Abstract
Rationale
Despite the high prevalence of nicotine use in humans, robust nicotine self-administration has been difficult to demonstrate in laboratory animals.
Objectives
A parametric analysis of nicotine self-administration was conducted to study its reinforcing effects in non-human primates.
Methods
Adult rhesus macaques (N=6) self-administered intravenous (IV) nicotine (0.001-0.1 mg/kg) under a fixed ratio (FR) 1 schedule of reinforcement during daily 90-min sessions. Next, the demand function relating drug intake and response cost was determined by increasing the FR across sessions during the availability of each of several unit doses of nicotine (0.0032-0.032 mg/kg/inj). The reinforcing effects of 0.01 mg/kg/inj cocaine and 1-g banana-flavored food pellets were also determined under similar testing conditions. Finally, the nicotine demand function was re-determined after approximately 8 months of daily IV nicotine self-administration.
Results
IV nicotine self-administration followed an inverted-U shaped pattern, with the peak number of injections maintained by 0.0032 mg/kg/inj. Self-administration of each reinforcer (food pellets, IV cocaine, and IV nicotine) decreased as FR size increased. Application of the exponential model of demand showed that the demand elasticity for nicotine was: 1) dose-dependent and lowest for 0.0032 mg/kg/inj); 2) for 0.0032 mg/kg/inj, similar to that of food pellets and significantly higher than cocaine; and 3) decreased after 8 months of daily nicotine self-administration.
Conclusions
These data show that, though high levels of nicotine self-administration can be achieved under simple FR schedules in nonhuman primates, its reinforcing effectiveness is dose-related but limited, and may increase over time.
Keywords: Nicotine self-administration, nonhuman primates, behavioral economics, fixed-ratio, exponential model of demand
Introduction
Tobacco use associated with nicotine addiction is a major contributor to many cancers, cardiovascular and pulmonary disease cases, and is the largest cause of preventable death in the United States (e.g., CDC, 2014). As with other addictive drugs, the ability of nicotine to maintain its intake depends upon both pharmacological and behavioral factors including the concentration (i.e., dose) of nicotine and its reinforcing effects under varying conditions of availability. In this regard, a better understanding of the reinforcing effectiveness of nicotine may accelerate the development of more effective treatment strategies to facilitate smoking cessation.
Notwithstanding the prevalence of nicotine use in humans, robust self-administration of nicotine has been difficult to demonstrate in laboratory studies using non-human primates (NHP). Further, the conditions that support nicotine self-administration have been inconsistent across studies and are poorly understood. For example, studies that have successfully established nicotine self-administration in NHP often have employed the delivery of non-contingent nicotine injections or have used subjects with extensive drug self-administration histories (reviewed by Le Foll et al. 2007; Goodwin et al. 2015). However, even such manipulations are not always sufficient, and many studies have failed to demonstrate intravenous (IV) nicotine self-administration above vehicle levels (Deneau and Inoki 1967; Ator and Griffiths 1983; Slifer and Balster 1985; de La Garza and Johanson 1987). Other variables such as speed of injection (Ator and Griffiths 1983; Wing and Shoaib 2013), schedule of reinforcement (Ator and Griffiths 1983; Gould et al. 2011; Mello and Newman 2011), and environmental context (Dougherty et al. 1981; Donny et al. 1998; Donny et al. 2000; Caggiula et al. 2002a; Caggiula et al. 2002b; Le Foll et al. 2007) also have been forwarded as critical determinants of nicotine self-administration, but their roles in the reinforcing effects of nicotine have not been clearly elucidated. In view of the difficulties in establishing IV nicotine self-administration in laboratory animals, it is not surprising that the reinforcing effectiveness of nicotine has been studied to a limited extent.
Recently, Koffarnus and Winger (2015) also addressed the issue of the reinforcing effectiveness of nicotine by studying the effects of increases in fixed-ratio response requirement or post-reinforcer time-out (TO) duration on the ability of nicotine to maintain responding in rhesus monkeys. In that study, nicotine served as a reinforcer in some, but not all, monkeys. A behavioral economic analysis applied to the FR data for the unit dose of nicotine that maintained peak responding (0.01 mg/kg/inj) found that nicotine maintained responding less effectively than that of a relatively high unit dose of cocaine (0.032 mg/kg/inj). Further, in some subjects, higher unit doses of nicotine (>0.01 mg/kg) suppressed remifentanil-maintained responding, consistent with early findings that, in addition to maintaining IV self-administration behavior, IV injections of nicotine also served as an effective punisher (Koffarnus and Winger 2015; see also Goldberg and Spealman 1983). On the basis of these findings, the authors concluded that the effects of nicotine were dose-dependent, i.e., it produced weak reinforcing effects at low unit doses and noxious effects at high unit doses.
Goldberg and colleagues have also found that nicotine maintains significant levels of self-administration behavior in drug-naïve squirrel monkeys responding under a 10 response fixed-ratio schedule of reinforcement (LeFoll et al, 2007; 2016; Justinova et al, 2015). After acquisition of nicotine self-administration and over approximately two years of daily nicotine access, experienced subjects reached relatively high breakpoints (about 600 lever-presses for each injection of nicotine) in a progressive ratio (PR) schedule of reinforcement (Le Foll et al, 2007). While PR breakpoints were not determined early in subjects’ experimental history, these data raise the possibility that nicotine’s reinforcing effects may change over time.
