Abstract
Objectives
To investigate changes in cartilage damage and bone marrow lesions (BMLs) on MRI in the patellofemoral and tibiofemoral joints over 7 years.
Methods
The Multicenter Osteoarthritis (MOST) Study is a cohort study of persons aged 50-79 years at baseline with or at high risk for knee osteoarthritis. Knees were eligible for the current study if they had knee MRI (1.0T) assessed for cartilage damage and BMLs at the baseline and 84-month visits. Knees were categorized as having MRI-detected structural damage (cartilage and BMLs) isolated to the patellofemoral joint (PFJ), isolated to the tibiofemoral joint (TFJ), mixed or no damage at baseline and 84-months. We determined the changes in PFJ and TFJ structural damage over 7 years and used logistic regression to assess the relation of baseline compartment distribution to incident isolated PFJ, isolated TFJ and mixed damage.
Results
Among 339 knees that had full-thickness cartilage loss isolated to the PFJ or TFJ at baseline, only 68 (20.1%) developed full-thickness cartilage loss in the other compartment while 271 (79.9%) continued to only have the initial compartment affected. Compared to knees without full-thickness cartilage damage (n=582), those with isolated TFJ and PFJ full-thickness cartilage damage had 2.7 (1.5, 4.9) and 5.8 (3.6, 9.6) times the odds of incident mixed full-thickness cartilage damage, respectively. Similar results were seen when using other definitions of MRI-defined structural damage.
Conclusions
Most knees with structural damage at baseline do not develop it in the other compartment. Knees that develop mixed structural damage are more likely to start with it isolated to the PFJ.
Keywords: knee osteoarthritis, MRI, pain
Introduction
Knee osteoarthritis (OA) can occur in either the patellofemoral joint (PFJ), the tibiofemoral joint (TFJ) or both. Little is known about the natural history of knee OA in regards to the compartment where disease begins and whether it tends to remain isolated to one compartment or subsequently develops in the other compartment. Knowledge about where OA starts and progresses to include both the PFJ and TFJ will provide information on targets for early intervention and prevention of disease burden. For example, recent studies have demonstrated that taping and bracing of the PFJ may improve knee pain and BMLS [1-3]. Future work is warranted to determine how these non-invasive treatment strategies affect PFJ and TFJ joint structures over time.
Duncan et al reported on the incidence, progression and sequence of development of radiographic OA in the PFJ and TFJ in symptomatic adults [4]. They concluded that OA starts in the PFJ with the development of TFJ OA over time. They proposed that isolated symptomatic PFJ OA may be a marker for future development of TFJ OA and thus a target for the early management of knee OA. A limitation of this study was the use of radiographs, which could have missed early changes in the OA disease process. To date, there are no published studies examining the natural history of OA development in the PFJ and TFJ evaluated by MRI, which is more sensitive than radiographs for identifying structural damage, in both the TFJ and PFJ [5]. MRI affords direct assessment of cartilage damage and bone marrow lesions (BMLs), which are hallmark structural features of OA [6]. Additionally, it is unknown if changes in the distribution of cartilage damage and BMLs in the PFJ and TFJ are related to changes in knee pain. Because the experience of pain ultimately brings individuals to seek treatment, knowledge of how pain relates to structural changes in the PFJ and TFJ will ultimately help to prioritize how treatments are developed for knee OA to prevent disease burden.
The purpose of this study was to investigate patterns of change in cartilage damage and bone marrow lesions (BMLs) in the patellofemoral and tibiofemoral joints over 7 years. Specifically, we describe which compartment tends to be involved first and whether disease that initially affects one compartment remains isolated or develops in the other compartment. A secondary aim was to investigate how changes in cartilage damage and BMLs among knee joint compartments relate to incident frequent knee pain.
Methods
Study Sample
Knees for the current study were from participants in the Multicenter OA (MOST) Study. 3,026 participants were recruited from Iowa City, Iowa and Birmingham, Alabama. The MOST cohort consists of older adults that have or are at risk of developing knee OA. Some subjects had knee pain and radiographic OA (52% with Kellgren Lawrence grade ≥2) at baseline where others were at high risk for developing knee pain and OA based on being overweight, or having a history of knee injury or surgery. Subjects were ineligible if they had bilateral knee replacements or rheumatoid/other inflammatory arthritis [7].
MRI Acquisition
Knee MRIs were acquired at the baseline and 84-month visits. A 1.0 Tesla extremity MRI system (OrthOne™, ONI Medical Systems Wilmington, MA) was used with a phased array knee coil to obtain the following sequences [8, 9]: Fat-suppressed fast spin echo intermediate weighted sequences in two planes, sagittal (TR 4800 ms, TE 35 ms, 3 mm slice thickness, 0 mm interslice gap, 32 slices, 288 × 192 matrix, 140 mm2 FOV, echo train length 8) and axial proton density weighted (TR 4680 ms, TE 13 ms, 3 mm slice thickness, 0 mm interslice gap, 20 slices, 288 × 192 matrix, 140 mm2 FOV, echo train length 8) and a STIR sequence in the coronal plane (TR 6650 ms, TE 15 ms, TI 100 ms, 3 mm slice thickness, 0 mm interslice gap, 28 slices, 256 × 192 matrix, 140 mm2 FOV, echo train length 8).
