR-PPD) compared to healthy comparison peripartum women (HCS). Our aim was to examine potential biological underpinnings of the aforementioned antepartum risk factors for PPD. Since antepartum depressive and anxiety symptoms may be an early manifestation of PPD for some women, we hypothesized that AR-PPD women would have an altered NAS profile and lower GABA concentrations as compared to HCS across the peripartum period and that the blood profiles would be correlated to depressive and anxiety symptoms as measured by clinician-assessed and subject self-report measures. Although there is strong clinical need identify predictive biomarkers of PPD, this study was designed to examine associations between plasma concentrations and peripartum symptomatology. An altered NAS profile could represent an abnormality in the metabolic pathway involved in the conversion of the GABAergic progesterone to pregnanolone and allopregnanolone by either of the 5–reductases and/or 3α-hydroxysteroid dehydrogenase, in exploratory analyses we examined peripartum NAS metabolite to precursor ratios.
2. MATERIAL AND METHODS
2.1 Subject selection
510 English-speaking pregnant subjects were consented and pre-screened with the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987) at 24–34 weeks gestational age as determined by first trimester ultrasound to determine eligibility and interest in the main longitudinal study. Of the 510 pre-screened subjects, 118 did not meet inclusion criteria, as delineated below, and 336, though meeting main inclusion criteria as ascertained by thorough medical record review, declined to participate due to lack of interest or time for research. A total of 56 eligible and interested nulliparous, primiparous or multiparous women between 18–40 years old were consented to the prospective study. Two groups were enrolled: (1) 24 HCS and (2) 32 AR-PPD women. The EPDS was used to assess peripartum depressive and anxiety symptoms (Cox et al., 1987; Lydsdottir et al., 2014) and a cut-off score of ≥10 was chosen to identify women with current depressive and anxiety symptomatology. The HCS group included women with an EPDS ≤5 and no current or past psychiatric diagnosis or family history of psychiatric illness, as ascertained by clinical and research interviews (First et al., 2001) conducted by a board-certified psychiatrist. As the EPDS is not sufficiently accurate in predicting risk of postpartum depressive symptoms alone (Meijer et al., 2014), the AR-PPD group included women who either had an EPDS score ≥10 (indicating current depressive and/or anxiety symptomatology) or, regardless of current EPDS score, a history of PPD or non-puerperal depression as determined by the Structured Clinical Interview for DSM-IV TR Disorders (SCID-IV), Patient Edition (First et al., 2001). Since antepartum anxiety and depression symptoms are associated with, or may represent the early presentation of postpartum depressive symptomatology, women who met criteria for an anxiety disorder or depressive disorder not otherwise specified were included in the AR-PPD group; however subjects who met SCID-IV criteria for a current major depressive episode (MDE) were excluded as the study was designed to identify a potential unifying plasma profile in those women at risk for developing a peripartum MDE.
Subjects were additionally excluded for: multiple gestation pregnancy, lifetime history of manic episode or any psychotic disorder, elevated suicidal risk, and alcohol, tobacco or substance abuse/dependence in the 6 months prior to study entry or any current use. Based on medical record review, subjects were excluded if they had any significant current medical illness. Available laboratory results including blood cell counts, oral glucose tolerance testing, chemistry panels, thyroid function tests and viral serology conducted for routine care were reviewed and were within normal limits. Prenatal vitamins and as needed over the counter antacids, antihistamines and stool softeners were allowed during the study. Concomitant use of pharmacotherapy with known psychotropic, GABAergic or neurosteroidotropic activity at any time during the study was not allowed. The University of Massachusetts Medical School Institutional Review Board (IRB) approved the study which was conducted between January 2010 and March 2013. All subjects provided written informed consent and each received monetary compensation for their participation.
2.2 Study procedures
Subjects were evaluated twice across pregnancy (i.e. between 27–34 weeks gestation (for study purposes named “Visit 1”) and 33–38 weeks gestation (“Visit 2”)) and in the postpartum (i.e. within 5 days after parturition (“Visit 3”) and between 2–9 weeks after parturition (“Visit 4”)). The 9-week postpartum cut-off was based on literature suggesting a postpartum onset definition of up to 6–8 weeks of delivery as optimal (Forty et al., 2006), with the additional week to allow research assessment completion. Serial mood and psychosocial assessments were completed at each of the four study visits. Blood samples for NAS and GABA analyses were obtained between 9–11 AM at all four study visits whenever possible and collected into tubes containing EDTA. Samples were centrifuged at 4,000 rpm for 15 minutes and plasma was stored at −80°C until analysis was completed by collaborators blind to the subject group assignment. Research assessments done at all 4 visits included: Structured Interview Guide for Hamilton Depression Rating Scale (HAM-D17) (Williams, 1988), Structured Interview Guide for Hamilton Anxiety Scale (HAM-A) (Hamilton, 1959; Shear et al., 2001), Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., 1987), Spielberger State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1970), Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), Sheehan Disability Scale (SDS) (Sheehan, 1983), subject weight in pounds (excluding Visit 3) and urine benzodiazepine test. Assessments done at Visit 1 included the SCID-IV and past medical history/demographics. Additional assessments done at the final postpartum study visit (Visit 4) included: SCID-IV, a labor and delivery questionnaire, a menses and breastfeeding recording form and Mother-to-Infant Bonding Scale (MIBS) (Taylor et al., 2005).
2.3 Neuroactive steroid and GABA quantification
Plasma concentrations of proneuroactive steroids (pregnenolone, progesterone, and deoxycorticosterone) and neuroactive steroids (5α- and 5β-dihydroprogesterone as well as 3α,5α- and 3α,5β-tetrahydroprogesterone) were determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Plasma GABA concentrations were determined by an additional LC-MS/MS assay. Details of both assays are in the Supplemental data online.
2.4 Urinary benzodiazepine detection
To determine the presence or absence of benzodiazepine use which could affect plasma NAS or GABA measurements, a urine sample was obtained at the time of each blood draw. The urinary benzodiazepine drug test (Innovacon, Inc., San Diego, CA) is a lateral flow chromatographic immunoassay for the qualitative detection of oxazepam (major metabolite) with a cut-off concentration of 300ng/mL. Common benzodiazepines (e.g. alprazolam, clonazepam, diazepam, etc.) are detected with the assay.
2.5 Statistical analysis
Baseline and postpartum characteristics and peripartum depression and anxiety ratings were compared between AR-PPD vs. HCS groups using Fisher’s exact test for categorical variables and Student’s independent samples t-test for continuous variables (with Satterthwaite adjustment for unequal variances, when appropriate).
