Abstract
Increasingly, the effects of copy number variation (CNV) in the genome on brain function and behaviors are recognized as means to elucidate pathophysiology of psychiatric disorders. Such studies require large samples and we characterized the neurocognitive profile of two cohorts of individuals with 22q11.2 deletion syndrome (22q11DS), the most common CNV associated with schizophrenia, in an effort to harmonize phenotyping in multi-site global collaborations. The Penn Computerized Neurocognitive Battery (PCNB) was administered to individuals with 22q11DS in Philadelphia (PHL; n=155, aged 12–40) and Tel Aviv (TLV; n=59, aged 12–36). We examined effect sizes of performance differences between the cohorts and confirmed the factor structure of PCNB performance efficiency in the combined sample based on data from a large comparison community sample. The cohorts performed comparably with notable deficits in executive function, episodic memory and social cognition domains that were previously associated with abnormal neuroimaging findings in 22q11DS. In mixed model analysis, while there was a main effect for site for accuracy (number of correct response) and speed (time to correct response) independently, there were no main site effects for standardized efficiency (average of accuracy and speed). The fit of a structural model was excellent indicating that PCNB tests were related to the targeted cognitive domains. Thus, our results provide preliminary support for the use of the PCNB as an efficient tool for neurocognitive assessment in international 22q11DS collaborations.
Keywords: 22q11.2 deletion syndrome, Penn Computerized Neurocognitive Battery, Neurocognition, Multisite, Structural model
1. Introduction
There is growing evidence that copy number variation (CNV) in the genome can impact brain function and may underlie some symptoms and cognitive deficits present in neuropsychiatric disorders (Malhotra & Sebat, 2012). The 22q11.2 deletion syndrome (22q11DS) is a common CNV, affecting approximately 1 in 4000 live births (Botto et al., 2003; Goodship, Cross, LiLing, & Wren, 1998). A hemizygous deletion of about 60 known genes (Drew et al., 2011) typically occurs de novo during meiotic recombination, mediated by low-copy repeats on the chromosome 22q11.2 region (Edelmann, Pandita, & Morrow, 1999; Shaikh et al., 2000). This mutation causes extensive phenotypic manifestations. Anomalies including congenital heart defect, thymic and palatal defects, abnormal facies and hypoparathyroidism are common (Bassett et al., 2005; Botto et al., 2003; McDonald-McGinn et al., 1999; Ryan et al., 1997). High incidences of cortical abnormalities (Bingham, Lynch, McDonald-McGinn, & Zackai, 1998; Jalbrzikowski et al., 2013; Lynch et al., 1995; Schaer et al., 2009; Schmitt, Vandekar, et al., 2014; Schmitt, Yi, et al., 2014; Tan, Arnone, McIntosh, & Ebmeier, 2009), developmental delays and learning difficulties in humans (Gerdes et al., 1999; Sobin et al., 2005; Swillen & McDonald-McGinn, 2015; Woodin et al., 2001) and analogous findings in animal models (Fenelon et al., 2013; Karayiorgou, Simon, & Gogos, 2010; Meechan, Tucker, Maynard, & LaMantia, 2009) have consistently implicated aberrant neurodevelopment in 22q11DS. Additionally, many neuropsychiatric conditions emerge during development. Attention deficit/hyperactivity disorder, autism spectrum disorder and anxiety disorders are prevalent in childhood, while mood disorders emerge during adolescence (Green et al., 2009; Schneider et al., 2014). Strikingly high rates of subthreshold psychotic features are observed in adolescents with 22q11DS (Esterberg, Ousley, Cubells, & Walker, 2013; Schneider et al., 2012; Stoddard, Niendam, Hendren, Carter, & Simon, 2010; Tang et al., 2014) and approximately 25% of affected individuals develop schizophrenia (Bassett & Chow, 2008). This 25-fold increased risk relative to the general population makes the 22q11.2 microdeletion one of the most significant copy number variations associated with schizophrenia (Hiroi et al., 2013; Rees et al., 2014; Xu et al., 2008).
Neurocognitive dysfunction is a central feature of non-22q11DS schizophrenia spectrum disorders – deficits in global cognitive function as well as in specific domains including memory, executive and social functioning have been well characterized (Kahn & Keefe, 2013). Impairments in these domains are also found in the clinical high-risk population (Cosway et al., 2000; Keefe et al., 2006) and family members of patients (Cannon et al., 2000), implicating such deficits as important endophenotypes of schizophrenia. Similarly, impairments in executive function, social cognition, non-verbal memory, working-memory and visual-spatial function have been reported in 22q11DS (Gerdes et al., 1999; Moss et al., 1999; Swillen et al., 1999). Notably, a decline in verbal IQ has been associated with the emergence of psychotic disorders in 22q11DS (Vorstman et al., 2015), a finding also present in the non-22q11DS schizophrenia spectrum disorders (Hedman, van Haren, van Baal, Kahn, & Hulshoff Pol, 2013). The deficits in comparable neurocognitive domains between the 22q11DS and non-deleted populations suggest that they may share defects in similar neural networks.