Semi-quantitative MRI Assessment
In MOST, one randomly selected knee per individual was selected to be read for MRI features. Two musculoskeletal radiologists (FWR, AG) used the Whole-Organ Magnetic Resonance Imaging Score (WORMS) [10] to assess cartilage morphology and bone marrow lesions (BMLs) [8, 9] in fourteen regions in the PFJ and TFJ. Inter-reader weighted kappa values for WORMS scores ranged from 0.62-0.78 [9]. Any cartilage damage was defined as a WORMS score ≥2, full-thickness cartilage damage was defined by WORMS scores of 2.5, 5, or 6, which denotes focal full thickness defects, different degrees of diffuse full-thickness damage, respectively. Any size BML was defined as WORMS scores of ≥1. At baseline and 7-year follow up, knees were categorized as having structural damage isolated to the PFJ, isolated to the TFJ, mixed (both PFJ and TFJ) or no damage in either compartment. We used three different definitions of structural damage: 1. Full-thickness cartilage damage (primary outcome), 2. Any cartilage damage, and 3. Any BML (Figures 1 and 2).
Figure 1.
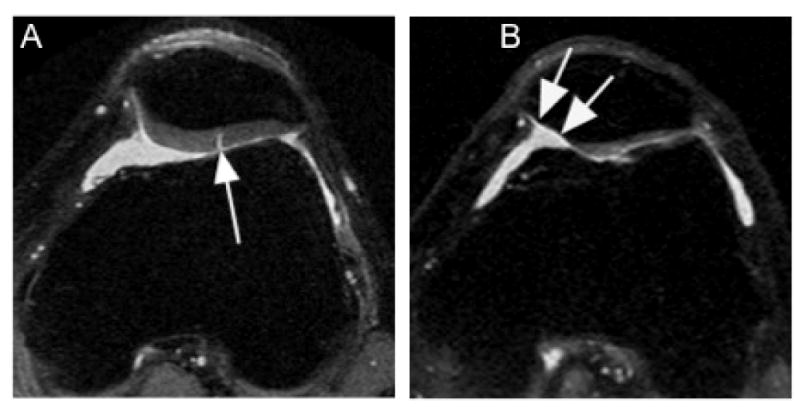
Isolated patello-femoral strucutral damage. A. Axial proton density-weighted image shows fissure-like full thickness defect at the lateral patellar facet (arrow). No additional structural damage was observed. B.Diiffuse and extensive but still isolated full thickness cartilage damage in the medial patellar facet is shown in this axial proton-density-weighted image (arrows).
Figure 2.
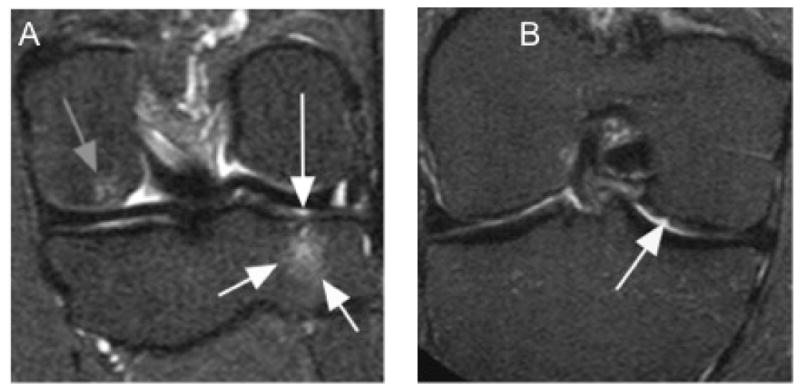
Isolated early tibio-femoral structural damage. A. Coronal STIR image shows bone marrow lesion at the medial tibial plateau (short white arrows) with adjacent superficial focal cartilage lesion (long white arrow). In addition there is a small bone marrow lesion at the medial femur (gray arrow). B. Another example shows a superficial cartilage defect at the central medial femur (arrow).
We then created the following categories of change in the compartmental distribution of structural damage: no damage, isolated PFJ, isolated TFJ and mixed at both time points (no change); incident isolated TFJ, incident isolated PFJ and incident mixed damage. We further divided the incident mixed group into knees that had no damage, isolated PFJ and isolated TFJ damage at baseline.
Frequent Knee Pain (FKP) Assessment
At the baseline and 84-month visits frequent knee pain was assessed in each knee by asking participants: “Do you have pain, aching or stiffness on most days of the month?”
Statistical Analysis
We first described the change in compartmental distribution of structural damage over 7 years using the definitions described above and used logistic regression to assess the relation of baseline compartment distribution to incident isolated PFJ, isolated TFJ and mixed damage, adjusting for age, sex and BMI. We then determined the relation of change of compartment distribution of structural damage over 7 years to incident frequent knee pain (knees were eligible if frequent knee pain was not present at baseline and present at 84-months) using logistic regression adjusting for age, sex and BMI. In sensitivity analyses we used a structural damage definition that required the presence of both cartilage damage and BMLs for a compartment to be considered to have structural damage. Results of this analysis were similar to the main analyses presented below and are not presented here.