We analyzed the longitudinal relationship between depression and anxiety symptoms and plasma concentrations of GABA and NAS across peripartum visits using generalized estimating equation (GEE) methods to control for the repeated subjects correlation at the four visit time points. Two sets of models were examined; the first were main effects models for each plasma/mood score relationship, with individual mood scores as the independent variable and plasma concentrations as the dependent variable, as acute depression and its successful treatment has been associated with changes in plasma concentrations of NAS. Additionally, we ran main effects models with risk group (AR-PPD vs. HCS) as the independent variable and plasma concentration as the dependent variable. The GEE models accounted for all measures taken for every subject and correlated the data between the independent and dependent variables at each visit across the study to estimate an average response over time for the study subjects.
To account for the timing during pregnancy or postpartum of the plasma measurement, we created a peripartum time variable, centering time around delivery date where timing prior to delivery (for study purposes, named “Visits 1 and 2”) was coded negatively and timing after delivery (“Visits 3 and 4”) was coded positively. All models were adjusted for this timing variable. We used an autoregressive covariance structure in our GEE models. For main effects models, P values are reported from a z test that a single regression coefficient was equal to 0 as well as from the overall Type 3 test of any difference among levels of a factor (such as risk group) adjusting for other variables in the model. Additionally, as part of exploratory analyses, we ran GEE models using the NAS metabolite to precursor ratios as outcome variables to determine if the ratios differed overall and separately by risk group across pregnancy (“Visits 1 and 2”) or the postpartum (“Visits 3 and 4”). All results are reported with the conventional critical significance level of p=0.05. Based on the suggestions of Rothman, we did not adjust for multiple comparisons in our analysis to avoid eliminating any indication of results from this preliminary study that may warrant further study, as well as to prevent an increase in the Type II error rate (Rothman, 1990). All analyses were conducted in SAS Version 9.3 (SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
3.1 Study population characteristics
A total of 56 women participated in the study. The average age was 31.9 years (± standard deviation [SD] 4.7) and the majority of subjects were white, non-Hispanic, employed and married. There were no between group differences in age, race, ethnicity, employment status, or number of children in household; however, AR-PPD women were more likely to be never married (p=0.041) and less educated (p=0.020) compared to HCS (Table 1). Overall, women were 32.1 weeks gestational age (±2.8 weeks) at study entry. Over half of the women had experienced one or more prior births; 44.6% of women were nulliparous at study entry. Prior to study entry, a majority (68.8%) of the AR-PPD women had a prior depression diagnosis; including 18.8% with a prior MDE with postpartum onset, and 25.0% had a prior anxiety diagnosis (Table 1). At the time of the first antepartum study visit, 18.8% of AR-PPD women had a depressive disorder NOS diagnosis and 56.3% had an anxiety disorder; no HCS women had either condition (p=0.03 for depression; p<.001 for anxiety). At the final postpartum study visit, 21.9% of AR-PPD women had a depressive disorder NOS or MDE and 53.1% had an anxiety disorder compared to no Axis I diagnoses in HCS women (p≤0.02 for both comparisons). At the final postpartum visit, all AR-PPD women with a depressive disorder NOS or MDE diagnosis had a past history of depression; 12.5% AR-PPD subjects developed an MDE with postpartum onset and 3.1% developed a new anxiety diagnosis.
Table 1.
Subject Characteristics at Study Entry and in the Postpartum
| Variable1 | AR-PPD (n=32) |
HCS (n=24) |
P-value2 |
|---|---|---|---|
| Study Entry | |||
| Age, mean (SD) | 32.73 (5.15) | 30.90 (4.03) | 0.1541 |
| Race, % | |||
| % Caucasian | 87.50 | 83.33 | 0.7131 |
| Ethnicity, % | |||
| % Not Hispanic or Latino | 75.00 | 91.67 | 0.1619 |
| Education level, % | |||
| High school diploma or less | 15.63 | 8.33 | 0.0200 |
| Partial or completed college | 68.75 | 41.67 | |
| Graduate/professional degree | 15.63 | 50.00 | |
| Marital status, % | |||
| Never married | 31.25 | 8.33 | 0.0410 |
| Married | 62.50 | 91.67 | |
| Divorced/separated/widowed | 6.25 | 0.00 | |
|
Employment status (currently employed), % |
65.63 | 75.00 | 0.7655 |
| Number of children in household | 0.3121 | ||
| 0 | 34.38 | 54.17 | |
| 1 | 46.88 | 29.17 | |
| 2+ | 18.75 | 16.67 | |
| Parity, % | |||
| Nulliparous | 40.63 | 50.00 | 0.4475 |
| Primiparous | 50.00 | 33.33 | |
| Multiparous | 9.38 | 16.67 | |
| Weight (lbs.), mean (SD) | 181.70 (32.78) | 174.50 (32.74) | 0.4183 |
| EPDS total score, mean (SD) | 8.94 (5.25) | 2.58 (2.04) | <.0001 |
| Depressive disorder NOS, % | 18.75 | 0.00 | 0.0321 |
| Anxiety disorder, % | 56.25 | 0.00 | <.0001 |
| Postpartum | |||
|
Gestational age at delivery (weeks), mean (SD) |
39.03 (1.27) | 39.48 (0.95) | 0.1685 |
| Delivery method, % | |||
| Vaginal | 68.75 | 70.83 | 0.9999 |
| Cesarean section | 21.88 | 25.00 | |
| Labor induction, % | 0.0890 | ||
| % Yes | 46.88 | 25.00 | |
| Breastfeeding status, % | |||
| Full or partial | 59.38 | 79.17 | 0.1403 |
| Bottle-feeding | 34.38 | 16.67 | |
|
Major depression with postpartum onset, % |
7.14 | 0.00 | 0.1268 |
| Depressive disorder NOS, % | 21.88 | 0.00 | 0.0160 |
| Anxiety disorder, % | 53.13 | 0.00 | <.0001 |
Unless otherwise indicated, data are expressed as number (percentage) of subjects. Percentages have been rounded and may not total 100.
P-values from Fisher's Exact Test for categorical variables and Student’s independent samples t-test for continuous variables (with Satterthwaite adjustment for unequal variances, when appropriate).
SD: Standard Deviation; AR-PPD: At-risk postpartum depression; HCS: Healthy comparison subject; EPDS: Edinburgh Postnatal Depression Scale
3.2 Depression and anxiety ratings across peripartum period
AR-PPD women scored higher compared to HCS women at each visit on HAM-D17, HAM-A, EPDS, STAI-S, SDS, and PSQI peripartum scale scores (Table 2). AR-PPD women also reported higher MIBS scores at Visit 4 compared to HCS women (AR-PPD 1.3 ± 1.8 vs. HCS 0.7 ± 1.0; data not shown).