Individuals with 22q11DS have abnormal brain development that is associated with cognitive deficits, providing a valuable model for investigating neural circuitry. Several studies have examined neural networks with functional MRI during task performance and in resting-state. Montojo et al found that individuals with 22q11DS had reduced activation of frontal cortical and basal ganglia regions that are associated with response inhibition during the Stop-signal task when compared to matched controls (Montojo et al., 2015). In another study, Montojo et al showed that individuals with 22q11DS had reduced activation of intraparietal sulcus and superior frontal sulcus during spatial working memory task compared to matched controls (Montojo et al., 2014). Additionally, Azuma et al found that patients with 22q11DS have reduced activation of fusiform-extrastriate cortices, anterior cingulate cortex and superomedial prefrontal cortices when viewing increasing intensity of expression of fear and disgust (Azuma et al., 2015). Notably, reduced activation of abovementioned brain regions that are implicated in inhibitory control, working memory and facial emotion processing were further correlated with levels of impulsivity, subthreshold psychotic symptoms and social difficulties, respectively (Azuma et al., 2015; Montojo et al., 2014; Montojo et al., 2015). Resting state studies have consistently found decreased connectivity within the default mode network (DMN) and lack of age-related maturation of these networks (Debbane et al., 2012; Padula et al., 2015; Schreiner et al., 2014). Taken together, the associated high genetic risk and deficits in neurocognitive function and neural networks make the 22q11DS a promising prodrome model for schizophrenia (Jonas, Montojo, & Bearden, 2014) that is investigated in an international collaboration (Vorstman et al., 2015).
Multicenter genomic studies are needed to generate large datasets for integration across different phenotypic units of analyses including neurocognition. Traditional neurocognitive batteries have several limitations in large-scale multicenter studies owing to their length, complexity of administration and scoring, and vulnerability of manual data handling. The Penn Computerized Neurocognitive Battery (PCNB) was developed to overcome these limitations and to facilitate comprehensive neurocognitive assessments in large-scale multicenter studies (Gur et al., 2010). The PCNB consists of 14 tests, designed to assess 5 neurocognitive domains: Executive Function, Episodic Memory, Complex Cognition, Social Cognition and Sensorimotor Speed (Gur et al., 2012). It takes approximately 1 hour to complete. Recently, it was utilized to characterize the neurocognitive performances of about 10,000 youths in the Philadelphia Neurodevelopmental Cohort (PNC) (Calkins et al., 2014). It has established psychometric properties validating its factor structure (Moore, Reise, Gur, Hakonarson, & Gur, 2015). Additionally, it was validated using functional neuroimaging (Roalf et al., 2014).
The PCNB has been applied in multiple genomic studies (Gur et al., 2015; Kos et al., 2015; Robinson et al., 2015) and more recently, in patients with the 22q11.2 deletion syndrome (22q11DS). We reported that predicted cognitive age, based on PCNB performance, was on average 2–3 years behind those of non-22q11DS individuals with other forms of developmental delays (R. E. Gur et al., 2014). Additionally, our recent analysis demonstrated that poor PCNB performance was associated with decreased overall functioning in individuals with 22q11DS (Yi et al., 2015). To examine a larger dataset required in a genetic study, we sought to harmonize our measures with other established sites with 22q11DS cohorts. We have translated PCNB into Hebrew, administered it to a 22q11DS cohort in Israel and conducted mixed model analysis to examine the effect of site on PCNB performance. We then compared the effect size of differences in performance between the Philadelphia (PHL) and Tel Aviv (TLV) 22q11DS cohorts. Finally, we confirmed the factor structure of PCNB efficiency in the combined sample based on our previous findings from the Philadelphia Neurodevelopmental Cohort (Moore et al., 2015).