Results
We restricted our analysis to knees that had knee MRI assessed for cartilage damage and BMLs at the baseline and 84-month study visits. In MOST one randomly selected knee from each subject who attended both the 60 and 84-month visits had their MRI read for cartilage damage and BMLs (n=1185 knees). Of these knees, 1012 and 762 knees had complete MRI readings at the baseline and 84-month visits for cartilage morphology and BMLs, respectively (Figure 3). Due to resource restrictions there were less knees that had BMLs assessed. Age, sex and BMI distribution for the entire MOST cohort and those included in the current study are presented in Table 1. Since our focus was on the development of new disease findings in compartments initially unaffected or isolated to one compartment, we excluded knees that at baseline already had involvement of both the PFJ and TFJ (mixed damage). 592, 91 and 130 knees were removed with mixed any cartilage damage, full-thickness damage and BMLs, respectively. We also excluded 67 knees where BMLs regressed or changed their compartment involvement because there were too few knees in each pattern of change (Supplemental Table). This left 420, 921 and 565 knees eligible for the any cartilage damage, full-thickness cartilage damage and BML analyses, respectively.
Figure 3. Knee eligibility flow chart.
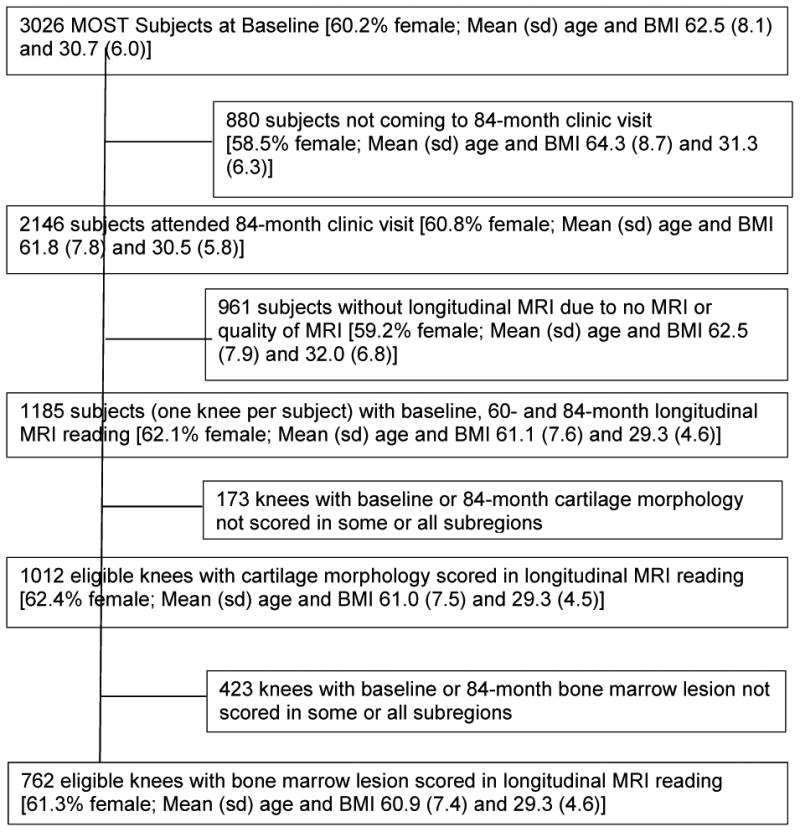
Table 1. Participant Characteristics.
| Overall MOST Cohort at baseline (n=3026) | Knees included in cartilage analysis (n=1012) | Knees included in BML* analysis (n=762) | |
|---|---|---|---|
| Age mean ± SD years | 62.5 (8.1) | 61.0 (7.5) | 60.9 (7.4) |
| Female (%) | 60.2% | 62.4% | 61.3% |
| BMI mean ± SD kg/m2 | 30.7 (6.0) | 29.3 (4.5) | 29.3 (4.6) |
BML=bone marrow lesion
Figure 4 shows the changes in PFJ and TFJ full-thickness cartilage damage over 84 months. The incidence of isolated PFJ, isolated TFJ and mixed full-thickness cartilage damage was 11.3, 13.9 and 5.3%, respectively. The remaining 69.3% did not develop full-thickness cartilage damage at follow-up. Among 339 knees that had full-thickness cartilage damage isolated to the PFJ or TFJ at baseline, only 68 (20.1%) developed full-thickness cartilage damage in the other compartment while 271 (79.9%) continued to only have the initial compartment affected with full-thickness cartilage damage. Among 68 knees that started with isolated full-thickness damage in the PFJ or TFJ and developed full-thickness cartilage damage in the other compartment, 48 (70.6%) and 20 (29.4%) started with isolated full-thickness damage in the PFJ and TFJ, respectively.
Figure 4. Changes in full-thickness cartilage damage in the patellofemoral and tibiofemoral joints over 84 months (n=921 knees).
