Abstract
Background
Perfluoroalkyl substances (PFAS) may affect breast development and decrease duration of breastfeeding, thus interfering with the health benefits of breastfeeding. We investigated the association between maternal PFAS exposure and breastfeeding duration.
Methods
We measured PFAS concentrations in maternal serum collected during pregnancy in 2003–2006. After delivery, women (n=336) completed standardized breastfeeding surveys every 3 months until ending breastfeeding or 36 months postpartum. We estimated relative risks (RRs) for ending any breastfeeding within 3 or 6 months postpartum by Poisson regression, adjusted for relevant confounding factors.
Results
Women in the 4th quartile of perfluorooctanoic acid (PFOA) serum concentration had 1.77 times the risk of ending any breastfeeding by 3 months (95% confidence interval (CI): 1.23, 2.54; p-trend=0.003) and 1.41 times the risk of ending any breastfeeding by 6 months (95%CI: 1.06, 1.87; p-trend=0.038), compared with women in the first quartile. Women in the 4th quartile of perfluorooctane sulfonic acid serum concentration had a marginally increased risk of discontinuing any breastfeeding by 3 months (RR=1.32; 95%CI: 0.97, 1.79; p-trend=0.065).
Conclusions
Maternal serum PFOA concentrations were inversely related to duration of any breastfeeding in this cohort, even after controlling for prior breastfeeding. These findings suggest that PFOA exposure may adversely affect breastfeeding duration and highlight the need to consider the potential adverse effects of maternal environmental chemical exposure on breastfeeding.
Keywords: Perfluoroalkyl substances, Perfluorooctanoic acid, Perfluorooctane sulfonic acid, Breastfeeding
1. Introduction
The American Academy of Pediatrics (AAP) recommends 6 months of exclusive breastfeeding and continued partial breastfeeding for 12 months or longer (AAP, 2012) to promote numerous associated short and long term health benefits for both mother and child (Ip et al., 2007). However, in the United States (US) in 2012 only 29.2% of mothers continue any breastfeeding until 12 months (National Immunization Survey 2015). Known barriers to breastfeeding include sociocultural, medical, nutritional, and infant level factors, including perceived inadequate milk supply, problems with latch, and insufficient support from health care providers, family, or workplace (Teich et al., 2014). However, exposure to environmental chemicals may interfere with hormones regulating initiation of lactation (e.g. prolactin) and milk production (e.g. oxytocin) (Lew et al., 2009; Rogan and Gladen, 1985).
Perfluoroalkyl substances (PFAS) are commonly used in oil and water resistant consumer products (e.g. non-stick cookware, food container coatings, textile treatments), fire-fighting foam, and industrial surfactants (Buck et al., 2011; European Food Safety Authority Panel on Contaminants in the Food Chain, 2008). Four PFAS are nearly ubiquitous in sera collected from the US population: perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS) (Jain, 2013; Woodruff et al., 2011). Toxicologic studies suggest that exposure to PFOA during pregnancy disrupts mammary gland differentiation and development (Tucker et al., 2015; White et al., 2007; Yang et al., 2009), delays epithelial involution (White et al., 2007), and may alter expression of placental prolactin-family hormone and milk protein genes (Suh et al., 2011; White et al., 2007). Only one prior epidemiologic study has assessed the effect of maternal PFOA and PFOS exposure on breastfeeding duration. Using data from the Danish National Birth Cohort, Fei et al. observed that greater concentrations of maternal plasma PFOA and PFOS during pregnancy were associated with shorter duration of breastfeeding among multiparous women (Fei et al., 2010). Collectively, these studies suggest that PFAS may have adverse effects, on duration of breastfeeding. However, Fei et. al did not control for prior breastfeeding duration (Fei et al., 2010), which is an important route of maternal PFAS excretion (Barbarossa et al., 2013; Mondal et al., 2014) and an important predictor of future breastfeeding success (Nagy et al., 2001; Whalen and Cramton, 2010).
We tested the hypothesis that greater maternal serum concentrations of PFAS during pregnancy are associated with a shorter duration of breastfeeding. We examined these associations, controlling for prior breastfeeding history, in a longitudinal cohort that had median serum PFOA concentrations about two-times higher than those of pregnant women who participated in the National Health and Nutrition Examination Survey (NHANES) (Braun et al., 2016), a nationally representative sampling of the US general population (Jain, 2013).
2. Materials and Methods
2.1 Study population
We used data from the Health Outcomes and Measures of the Environment (HOME) Study, a prospective pregnancy and birth cohort designed to examine the impact of early life environmental chemical exposures (Braun et al., 2014; Geraghty et al., 2008). Pregnant women were recruited from nine prenatal clinics associated with three hospitals in the Cincinnati, Ohio area between March 2003 and January 2006. At baseline, women were eligible to participate if they were pregnant (16±3 weeks gestation), >18 years old, English speakers, living in a home built before 1978, intending to continue prenatal care and deliver at a study-affiliated obstetric practice, and had no history of HIV infection. Women taking medication for seizures or thyroid disorders were not eligible to participate. All women provided written informed consent, and the institutional review boards of Cincinnati Children’s Hospital Medical Center, the cooperating delivery hospitals, and the Centers for Disease Control and Prevention (CDC) approved the study protocol.
Of the 468 women who initially enrolled in the study, 67 dropped out before delivery. We excluded nine sets of twins, three stillborn children, and three children with congenital anomalies. Among the remaining 386 singleton births, 357 (93%) mothers provided serum samples; of these, 336 (87%) completed follow-up surveys regarding breastfeeding practices and had complete covariate information.