2. Methods
2.1. Participants
2.1.1. Philadelphia 22q11DS cohort
The data for the current study was drawn from the PHL 22q11DS cohort established through the Brain-Behavior and Genetic Studies of the 22q11DS, a collaborative project between the Children’s Hospital of Philadelphia (CHOP) and University of Pennsylvania. The study aims to characterize the developmental trajectory of 22q11DS and has been recruiting individuals with 22q11DS from the “22q and You Center” at CHOP and nationally through social media. Inclusion criteria were: ability to provide informed consent/assent, proficiency in English, medical stability, and IQ ≥70 by available records or standardized score ≥70 on the reading section of the Wide Range Achievement Test-IV (Wilkinson & Robertson, 2006). Exclusion criteria were: pervasive developmental disorder (PDD), IQ <70, medical conditions that may affect brain function (e.g., uncontrolled seizures, head trauma, brain tumors or infections). Individuals with PDD or IQ <70 were excluded to increase the reliability of the assessments and generalizability of findings to the non-22q11DS population. At the time of this study, approximately 260 participants had been enrolled and received PCNB. The 22q11.2 deletion status for all participants was confirmed using multiplex ligation-dependent probe amplification (Jalali et al., 2008). University of Pennsylvania and CHOP Institutional Review Boards approved all study procedures associated with the PHL cohort. All participants or parents in the PHL cohort provided informed consent/assent prior to participating in the study.
2.1.2. Tel Aviv 22q11DS cohort
TLV 22q11DS cohort was recruited from the Behavioral Neurogenetics Center, Sheba Medical Center, Tel Aviv University, Israel. This center receives referrals of individuals with 22q11DS from across Israel. For the current study, 59 individuals with 22q11DS were recruited and received the Hebrew version of PCNB. Inclusion criteria were FSIQ ≥ 60, ability to provide informed consent/assent, proficiency in Hebrew, lack of medical conditions that may affect brain function (e.g., brain tumors and uncontrolled seizures). The 22q11.2 deletion status in TLV cohort was confirmed with a fluorescent in situ hybridization and multiple ligation-dependent probe amplification tests (Michaelovsky et al., 2012). The Institutional Review Board of Sheba Medical Center approved all procedures relevant to the TLV cohort. All participants or parents in the TLV cohort provided informed consent/assent prior to participating in the study.
2.1.3. A subset of PHL cohort for the current study
The PHL 22q11DS cohort was demographically different from the TLV cohort. The age range for TLV cohort was 12 to 36 years while the PHL cohort’s age range was 8 to 52 years. Additionally, the TLV cohort was comprised of all Caucasians. Therefore, to demographically match the two samples, younger (<12 years) and older (>39 years) and all non-Caucasian participants from the PHL cohort were excluded in the current study. The resultant PHL sample (n=155) had comparable demographic features in age, sex, race, participant education, and handedness (all, p >0.05) to TLV sample (Table 1). The parental education level, the highest number of years in a formal education setting attained by either parent, was significantly higher in the PHL cohort (p <0.0001).
Table 1.
Demographic characteristic of 22q11DS cohorts.
| Philadelphia N = 155 |
Tel Aviv N = 59 |
p | |
|---|---|---|---|
| Age, mean±SD | 19.7±6.3 | 21.4±6.3 | 0.08 |
| Sex (%) | 1.0 | ||
| Male | 82 (52.9) | 31 (52.5) | |
| Female | 73 (47.1) | 28 (47.5) | |
| Race (%) | |||
| Caucasian | 155 (100) | 59 (100) | |
| Participant Education mean±SD | 10.3±3.0 | 11.0±2.2 | 0.12 |
| Parental Education1 mean±SD | 15.6±2.3 | 14.1±2.3 | <0.0001* |
| Handedness (%) | 0.08 | ||
| Right | 130 (83.9) | 43 (72.9) | |
| Left/Bilateral | 25 (16.1) | 16 (27.1) |
The highest number of years in a formal school setting attained by either parent. The p-values for Age, Participant and Parent Education were calculated using ANOVA; the p-values for sex and handedness were calculated using Chi-square test.
p <0.05 (two-tailed).
2.2. Penn Computerized Neurocognitive Battery (PCNB)
2.2.1. PCNB
A detailed description of the PCNB is reported elsewhere (Gur et al., 2012; Gur et al., 2010; Moore et al., 2015). Briefly, PCNB is a 1-hour computerized battery consisting of 14 tests assessing five neurocognitive domains: Executive Function, Episodic Memory, Complex Cognition, Social Cognition and Sensorimotor speed. Each test provides measures of both accuracy (number of correct responses) and speed (median time for correct responses) except Sensorimotor Processing and Motor Speed tests that provide only the speed measure. Efficiency score is calculated by averaging the accuracy and speed scores of each test. Executive Function domain includes Abstraction & Mental-Flexibility, Attention and Working Memory tests; Episodic Memory includes Verbal, Facial, and Spatial Memory tests; Complex Cognition is measured by Language Reasoning, Non-Verbal Reasoning and Spatial Processing tests; Social Cognition is assessed by the Emotion Identification, Emotion Differentiation and Age Differentiation tests and Sensorimotor Speed is examined by Motor Speed and Sensorimotor Speed tests.