The present studies were conducted to both confirm and extend the above investigations of the reinforcing effectiveness of nicotine by examining the roles of dose and self-administration history using NHP. First, the dose-response function for IV nicotine self-administration under a simple fixed ratio (FR) 1; time-out (TO) 10-sec schedule of reinforcement was determined. The response requirement (i.e., FR) contingency for several doses of nicotine (0.0032-0.032 mg/kg/inj) was then manipulated so as to obtain data for measuring reinforcing effectiveness using a behavioral economic demand analysis (i.e., Exponential Model of Demand). Previous studies have suggested that reinforcing effectiveness is independent of dose of the drug self-administered (Bickel et al. 1990; Hursh and Winger 1995), however, the reinforcing effects of higher unit doses of nicotine might be diminished by its reported noxious effects (Fowler and Kenny 2014; Koffarnus and Winger 2015; Goldberg and Spealman 1983). The reinforcing effects of nicotine also were compared with that of another self-administered drug (cocaine) and a nondrug reinforcer (banana-flavored food pellets). Finally, as other studies have found that the reinforcing effectiveness of a drug may change over the course of prolonged or repeated exposure (Christensen et al. 2008b; Wade-Galuska et al. 2011), the demand curve for 0.01 mg/kg/inj nicotine was re-determined after approximately 8 months of nicotine self-administration to evaluate the stability of nicotine’s reinforcing effectiveness over time.
Methods
Subjects
Six adult male (n=3) and female (n=3) rhesus monkeys (Macaca mulatta), weighing between 5 and 9 kg, served as subjects and were housed within a vivarium in well-ventilated stainless steel chambers (64 × 64 × 79 cm). All subjects had self-administered nicotine for two to three years prior to the initiation of the present studies (three of the subjects also had previously self-administered cocaine). Monkeys were provided with a high protein chow (Purina Monkey Chow, St. Louis, MO) and fresh fruit and vegetables daily in addition to food pellets earned during operant sessions; water was freely available at all times. A 12-hr light-dark cycle was in effect (lights on 8:00AM – 8:00PM) except as noted below. Animal maintenance and husbandry were conducted in accordance with the guidelines issued by the Institute of Laboratory Animal Resources (ILAR-NRC, 2010) and the NIH Office of Laboratory Animal Welfare (OLAW). The facility is licensed by the U.S. Department of Agriculture; the protocol used in these studies was fully approved by the Institutional Animal Care and Use Committee at McLean Hospital.
Apparatus
Each chamber was equipped with a custom-designed operant response panel containing visual stimuli and response keys. An externally mounted pellet dispenser (Gerbrands Model G5210, Arlington, MA) and two syringe pumps (Model 981210, Harvard Apparatus, Inc., South Natick, MA) that could be connected to the two lumens of the double-lumen catheter were mounted on shelving above the chamber. Banana-flavored food pellets (1-g; Formula 4TUR, Purina Mills Test Diet, Richmond, IN) were delivered to a food cup attached to the lower left front of the chamber. All experimental events were programmed on a desktop PC connected to the chambers via a Med Associates interface (Georgia, VT).
Self-Administration Procedures
Drug Self-Administration
After initial training to respond for food pellets, subjects were surgically prepared with chronic indwelling double-lumen catheters as described previously (Kohut et al. 2013). Monkeys responded during daily 120-min sessions that consisted of components of food and nicotine availability. During the first component, red lights illuminated the center response key, signaling the availability of 1-g banana-flavored food pellets for 30-min. During the second (IV self-administration) component, green lights illuminated the center response key, signaling the availability of IV injections of nicotine (0.01 mg/kg/inj) or saline for 90-min. This unit dose of nicotine was found to produce stable and relatively high levels of self-administration behavior in preliminary studies and, consequently, served as the maintenance dose. Initially, both food pellets and IV injections were available under a fixed ratio (FR) 1: time-out (TO) 10-s schedule of reinforcement. During TO periods, all lights were off (i.e., no cue light was paired with nicotine delivery) and responding had no scheduled consequences. Monkeys were limited to 25 food pellets but could earn an unlimited number of nicotine injections during the session.
Once responding for the maintenance dose of nicotine was stable and reliable across sessions, the dose-response curve for self-administration of IV nicotine (0.001 – 0.1 mg/kg/inj) was determined in all subjects. Each dose was available for 2-3 consecutive sessions before presentation of the next dose; doses of nicotine were presented in an irregular order that varied across subjects. After self-administration of vehicle and a range of nicotine doses were evaluated, monkeys were returned to the maintenance dose, and the effect of changes in FR requirement was studied.