2.2 Serum PFAS concentrations
We collected blood samples from women at ~16 and ~26 weeks gestation and at delivery. For women who had insufficient serum volume to quantify PFAS concentrations in their 16 week sample, we used the 26 week (n=33, 10%), or delivery sample (n=16, 5%). We used online solid phase extraction coupled to high performance liquid chromatography-isotope dilution tandem mass spectrometry to measure serum PFOA, PFOS, PFNA, and PFHxS concentrations (Kato et al., 2011), which were detectable in all samples. The limits of detection were 0.1 ng/mL (PFOA, PFHxS), 0.2 ng/mL (PFOS), and 0.082 ng/mL (PFNA). Every analytic batch included reagent blanks and quality control (QC) materials. The coefficients of variation of repeated measurements of the QC materials were ~6%.
2.3 Duration of breastfeeding
After delivery, women completed standardized interviewer-administered surveys by phone about breastfeeding practices every 3 months until breastfeeding was discontinued or the child’s third year birthday, whichever came first. The surveys included questions about the introduction of water, juice or other liquids, formula, non-human milk, and solid foods into the infant diet. Women also provided information about the duration of any breastfeeding of their previous children. We defined any breastfeeding as the duration in months that the mother reported any extent of breastfeeding, irrespective of supplementation with formula, liquids, or solid foods. We used the AAP guidelines to define exclusive breastfeeding, which indicate that breast milk may not be supplemented (including water, juice, non-human milk, formula, or solid foods) with the exception of vitamins, minerals, and medications (AAP, 2012). We set the duration of exclusive breastfeeding to each infant’s age at the earliest of first use of formula, introduction of water, juice, or solid foods; we excluded 19 participants with missing data related to introduction of liquids and solid foods from the exclusive breastfeeding analyses. Our primary outcomes were the termination of any breastfeeding by 3 or 6 months postpartum and termination of exclusive breastfeeding by 3 months postpartum. We chose these cutoffs for our participants because relatively few infants were exclusively breastfed for ≥3 months (n=44) or continued to receive any breastmilk for ≥12 months (n=83) (AAP, 2012).
2.4 Covariates
During the second trimester, demographic, socioeconomic, perinatal, and behavioral factors, as well as reproductive and medical histories, were collected using a computer-assisted questionnaire administered by trained research staff.
2.5 Statistical analysis
First, we examined univariate statistics of PFAS concentrations, duration of any breastfeeding, and potential covariates. We then conducted bivariate analyses to assess associations among PFAS concentrations, breastfeeding duration, and covariates, applying a log(2) transformation to PFAS concentrations and a natural log transformation for duration of any breastfeeding, due to their skewed distribution. Second, because the outcome under study (ending breastfeeding) was not rare and data were collected prospectively, we used multivariable Poisson regression models with robust standard errors to estimate the relative risk (RR) (Zou, 2004) of ending any breastfeeding by 3 or 6 months and exclusive breastfeeding by 3 months. Potential confounding variables were initially identified based on known associations with either PFAS or breastfeeding duration. Covariates were added to the final regression models if they were associated with both log(2)-transformed serum PFOA and natural log-transformed duration of any breastfeeding (p<0.20) in the bivariate analyses. The final multivariable models were adjusted for: maternal age at delivery, race/ethnicity, marital status, household income, parity, maternal serum cotinine during pregnancy (a sensitive and specific biomarker of secondhand and active tobacco smoke exposure (Bernert et al., 2009), any alcohol use during pregnancy, total weeks of breastfeeding previous children, and gestational week at blood draw. Third, we used unadjusted Kaplan-Meier curves to visually assess time to discontinuation of any breastfeeding according to PFAS quartiles. Finally, we created covariates-adjusted multivariable Cox proportional hazards models to assess duration of any breastfeeding according to PFAS concentrations. The proportional hazards assumption was valid for follow-up through 12 months, so we censored women that continued any breastfeeding for >12 months in these models (n=72, range=12.3–35 months).
2.6 Sensitivity analyses
We stratified by parity to assess whether parity status might modify the association between PFAS and breastfeeding. Because serum PFOA and PFOS are correlated (Spearman rho=0.59; p<0.01) in the HOME Study cohort and Fei et al. observed that both were associated with reduced breastfeeding duration (Fei et al., 2010), we included both PFOA and PFOS in the same statistical model to determine whether a particular PFAS was driving any of the observed associations. We additionally performed an analysis excluding the 63 women who did not initiate breastfeeding. We repeated the analyses, excluding 16 maternal-child pairs in which the infant was admitted to the NICU after delivery. We compared results from the Cox proportional hazards models to those from Weibull accelerated failure time models, appropriate for interval censored longitudinal data, and results were similar (data not shown). Approximately one third of the women in our study reported that they terminated breastfeeding earlier than they had intended (n=102). Women were allowed to provide up to five reasons why they discontinued breastfeeding. Most women reported one (51.0%) or 2 reasons (35.3%) for ending breastfeeding, with a total of 171 reasons provided by the 102 women. We calculated and plotted the geometric mean (GM) and 95% confidence interval (CI) for PFOA concentrations across the reported reasons for stopping breastfeeding. Additionally, we performed analysis of variance (ANOVA) of log(2)-transformed PFOA by reason for ending to determine whether PFOA serum concentration was associated with reason for ending.