2.2.2. PCNB translation into Hebrew
For the administration of PCNB in the TLV cohort, a linguist proficient in both English and Hebrew first translated the instruction of each test into Hebrew. Upon review by senior bilingual researchers (D.G., R.E.G. & R.C.G.), they were field tried on three non-22q11DS healthy participants and adjustments were made, prior to administration to 22q11DS participants.
The Hebrew version of PCNB consisted of the same tests found in the English version with the exception of omitting tests that required translation of test stimuli in addition to instructions. These included omission of 30 stimuli from Attention test that contained Latin alphabets and the entire Verbal Memory and Language Reasoning tests.
2.2.3. PCNB data preparation
For each PCNB test, based on its distribution, outliers (more than 3 SD away from mean) were removed prior to analysis. For Attention, because the total number of items administered in the TLV cohort was 30 (just the stimuli containing Arabic numerals) and in PHL sample it was 60 (both Arabic numerals and Latin alphabets), the proportion of correct responses out of total items administered was used for accuracy score. For the current study, only participants who completed >50% of the PCNB were included. Approximately, 5% of PHL cohort and none of TLV cohorts were excluded for this reason.
2.3. Statistical analyses
For linear mixed effect model analysis, the accuracy, speed and efficiency scores for each PCNB test in both PHL and TLV 22q11DS samples were first z-transformed using the mean and SD of demographically-matched typically developing sample from PNC as previously described (Gur et al., 2012). The z-transformation allows a comparison of PCNB performance measures across each test and cognitive domain. For consistency, higher z-scores always reflect better performance (e.g., z-scores of response times were multiplied by −1 so that z-score of +1 and −1 indicates a score that is one SD better or worse than the mean, respectively). Three independent mixed model analyses (PROC MIXED procedure) with z-transformed accuracy, speed and efficiency as outcome variables were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, NC). The mixed model allows for subjects to be included who are missing one or more of the PCNB values. Each analysis included fixed effects for Site with Parental Education and the interaction term as covariates. Parental Education was entered as a covariate as it is known to affect PCNB performance (R. E. Gur et al., 2014) and was significantly different between the two sites. The random effect for subject accounted for the repeated measurements from each subject.
Comparison of demographic features, effect size calculations and paired t-test of accuracy and speed scores for each PCNB test between PHL and TLV cohorts were conducted using JMP, version 11 (SAS Institute Inc., Cary, NC). For effect size, Cohen’s d (Cohen, 1977) was calculated by subtracting the PHL mean efficiency score from the TLV mean efficiency score (i.e., mean difference = TLV – PHL) and dividing the difference by a pooled SD. Thus, a positive Cohen’s d indicates that the TLV cohort did better than PHL while a negative Cohen’s d indicates that the TLV cohort did worse than PHL in a given test. For t-test, 2-tailed equal variance test with a significance level of p <0.05 was used except where indicated otherwise.
To assess the factor structure of the PCNB, we performed a confirmatory factor analysis using the PCNB efficiency scores and based on the bifactor (Holzinger, 1937; Reise, Moore, & Haviland, 2010) structure presented in Moore et al. (2015). However, note that our model differs slightly from Moore et al. (2015) insofar as our cross-site battery did not include a Language Reasoning test or Verbal Memory test. The data from the combined sample was used. The analysis was conducted with Mplus, version 7 (Muthén & Muthén, 2013) using maximum likelihood estimation.
3. Results
3.1. Neurocognitive profile across PCNB domains
To examine the neurocognitive profiles across tests between the two cohorts, we compared the z-transformed efficiency scores (Figure 1). Overall, both cohorts had impaired performance, reflected by negative z-scores across all cognitive domains assessed. The pattern of performance was similar between the PHL and TLV cohorts with the lowest performance in Emotion Identification (−1.63 in PHL and −2.28 in TLV) and the best performance in Spatial Processing (−0.44 in PHL and −0.45 in TLV). Additionally, both cohorts exhibited impaired performance in two of the Executive Function domain tests, Attention (−1.26 in PHL and −0.7 in TLV) and Working Memory (−0.8 in PHL and −1.2 in TLV), as well as in Facial Memory (−1.2 in PHL and −1.3 TLV), a test in the Episodic Memory domain.