The FR size was increased in both drug and food components across multiple sessions in an ascending order: 1, 3, 6, 10, 18, 30, 60, 100, 180, 300, etc. Each manipulation of FR value was studied for at least two sessions and until responding was stable. Responding at each FR value was considered stable if the number of injections in each of two consecutive sessions was within 25% of the two-day mean. When stable responding after an FR change resulted in ≤ 10 injections in two consecutive sessions, the response requirement was reset to FR1 and the subject was returned to the maintenance dose of nicotine (0.01 mg/kg/inj). Manipulation of FR value was repeated with saline and other nicotine doses (0.0032 and 0.032 mg/kg/inj) under identical conditions. Finally, similar procedures were conducted to evaluate the reinforcing strength of the unit dose of 0.01 mg/kg cocaine and, separately, 1 g banana-flavored food pellets (described below). The unit dose of cocaine was chosen because we previously found that it produced peak levels of IV self-administration behavior under similar schedule conditions (see Bergman et al. 2012; Kohut et al. 2013). A limit of 200 inj was placed on self-administration of 0.01 mg/kg/inj cocaine to reduce the likelihood of toxic effects. Following the evaluation of demand for all doses of nicotine, 0.01 mg/kg cocaine, and saline, the demand function for 0.01 mg/kg/inj nicotine was determined again in a subset of subjects (n=4) approximately 8 months following the initial determination to evaluate the stability of nicotine’s reinforcing effects over time.
Food-maintained Responding
After completion of the nicotine and cocaine self-administration studies described above, ongoing laboratory renovations required modification of procedures to systematically study food-maintained responding under controlled conditions. Briefly, each subject’s double lumen catheter was replaced with a single lumen catheter under aseptic conditions and attached to an intravenous access port placed subcutaneously at the mid-lumbar region. Subjects next were trained to respond for 1-g banana-flavored food pellets when seated in standard primate chairs (Primate Products, Immokalee, FL) within a custom-designed, ventilated, sound-attenuating chamber. The same operant response panel used in the previous studies in the home cage was mounted to the front of the chamber at a height and position that was easily within reach of the seated subject, and a food receptacle was located directly below the response panel. Experiments were conducted in 10-min daily sessions in which the number of food pellets was restricted to 50 in order to limit the role of satiety on food-maintained responding. Responding was initially maintained under an FR1: TO 10 sec schedule of reinforcement as described above. Once responding was stable, the FR value was increased according to the following ascending steps: 1, 3, 10, 30, 100, 300.
Data Analysis
Data collected from each session included the number of responses and the number of reinforcement deliveries; the average of data for the last two days in each condition was used for further analysis. The primary dependent variables for analysis were intake of nicotine, cocaine, saline, and consumption of food pellets (the total number of completed ratios or total mg/kg/session) under each FR condition and demand elasticity (see below).
A one-way analysis of variance (ANOVA) for repeated measures with Dunnet’s post-hoc analyses was used to compare the number of injections of different nicotine doses that were self-administered under the FR1 schedule and, on this basis, identify doses of nicotine that were self-administered at levels higher than those observed for saline.
The demand curve analysis employed the Exponential Model of Demand (Hursh and Silberberg 2008; Hursh 2014). Normalized demand curves were generated, with the point of origin on the log-log curve set to 100 (Q0=100). To compare demand between various doses of nicotine and between nicotine with that of cocaine and food, consumption/intake (Q) and price (P) were derived from analyses of mean demand curves and then normalized to values at FR1 with the equations Q = 100*(YFRx/YFR1) and P = (FR*YFR1)/100, respectively. Normalized values for both analyses were then fit to the Exponential Model of Demand Equation, logQ = logQ0 + k (e −αP − 1). The term k denotes the range of the exponential and was experimentally derived as 1.4, which was used for all analyses. This value is similar to those used in related studies in rhesus monkeys (Koffarnus et al. 2011; Koffarnus and Winger 2015). The symbol α defines a free parameter that quantifies elasticity of demand as a measure of the rate of change in elasticity across the function.
Demand curves were based on the group means for intake at each FR. FR sizes in which fewer than half of the subjects responded were excluded from the group demand analysis to limit the influence of individual subject data on demand curves. Six subjects completed demand curves for each dose of nicotine whereas five and four subjects completed demand curves for, respectively, cocaine and saline. Data from one subject were excluded from saline calculations because number of saline injections earned was less than 10 at FR1, precluding further analysis. Consequently, demand curves were considered to be significantly different only if the ± 95% CIs for α did not overlap. The Exponential Model of Demand was calculated using GraphPad Prism to obtain k, α (± 95% CI), and R2.
Drugs
(−)-Nicotine hydrogen tartrate was obtained commercially (Sigma-Aldrich, St. Louis, MO) and solubilized in sterile water buffered with NaOH to achieve a pH of 6–7. Cocaine HCl was provided by the National Institute on Drug Abuse (Rockville, MD, USA). Nicotine and cocaine doses were calculated on the basis of, respectively, base and salt weights. Each unit dose was delivered at an injection speed of 0.1 ml/sec and volume per injection was adjusted to deliver the different unit doses of nicotine. All drugs were dissolved in sterile water and were filtered with a syringe-driven 0.22-micron filter (Millipore Corporation, Billerica, MA).