3. Results
The majority of the 336 mothers included in the current study were between 25 and 35 years old at delivery (61%), non-Hispanic white (62.5%), with a bachelors or graduate/professional degree (50.3%), married (66.7%), and multiparous (56.8%). Few women were active smokers (9.2%) or consumed alcohol more than once a month during pregnancy (14.3%). Most mothers delivered their infants vaginally (71.7%) and slightly more than half of the infants were female (53.3%) (Table 1). As previously described, median PFOA concentrations were about two times higher among HOME Study women (5.5 ng/mL, Table 1) than the median reported among 174 pregnant women in NHANES 2003–2004 and 2005–2006 (2.3 ng/mL), though levels of PFOS, PFNA, and PFHxS were fairly comparable among HOME Study women and NHANES (Braun et al., 2016).
Table 1.
Maternal serum perfluorooctanoic acid (PFOA) concentrations and duration of any breastfeeding by maternal sociodemographic and perinatal factors among Cincinnati, OH women (2003–2006)
| Covariate | n (%) | Maternal Serum PFOA (ng/mL) | Any Breastfeeding (months) | ||
|---|---|---|---|---|---|
| Median (25th–75th) | p-value a | Median (25th–75th) | p-value b | ||
| Overall | 336 (100) | 5.5 (3.8–7.7) | 3.9 (0.5–11.0) | ||
|
| |||||
| Maternal Age | |||||
| 18–25 years | 78 (23.2) | 6.1 (4.8–7.8) | 0.9 (0.0–3.1) | ||
| 25–35 years | 205 (61.0) | 5.2 (3.7–7.5) | 6.0 (1.0–12.5) | ||
| >35 years | 53 (15.8) | 5.7 (3.9–9.0) | 4.3 (1.3–10.0) | ||
| 0.204 | <0.001 | ||||
| Maternal Race | |||||
| White | 210 (62.5) | 5.9 (3.8–8.8) | 7.0 (2.0–12.8) | ||
| Black | 106 (31.5) | 5.0 (3.7–6.3) | 0.8 (0.0–3.0) | ||
| Other | 20 (6.0) | 5.9 (4.2–9.3) | 2.0 (0.9–6.9) | ||
| 0.004 | <0.001 | ||||
| Maternal Education | |||||
| High School or less | 78 (23.2) | 5.2 (3.9–7.5) | 0.1 (0.0–3.0) | ||
| Tech school or some college | 89 (26.5) | 5.7 (3.9–7.7) | 2.0 (0.5–8.0) | ||
| Bachelors or more | 169 (50.3) | 5.6 (3.7–7.9) | 7.5 (2.5–13.0) | ||
| 0.870 | <0.001 | ||||
| Marital Status | |||||
| Not Married | 112 (33.3) | 5.3 (3.9–7.2) | 0.8 (0.0–3.0) | ||
| Married | 224 (66.7) | 5.8 (3.7–8.2) | 6.9 (1.8–12.9) | ||
| 0.184 | <0.001 | ||||
| Household Income | |||||
| >$80,000 | 95 (28.3) | 6.0 (3.7–9.3) | 7.0 (2.0–12.0) | ||
| $40–80,000 | 108 (32.1) | 5.7 (4.1–7.7) | 6.0 (1.6–13.0) | ||
| $20–40,000 | 60 (17.9) | 5.5 (4.0–7.2) | 2.9 (0.5–8.5) | ||
| <$20,000 | 73 (21.7) | 5.0 (3.6–7.1) | 0.2 (0.0–3.0) | ||
| 0.192 | <0.001 | ||||
| Nulliparous | |||||
| Yes | 145 (43.2) | 6.6 (5.3–9.4) | 5.0 (1.0–10.0) | ||
| No | 191 (56.8) | 4.6 (3.4–6.6) | 3.0 (0.1–12.0) | ||
| <0.001 | 0.039 | ||||
| Body Mass Index (kg/m2) c | |||||
| <25 | 144 (42.9) | 5.7 (3.9–7.8) | 4.1 (0.8–12.0) | ||
| 25–30 | 113 (33.6) | 5.7 (3.8–8.1) | 5.8 (0.8–12.0) | ||
| >30 | 79 (23.5) | 5.1 (3.7–7.5) | 1.5 (0.0–8.0) | ||
| 0.476 | 0.033 | ||||
| Serum Cotinine (ng/mL) d | |||||
| <0.015 (unexposed) | 124 (36.9) | 5.1 (3.5–6.9) | 8.5 (2.8–13.3) | ||
| 0.015 – 3 (secondhand) | 181 (53.9) | 6.1 (4.0–8.8) | 2.0 (0.0–8.0) | ||
| >3 ng/mL (active) | 31 (9.2) | 4.8 (4.0–5.5) | 0.8 (0.0–6.5) | ||
| 0.046 | <0.0001 | ||||
| Alcohol During Pregnancy e | |||||
| Never | 184 (54.8) | 5.3 (3.7–7.1) | 3.0 (0.0–9.9) | ||
| ≤1/month | 104 (31.0) | 5.8 (3.6–7.6) | 5.8 (1.1–12.9) | ||
| >1/month | 48 (14.3) | 6.4 (4.5–9.2) | 3.4 (0.2–8.4) | ||
| 0.052 | 0.011 | ||||
| Prior Breastfeeding (weeks) f | |||||
| 0 (Nulliparous) | 145 (43.2) | 6.6 (5.3–9.4) | 5.0 (1.0–10.0) | ||