Table 2.
Peripartum Psychometric Scale Total Scores (mean ± SD)
| n | HAM-D17 | HAM-A | EPDS | STAI-S | SDS | PSQI | |
|---|---|---|---|---|---|---|---|
|
Antepartum Visit 1 |
|||||||
| AR-PPD | 3 2 |
8.97 ± 4.84 |
14.88 ± 6.24 |
8.94 ± 5.25 |
32.06 ± 8.11 |
6.97 ± 7.23 |
8.75 ± 3.21 |
| HCS | 2 4 |
3.08 ± 2.00 |
6.13 ± 2.44 |
2.58 ± 2.04 |
26.21 ± 4.94 |
1.25 ± 2.05 |
4.67 ± 2.57 |
|
Antepartum Visit 2 |
|||||||
| AR-PPD | 2 6 |
9.42 ± 4.14 |
13.88 ± 6.12 |
8.54 ± 5.34 |
35.00 ± 11.36 |
7.92 ± 7.69 |
8.81 ± 4.20 |
| HCS | 2 1 |
3.19 ± 2.56 |
4.81 ± 4.31 |
1.81 ± 1.94 |
27.33 ± 7.10 |
2.05 ± 4.15 |
4.57 ± 2.84 |
|
Postpartum Visit 3 |
|||||||
| AR-PPD | 3 0 |
9.69 ± 4.70 |
12.93 ± 7.00 |
6.57 ± 4.27 |
33.07 ± 11.09 |
6.00 ± 5.84 |
9.23 ± 3.68 |
| HCS | 2 2 |
4.45 ± 3.33 |
5.75 ± 3.58 |
1.41 ± 1.99 |
27.50 ± 6.47 |
1.27 ± 2.14 |
6.00 ± 3.21 |
|
Postpartum Visit 4 |
|||||||
| AR-PPD | 3 0 |
6.54 ± 5.21 |
8.24 ± 6.36 |
5.87 ± 5.15 |
30.66 ± 7.97 |
3.79 ± 4.89 |
7.55 ± 2.65 |
| HCS | 2 3 |
2.91 ± 2.11 |
2.87 ± 2.78 |
1.04 ± 1.07 |
25.09 ± 4.97 |
1.17 ± 3.27 |
6.30 ± 2.58 |
Data was collected across peripartum time where Visit 1 occurred during 27–34 weeks GA, Visit 2 occurred during 33–38 weeks GA, Visit 3 occurred within 5 days after parturition and Visit 4 during 2–9 weeks after parturition.
AR-PPD: At-risk postpartum depression; HCS: Healthy comparison subject; HAM-D17 Hamilton Depression Scale; HAM-A: Hamilton Anxiety Scale; EPDS: Edinburgh Postnatal Depression Scale; STAI-S: Spielberger State-Trait Anxiety Inventory; SDS: Sheehan Disability Scale; PSQI: Pittsburgh Sleep Quality Index
3.3 Primary analyses of peripartum plasma neuroactive steroid and γ-aminobutyric acid concentrations by risk group
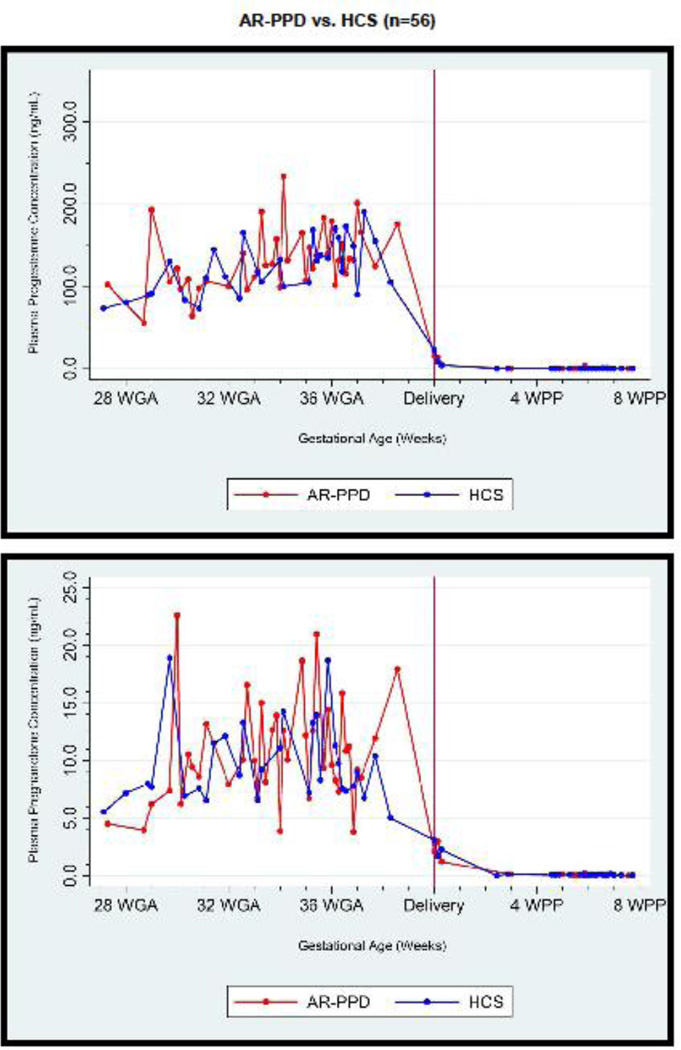
Average plasma concentrations by peripartum time by risk group for progesterone, pregnanolone and GABA are displayed in Figure 1. GABA concentration increased on average by 0.97 ng/mL per visit. Main effects models examining the relationship of plasma concentrations by risk group or by mood score for all subjects (in separate models) across peripartum time points are reported in Supplemental Table 1. Main effects models examining the relationship between depressive and anxiety symptoms and plasma concentrations across peripartum time points showed that plasma GABA concentration was 1.9 ± 0.7 ng/mL (p=0.004) lower and plasma progesterone and pregnanolone were 15.8 ± 7.5 and 1.5 ± 0.7 ng/mL higher in AR-PPD women across time as compared to HCS, respectively (p=0.04 for progesterone; p=0.03 for pregnanolone). HAM-D17 was negatively associated with GABA concentration (β=−0.14 ± 0.05, p=0.01) and positively associated with pregnanolone (β=0.16 ± 0.06, p=0.01) concentration. STAI-S was positively associated with pregnanolone (β=0.11 ± 0.04, p=0.004), allopregnanolone (β=0.13 ± 0.05, p=0.006) and pregnenolone (β=0.02 ± 0.01, p=0.04) concentrations. HAM-A was negatively associated with GABA concentration (β=−0.12 ± 0.04, p=0.004) and positively associated with pregnanolone (β=0.11 ± 0.05, p=0.05).