Results
Nicotine Self-administration
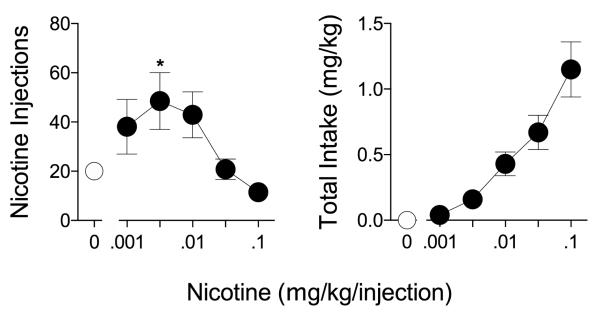
Intravenous injections of nicotine maintained self-administration behavior in all subjects under an FR1: TO 10-sec schedule of reinforcement. Nicotine self-administration (shown in Figure 1) described an inverted-U shaped function, with the number of injections peaking during availability of the unit dose of 0.0032 mg/kg/inj nicotine (48.5 +/− 11.53 inj). Total daily intake (mg/kg/day) increased monotonically as a function of dose, and maximal session intake occurred during availability of the highest unit dose of nicotine (i.e., 0.1 mg/kg/inj; 1.15 ± 0.21 mg/kg/session). Physiological responses to nicotine such as salivation and vomiting were not observed after self-administration of high doses of nicotine though, anecdotally, skin tone appeared to pale in most subjects.
Figure 1. IV Nicotine self-administration under an FR1 schedule of reinforcement.
(Left panel) Mean number of nicotine injections (±SEM) and (Right panel) total nicotine intake in mg/kg earned per session. Each data point represents the mean of two sessions at each dose of nicotine or saline vehicle in five rhesus monkeys. * p < 0.05 vs. saline self-administration.
Behavioral Economic Analyses
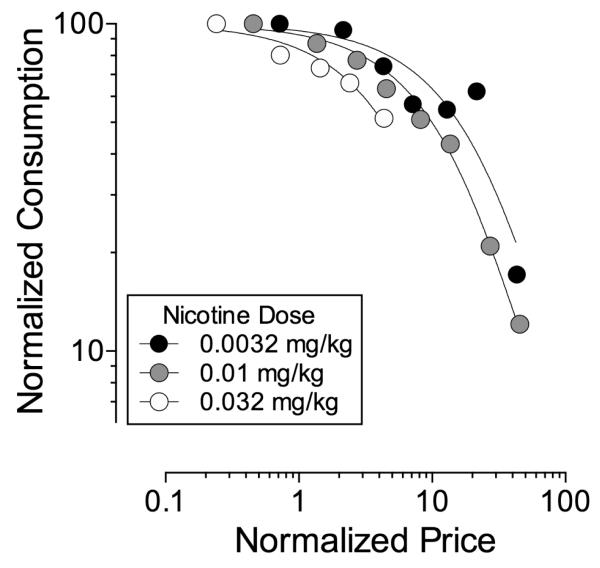
Demand curves in Figure 2 show that intake of each unit dose of nicotine (0.0032, 0.01, and 0.032 mg/kg) decreased as price increased. In some cases, transient increases in responding that subsequently dissipated were observed after an FR change at the lower FR values. The exponential model of demand provides a good fit of the data for all unit doses of nicotine (R2s = 0.84 - 0.99). Moreover, a comparison of results across doses of nicotine showed that demand elasticity (i.e., α) differed as a function of nicotine dose, with α for the dose of 0.0032 greater than unit doses of 0.01 and 0.032 mg/kg/inj and α for the dose of 0.01 greater than 0.032 mg/kg (see Table 1).
Figure 2. Normalized demand curves for IV nicotine as a function of unit price.
Each data point represents the mean number of nicotine injections earned at that price in six rhesus monkeys. Lines represent best-fit for each data set determined using the Exponential Model of Demand. Statistical details shown in Table 1.
Table 1.
Comparison of demand elasticity (α) and R2 values for several doses of self-administered nicotine.