| 0 (Parous) | 46 (13.7) | 5.1 (4.0–6.6) | 0.0 (0.0–0.5) | ||
| <15 | 49 (14.6) | 6.0 (4.3–7.5) | 1.0 (0.1–2.8) | ||
| 15–52 | 48 (14.3) | 4.8 (3.8–6.9) | 6.8 (3.1–12.0) | ||
| >52 | 48 (14.3) | 3.2 (2.4–4.2) | 13.0 (10.5–15.0) | ||
| <0.001 | <0.0001 | ||||
| Caesarian Section Delivery | |||||
| No | 241 (71.7) | 5.5 (3.7–7.6) | 3.8 (0.5–11.0) | ||
| Yes | 95 (28.3) | 5.9 (3.9–8.2) | 5.0 (0.5–11.0) | ||
| 0.667 | 0.949 | ||||
| Infant Sex | |||||
| Female | 179 (53.3) | 5.7 (3.9–8.1) | 3.5 (0.5–10.0) | ||
| Male | 157 (46.7) | 5.5 (3.7–7.5) | 5.0 (0.5–12.0) | ||
| 0.403 | 0.861 | ||||
p-values from linear regression of log(2)-transformed PFOA concentrations on each covariate
p-values from linear regression of natural log-transformed duration of any breastfeeding on each covariate
Body Mass Index measured at 16 weeks gestation
The mean of serum cotinine measured in maternal serum at 16 weeks, 26 weeks, and delivery (Bernert et al. 2009)
Self-reported consumption of alcohol during pregnancy
Total number of self-reported weeks of breastfeeding across previous children
Duration of breastfeeding of previous children was inversely correlated with maternal serum PFOA concentration during the study pregnancy (Spearman’s rho=−0.372; p-value <0.001) and directly correlated with duration of any breastfeeding following the study pregnancy (Spearman’s rho=0.373; p-value <0.001). Over 80% of women (81.3%) initiated breastfeeding. Among women initiating breastfeeding, the median duration of exclusive breastfeeding was 0.07 months (interquartile range (IQR): 0.03–0.8), and most ended exclusive breastfeeding within 1 month of delivery (78.0%). The median duration of any breastfeeding among initiators was 6 months (IQR: 2.0–12.5), and 47.3% ended any breastfeeding by 6 months. One fourth of infants in the analytic sample continued to receive some breast milk for at least 12 months (24.7%). Mothers that continued any breastfeeding for 12 or more months had lower median PFOA (4.4 ng/mL) and PFOS (12.9 ng/mL) serum concentrations than mothers that initiated breastfeeding but discontinued any breastfeeding by 3 months (PFOA: 6.7 ng/mL; PFOS 15.4 ng/mL) (Table 2).
Table 2.
Median serum concentrations of perfluoroalkyl substances (PFAS) among all women and stratified by duration of any breastfeeding
| Median (25th–75th) | |||||
|---|---|---|---|---|---|
| n | PFOA (ng/mL) | PFOS (ng/mL) | PFNA (ng/mL) | PFHxS (ng/mL) | |
| All women | 336 | 5.5 (3.8–7.7) | 13.9 (9.6–18.2) | 0.9(0.7–1.2) | 1.5 (0.9–2.3) |
| Any breastfeeding (months) | |||||
| 0 | 63 | 5.5 (4.4–7.2) | 12.8 (8.9–17.2) | 0.9 (0.7–1.2) | 1.3 (0.8–2.2) |
| >0 and <3 | 85 | 6.7 (4.7–9.0) | 15.4 (12.3–19.7) | 1.0 (0.7–1.2) | 1.5 (1.0–2.6) |
| ≥3 and <6 | 44 | 5.8 (4.1–7.3) | 13.3 (10.0–19.6) | 1.0 (0.7–1.4) | 1.6 (1.0–2.8) |
| ≥6 and <12 | 61 | 5.5 (3.7–8.4) | 13.3 (9.0–18.1) | 1.0 (0.8–1.2) | 1.3 (0.9–2.2) |
| ≥12 | 83 | 4.4 (3.1–6.4) | 12.9 (9.5–16.8) | 0.9 (0.7–1.1) | 1.6 (0.9–2.2) |
We observed a monotonic dose-response relation between increasing maternal PFOA and risk of ending any breastfeeding prior to 3 months and 6 months (Table 3). Women in the highest quartile of serum PFOA had 77% greater risk of ending any breastfeeding prior to 3 months (95% CI: 1.23, 2.54; p-trend=0.003) and 41% greater risk of ending any breastfeeding by 6 months (95% CI: 1.06, 1.87; p-trend=0.038), compared with women in the lowest quartile.
Table 3.