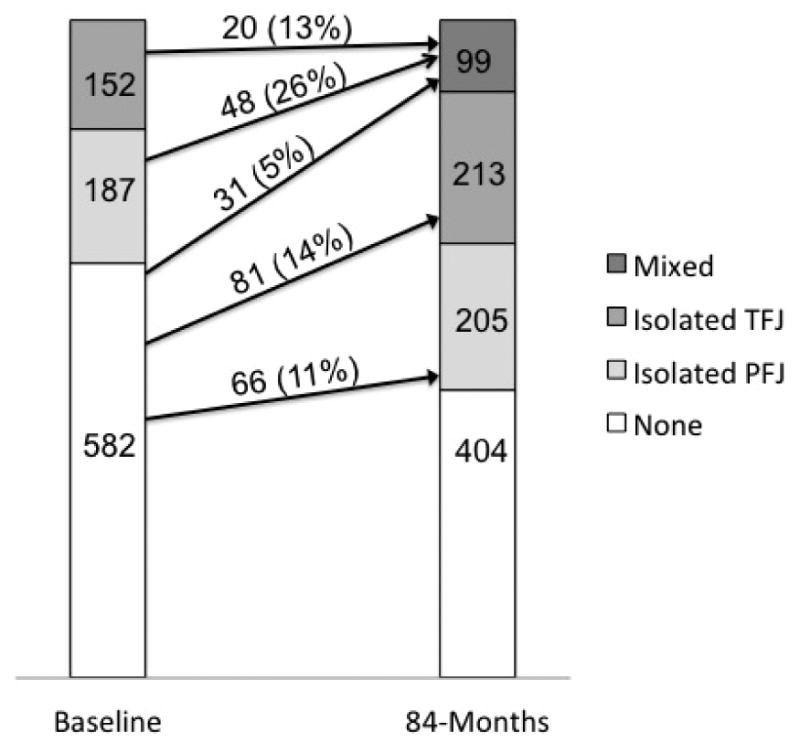
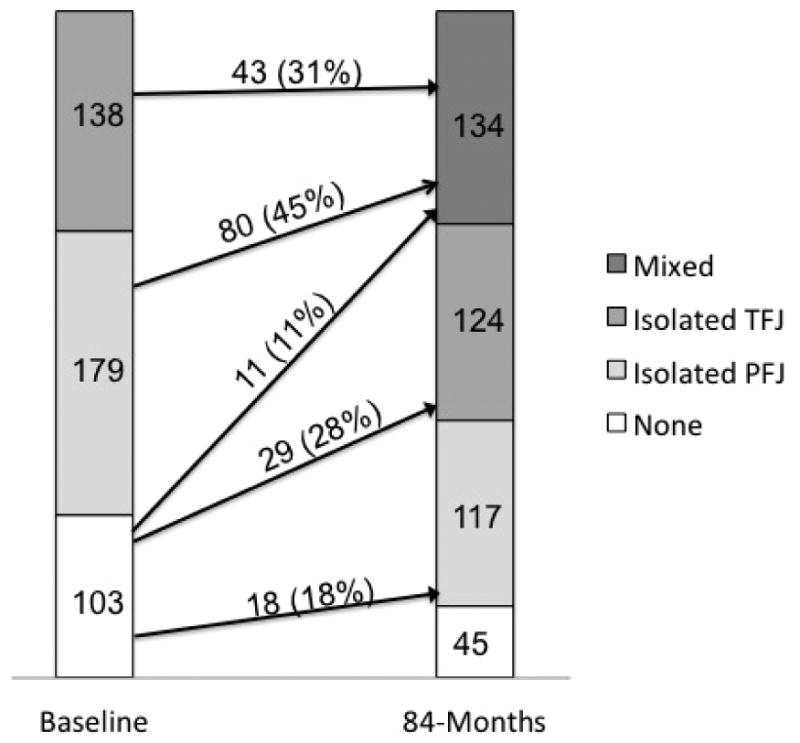
Figure 5 shows the changes in PFJ and TFJ any cartilage damage over 84 months. The incidence of isolated PFJ, isolated TFJ and mixed any cartilage damage was 17.5, 28.2 and 10.7%, respectively. The remaining 43.7% did not develop any cartilage damage at follow up. Among 317 knees that had any cartilage damage isolated to the PFJ or TFJ at baseline, 123 (38.8%) developed any cartilage damage in the other compartment while 194 (61.2%) continued to have only the initial compartment affected with any cartilage damage. Among 123 knees that started with isolated any damage in the PFJ or TFJ and developed any cartilage damage in the other compartment, 80 (65.0%) and 43 (35.0%) started with isolated any cartilage damage in the PFJ and TFJ, respectively.
Figure 5. Changes in any cartilage damage in the patellofemoral and tibiofemoral joints over 84 months (n=420 knees).