| Nicotine Dose | α (± 95% CIs) |
R2 |
|---|---|---|
| 0.0032 mg/kg | 0.00015 *# (9.7 × 10−5 − 0.00021) |
0.86 |
| 0.01 mg/kg | 0.00024 * (0.00022 – 0.00026) |
0.99 |
| 0.032 mg/kg | 0.00057 (0.00044 – 0.00069) |
0.94 |
Significantly different from 0.032 mg/kg;
Significantly different from 0.01 mg/kg
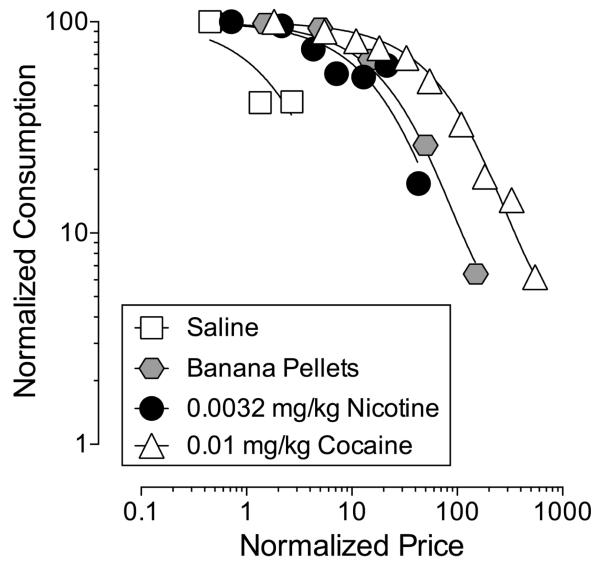
A major goal of the present study was to compare the reinforcing effectiveness of the unit dose of nicotine (0.0032 mg/kg/inj) that produced the highest levels of self-administration behavior with the reinforcing effects of a highly effective unit dose of IV cocaine (0.01 mg/kg/inj) and a non-drug reinforcer, 1-g banana-flavored food pellets. Data for cocaine- and food-maintained responding are presented as normalized demand curves in Figure 3. Data for 0.0032 mg/kg/inj nicotine (lowest demand elasticity of nicotine doses studied) and saline are included for comparison. Like nicotine, the number of cocaine injections and food pellets earned/session decreased as a function of FR requirement. However, as can be seen in comparing the position of the demand functions along the abscissa, baseline levels of IV cocaine self-administration were maintained over a wider range of FR values than IV nicotine self-administration. The exponential model of demand provided a good fit of the data; see R2 in Table 2. Table 2 shows demand elasticity (i.e., α) for each reinforcer; demand elasticity for 0.0032 mg/kg nicotine and food pellets was similar and significantly greater than for 0.01 mg/kg cocaine; demand elasticity was lower for all reinforcers compared to saline.
Figure 3. Normalized demand curves for IV saline, nicotine, and cocaine and 1-g banana flavored food pellets.
Each data point represents the mean of two sessions for nicotine (n=6), cocaine (n=5), saline (n=4), and food pellets (n=6). Data from one subject was excluded from the saline curve because number of saline injections was less than 10 at FR1 and a demand curve could not be generated. Lines represent best-fit for each data set determined using the Exponential Model of Demand. Statistical details shown in Table 2.
Table 2.
Comparison of demand elasticity (α) and R2 values for each reinforcer studied.
| Reinforcer | α (± 95% CIs) |
R2 |
|---|---|---|
| Nicotine (0.0032 mg/kg) | 0.00015 (9.7×10−5 − 0.00021) |
0.86 |
| Cocaine (0.01 mg/kg) | 3.6×10−5
* (3.1×10−5 – 4.0×10−5) |
0.98 |
| Banana-Flavored Food Pellets (1 g) | 0.00011 (9.7×10−5 − 0.00013) |
0.99 |
| Saline | 0.0014 # (−0.00034 – 0.0032) |
0.68 |
different from nicotine, banana-flavored food pellets, and saline;
different from nicotine, cocaine, and banana-flavored food pellets.
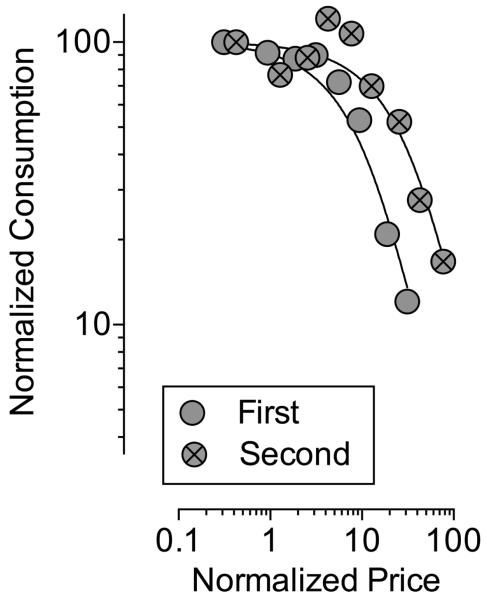
The reinforcing effectiveness of 0.01 mg/kg nicotine was re-determined after approximately 8 months in four subjects that continued to self-administer nicotine on a daily basis throughout this period. When compared to the first determination of demand for 0.01 mg/kg nicotine, demand elasticity after 8 months (second determination) was shifted to the right suggesting an increase in reinforcing effectiveness over time; see Table 3 and Figure 4.
Table 3.
Comparison of demand elasticity (α) and R2 values for 0.01 mg/kg nicotine before and after an 8-month history of nicotine self-administration.
| 0.01 mg/kg Nicotine | α (± 95% CIs) |
R2 |
|---|---|---|
| First Determination | 0.00031 (0.00025 - 0.00037) |
0.97 |
| Second Determination | 0.00010 * (7.5 × 10−5 – 0.00013) |
0.92 |
Significantly different from First Determination
Figure 4. Effect of self-administration history on demand for IV 0.01 mg/kg/inj nicotine.
Each data point represents the mean number of nicotine injections earned at that unit price in four rhesus monkeys. Lines represent the best-fit for each data set determined using the Exponential Model of Demand. Demand curve for the first determination includes only data from subjects that completed the second determination. Statistical details shown in Table 3.