Adjusted relative risks (RR) for stopping any breastfeeding before 3 and 6 months by quartiles of maternal serum perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) concentrations
| PFAS (ng/mL) | Stopped Any Breastfeeding by 3 months | Stopped Any Breastfeeding by 6 months | ||
|---|---|---|---|---|
| n/N a | RR (95% CI)b | n/N a | RR (95% CI)b | |
| PFOA | ||||
| <3.8 | 21/83 | 1.00 (reference) | 30/83 | 1.00 (reference) |
| 3.8–5.4 | 37/79 | 1.32 (0.92, 1.88) | 49/79 | 1.25 (0.96, 1.62) |
| 5.5–7.6 | 47/86 | 1.63 (1.16, 2.28) | 59/86 | 1.38 (1.06, 1.79) |
| >7.6 | 43/88 | 1.77 (1.23, 2.54) | 54/88 | 1.41 (1.06, 1.87) |
| p-trend | 0.003 | 0.038 | ||
| PFOS | ||||
| <9.6 | 33/82 | 1.00 (reference) | 41/82 | 1.00 (reference) |
| 9.6–13.8 | 34/86 | 1.08 (0.79, 1.46) | 50/86 | 1.17 (0.93, 1.48) |
| 13.9–18.1 | 40/84 | 1.39 (1.04, 1.88) | 47/84 | 1.16 (0.91, 1.48) |
| >18.1 | 41/84 | 1.32 (0.97, 1.79) | 54/84 | 1.25 (0.98, 1.58) |
| p-trend | 0.065 | 0.111 | ||
Number stopping/number in quartile
Adjusted for continuous maternal age at delivery, household income, total weeks of prior breastfeeding, and gestational week at blood draw, and indicator variables for marital status (married or unmarried), race (White, Black, Other), parity (nulliparous v. parous), maternal serum cotinine during pregnancy (<0.015, 0.0015–3, or >3 ng/mL), and alcohol use during pregnancy (yes v. no)
We observed a marginal trend between higher maternal serum PFOS and stopping breastfeeding by 3 months (p-trend=0.065), with women in the highest quartile having 32% greater risk of ending any breastfeeding by 3 months compared to women in the lowest quartile (95% CI: 0.97, 1.79) (Table 3). PFNA and PFHxS were not related to ending any breastfeeding by 3 or 6 months (Supplemental Table 1). None of the PFAS we examined were associated with termination of exclusive breastfeeding by 3 months of age (Supplemental Table 2).
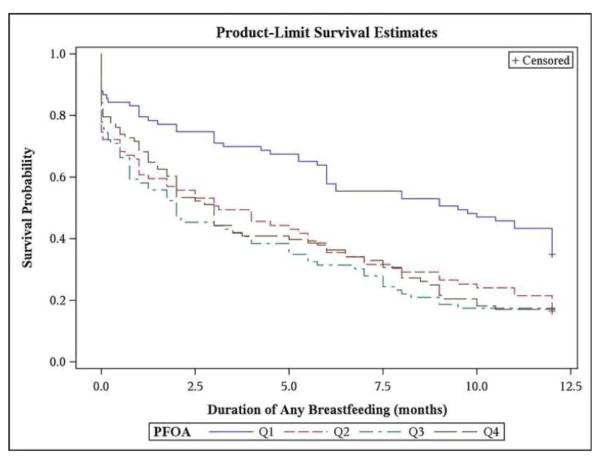
Visualization of time to ending any breastfeeding via Kaplan-Meier curves suggested that women with PFOA <3.8 ng/mL (lowest quartile) generally had longer durations of any breastfeeding compared to women in the upper three quartiles; however, duration of any breastfeeding was not markedly different across the upper three quartiles of maternal serum PFOA concentrations (Figure 1). In multivariable Cox-proportional hazards models, women in the upper three quartiles of PFOA (≥3.8 ng/mL) had a 30% greater hazard of ending any breastfeeding in the first 12 months compared to those in the first quartile (95% CI: 0.94, 1.79). Additionally, for each doubling of maternal serum PFOA, the hazard of ending any breastfeeding in the first 12 months increased by 13% (95% CI: 0.95, 1.35).
Figure 1.
Time to ending any breastfeeding within the first 12 months following delivery by quartiles of maternal serum perfluorooctanoic acid [PFOA ng/mL: Q1 <3.8 ng/mL, Q2 3.8–5.4, Q3 5.5–7.6, Q4 >7.6 ng/mL] from unadjusted Kaplan-Meier estimates.
There were no substantial differences in the associations between PFOA or PFOS and risk of ending any breastfeeding at 3 or 6 months among nulliparous versus parous women (Supplemental Figure 1). We observed the same pattern of results between PFOA concentrations and risk of ending any breastfeeding by 3 or 6 months of age when we included PFOA and PFOS in the same model. However, the association between PFOS concentrations and risk of ending any breastfeeding by 3 or 6 months were attenuated toward the null (Supplemental Table 3). For PFOA and PFOS, the magnitude of the observed associations were stronger and the overall pattern of results was similar for all PFAS when we excluded the 63 women that did not initiate breastfeeding (Supplemental Table 4). Restriction to maternal-child pairs in which the infant had not been admitted to the NICU did not substantially alter the pattern or magnitude of the observed results (data not shown). Log(2)-transformed PFOA serum concentrations were similar across women’s reported reason for ending breastfeeding (ANOVA p-value for difference across groups =0.73) (Table 4; Supplemental Figure 2).
Table 4.