FIGURE 1.
Average plasma progesterone, pregnanolone and GABA concentration (ng/mL) at pregnancy and postpartum time points. Subject results demonstrate significantly increased progesterone and pregnanolone and lower γ-aminobutyric acid (GABA) plasma concentrations in women at-risk for postpartum depression (AR-PPD; n=32) versus healthy control subjects (HCS; n=24) across the peripartum time period. Data is shown across peripartum time and represents data from Visit 1 (27–34 weeks GA), Visit 2 (33–38 weeks GA), Visit 3 (within 5 days after parturition) and Visit 4 (2–9 weeks after parturition). Please see On-line Supplemental Table 2: Peripartum GABA and NAS concentrations for sample size and mean ± standard deviation values when plasma concentration data was grouped into antepartum and postpartum “study visits.” WGA: gestational age in weeks; WPP: postpartum time in weeks
Additionally, we examined the correlation between GABA and NAS using similar GEE models. We did not find any relationships between plasma GABA concentrations and individual NAS values.
3.4 Exploratory analyses of antepartum and postpartum plasma neuroactive steroid metabolite to precursor ratios
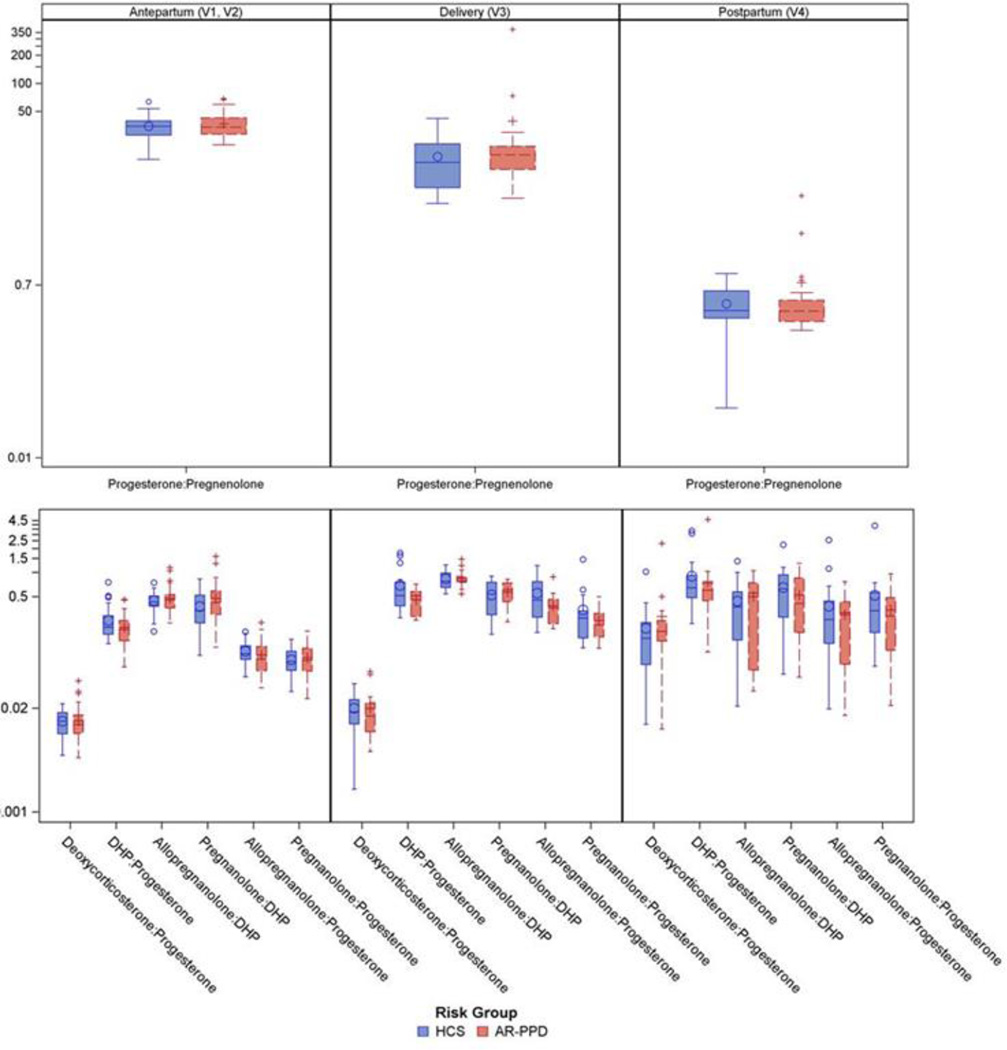
In an exploratory analysis, seven NAS metabolite to precursor ratios were examined by risk group (Figure 2). During pregnancy (Visits 1 and 2), GEE models revealed that progesterone: pregnenolone, deoxycorticosterone: progesterone, and dihydroprogesterone: progesterone increased significantly for all subjects, while allopregnanolone: dihydroprogesterone, pregnanolone: dihydroprogesterone, and pregnanolone: progesterone decreased significantly over all subjects (p≤0.001 for all). During pregnancy, AR-PPD subjects had an average increase of 0.10 (±0.05) ng/mL in the pregnanolone: dihydroprogesterone ratio (p=0.053) and an average decrease of 0.05 (±0.03) ng/mL in the ratio of dihydroprogesterone: progesterone (p=0.057) compared to HCS.
FIGURE 2.
Peripartum neuroactive steroid metabolite to precursor ratios
Note: Mean ratio values shown are from raw data values with standard deviation. The Y-axis is scaled logarithmically. AR-PPD: at-risk postpartum depression; HCS: healthy control subject; DHP: dihydroprogesterone
During the postpartum (Visits 3 and 4), deoxycorticosterone: progesterone and pregnanolone: progesterone ratios increased significantly for all subjects, while progesterone: pregnenolone, allopregnanolone: dihydroprogesterone, and allopregnanolone: progesterone ratios significantly decreased (p<0.05 for all). There were no differences in NAS metabolite: precursor ratios between risk groups over the postpartum period.
Supplemental Table 2 depicts peripartum plasma γ-aminobutyric acid (ng/mL) and neuroactive steroid concentrations (ng/mL) (mean±SD) within each “study visit” so mean concentrations can be compared in future studies even though study visits were across the peripartum period and not narrowly defined by gestational age for study feasibility.