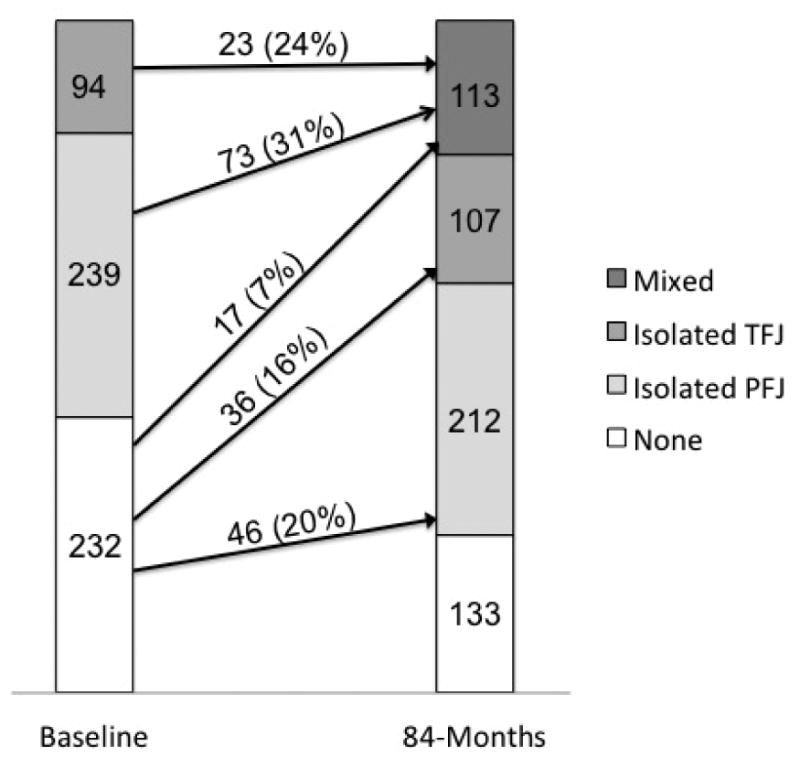
Figure 6 shows the changes in PFJ and TFJ BMLs over 84 months. The incidence of isolated PFJ, isolated TFJ and mixed BMLs was 19.8, 15.5 and 7.3%, respectively. The remaining 57.3% did not develop a BML at follow up. Among 333 knees that had BMLs isolated to the PFJ or TFJ at baseline, only 96 (28.8%) developed BMLs in the other compartment while 237 (71.7%) continued to only have the initial compartment affected with BMLs. Among 96 knees that started with isolated BMLs in the PFJ or TFJ and developed a BML in the other compartment, 73 (76.0%) and 23 (24.0%) started with a BML in the PFJ and TFJ, respectively.
Figure 6. Changes in BMLs in the patellofemoral and tibiofemoral joints over 84 months (n=565 knees).
The relation of baseline compartment distribution to incident isolated PFJ, isolated TFJ and mixed damage is presented in Table 2. Compared to knees without full-thickness cartilage damage, those with isolated TFJ full-thickness cartilage damage had 2.7 (1.5, 4.9) times the odds of incident mixed full-thickness cartilage damage and those with isolated PFJ full-thickness cartilage damage had 5.8 (3.6, 9.6) times the odds of developing mixed full-thickness cartilage damage. When directly comparing knees with baseline full-thickness damage in the TFJ to those with full-thickness damage in the PFJ,, those with isolated damage in the PFJ had 2.1 (1.2, 3.9) times the odds of developing mixed full-thickness cartilage damage (result not in table). Similar results were seen when using the any cartilage damage and any BML definition of structural damage.
Table 2. Relation of baseline compartment status to follow-up compartment status.
| MRI Feature | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Full-thickness cartilage damage | Any cartilage damage | Any BML | |||||
|
| |||||||
| Baseline Status | Follow Up Status | Incidence | Adjusted OR* | Incidence | Adjusted OR* | Incidence | Adjusted OR* |
| No damage | Incident PFJ damage | 97/582<br>(16.7%) | Reference | 29/103<br>(28.2%) | Reference | 63/232<br>(27.2%) | Reference |
| Isolated TFJ damage | 20/152<br>(13.2%) | 0.80<br>(0.47, 1.3) | 43/138<br>(31.2%) | 1.2<br>(0.65, 2.1) | 23/94<br>(24.5%) | 0.84<br>(0.48, 1.5) | |
|
| |||||||
| No damage | Incident TFJ damage | 112/582<br>(19.2%) | Reference | 40/103<br>(38.8%) | Reference | 53/232<br>(22.8%) | Reference |
| Isolated PFJ damage | 48/187<br>(25.7%) | 1.3<br>(0.89, 2.0) | 80/179<br>(44.7%) | 1.2<br>(0.75, 2.1) | 73/239<br>(30.5%) | 1.3<br>(0.86, 2.0) | |
|
| |||||||
| No damage | Incident mixed damage | 31/582<br>(5.3%) | Reference | 11/103<br>(10.7%) | Reference | 17/232<br>(7.3%) | Reference |
| Isolated PFJ damage | 48/187<br>(25.7%) | 5.8<br>(3.6, 9.6) | 80/179<br>(44.7%) | 6.5<br>(3.2, 13.1) | 73/239<br>(30.5%) | 5.4<br>(3.1, 9.7) | |
| Isolated TFJ damage | 20/152<br>(13.2%) | 2.7<br>(1.5, 4.9) | 43/138<br>(31.2%) | 4.0<br>(1.9, 8.4) | 23/94<br>(24.5%) | 4.2<br>(2.1, 8.4) | |
Adjusted for age, sex and BMI
Of knees that developed full-thickness cartilage damage in PFJ and/or TFJ at 7 years, 24% (38/158) reported the incidence of FKP at 7 years. The corresponding figure for those developing BMLs at 7 years was 19% (27/140). In general, after adjustment for age, gender, and baseline BMI, there were no strong associations between the pattern of incident structural damage (PFJ, TFJ or mixed) and incident FKP at 7 years (Table 3). Additionally, there were low numbers of events to estimate the relationship between patterns of incident structural damage and incident FKP at 7 years, especially for the any cartilage damage definition. Knees that had developed incident isolated TFJ full-thickness cartilage damage at 7 years had the highest rate of FKP at 7 years and this pattern was associated with a two-fold increase in the odds of incident FKP compared to knees that had no full-thickness cartilage damage at both time points (adjusted OR 2.2 (1.2, 4.2)).