Geometric mean and 95% confidence interval for maternal serum perfluorooctanoic acid (PFOA) concentrations by reason for terminating breastfeeding
| Reported Reason | n a | PFOA ng/mL GM (95% CI)b |
|---|---|---|
| Made insufficient milk | 49 | 5.9 (4.9, 7.1) |
| Sore nipples or breasts | 9 | 5.4 (2.4, 12.5) |
| Too tired | 7 | 5.5 (2.0, 15.6) |
| Returned to work or school | 21 | 5.5 (4.4, 7.0) |
| Emotional difficulty | 10 | 8.3 (5.3, 13.2) |
| Not enough support from family/doctors | 5 | 9.2 (3.4, 24.9) |
| Took medicine incompatible with breastfeeding | 10 | 7.5 (5.1, 11.0) |
| Infant did not feed well | 22 | 6.5 (4.9, 8.6) |
| Infant not gaining weight or not tolerating breast milk | 13 | 4.6 (2.3, 9.3) |
| Other | 25 | 6.0 (4.7, 7.6) |
| ANOVA p-value c | 0.73 |
Women that reported ending breastfeeding earlier than they intended (n=102) were given the opportunity to report up to five reasons for stopping breastfeeding, thus the presented reasons for stopping are not mutually exclusive
The geometric mean of serum PFOA for all women in the analytic sample was 5.5 ng/mL (n=336)
p-value from Analysis of Variance (ANOVA) of log(2)-transformed PFOA on reason for ending breastfeeding
4. Discussion
Our results suggest that higher maternal serum PFOA concentrations during pregnancy are associated with shorter duration of any breastfeeding in the HOME Study cohort. Previous research suggests that both PFOA and PFOS are inversely associated with duration of any breastfeeding among multiparous women (Fei et al., 2010). However, Fei et al. were unable to control for previous breastfeeding (Fei et al., 2010), a route of maternal PFAS excretion (Barbarossa et al., 2013; Mondal et al., 2014) and factor influencing breastfeeding practices in subsequent pregnancies (Nagy et al., 2001; Whalen and Cramton, 2010), thus raising the concern that the associations observed among multiparous women may have been non-causal (Fei et al., 2010). Indeed, when prior breastfeeding was not included in our multivariable models the magnitude of the RR’s tended to be inflated. For example, women in the highest quartile of serum PFOA had 2.42 times the risk of ending any breastfeeding prior to 3 months (95% CI: 1.64, 3.58; p-trend<0.001) and 1.93 times the risk of ending any breastfeeding by 6 months (95% CI: 1.41, 2.64; p-trend<0.001), compared with women in the lowest quartile (Supplemental Table 5). We observed an association between higher maternal PFOA serum concentration and shorter duration of any breastfeeding even after adjustment for previous breastfeeding, providing additional evidence suggesting that PFOA may reduce breastfeeding duration.
In contrast to the study by Fei et al. (2010), we did not observe a clear association between PFOS and duration of any breastfeeding, though risk of ending any breastfeeding by 3 months was marginally elevated among women in the highest PFOS quartile (>18.1 ng/mL). Although the median PFOA level (5.5 ng/mL) in our study was comparable to the median in the Danish study (5.2 ng/mL), the median PFOS plasma concentration in the Danish study was substantially higher (33.3 ng/mL) (Fei et al., 2010) than the median in our study (13.9 ng/mL). Higher concentrations of PFOS than observed in our population may be necessary to adversely affect breastfeeding duration. Alternatively, the inverse association between maternal PFOS and duration of breastfeeding in the prior research may have been confounded by lack of control for previous breastfeeding experience or PFOA co-exposure. Our sensitivity analysis suggested that the association we observed between PFOS and ending any breastfeeding by three months may have been driven by PFOA, as the association between PFOS and breastfeeding was attenuated when both PFOA and PFOS were included in the same model. The Spearman correlation between maternal serum PFOA and PFOS concentrations is relatively strong among women in the HOME Study, further suggesting that PFOA co-exposure may be responsible for the observed association with PFOS.
Both PFOA and PFOS were inversely related to duration of exclusive breastfeeding in the Danish National Birth Cohort (Fei et al., 2010). However, we did not observe any associations between PFAS and exclusive breastfeeding in the HOME Study cohort. In the Danish cohort, the median duration of exclusive breastfeeding was 3.9 months (IQR: 2.9–4.9), whereas the majority of women in the HOME Study breastfed exclusively for less than one month (82.3%, inclusive of women who did not initiate breastfeeding). Fei et al. used a slightly relaxed definition of exclusive breastfeeding, allowing for supplementation of breast milk with water (Fei et al., 2010), which may have inflated the duration of exclusive breastfeeding in their study. Additionally, maternity leave policies in the US and Denmark differ. Women in the US are guaranteed only 12 weeks of maternity leave, often unpaid, if they meet certain conditions (The Lancet, 2014), whereas women in Denmark are entitled to 6 months of paid maternity leave (Fei et al., 2010). Such systemic factors as well as environmental exposures may play a role in the rates of exclusive breastfeeding. Although, the rates of exclusive breastfeeding were quite low in our population, patterns of any breastfeeding in our cohort are very comparable to those observed in the broader population of the US (Supplemental Table 6) (National Immunization Survey 2015).
We did not observe associations of either PFNA or PFHxS with duration of any breastfeeding. Rodent studies suggest that several of the adverse health outcomes associated with PFOA exposure are related to disruption of the peroxisome proliferator activated receptor-α, whereas other PFAS may influence different underlying biological mechanisms (Abbott, 2009; Abbott et al., 2009; Abbott et al., 2007). To the best of our knowledge, the association between PFNA and PFHxS and duration of breastfeeding has not been previously assessed in an epidemiologic study. Evidence from animal studies suggests that PFOA may adversely affect multiple biological mechanisms involved in maternal mammary gland and lactational development. Experimental research using mice shows that PFOA exposure during pregnancy delays epithelial involution and lactational differentiation in dams (White et al., 2007). Additional evidence suggests that PFOA reduces mRNA levels of placental lactogen-II and prolactin like protein-E and –F in a dose-dependent fashion (Suh et al., 2011), suggesting potential hormonal pathways by which PFOA could impact lactation. PFOA exposure may also influence expression of milk protein genes (White et al., 2007), thus adversely impacting the nutritional quality or taste of milk and making it less appealing to offspring. It is presently unknown whether PFOA exposure interferes with initiation of lactation, is related to either poor quantity/quality of breast milk, or potentially decreases duration of breastfeeding through other mechanisms in humans.