Figure 1.
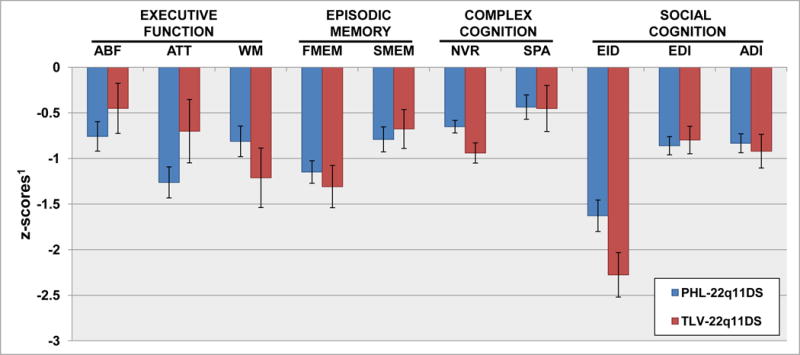
Neurocognitive profile across PCNB domain in PHL and TLV 22q11DS cohorts.
1Efficiency (average of accuracy and speed) score on each PCNB test was z-transformed using demographically-matched typically developing sample from a large community sample. Each cognitive domain is assessed by their corresponding PCNB tests: Executive Function – Abstraction and Mental-Flexibility (ABF), Attention (ATT) and Working-Memory (WM), Episodic Memory – Facial Memory (FMEM) and Spatial Memory (SMEM), Complex Cognition – Non-Verbal Reasoning (NVR) and Spatial Processing (SPA), Social Cognition – Emotion Identification (EID), Emotion Differentiation (EDI) and Age Differentiation (ADI)
3.2. Linear mixed effect model analysis of standardized PCNB measures
To examine the effect of site on neurocognitive profile, we conducted mixed effect model with z-transformed efficiency score as an outcome variable and site as fixed effect and parental education and its interaction with site as covariates (Table 2). There was no significant main effect by site (F1,202 = 0.31, p = 0.58) or parental education (F7,196 =1.64, p = 0.12). The effect remained non-significant for site even when parental education (F1,195 =0.07, p = 0.79) and site x parental education interaction term (F1,188 = 1.65, p = 0.20) were entered as covariates. To examine whether there was a site effect on accuracy and speed measures, we conducted additional mixed effect model analysis with accuracy and speed as outcome variables (Table S1). We found that there were significant site effects for both accuracy (F1,202 = 19.53, p<0.001) and speed (F1,202 = 13.99, p = 0.0002). The effects remained significant even when parental education (Accuracy: F1,195 = 11.82, p = 0.0007; Speed: F1,195 = 14.03, p =0.0002) and site x parental education interaction term (Accuracy: F1,188 = 12.16, p =0.0006; Speed: F1,188 = 7.30, p = 0.0075) were entered as covariates (Table S1).
Table 2.
Linear mixed model analysis of efficiency across PCNB tests.
| Efficiency | F | df | p1 | |
|---|---|---|---|---|
| Main effect | Site | 0.31 | 1, 202 | 0.58 |
| Covariate | Parental education | 0.07 | 1, 195 | 0.79 |
| Parental education × site | 1.65 | 1, 188 | 0.20 |
The p-values were calculated in mixed model analysis with z-transformed efficiency (average of accuracy and speed) scores as an outcome variable with fixed effect for site with parental education and site × parental education interaction term as covariates. The random effect for subjects accounted for repeated measurements.
3.3. Comparison of PCNB Accuracy between PHL and TLV 22q11DS cohorts
The accuracy on most PCNB tests was similar between the two cohorts (Table 3). In all tests, the effect sizes ranged from small (Face Memory, Cohen’s d = −0.15) to medium (Emotion Identification, d = −0.61). The PHL cohort performed better than the TLV cohort (negative Cohen’s d) in all tests except in Abstraction and Mental-Flexibility and Attention. The t-test was significant for Abstraction and Mental-Flexibility (p = 0.01), Non-Verbal Reasoning (p = 0.01) Age Differentiation (p = 0.02), Working Memory (p = 0.006), Emotion Identification (p = 0.0008) and Spatial Processing (p <0.0001). However, when Bonferroni correction for multiple comparisons was applied (Bonferroni corrected p = 0.004), only Emotion Identification and Spatial Processing remained significant. There were no significant differences between the two cohorts in any other tests.
Table 3.