4. DISCUSSION
We tested the primary hypothesis that women with risk factors for the development of PPD would have an altered plasma NAS profile and lower GABA concentrations compared to HCS women across the peripartum period and these plasma profiles would be associated with depressive and anxiety symptomatology. Our study yielded two main novel findings: (1) mean peripartum progesterone and pregnanolone concentrations were higher in AR-PPD women compared to HCS with pregnanolone positively associated with HAM-D17 and HAM-A and (2) peripartum GABA concentrations were lower in AR-PPD women compared to HCS and negatively associated with HAM-D17 and HAM-A scores.
In our study, all subjects at risk for PPD had a history of MDD or PPD and/or current depressive and anxiety symptomatology in the mild to moderate range. Symptomatology was significantly greater in AR-PPD compared to HCS women at every peripartum time point including the SDS, a measure of functional impairment. Reported sleep quality, as measured by the PSQI, was also significantly worse in the AR-PPD group across all visits except for the final postpartum visit. Although women reported functional impairment, depressive and anxiety symptomatology severity and SCID-IV diagnoses were fairly stable across the peripartum period studied. Four ARPPD subjects developed worsening symptomatology that met SCID-IV criteria for a MDE at the final postpartum study visit and one AR-PPD subject with no past history of anxiety developed a new postpartum anxiety disorder.
Our findings of elevated progesterone, the precursor to 5β-dihydroprogesterone, and pregnanolone, its metabolite, in the AR-PPD women are consistent with previous studies as mentioned in the introduction (Buckwalter et al., 1999; Pearson Murphy et al., 2001). Given that allopregnanolone was not additionally elevated, the finding of elevated pregnanolone does not appear to be due to elevated concentrations of its precursor, progesterone and may represent evidence of altered 5β–reductase and/or 3α-hydroxysteroid dehydrogenase function, though the exploratory analysis of NAS:metabolite ratios did not meet statistical significance, which would have given stronger evidence of this possibility. Our study could not confirm or refute a previous report of elevated 5α-dihydroprogesterone in antepartum major depression (Pearson Murphy et al., 2001) as we report a combined measurement of 5α-dihydroprogesterone and 5β-dihydroprogesterone.
In exploratory analyses, we examined NAS metabolite to precursor ratios as an index of enzymatic conversion of pregnenolone to progesterone and progesterone to deoxycorticosterone, DHP, allopregnanolone or pregnanolone. Although none of the between-group comparisons met statistical significance level of p≤0.050, ratios of pregnanolone to dihydroprogesterone and dihydroprogesterone to progesterone during pregnancy in AR-PPD appear to mirror a pattern consistent with a previous report of an increased ratio of pregnanolone to progesterone in non-peripartum women with a history of depression after exposure to a single administration of oral progesterone (Girdler et al., 2012). Given that the majority of the AR-PPD women had a history of depression and were exposed to elevated concentrations of progesterone throughout gestation, this pattern of ratios, if found statistically significant in a larger replication sample, could be a marker of mood susceptibility during times of physiologic changes in progesterone, or simply the presence of a past depressive episode. We did not find an altered ratio of allopregnanolone to progesterone as did the aforementioned Girdler et. al study. Our findings may differ slightly from this study, as AR-PPD subjects were more symptomatic and it may be that the duration of progesterone exposure (i.e. prolonged gestational exposure vs. acute single dose administration) differentially affects biosynthetic pathway functioning. Future studies will isolate the 5α- and 5β-dihydroprogesterone isomers to more precisely determine if the altered NAS profile is due to an underlying abnormality in the pathway involved in the conversion of the GABAergic progesterone to pregnanolone or allopregnanolone by 5α and 5β–reductase and/or 3α-hydroxysteroid dehydrogenase.
The inability to sufficiently adapt to chronic changes in NAS production may result in changes in the balance of neuronal excitability and inhibition and contribute to the development of hormone-sensitive affective disorders (Deligiannidis et al., 2013; Maguire and Mody, 2009). The differences we report in peripartum NAS concentrations could represent differential metabolism within the progesterone-based biosynthetic pathway, either peripherally and/or in the brain, though they are regulated differently (Paul and Purdy, 1992). Differences in NAS concentrations could affect further downstream interactions with glutamic acid decarboxylase (GAD), which catalyzes glutamate decarboxylation to GABA, as there is evidence that NAS can induce GABA synthesis via GAD (Magnaghi et al., 2010) and that changes in GAD mRNA expression are associated with changes in brain GABA and glutamate levels across the peripartum period (Zhao and Gammie, 2014). Additionally, there is increasing evidence that abnormalities in GABAAR neuroplasticity, changes driven by chronic NAS exposure, alter the balance of neuronal excitability and inhibition (Maguire and Mody, 2008) in animal models. Altered NAS concentrations could affect downstream GABAergic inhibitory tone within the hypothalamus (Herman and Cullinan, 1997; Makara and Stark, 1974) affecting HPA axis function, evidenced by current depressive and anxiety symptomatology as measured by the HAM-D and HAM-A. This is consistent with a growing literature, as reviewed by Crowley & Girdler (2014), supporting NAS, GABAergic and HPA axis dysregulation in stress-related psychiatric disorders including MDD and PMDD (Crowley and Girdler, 2014).
We are not aware of any previous studies which measured peripartum plasma GABA concentrations. In contrast to the dramatic decrease in all measured NAS at the first postpartum visit, GABA concentrations continued to rise in all subjects through the final postpartum study visit and differed between groups across the peripartum period. Why GABA concentrations rose across the peripartum period requires further study. Our finding of lower peripartum plasma GABA concentrations in the AR-PPD women is consistent with findings in non-puerperal MDD (Sanacora et al., 1999) and in women with PMDD and a prior history of MDD (Halbreich et al., 1996) where plasma GABA concentrations are lower throughout the menstrual cycle compared to comparison women. Although NAS were not measured in the aforementioned Halbreicht et. al. study (Halbreich et al., 1996), plasma GABA levels were low during the symptomatic late luteal phase when progesterone is elevated as compared to the follicular phase. This supports our finding of elevated progesterone and pregnanolone and lower GABA concentrations in symptomatic peripartum women. As it has been postulated that decreased GABA function may represent a common endophenotype between MDD and PMDD (Halbreich et al., 1996), we did not expect peripartum changes in NAS to be correlated with changes in GABA. Given that all AR-PPD subjects had a history of MDD or PPD and/or current peripartum symptomatology, it is not clear if the finding of lower peripartum plasma GABA is a state or trait biomarker for depression, peripartum or otherwise. Very few AR-PPD subjects had a history of PMDD so further sub-analysis was not possible. A single, cross-sectional study demonstrated that CSF GABA concentration in healthy pregnant women was lower than non-pregnant comparison women (Altemus et al., 2004). Given that we did not examine pre-partum or postpartum plasma GABA concentrations beyond 8 weeks, we cannot determine when in the postpartum plasma GABA would become comparable to that of non-pregnant comparison women. Future studies should compare plasma GABA in peripartum and non-peripartum cycling females to understand the potential dynamic changes in this neurotransmitter during times of altered NAS production. For example, if peripartum plasma GABA concentrations are lower than healthy non-peripartum women, as seen in CSF GABA, does this altered GABAergic state, facilitated by pregnancy, represent a period of risk for peripartum women (Epperson et al., 2006)? Ideally, future studies should examine plasma and CSF given a mixed literature on the correlation of plasma to CSF GABA concentrations.