Table 3. Relation of changes in compartment distribution of structural damage over 7 years to incident frequent knee pain (FKP).
| MRI Feature | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Any cartilage damage | Full-thickness cartilage damage | Any BML | ||||
|
| ||||||
| Pattern of change | Incident FKP | Adjusted OR* | Incident FKP | Adjusted OR* | Incident FKP | Adjusted OR* |
| No damage | 4/38<br>(10.5%) | Reference | 50/319<br>(15.7%) | Reference | 14/106<br>(13.2%) | Reference |
| Incident isolated PFJ damage | 2/12<br>(16.7%) | 1.6<br>(0.26, 10.3) | 9/49<br>(18.4%) | 1.1<br>(0.50, 2.5) | 8/34<br>(23.5%) | 1.9<br>(0.71, 5.1) |
| Incident isolated TFJ damage | 1/22<br>(4.6%) | 0.4<br>(0.04, 3.8) | 18/58<br>(31.0%) | 2.2<br>(1.2, 4.2) | 7/30<br>(23.3%) | 1.7<br>(0.61, 4.8) |
| Incident mixed damage | 16/95<br>(16.8%) | 1.5<br>(0.48, 5.0) | 15/64<br>(23.4%) | 1.5<br>(0.78, 2.9) | 12/76<br>(15.8%) | 0.9<br>(0.38, 2.1) |
Adjusted for age, sex and BMI
Discussion
Over 7 years of follow-up, full-thickness cartilage damage, any cartilage damage and BMLs developed in the other compartment in the knee when present in one compartment at baseline 20, 39 and 28% of the time (i.e., a majority of knees do not develop damage in the other compartment). Furthermore, most knees that developed mixed disease started with damage isolated to the PFJ. Our findings confirm previous radiographic data that have suggested that development of preventative and therapeutic strategies (i.e., taping, bracing, etc.) targeting the PFJ are warranted and may decrease the disease burden of knee OA [4].
Most knees that had isolated structural damage in one compartment at baseline did not develop it in the other compartment over 7 years. This finding could reflect that certain individuals have risk factors for disease in one compartment and not the other. For example, those individuals that have stable isolated PFJ damage over time may have specific risk factors for PFJ OA (e.g., patella alta, trochlear dysplasia, PFJ malalignment, etc.). Additionally, we may speculate that PFJ and TFJ OA are distinct disease processes and mixed disease only occurs in those that either have risk factors for both PFJ and TFJ disease or have maladaptive compensations to disease in one compartment that contribute to increased risk for development of disease in the other compartment. Similarly, while we did not assess changes between the medial and lateral TFJ (our objective was to look at changes between the PFJ and TFJ), it is likely that medial and lateral TFJ OA are distinct disease processes and highly driven by frontal plane alignment. This is evidenced by the relation of varus alignment to medial TFJ OA and the relation of valgus alignment to lateral TFJ OA. Furthermore, varus (valgus) alignment has protective relationship for lateral (medial) TFJ OA and it is likely that once you have medial or lateral TFJ OA the joint compartment affected will continue to progress and incident damage in the other compartment is less likely [11].
While several studies have suggested OA may start in the PFJ, the current study is the first longitudinal study using MRI features of OA to address this question. Duncan reported isolated radiographic PFJ OA was more common than isolated TFJ OA (24% versus 4%) at baseline; over three years the incidence of isolated PFJ OA was more common than the incidence of isolated TFJ OA (17% versus 14%). Among those that developed incident mixed disease (n=55), 13 (24%), 32 (58%) and 10 (18%) started with no OA, isolated PFJ OA and isolated TFJ OA, respectively [4, 12]. In the Framingham study using various definitions of cartilage loss and BMLs, we found isolated PFJ damage was more common than isolated TFJ damage[13]. Sharma et al. found in knees without radiographic OA more knees with cartilage damage in the PFJ than the TFJ (47% versus 18%). In this study isolated PFJ BMLs were more common than isolated TFJ BMLs (34% versus 9%) [14]. The results of the current study are consistent with the findings of Duncan where mixed OA was most likely to start as isolated PFJ OA.