Interestingly, prenatal exposure to PFOA decreases mammary epithelial branching and duct epithelial growth (White et al., 2007) and can substantially delay mammary gland development among female mouse pups (Tucker et al., 2015). These delays are potentially mediated by effects of PFOA on the ovary, such as disruption of mammary growth factors via decreased expression of estrogen or progesterone (Zhao et al., 2012). Gestational PFOA exposure negatively influences mammary gland development and differentiation across multiple generations of mice (White et al., 2011), raising concerns for intergenerational effects of such exposure in human populations.
A major strength of our study was the use of prospectively collected exposure, outcome, and covariate data. While a complex set of sociocultural, maternal, infant, societal, and other external factors influence when women discontinue breastfeeding, we were able to control for many pertinent factors known to influence breastfeeding practices, including parity, race/ethnicity, education, marital status, smoking, and prior breastfeeding experience (Whalen and Cramton, 2010). Additionally, our sensitivity analysis suggests that PFOA serum concentrations were similar across women’s reported reasons for ending breastfeeding, which provides some reassurance that the observed associations between PFOA and ending any breastfeeding are not confounded by such factors. However, there is also potential for residual confounding by unknown or unmeasured co-exposures that may be related to PFAS exposure and breastfeeding. Women in our cohort have higher average serum PFOA concentrations compared to pregnant women who participated in NHANES (Braun et al., 2016), which allowed us to examine the dose-response relation at high levels within the range pertinent to the general population. Higher serum PFOA concentrations among women in our cohort may be the result of drinking water contaminated with PFOA released by the DuPont Washington Works plant in Parkersburg, West Virginia, which is located ~250 miles upstream of Cincinnati on the Ohio River and was emitting over 80,000 lbs of PFOA into the environment per year as recently as 2000 (Emmett et al., 2006; Frisbee et al., 2009). These higher than average exposure levels should be carefully considered when generalizing our results to the broader population of the US. We also had a low prevalence of Hispanic women in our study. Hispanic men and women tend to have lower concentrations of PFOA and PFOS than either non-Hispanic Whites or Blacks (Calafat et al., 2007), and in the US, Hispanic women have the highest rates of both initiation and continuation of breastfeeding (Jones et al., 2015).
5. Conclusions
Our findings suggest that maternal serum PFOA concentrations during pregnancy may be related to decreased duration of breastfeeding. These results are consistent with the findings of the one prior epidemiologic study assessing the effect of PFOA on duration of breastfeeding (Fei et al., 2010). Maternal serum PFAS concentrations were not associated with exclusive breastfeeding in our study, suggesting that other factors may have been responsible for low rates of exclusive breastfeeding in this cohort. Although there are certainly social and cultural barriers to breastfeeding, our observations highlight the relevance of environmental risk factors that may adversely influence duration of breastfeeding.
Supplementary Material
Highlights.
Perfluoroalkyl substances (PFAS) are persistent endocrine disrupting chemicals
Maternal serum perfluorooctanoic acid (PFOA) influences breastfeeding duration
Greater PFOA increased risk of ending any breastfeeding by 3 or 6 months postpartum
PFAS were not associated with duration of exclusive breastfeeding in this cohort
Study improves upon prior research by controlling for prior breastfeeding duration
Acknowledgments
This work was supported by NIEHS grants R00 ES020346, R01 ES024381, PO1 ES11261, R01 ES014575, R01 ES020349, and R01 ES025214. We acknowledge K. Kato, C. Dunbar, T. Jia, J.T. Bernert for technical assistance.