Comparison of accuracy on PCNB between PHL and TLV 22q11DS Cohorts.
| Penn Computerized Neurocognitive Battery (Neurocognitive Domain/Individual Tests) |
Accuracy1 mean, (SD) |
Cohen’s d2 | 95% CI | t | p | |
|---|---|---|---|---|---|---|
| Philadelphia | Tel Aviv | |||||
| Executive Function | ||||||
| Abstraction & Mental-Flexibility | 1.57 (0.91) | 2.00 (1.11) | 0.46 | 0.10, 0.79 | −2.54† | 0.01* |
| Attention | 24.06 (3.95) | 25.08 (5.57) | 0.23 | −0.32, 2.37 | 1.50 | 0.14 |
| Working Memory | 16.00 (3.69) | 14.30 (3.81) | −0.46 | −2.91, −0.50 | −2.82† | 0.006* |
| Episodic Memory | ||||||
| Face Memory | 24.85 (3.48) | 24.31 (3.57) | −0.15 | −1.61, 0.52 | −1.00 | 0.32 |
| Spatial Memory | 13.47 (2.54) | 12.67 (2.70) | −0.31 | −1.58, −0.01 | −1.99 | 0.05 |
| Complex Cognition | ||||||
| Non-Verbal Reasoning | 7.03 (4.00) | 5.50 (3.59) | −0.39 | −2.72, −0.34 | −2.55† | 0.01* |
| Spatial Processing | 6.91 (4.57) | 4.33 (3.25) | −0.60 | −3.79, −1.36 | −4.21† | <0.0001** |
| Social Cognition | ||||||
| Emotion Identification | 28.63 (4.36) | 25.75 (5.66) | −0.61 | −4.52, −1.23 | −3.47† | 0.0008** |
| Emotion Differentiation | 22.28 (4.66) | 20.91 (4.61) | −0.30 | −2.82, 0.07 | −1.87 | 0.06 |
| Age Differentiation | 20.69 (4.84) | 18.78 (5.23) | −0.39 | −3.53, −0.30 | −2.36† | 0.02* |
Accuracy reflects the number of correct responses on each test.
Negative Cohen’s d indicates better performance in PHL relative to TLV cohort and positive d indicates better performance in TLV compared to PHL cohort.
p <0.05 (two-tailed), but non-significant after Bonferroni correction (i.e., p >Bonferroni corrected p of 0.005);
p <0.005 (two-tailed), significant even after Bonferroni correction.
3.4. Comparison of PCNB Speed between PHL and TLV 22q11DS cohorts
The two cohorts performed comparably in speed (Table 4). The effect size of differences ranged from small (Abstraction and Mental-Flexibility, d = −0.05) to medium (Emotion Identification, d = −0.5). There was no large effect size of difference between the two samples. In seven out of twelve tests (Abstraction and Mental-Flexibility, Working Memory, Face Memory, Non-Verbal Reasoning, Spatial Processing, Emotion Identification, Age Differentiation), PHL cohort performed better than the TLV cohort while in the remaining five tests (Attention, Spatial Memory, Emotion Differentiation, Motor, Sensorimotor), TLV outperformed the PHL cohort. While t-test was significant for Sensorimotor (p = 0.03), Face Memory (p = 0.02), Spatial Memory (p = 0.01) and Emotion Identification (p = 0.01), they did not remain significant when Bonferroni correction was applied (all p > Bonferroni corrected p of 0.004).
Table 4.
Comparison of speed on PCNB between PHL and TLV 22q11DS Cohorts.