If the disease process for mixed knee OA does start in the PFJ, treatment and prevention strategies that specifically target the PFJ are warranted. To date there is a lack of randomized controlled trials for treatments for PFJ OA. The few randomized controlled trials investigating interventions for PFJ OA have focused on bracing or taping interventions [2, 3, 15]. Patellofemoral pain is common in younger individuals and although cartilage or bone damage is not prevalent, these individuals have increased PFJ cartilage stress [16], bone strain [17] and bone water content [18, 19]. These abnormalities in the PFJ cartilage and bone may be a precursor to the development of OA in the PFJ. There is some suggestion in the literature that individuals with patellofemoral pain go on to develop PFJ OA [20-23]. Focusing prevention strategies in these individuals may decrease the burden of OA in the later years of their lives.
In general we found no evidence of a strong relation between changes in the compartment distribution of structural damage and incident knee pain. This could be due to the fact that we only studied cartilage damage and BMLs and there are other features of OA on MRI that may be related to pain. However, we focused our analyses on cartilage damage and BMLs because we could specifically attribute them to the PFJ and TFJ. We are unaware of other studies that have investigated changes in compartment distribution of OA with changes in knee pain; however, other studies have investigated the presence of structural damage in knee compartments with incident knee pain/symptoms. Sharma and colleagues found that in knees without radiographic OA from the Osteoarthritis Initiative, cartilage damage (isolated PFJ and mixed) and BMLs (isolated PFJ and mixed) were associated with incident persistent symptoms [14]. The significance of these lesions in this population also confirms that the PFJ is important in the knee OA disease process. We also recognize that the use of pain medications could confound the association between joint damage and knee pain and is a recognized limitation of the current study.
There are limitations to our study. We recognize that there are other MRI features of OA that are relevant for disease incidence and progression (meniscal lesions, synovitis, joint effusion etc.) that we have not assessed. However, we cannot specifically attribute these features to the PFJ or TFJ. We also recognize that these features may also be related to the pain process in OA. However, the temporal relation of these features with cartilage damage and BMLs in the PFJ and TFJ is unknown and for this reason adjustment for these features would not be appropriate. We also did not adjust for additional potential mechanical risk factors for OA (e.g., meniscal lesions, frontal plane alignment, etc.) because our aim was not to identify risk factors for various patterns of knee OA over time. We assessed any cartilage damage, full thickness damage and BMLs as separate predictors but acknowledge that knees may have had both features present. Using a combined definition in sensitivity analyses we found similar results. We also recognize that not all individuals attended the 84-month visit or had MRI assessed at baseline and 84 months because of financial restrictions or total knee replacement and there may be differential loss to follow up in MOST. To address this we provided the age, sex and BMI for all baseline subjects and subjects included in our study (Table 1) where we see a similar age, sex and BMI distribution.
In summary, over 7 years of follow-up full-thickness cartilage damage, any cartilage damage and BMLs developed in the other compartment in the knee when present in one compartment at baseline 20, 39 and 28% of the time (i.e., a majority of knees do not develop damage in the other compartment). Furthermore, most knees that develop cartilage damage and BMLs in the other compartment start with damage isolated to the PFJ, suggesting that mixed disease may begin in the PFJ. Prevention and treatment strategies targeting the PFJ are lacking and are needed to decrease the disease burden of knee OA.
Supplementary Material
Supplemental Table. Patterns of BML Regression (n=67)
Acknowledgments
The authors would like to thank the MOST study participants and clinic staff as well as the coordinating center at UCSF.
Role of Funding Source: The Multicenter Osteoarthritis Study was funded by the NIH (U01-AG18820, U01-AG18832, U01-AG18947, U01-AG19069 and AR-47785). Dr. Stefanik's work was supported by an Investigator Award from the Rheumatology Research Foundation. Funding sources had no role in the study design, collection, analysis and interpretation of the data or the decision to submit the manuscript for publication.
Footnotes
Author Contributions: All authors contributed to the conception and design of the study, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, and approved the final version submitted. Dr. Stefanik takes responsibility for the integrity of the work as a whole, from inception to finished article.
Competing Interests: Dr. Guermazi is President, shareholder Boston Imaging Core Lab (BICL), LLC; and Consultant to MerckSerono, TissueGene, OrthoTrophix and Genzyme. Dr. Roemer is CMO, shareholder Boston Imaging Core Lab (BICL), LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ali Guermazi, Email: ali.guermazi@bmc.org.
Frank W. Roemer, Email: frank.roemer@klinikum-augsburg.de.
George Peat, Email: g.m.peat@keele.ac.uk.
Jingbo Niu, Email: niujp@bu.edu.
Neil A. Segal, Email: neil-nsegal@kumc.edu.
Cora E. Lewis, Email: celewis@uabmc.edu.
Michael Nevitt, Email: mnevitt@psg.ucsf.edu.
David T. Felson, Email: dfelson@bu.edu.