Strengths of this study include stringent inclusion/exclusion criteria; subjects underwent thorough research diagnostic and symptomatic evaluations. The EPDS is widely utilized and validated in the peripartum population to screen for depressive and anxiety symptomatology and identifies women with various psychiatric diagnoses (Lydsdottir et al., 2014). In addition to the SCID-IV, we utilized both research-clinician administered instruments as well as self-report measures. We hypothesized that sole use of the EPDS, which measures both depression and anxiety, may obscure hypothesized relationships with NAS and GABA: in this study, plasma concentrations were more often associated with symptoms as measured by the HAM-D17 and HAM-A rather than the EPDS. An additional strength is the use of an ultra-sensitive LC-MS/MS assay (Auchus, 2014) to quantify plasma GABA and NAS in the same subjects repeatedly across time. To our knowledge, this study represents the largest prospective study of its kind to date.
We designed the study with an approach consistent with the NIMH Research Domain Criteria (Cuthbert and Insel, 2013) and included a heterogeneous at-risk population. However, since the AR-PPD group included a variety of clinical at-risk phenotypes, the underlying neuroendocrine mechanisms for each may differ (Schiller et al., 2015) and inclusion as a group obscures the ability to identify potential differences in NAS and GABA profiles for individual clinical phenotypes (e.g. euthymic peripartum women with a history of PPD vs. symptomatic peripartum women with no prior history of PPD vs. symptomatic peripartum women with a history of MDD, etc.). Importantly, despite the heterogeneity in risk, we were able to identify altered peripartum NAS and GABA profiles in an at-risk sample. Additionally, although we pre-screened a diverse ethnic and racial pregnant population, women who ultimately were interested in and participated in the study were mainly white, non-Hispanic women, which could limit the generalizability of the results.
In our study, few women developed a SCID-IV diagnosis of MDE with post-partum onset, likely due to the strict study inclusion and exclusion criteria required for valid NAS and GABA measurement. Thus our study excluded many women with additional important risk factors for the development of peripartum depressive and anxiety disorders, including: current psychotropic medication use, recent substance abuse and active medical disorders. Additionally, some women may have developed a MDE beyond the postpartum time frame under study which was restricted to when women could feasibly remain off of hormonal contraceptives as their use would have invalidated the blood GABA and NAS data. Given the small number of women who developed an MDE with post-partum onset, it was not possible to examine how this subset of women may differ from those women AR-PPD who did not. Future studies should examine NAS and GABA profiles in individual risk phenotypes and across a broader range of symptom severity. Also, given that NAS biosynthesis, metabolism and function change across the peripartum period (Hill et al., 2007), future studies should examine blood levels at more tightly defined study visit time frames as compared to examining levels across the peripartum time period. Our across-peripartum time approach in concert with the variable number of women sampled at each gestational age time point, likely added to observed inter-subject variability in plasma NAS levels.
Understanding the interactions of NAS with the GABAergic system across the peripartum period is critical to understanding potential neuroendocrine mechanisms of risk for peripartum depressive and anxiety disorders. This study builds upon limited research in the role of NAS and GABA in peripartum depression and anxiety in a novel and understudied population, women at-risk for developing PPD.
CONTRIBUTORS
Dr. Deligiannidis designed and directed the study, performed subject visits, wrote the protocol, interpreted results, managed the literature searches and wrote the first draft of the manuscript. Ms. Kroll-Desrosiers and Dr. Bruce Barton designed and executed the statistical analyses and contributed to writing of the manuscript. Dr. Jaitly and Ms. Svenson performed subject visits, managed institutional review board and study documents, created the study database and assisted with manuscript edits. Dr. Hall assisted with interpretation of the data and manuscript edits. Dr. Rothschild assisted with interpretation of the data and assisted with conduct of the study and manuscript edits. All authors contributed to and have approved the final manuscript.
Supplementary Material
Highlights.
Peripartum progesterone and pregnanolone concentrations were higher in at-risk women.
Peripartum GABA concentrations were lower in at-risk women.
Peripartum GABA concentration increased across peripartum time in both groups
Acknowledgments
We thank Dr. Peter J. Schmidt for his thoughtful comments during preparation of the manuscript and gratefully thank the research subjects for their participation. We thank Dr. Robert H. Purdy, in memoriam, for providing several of the steroid standards used in the analyses.
ROLE OF THE FUNDING SOURCE
This study was supported by National Center for Advancing Translational Sciences, National Institutes of Health Grant (UL1TR000161) and National Institutes of Health Grant (5K23MH097794). Mass spectrometry instrumentation was supported by funding from an NIH Shared Instrumentation Grant (1S10RR027107).
The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The sponsoring agency had no further role in the study design and analysis, the writing of the report, or the decision to submit the paper for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position of the NIH.
Dr. Deligiannidis has received research support from the National Center for Advancing Translational Sciences, National Institutes of Health (UL1TR000161), National Institute of Mental Health (5K23MH097794), SAGE Therapeutics, Worcester Foundation for Biomedical Research and receives royalties from an NIH Employee Invention. Dr. Rothschild has received research support from the National Institute of Mental Health ((5U01MH062624), Cyberonics, St. Jude Medical, AssureRx, Alkermes, Janssen, and Takeda; has served as a consultant to GlaxoSmithKline, Eli Lilly, OmniCare, Pfizer, and Sunovian, has received royalties for the Rothschild Scale for Antidepressant Tachyphylaxis (RSAT)™, has received royalties from Up-to-Date, and has received royalties from American Psychiatric Press, Inc. for Psychoneuroendocrinology: The Scientific Basis of Clinical Practice (2003), Clinical Manual for Diagnosis and Treatment of Psychotic Depression (2009), The Evidenced-Based Guide to Antipsychotic Medications (2010), and The Evidenced-Based Guide to Antidepressant Medications (2011).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work described in this paper was presented, in part, as a poster presentation at the Society of Biological Psychiatry (SOBP) 70th Annual Meeting, Toronto, Canada on May 12, 2015 and in part, at the American College of Neuropsychopharmacology (ACNP) 54th Annual Meeting, Hollywood, Florida on December 7, 2015.