Discussion
The present data illustrating that nicotine self-administration under an FR1: TO 10-sec schedule of IV injections can be described by an inverted U-shaped function are consistent with previous studies of the reinforcing effects of nicotine and other self-administered drugs (Schuster and Thompson 1969). Peak values for responding and number of IV injections were observed during the availability of the unit dose of 0.0032 mg/kg nicotine. Although self-administration of nicotine decreased as the unit dose increased on the descending segment of the bitonic dose-response function, total session intake was monotonically dose-dependent, with maximal intake of approximately 1.15 mg/kg during the availability of 0.1 mg/kg nicotine. The shape of the IV nicotine dose-effect curve in NHP in the present study is similar to that obtained with comparable doses of nicotine (0.1 - 3.0 mg/70 kg) in recent studies of IV nicotine self-administration in human subjects (Harvey et al. 2004; Sofuoglu et al. 2007). Also, nicotine intake in the present study is comparable to the level of nicotine intake for the average smoker, i.e., about 1-2 cigarettes/hr, which delivers 1-4 mg nicotine/hr (Benowitz and Jacobs, 1984). Taken together, these data suggest that, for nicotine, the dose range and intake levels in NHP are comparable to those in the human population. Unfortunately, the absence of plasma levels of nicotine in the present studies preclude a direct comparison with data from human tobacco users.
Inspection of the pattern of nicotine self-administration in cumulative records for individual subjects indicates that subjects frequently responded in bursts, with each burst followed by a period of no responding. This pattern is similar to that previously reported in baboons responding for nicotine under an FR2: TO 15-sec schedule of reinforcement (Ator and Griffiths 1983) and contrasts with the regularly-spaced injections commonly observed for IV self-administration of optimal unit doses of cocaine in NHP. The burst pattern of responding for nicotine suggests that density of reinforcement was an important factor in determining overall levels of intake in the present studies. That is, when the response requirement and inter-injection intervals were relatively low, the intake of sufficient levels of nicotine early in the session may have encouraged continued responding; on the other hand, when the response requirement was increased and, consequently, the increased inter-injection interval increased, less nicotine was taken early in the session and nicotine did not maintain responding. These findings differ from those of Koffarnus and Winger (2015) in which responding for nicotine increased when post-reinforcer timeout duration was increased. The reasons for such differences in the two studies are unclear but may be related to the more extensive nicotine self-administration history of subjects in the present study, which may have resulted in better regulation of nicotine intake.
A major aim of the present studies was to quantitatively evaluate the reinforcing effectiveness of unit doses of IV nicotine that maintained maximal levels of self-administration behavior (0.0032-0.01 mg/kg/inj). Nicotine self-administration in the present study was highly sensitive to changes in price, consistent with the results of recent studies in NHP (Koffarnus and Winger 2015). In this regard, behavioral economic theories generally posit a functional equivalence between response requirement and dose manipulations in drug self-administration studies, which suggests that reinforcing effectiveness is constant across unit doses (Hursh et al. 1988; Bickel et al. 1990). Support for this idea has come from studies in human subjects in which reinforcing strength was constant in cigarette smokers that responded for 1, 2, or 4 puffs from a cigarette (Bickel et al. 1991); see also (DeGrandpre et al. 1992). Other laboratory studies also find this relationship to generally hold across self-administered drugs, though exceptions have been noted (see Bickel et al, 1990). Of interest, in cases for which different doses of a drug are better fit with different demand curves (i.e., ketamine Moreton et al. 1983; phencyclidine, Marquis et al. 1989; and cocaine, Winger, 1993), lower, not higher, doses do not conform to the unit price analysis. In contrast, our results suggest that lower doses of nicotine have greater reinforcing effectiveness than higher doses. Taken together, these findings suggest that theoretical price analysis may not fully account for non-specific behavioral effects of a drug such as nicotine that may influence its self-administration. For example, it is well established that the same dose of nicotine can produce both reinforcing and aversive or negative effects in both laboratory animals and human subjects (Spealman 1983; Goldberg et al. 1981; Spealman and Goldberg 1982; Goldberg et al. 1983; Koffarnus and Winger 2015; Jones et al. 1999). Possibly, the apparently greater reinforcing strength of lower than higher unit doses of nicotine in the present studies may reflect the emergence of concurrent reinforcing and noxious effects produced by higher levels of nicotine intake. From this perspective, the highest number of puffs in the study mentioned above (4 puffs; Bickel et al. 1991) may not have sufficed to produce a noxious effect of nicotine that otherwise might modify its reinforcing effectiveness.
The demand analysis of the present data suggesting that nicotine is a weaker reinforcer than cocaine is consistent with previous studies in which the two drugs were directly compared in rodents, beagle dogs, and human subjects; see also (Koffarnus and Winger 2015). For example, in studies of concurrent access to nicotine (8, 25, 75 ug/kg) and cocaine (267 or 800 ug/kg), Manzardo et al showed that rats chose both doses of cocaine more often than any dose of nicotine (Manzardo et al. 2002). In beagle dogs, nicotine maintained lower levels of self-administration under an FR schedule as well as lower PR breakpoints when compared with cocaine (Risner and Goldberg, 1983). Similarly, despite a faster onset of action and greater increases in subjective reports of “Rush,” “Good Effects,” and “High”, human subjects reported higher ratings of drug-liking for IV cocaine than IV nicotine, and were willing to pay twice as much for cocaine than for nicotine (Jones et al. 1999).