References
- 1.Callaghan MJ, Parkes MJ, Hutchinson CE, Gait AD, Forsythe LM, Marjanovic EJ, et al. A randomised trial of a brace for patellofemoral osteoarthritis targeting knee pain and bone marrow lesions. Ann Rheum Dis. 2015;74(6):1164–70. doi: 10.1136/annrheumdis-2014-206376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crossley KM, Marino GP, Macilquham MD, Schache AG, Hinman RS. Can patellar tape reduce the patellar malalignment and pain associated with patellofemoral osteoarthritis? Arthritis Rheum. 2009;61(12):1719–25. doi: 10.1002/art.24872. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, Harvey W, Gross KD, Felson D, McCree P, Li L, et al. A randomized trial of patellofemoral bracing for treatment of patellofemoral osteoarthritis. Osteoarthritis Cartilage. 2011;19(7):792–800. doi: 10.1016/j.joca.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan R, Peat G, Thomas E, Hay EM, Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70(11):1944–8. doi: 10.1136/ard.2011.151050. [DOI] [PubMed] [Google Scholar]
- 5.Guermazi A, Roemer FW, Burstein D, Hayashi D. Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther. 2011;13(6):247. doi: 10.1186/ar3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guermazi A, Zaim S, Taouli B, Miaux Y, Peterfy CG, Genant HG. MR findings in knee osteoarthritis. Eur Radiol. 2003;13(6):1370–86. doi: 10.1007/s00330-002-1554-4. [DOI] [PubMed] [Google Scholar]
- 7.Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The Multicenter Osteoarthritis Study: opportunities for rehabilitation research. PM&R. 2013;5(8):647–54. doi: 10.1016/j.pmrj.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roemer FW, Guermazi A, Lynch JA, Peterfy CG, Nevitt MC, Webb N, et al. Short tau inversion recovery and proton density-weighted fat suppressed sequences for the evaluation of osteoarthritis of the knee with a 1.0 T dedicated extremity MRI: development of a time-efficient sequence protocol. Eur Radiol. 2005;15(5):978–87. doi: 10.1007/s00330-004-2608-6. [DOI] [PubMed] [Google Scholar]
- 9.Javaid MK, Lynch JA, Tolstykh I, Guermazi A, Roemer F, Aliabadi P, et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: the most study. Osteoarthritis Cartilage. 2010;18(3):323–8. doi: 10.1016/j.joca.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Sharma L, Song J, Dunlop D, Felson D, Lewis CE, Segal N, et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940–5. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan RC, Hay EM, Saklatvala J, Croft PR. Prevalence of radiographic osteoarthritis--it all depends on your point of view. Rheumatology. 2006;45(6):757–60. doi: 10.1093/rheumatology/kei270. [DOI] [PubMed] [Google Scholar]
- 13.Stefanik JJ, Niu J, Gross KD, Roemer FW, Guermazi A, Felson DT. Using magnetic resonance imaging to determine the compartmental prevalence of knee joint structural damage. Osteoarthritis Cartilage. 2013;21(5):695–9. doi: 10.1016/j.joca.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma L, Chmiel JS, Almagor O, Dunlop D, Guermazi A, Bathon JM, et al. Significance of preradiographic magnetic resonance imaging lesions in persons at increased risk of knee osteoarthritis. Arthritis Rheumatol. 2014;66(7):1811–9. doi: 10.1002/art.38611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crossley KM, Vicenzino B, Pandy MG, Schache AG, Hinman RS. Targeted physiotherapy for patellofemoral joint osteoarthritis: a protocol for a randomised, single-blind controlled trial. BMC Musculoskelet Disord. 2008;9:122. doi: 10.1186/1471-2474-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrokhi S, Keyak JH, Powers CM. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthritis Cartilage. 2011;19(3):287–94. doi: 10.1016/j.joca.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho KY, Keyak JH, Powers CM. Comparison of patella bone strain between females with and without patellofemoral pain: a finite element analysis study. J Biomech. 2014;47(1):230–6. doi: 10.1016/j.jbiomech.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Ho KY, Hu HH, Colletti PM, Powers CM. Recreational runners with patellofemoral pain exhibit elevated patella water content. Magn Reson Imaging. 2014;32(7):965–8. doi: 10.1016/j.mri.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Ho KY, Hu HH, Colletti PM, Powers CM. Running-induced patellofemoral pain fluctuates with changes in patella water content. Eur J Sport Sci. 2013 doi: 10.1080/17461391.2013.862872. [DOI] [PubMed] [Google Scholar]
- 20.Utting MR, Davies G, Newman JH. Is anterior knee pain a predisposing factor to patellofemoral osteoarthritis? Knee. 2005;12(5):362–5. doi: 10.1016/j.knee.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Hinman RS, Lentzos J, Vicenzino B, Crossley KM. Is patellofemoral osteoarthritis common in middle-aged people with chronic patellofemoral pain? Arthritis Care Res (Hoboken) 2014;66(8):1252–7. doi: 10.1002/acr.22274. [DOI] [PubMed] [Google Scholar]
- 22.Crossley KM. Is patellofemoral osteoarthritis a common sequela of patellofemoral pain? Br J Sports Med. 2014;48(6):409–10. doi: 10.1136/bjsports-2014-093445. [DOI] [PubMed] [Google Scholar]
- 23.Thomas MJ, Wood L, Selfe J, Peat G. Anterior knee pain in younger adults as a precursor to subsequent patellofemoral osteoarthritis: a systematic review. BMC Musculoskelet Disord. 2010;11:201. doi: 10.1186/1471-2474-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Patterns of BML Regression (n=67)