| Penn Computerized Neurocognitive Battery (Neurocognitive Domain/Individual Tests) |
Speed1 mean, (SD) |
Cohen’s d3 | 95% CI | t | p | |
|---|---|---|---|---|---|---|
| Philadelphia | Tel Aviv | |||||
| Executive Function | ||||||
| Abstraction & Mental-Flexibility | −2526.76 (1107.87) | −2575.28 (858.23) | −0.05 | −386.98, 289.93 | −0.28 | 0.78 |
| Attention | −481.25 (63.38) | −465.98 (111.52) | 0.19 | −8.84, 39.37 | 1.25 | 0.21 |
| Working Memory | −511.30 (134.69) | −542.23 (213.85) | −0.19 | −81.27,19.41 | −1.21 | 0.23 |
| Episodic Memory | ||||||
| Face Memory | −2029.58 (639.37) | −2425.34 (1905.32) | −0.35 | −738.92, −52.60 | −2.27 | 0.02* |
| Spatial Memory | −1930.57 (586.00) | −1691.40 (580.86) | 0.41 | 61.12, 417.23 | 2.66† | 0.01* |
| Complex Cognition | ||||||
| Non-Verbal Reasoning | −6424.37 (3385.83) | −7457.06 (4440.08) | −0.28 | −2244.4, 179.00 | −1.68 | 0.09 |
| Spatial Processing | −9729.60 (3918.43) | −10756.60 (4593.70) | −0.25 | −2457.70, 403.50 | −1.42 | 0.16 |
| Social Cognition | ||||||
| Emotion Identification | −2237.26 (594.78) | −2588.21 (935.92) | −0.50 | −615.71, −86.19 | −2.64† | 0.01* |
| Emotion Differentiation | −3171.20 (1031.92) | −3065.80 (779.81) | 0.11 | −197.89, 408.73 | 0.69 | 0.49 |
| Age Differentiation | −2744.00 (1224.68) | −2853.10 (1443.17) | −0.08 | −510.07, 291.80 | −0.54 | 0.59 |
| Sensorimotor Processing | ||||||
| Motor2 | 97.99 (17.66) | 102.75 (17.75) | 0.27 | −1.01, 10.52 | 1.63 | 0.11 |
| Sensorimotor | −780.36 (231.15) | −704.24 (215.66) | 0.34 | 8.58, 143.67 | 2.23† | 0.03* |
Speed is calculated by multiplying the response time, a median time in milliseconds to correct response, by −1 so that higher value indicates worse performance.
Motor test measures a total number of spacebar-tapping in one minute.
Negative Cohen’s d indicates better performance in PHL compared to TLV cohort while positive d indicates better performance in TLV compared to PHL cohort.
p <0.05 (two-tailed), but non-significant after Bonferroni correction (i.e., p >Bonferroni corrected p of 0.004).
3.5. Confirmatory bifactor model of PCNB efficiency scores in the PHL 22q11DS cohort
Figure 2 shows the confirmatory bifactor model of the PCNB efficiency scores. The fit of the model was good (Comparative Fit Index = 0.95; Root Mean-Square Error of Approximation = 0.056 ±0.021; Standardized Root Mean-Square Residual = 0.041). Results are standardized such that the variances of the factors are 1.00, and all coefficients are significant at the p <0.05 level except where indicated by†. The contribution to the general factor was highest for Non-verbal Reasoning, Emotion Differentiation, Emotion Identification, and Spatial Processing with loadings of 0.65, 0.59, 0.59, and 0.56, respectively. By contrast, Attention and Spatial Memory contributed the least to the general factor, with loadings of 0.33 and 0.38, respectively. Additionally, Social Cognition and Memory factors were non-significant (p >0.05 for all loadings).
Figure 2.
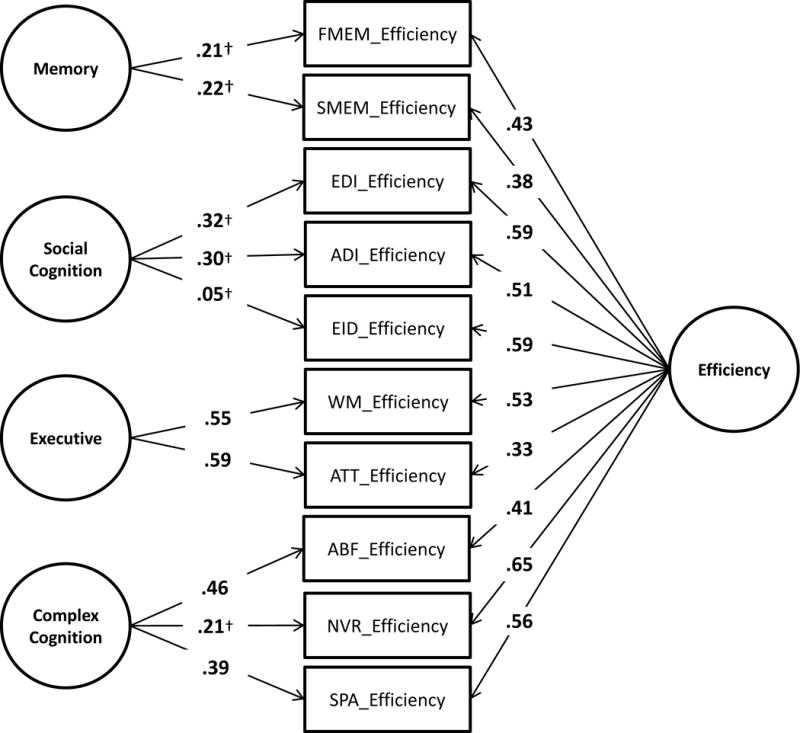
Confirmatory bifactor model of PCNB efficiency in 22q11DS. Results are standardized such that the variance of the latent variables is 1.00; all path coefficients are significant at the p <0.05 level except where indicated by†; Comparative Fit Index (CFI) = 0.95; Root Mean-Square Error of Approximation (RMSEA) = 0.056 ± 0.021; Standardized Root-Mean Residual (SRMR) = 0.041; FMEM = Facial Memory; SMEM = Spatial Memory; EDI = Emotion Differentiation; AGD = Age Differentiation; EID = Emotion Identification; WM = Working Memory; ATT = Attention; ABF = Abstraction and Mental Flexibility; NVR = Non-Verbal Reasoning; SPA = Spatial Processing.