Abbreviations
- AAP
American Academy of Pediatrics
- ANOVA
Analysis of Variance
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- HOME
Health Outcomes and Measures of the Environment
- IQR
Interquartile Range
- NHANES
National Health and Nutrition Examination Survey
- NICU
Neonatal Intensive Care Unit
- PFAS
Perfluoroalkyl substances
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctane sulfonic acid
- PFNA
Perfluorononanoic acid
- PFHxS
Perfluorohexane sulfonic acid
- QC
Quality Control
- RR
Relative risk
- US
United States
Footnotes
Financial Disclosure: Dr. Lanphear has served as an expert witness and a consultant to the California Attorney General’s Office for the plaintiffs in a public nuisance case related to childhood lead poisoning, but he has not personally received any compensation for these services. Dr. Lanphear has also served as a paid consultant on a United States Environmental Protection Agency research study related to childhood lead poisoning. Dr. Braun received financial compensation for conducting a re-analysis of a study of child lead exposure for the plaintiffs in a public nuisance case related to childhood lead poisoning. None of these activities are directly related to the present study. The other authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AAP. (American Academy of Pediatrics Section on Breastfeeding). Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Reproductive Toxicology (Elmsford, NY) 2009;27:246–257. doi: 10.1016/j.reprotox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Abbott BD, et al. Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPAR alpha) in the mouse. Reproductive Toxicology (Elmsford, NY) 2009;27:258–265. doi: 10.1016/j.reprotox.2008.05.061. [DOI] [PubMed] [Google Scholar]
- Abbott BD, et al. Perfluorooctanoic acid induced developmental toxicity in the mouse is dependent on expression of peroxisome proliferator activated receptor-alpha. Toxicological Sciences: An Official Journal of the Society of Toxicology. 2007;98:571–581. doi: 10.1093/toxsci/kfm110. [DOI] [PubMed] [Google Scholar]
- Barbarossa A, et al. Perfluoroalkyl substances in human milk: a first survey in Italy. Environ Int. 2013;51:27–30. doi: 10.1016/j.envint.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Bernert JT, et al. Interlaboratory comparability of serum cotinine measurements at smoker and nonsmoker concentration levels: a round-robin study. Nicotine Tob Res. 2009;11:1458–66. doi: 10.1093/ntr/ntp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 2016;24:231–7. doi: 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, et al. Early-life bisphenol a exposure and child body mass index: a prospective cohort study. Environ Health Perspect. 2014;122:1239–45. doi: 10.1289/ehp.1408258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7:513–41. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, et al. Serum concentrations of 11 polyfluoroalkyl compounds in the u.s. population: data from the national health and nutrition examination survey (NHANES) Environmental Science & Technology. 2007;41:2237–2242. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- Emmett EA, et al. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. Journal of Occupational and Environmental Medicine/American College of Occupational and Environmental Medicine. 2006;48:759–770. doi: 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority Panel on Contaminants in the Food Chain. Perfluoroctane sulfonate, perfluorooctanoic acid and their salts: Scientific opinion of the panel on contaminants in the food chain. EFSA Journal. 2008;653:1–131. doi: 10.2903/j.efsa.2008.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, et al. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scandinavian Journal of Work, Environment & Health. 2010;36:413–21. doi: 10.5271/sjweh.2908. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, et al. The C8 health project: design, methods, and participants. Environ Health Perspect. 2009;117:1873–82. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraghty SR, et al. Reporting individual test results of environmental chemicals in breastmilk: potential for premature weaning. Breastfeed Med. 2008;3:207–13. doi: 10.1089/bfm.2008.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep) 2007:1–186. [PMC free article] [PubMed] [Google Scholar]
- Jain RB. Effect of pregnancy on the levels of selected perfluoroalkyl compounds for females aged 17–39 years: data from National Health and Nutrition Examination Survey 2003–2008. J Toxicol Environ Health A. 2013;76:409–21. doi: 10.1080/15287394.2013.771547. [DOI] [PubMed] [Google Scholar]
- Jones KM, et al. Racial and ethnic disparities in breastfeeding. Breastfeed Med. 2015;10:186–96. doi: 10.1089/bfm.2014.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, et al. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218:2133–7. doi: 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- Lew BJ, et al. Activation of the aryl hydrocarbon receptor during different critical windows in pregnancy alters mammary epithelial cell proliferation and differentiation. Toxicol Sci. 2009;111:151–62. doi: 10.1093/toxsci/kfp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, et al. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect. 2014;122:187–92. doi: 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E, et al. Breastfeeding duration and previous breastfeeding experience. Acta Paediatr. 2001;90:51–6. doi: 10.1080/080352501750064879. [DOI] [PubMed] [Google Scholar]
- CDC National Immunization Surveys. Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; Atlanta, GA: 2015. National Immunization Survey Breastfeeding among U.S. Children Born 2002–2012. [Google Scholar]
- Rogan WJ, Gladen BC. Study of human lactation for effects of environmental contaminants: the North Carolina Breast Milk and Formula Project and some other ideas. Environ Health Perspect. 1985;60:215–21. doi: 10.1289/ehp.8560215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh CH, et al. Perfluorooctanoic acid-induced inhibition of placental prolactin-family hormone and fetal growth retardation in mice. Mol Cell Endocrinol. 2011;337:7–15. doi: 10.1016/j.mce.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Teich AS, et al. Women’s perceptions of breastfeeding barriers in early postpartum period: a qualitative analysis nested in two randomized controlled trials. Breastfeed Med. 2014;9:9–15. doi: 10.1089/bfm.2013.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet. The future of paid family leave in the USA. Lancet. 2014;384:2. doi: 10.1016/s0140-6736(14)61101-6. [DOI] [PubMed] [Google Scholar]
- Tucker DK, et al. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod Toxicol. 2015;54:26–36. doi: 10.1016/j.reprotox.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen B, Cramton R. Overcoming barriers to breastfeeding continuation and exclusivity. Curr Opin Pediatr. 2010;22:655–63. doi: 10.1097/MOP.0b013e32833c8996. [DOI] [PubMed] [Google Scholar]
- White SS, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96:133–44. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- White SS, et al. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ Health Perspect. 2011;119:1070–6. doi: 10.1289/ehp.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, et al. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, et al. Differential effects of peripubertal exposure to perfluorooctanoic acid on mammary gland development in C57Bl/6 and Balb/c mouse strains. Reprod Toxicol. 2009;27:299–306. doi: 10.1016/j.reprotox.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, et al. Perfluorooctanoic acid effects on ovaries mediate its inhibition of peripubertal mammary gland development in Balb/c and C57Bl/6 mice. Reprod Toxicol. 2012;33:563–76. doi: 10.1016/j.reprotox.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.