Of interest, the reinforcing strength of nicotine, although lower than cocaine’s, was similar to that of 1-g banana-flavored food pellets in the present study. Studies in rodents have shown that demand for food often exceeds that for IV injections of drugs, including cocaine, when all food is earned in the behavioral session, i.e., in a closed economy (Christensen et al. 2008a; Christensen et al. 2008b). However, as might be expected, the reinforcing strength of food decreases proportionally with the amount of food available outside of behavioral sessions, i.e., in an open economy (see also Hursh and Roma 2013). In the present studies, food was available ad libitum outside the behavioral session; it is likely that the position of the demand curve relative to nicotine would change if food were available under more restrictive conditions. Other factors such as session length (i.e., 10 min food vs. 90 min drug) and experimental conditions (i.e., home cage vs. chaired experiments) may have played a role in determining demand for food. Regardless, the present data provide a foundation for future studies to evaluate various factors (e.g., treatment with candidate medications, chronic drug exposure and withdrawal, etc.) that may alter demand for drug (i.e., nicotine, cocaine) and non-drug (i.e., food) reinforcers.
There has been relatively little investigation of how the reinforcing effectiveness of a self-administered drug, as quantified by demand analysis, may change over the course of the subject’s self-administration history. Changes in demand for IV cocaine over time have been reported in self-administration studies in rats (Christensen et al. 2008b). Also, it has been suggested that demand for nicotine in human subjects can increase over time and may vary directly with the level of nicotine dependence (Chase et al, 2013). Consistent with these reports, our data indicate that demand for nicotine may have increased over the course of nicotine exposure. In the present studies, the nicotine demand curves were established initially in all subjects and then again approximately 8 months during which monkeys were self-administering nicotine on a near-daily basis. Nicotine intake during 90-min daily sessions remained relatively stable in individual subjects over this time, and, given the absence of escalating intake or signs of withdrawal, it is unlikely that nicotine dependence developed in these subjects. However, the suggestion that nicotine demand varies directly with dependence is an intriguing one and deserves further investigation in individual subjects in which the duration and extent of chronic exposure to nicotine are systematically varied.
Acknowledgements
This work was supported by grants K01-DA039306 and R01-DA026892 from the National Institute on Drug Abuse, National Institute of Health. We thank Claire Barkin and George Anderson for excellent technical assistance. We also thank Dr. Peter G. Roma for helpful discussions regarding the demand analysis.
Footnotes
The authors have no conflicts of interest to declare.
References
- Ator NA, Griffiths RR. Nicotine self-administration in baboons. Pharmacology, Biochemistry and Behavior. 1983;19:993–1003. doi: 10.1016/0091-3057(83)90406-9. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Bergman J, Roof RA, Furman CA, et al. Modification of cocaine self-administration by buspirone (buspar®): potential involvement of D3 and D4 dopamine receptors. Int J Neuropsychopharm. 2012;16:445–458. doi: 10.1017/S1461145712000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sciences. 1990;47:1501–1510. doi: 10.1016/0024-3205(90)90178-T. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Hughes JR, Higgins ST. Behavioral economics of drug self-administration. II. A unit-price analysis of cigarette smoking. J Exp Anal Behav. 1991;55:145–154. doi: 10.1901/jeab.1991.55-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula A, Donny E, Chaudhri N, et al. Importance of nonpharmacological factors in nicotine self-administration. Physiology & Behavior. 2002;77:683–687. doi: 10.1016/S0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula A, Donny E, White A, et al. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Chase HW, MacKillop J, Hogarth L. Isolating behavioural economic inidices of demand in relation to nicotine dependence. Psychopharmacology. 2013;226:371–380. doi: 10.1007/s00213-012-2911. [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, et al. Essential value of cocaine and food in rats: tests of the exponential model of demand. Psychopharmacology. 2008a;198:221–229. doi: 10.1007/s00213-008-1120-0. [DOI] [PubMed] [Google Scholar]
- Christensen CJ, Silberberg A, Hursh SR, et al. Demand for cocaine and food over time. Pharmacology, Biochemistry and Behavior. 2008b;91:209–216. doi: 10.1016/j.pbb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK, Hughes JR, Higgins ST. Behavioral economics of drug self-administration. III. A reanalysis of the nicotine regulation hypothesis. Psychopharmacology. 1992;108:1–10. doi: 10.1007/BF02245277. [DOI] [PubMed] [Google Scholar]
- de La Garza De R, Johanson CE. The effects of food deprivation on the self-administration of psychoactive drugs. Drug Alcohol Depend. 1987;19:17–27. doi: 10.1016/0376-8716(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Deneau GA, Inoki R. Nicotine self-administration in monkeys. Annals of the New York Academy of Sciences. 1967;142:277–279. [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, et al. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, et al. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Dougherty J, Miller D, Todd G, Kostenbauder HB. Reinforcing and other behavioral effects of nicotine. Neuroscience and Biobehavioral Reviews. 1981;5:487–495. doi: 10.1016/0149-7634(81)90019-1. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 2014;76:533–544. doi: 10.1016/j.neuropharm.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Suppression of behavior by intravenous injections of nicotine or by electric shocks in squirrel monkeys: effects of chlordiazepoxide and mecamylamine. J Pharmacol Exp Ther. 1983;224:334–340. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Risner ME, Henningfield JE. Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacology, Biochemistry and Behavior. 1983;19:1011–1020. doi: 10.1016/0091-3057(83)90408-2. [DOI] [PubMed] [Google Scholar]