CONFLICT OF INTEREST
None. The authors of this manuscript do not have conflicts of interest relevant to the subject of this manuscript.
Drs. Mo, Nguyen, Jaitly, Hall, Barton and Shaffer and Ms. Kroll-Desrosiers and Ms. Svenson report no financial disclosures.
Supplementary material associated with this article can be found in the online version at the Psychoneuroendocrinology website.
Contributor Information
Kristina M. Deligiannidis, Email: kristina.deligiannidis@umassmemorial.org.
Aimee R. Kroll-Desrosiers, Email: aimee.kroll@umassmed.edu.
Shunyan Mo, Email: shunyan.mo@umassmed.edu.
Hien P. Nguyen, Email: hien.nguyen@umassmed.edu.
Abby Svenson, Email: abbysve@gmail.com.
Nina Jaitly, Email: nina.jaitly@nih.gov.
Janet E. Hall, Email: janet.hall@nih.gov.
Bruce A. Barton, Email: bruce.barton@umassmed.edu.
Anthony J. Rothschild, Email: anthony.rothschild@umassmemorial.org.
Scott A. Shaffer, Email: scott.shaffer@umassmed.edu.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-5. Fifth. Washington, D.C.: American Psychiatric Publishing; 2013. [Google Scholar]
- Altemus M, Fong J, Yang R, Damast S, Luine V, Ferguson D. Changes in cerebrospinal fluid neurochemistry during pregnancy. Biol Psychiatry. 2004;56:386–392. doi: 10.1016/j.biopsych.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Auchus RJ. Steroid assays and endocrinology: best practices for basic scientists. Endocrinology. 2014;155:2049–2051. doi: 10.1210/en.2014-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Concas A, Serra M, Biggio G. Stress and neurosteroids in adult and aged rats. Experimental gerontology. 1998;33:697–712. doi: 10.1016/s0531-5565(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br J Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini WH, Nurnberger JI, Jr, Hare T, Gershon ES, Post RM. Plasma and CSF GABA in affective illness. Br J Psychiatry. 1982;141:483–487. doi: 10.1192/bjp.141.5.483. [DOI] [PubMed] [Google Scholar]
- Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab. 2005;90:695–699. doi: 10.1210/jc.2004-1388. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bohlen P, Huot S, Palfreyman MG. The relationship between GABA concentrations in brain and cerebrospinal fluid. Brain Res. 1979;167:297–305. doi: 10.1016/0006-8993(79)90824-2. [DOI] [PubMed] [Google Scholar]
- Buckwalter JG, Stanczyk FZ, McCleary CA, Bluestein BW, Buckwalter DK, Rankin KP, Chang L, Goodwin TM. Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendocrinology. 1999;24:69–84. doi: 10.1016/s0306-4530(98)00044-4. [DOI] [PubMed] [Google Scholar]
- Buttner MM, Mott SL, Pearlstein T, Stuart S, Zlotnick C, O'Hara MW. Examination of premenstrual symptoms as a risk factor for depression in postpartum women. Arch Womens Ment Health. 2013;16:219–225. doi: 10.1007/s00737-012-0323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Crowley SK, Girdler SS. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology (Berl) 2014;231:3619–3634. doi: 10.1007/s00213-014-3572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, Kosma CA, Rothschild AJ, Moore CM. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res. 2013;47:816–828. doi: 10.1016/j.jpsychires.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, Rothman DL, Mason GF. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186:425–433. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- Eser D, Romeo E, Baghai TC, di Michele F, Schule C, Pasini A, Zwanzger P, Padberg F, Rupprecht R. Neuroactive steroids as modulators of depression and anxiety. Neuroscience. 2006;138:1041–1048. doi: 10.1016/j.neuroscience.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Butler IJ, Enna SJ. Effect of drugs on rat brain, cerebrospinal fluid and blood GABA content. J Neurochem. 1979;33:29–33. doi: 10.1111/j.1471-4159.1979.tb11702.x. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Smith LA, Seifert WE, Caprioli RM, Enna SJ. Measurement of gamma-aminobutyric acid (GABA) in blood. Life Sci. 1978;22:2121–2128. doi: 10.1016/0024-3205(78)90456-3. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York, NY: New York State Psychiatric Institute; 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition. [Google Scholar]
- Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G. Molecular and functional adaptation of the GABA(A) receptor complex during pregnancy and after delivery in the rat brain. Eur J Neurosci. 1998;10:2905–2912. doi: 10.1111/j.1460-9568.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- Forty L, Jones L, Macgregor S, Caesar S, Cooper C, Hough A, Dean L, Dave S, Farmer A, McGuffin P, Brewster S, Craddock N, Jones I. Familiality of postpartum depression in unipolar disorder: results of a family study. Am J Psychiatry. 2006;163:1549–1553. doi: 10.1176/ajp.2006.163.9.1549. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3alpha-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21:268–279. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37:543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Petty F, Yonkers K, Kramer GL, Rush AJ, Bibi KW. Low plasma gamma-aminobutyric acid levels during the late luteal phase of women with premenstrual dysphoric disorder. Am J Psychiatry. 1996;153:718–720. doi: 10.1176/ajp.153.5.718. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hill M, Cibula D, Havlikova H, Kancheva L, Fait T, Kancheva R, Parizek A, Starka L. Circulating levels of pregnanolone isomers during the third trimester of human pregnancy. J Steroid Biochem Mol Biol. 2007;105:166–175. doi: 10.1016/j.jsbmb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Hill M, Parizek A, Bicikova M, Havlikova H, Klak J, Fait T, Cibula D, Hampl R, Cegan A, Sulcova J, Starka L. Neuroactive steroids, their precursors, and polar conjugates during parturition and postpartum in maternal and umbilical blood: 1. Identification and simultaneous determination of pregnanolone isomers. J Steroid Biochem Mol Biol. 2000;75:237–244. doi: 10.1016/s0960-0760(00)00192-8. [DOI] [PubMed] [Google Scholar]