- Goldberg Spealman R, Goldberg D. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Hiranita T, Paule MG. The reinforcing effects of nicotine in humans and nonhuman primates: A review of intravenous self-administration evidence and future directions for research. Nicotine & Tobacco Research; 2015. [DOI] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Nader SH, Nader MA. Effects of varenicline on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2011;339:678–686. doi: 10.1124/jpet.111.185538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, et al. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology. 2004;175:134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral Economics and the Analysis of Consumption and Choice. In: McSweeney FK, Murphy ES, editors. The Wiley Blackwell Handbook of Operant and Classical Conditioning. John Wiley & Sons, Ltd; Oxford, UK: 2014. [DOI] [Google Scholar]
- Hursh SR, Raslear TG, Shurtleff D, et al. A cost-benefit analysis of demand for food. J Exp Anal Behav. 1988;50:419–440. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Beh. 2013;99:98–124. doi: 10.1002/jeab.7. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Garrett BE, Griffiths RR. Subjective and physiological effects of intravenous nicotine and cocaine in cigarette smoking cocaine abusers. J Pharmacol Exp Ther. 1999;288:188–197. [PubMed] [Google Scholar]
- Justinova Z, Panlilio LV, Moreno-Sanz G, Redhi GH, Auber A, Secci ME, et al. Effects of fatty acid amide hydrolase (FAAH) inhibitors in non-human primate models of nicotine reward and relapse. Neuropsychopharmacology. 2015;40:2185–2197. doi: 10.1038./npp.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Hall A, Winger G. Individual differences in rhesus monkeys' demand for drugs of abuse. Addiction Biology. 2011;17:887–896. doi: 10.1111/j.1369-1600.2011.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Winger G. Individual differences in the reinforcing and punishing effects of nicotine in rhesus monkeys. Psychopharmacology. 2015;232:2393–2403. doi: 10.1007/s00213-015-3871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut SJ, Fivel PA, Mello NK. Differential effects of acute and chronic treatment with the α2-adrenergic agonist, lofexidine, on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend. 2013;133:593–599. doi: 10.1016/j.drugalcdep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS ONE. 2007;2:e230. doi: 10.1371/journal.pone.0000230.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Chefer SI, Kimes AS, Stein EA, Goldberg SR, Mukhin AG. Impact of short access nicotine self-administration of alpha4beta2 nicotinic acetylcholine receptors in non-human primates. Psychopharmacology. doi: 10.1007/s00213-016-4250-9. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzardo AM, Stein L, Belluzzi JD. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Research. 2002;924:10–19. doi: 10.1016/S0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Webb MG, Moreton JE. Effects of fixed ratio size and dose on phencyclidine self-administration by rats. Psychopharmacology. 1989;97:179–182. doi: 10.1007/BF00442246. [DOI] [PubMed] [Google Scholar]
- Mello NK, Newman JL. Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine + nicotine combinations in rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:203–214. doi: 10.1037/a0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreton JE, Meisch RA, Stark L, Thompson T. Ketamine self-administration by the rhesus monkey. J Pharmacol Exp Ther. 1977;203:303–309. [PubMed] [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office of Smoking and Health . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); Atlanta (GA): 2014. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003;70:39–52. doi: 10.1016/S0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- Schuster CR, Thompson T. Self Administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- Slifer BL, Balster RL. Intravenous self-administration of nicotine: with and without schedule-induction. Pharmacology, Biochemistry and Behavior. 1985;22:61–69. doi: 10.1016/0091-3057(85)90487-3. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Yoo S, Hill KP, Mooney M. Self-Administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology. 2007;33:715–720. doi: 10.1038/sj.npp.1301460. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Maintenance of behavior by postponement of schedule injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1983;227:154–159. [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Maintenance of schedule-controlled behavior by intravenous injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1982;223:402–408. [PubMed] [Google Scholar]
- Wade-Galuska T, Galuska CM, Winger G. Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. J Exp Anal Behav. 2011;95:75–89. doi: 10.1901/jeab.2011.95-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Effect of infusion rate on intravenous nicotine self-administration in rats. Behavioural Pharmacology. 2013;24:517–522. doi: 10.1097/FBP.0b013e3283644d58. [DOI] [PubMed] [Google Scholar]
- Winger G. Fixed-ratio and time-out changes on behavior maintained by cocaine or methohexital in rhesus monkeys: I. Comparison of reinforcing strength. Experimental and Clinical Psychopharmacology. 1993;1:142. [Google Scholar]