- Hill M, Parizek A, Klak J, Hampl R, Sulcova J, Havlikova H, Lapcik O, Bicikova M, Fait T, Kancheva R, Cibula D, Pouzar V, Meloun M, Starka L. Neuroactive steroids, their precursors and polar conjugates during parturition and postpartum in maternal and umbilical blood: 3.3beta-hydroxy-5-ene steroids. J Steroid Biochem Mol Biol. 2002;82:241–250. doi: 10.1016/s0960-0760(02)00188-7. [DOI] [PubMed] [Google Scholar]
- Loscher W. GABA in plasma and cerebrospinal fluid of different species. Effects of gamma-acetylenic GABA, gamma-vinyl GABA and sodium valproate. J Neurochem. 1979;32:1587–1591. doi: 10.1111/j.1471-4159.1979.tb11104.x. [DOI] [PubMed] [Google Scholar]
- Loscher W, Frey HH. Transport of GABA at the blood-CSF interface. J Neurochem. 1982;38:1072–1079. doi: 10.1111/j.1471-4159.1982.tb05350.x. [DOI] [PubMed] [Google Scholar]
- Loscher W, Rating D, Siemes H. GABA in cerebrospinal fluid of children with febrile convulsions. Epilepsia. 1981;22:697–702. doi: 10.1111/j.1528-1157.1981.tb04143.x. [DOI] [PubMed] [Google Scholar]
- Lydsdottir LB, Howard LM, Olafsdottir H, Thome M, Tyrfingsson P, Sigurdsson JF. The mental health characteristics of pregnant women with depressive symptoms identified by the Edinburgh Postnatal Depression Scale. J Clin Psychiatry. 2014;75:393–398. doi: 10.4088/JCP.13m08646. [DOI] [PubMed] [Google Scholar]
- Magnaghi V, Parducz A, Frasca A, Ballabio M, Procacci P, Racagni G, Bonanno G, Fumagalli F. GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J Neurochem. 2010;112:980–990. doi: 10.1111/j.1471-4159.2009.06512.x. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Makara GB, Stark E. Effects of gamma-aminobutyric acid (GABA) and GABA antagonist drugs on ACTH release. Neuroendocrinology. 1974;16:178–190. doi: 10.1159/000122564. [DOI] [PubMed] [Google Scholar]
- Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, Cintron D, Thompson KD, Khine KK, Schmidt PJ. 5alpha-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder. Neuropsychopharmacology. 2016;41:1093–1102. doi: 10.1038/npp.2015.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer JL, Beijers C, van Pampus MG, Verbeek T, Stolk RP, Milgrom J, Bockting CL, Burger H. Predictive accuracy of Edinburgh postnatal depression scale assessment during pregnancy for the risk of developing postpartum depressive symptoms: a prospective cohort study. BJOG : an international journal of obstetrics and gynaecology. 2014;121:1604–1610. doi: 10.1111/1471-0528.12759. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Boschloo L, Jones I, Sullivan PF, Penninx BW. The EPDS-Lifetime: assessment of lifetime prevalence and risk factors for perinatal depression in a large cohort of depressed women. Arch Womens Ment Health. 2013;16:465–473. doi: 10.1007/s00737-013-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, Ericksen J, Ellwood D, Buist A. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–157. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Mostallino MC, Sanna E, Concas A, Biggio G, Follesa P. Plasticity and function of extrasynaptic GABA(A) receptors during pregnancy and after delivery. Psychoneuroendocrinology. 2009;34(Suppl 1):S74–S83. doi: 10.1016/j.psyneuen.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum "blues". Obstet Gynecol. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Parizek A, Hill M, Kancheva R, Havlikova H, Kancheva L, Cindr J, Paskova A, Pouzar V, Cerny I, Drbohlav P, Hajek Z, Starka L. Neuroactive pregnanolone isomers during pregnancy. J Clin Endocrinol Metab. 2005;90:395–403. doi: 10.1210/jc.2004-0444. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pearson Murphy BE, Steinberg SI, Hu FY, Allison CM. Neuroactive ring A-reduced metabolites of progesterone in human plasma during pregnancy: elevated levels of 5 alpha-dihydroprogesterone in depressed patients during the latter half of pregnancy. J Clin Endocrinol Metab. 2001;86:5981–5987. doi: 10.1210/jcem.86.12.8122. [DOI] [PubMed] [Google Scholar]
- Petty F. Plasma concentrations of gamma-aminobutyric acid (GABA) and mood disorders: a blood test for manic depressive disease? Clin Chem. 1994;40:296–302. [PubMed] [Google Scholar]
- Petty F, Kramer G, Feldman M. Is plasma GABA of peripheral origin? Biol Psychiatry. 1987;22:725–732. doi: 10.1016/0006-3223(87)90204-6. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. 2015;20:48–59. doi: 10.1017/S1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Loscher W. Plasma and cerebrospinal fluid gamma-aminobutyric acid in neurological disorders. Journal of neurology, neurosurgery, and psychiatry. 1982;45:931–935. doi: 10.1136/jnnp.45.10.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, Pollack MH, Chandler L, Williams J, Ali A, Frank DM. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depress Anxiety. 2001;13:166–178. [PubMed] [Google Scholar]
- Sheehan DV. The Anxiety Disease. New York: Charles Scribner & Sons; 1983. [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smolen A, Smolen TN, Han PC. Alterations in regional brain GABA concentration and turnover during pregnancy. Pharmacol Biochem Behav. 1993;44:63–69. doi: 10.1016/0091-3057(93)90281-w. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. State Trait Anxiety Inventory. Mind Garden Inc. 1970 [Google Scholar]
- Taylor A, Atkins R, Kumar R, Adams D, Glover V. A new Mother-to-Infant Bonding Scale: links with early maternal mood. Arch Womens Ment Health. 2005;8:45–51. doi: 10.1007/s00737-005-0074-z. [DOI] [PubMed] [Google Scholar]
- Uhlhaas S, Lange H, Wappenschmidt J, Olek K. Free and conjugated CSF and plasma GABA in Huntington's chorea. Acta Neurol Scand. 1986;74:261–265. doi: 10.1111/j.1600-0404.1986.tb03511.x. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, Hanusa BH, Findling RL, Rapport D. Prevention of recurrent postpartum depression: a randomized clinical trial. J Clin Psychiatry. 2001;62:82–86. doi: 10.4088/jcp.v62n0202. [DOI] [PubMed] [Google Scholar]
- Zhao C, Gammie SC. Glutamate, GABA, and glutamine are synchronously upregulated in the mouse lateral septum during the postpartum period. Brain Res. 2014;1591:53–62. doi: 10.1016/j.brainres.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.