4. Discussion
The 22q11DS is an informative risk model for schizophrenia and provides a unique opportunity to examine cognitive endophenotypes as well as their association to genetic findings. Increasingly, large multicenter studies are conducted in genomic investigations to provide adequate sample size for gene discovery. This is also true in the 22q11DS field and there is an on-going effort to harmonize assessments across multiple sites through the International 22q11.2 Brain Behavior Consortium. Notably, because PCNB has been used in other relevant populations including clinical high risk for psychosis and family members of schizophrenia (Gur et al., 2015; R. C. Gur et al., 2014), use of PCNB in 22q11DS should allow a comparison across multiple at-risk populations. In the present study, we have administered a Hebrew translation of the PCNB to a 22q11DS cohort in Tel Aviv (TLV), Israel and compared their neurocognitive profile to one of the largest 22q11DS cohorts based in Philadelphia (PHL), United States.
In agreement with others, we found clear neurocognitive impairments across multiple domains in 22q11DS. In particular, marked deficits in Executive Function, Episodic Memory and Social Cognition domains were observed. Notably, there was no significant site effect on the neurocognitive profile. We have previously demonstrated that higher parental education was associated with better performance on PCNB (R. E. Gur et al., 2014) and this may be due to parental education being an indirect measure of having more resources and higher socioeconomic status (Shashi et al., 2010). As we did not find a significant main effect for parental education or for site with parental education and interaction term entered as covariates, our study showed a minimal mediating effect of parental education. Additionally, because we did not measure socioeconomic status, the current study could not directly examine its potential effect on neurocognitive function but can be investigated in future efforts.
An overall analysis indicated no site effect for efficiency, but significant effect was found when accuracy and speed were analyzed separately. Indeed, while a majority of tests did not survive the Bonferroni correct, there were six tests (two surviving the Bonferrorni correction and four reaching p <0.05) in accuracy and four (all with p <0.05, but not surviving Bonferroni correction) in speed that differed between two samples. The efficiency score, accounting for both accuracy and speed, is an index for the optimal decision making process in terms of the accuracy/speed trade-off and it reflects the integration of neural processes for producing an accurate and timely response to a stimulus. The presence of main effects for accuracy and speed, but not for efficiency, suggests that while the two sites are performing differently in accuracy and speed in some of tests, when the two measures are combined, the accuracy/speed trade-off is such that it “cancels out” these differences. It is possible that differences observed in accuracy and speed could be due to differences in the presence of psychopathology in two sites. For example, individuals with anxiety disorders, which are common in 22q11DS, may perform better in accuracy than in speed as they are more likely to focus on getting the correct answer to avoid negative prompts (e.g., “incorrect answer, please try again”) that can be perceived as threatening (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van, 2007). In contrast, individuals with hyperactivity and impulsivity, also equally common in 22q11DS, may do better in speed than in accuracy as they are more likely to be quick with their responses in general. It would be important to examine the effect of psychopathology on PCNB performance and we are currently examining this subject.
The biggest differences between the two sites were in Accuracy for Emotion Identification (Cohen’s d = −0.61) and Spatial Processing (d = −0.60) with PHL cohort outperforming TLV in both tests. The Emotion Identification test requires a participant to identify one of four emotions (happy, sad, anger, fear) and neutral faces. The faces are presented one at a time, and the participant is asked to identify the emotion displayed from the set of four listed. As the facial stimuli are balanced for sex, age, and race (Carter et al., 2009; Gur et al., 2002), it is possible that the better performance in the PHL sample is due to greater familiarity with diverse races. Previous studies have demonstrated cultural effects on performance (Pinkham et al., 2008) and differential activation of neural networks during emotion identification task (Derntl et al., 2012; Han & Ma, 2014), perhaps partly due to the effect of cultural practices and experiences in shaping the neural correlates of social cognition. In 22q11DS, reduced activation of fusiform-extrastriate cortices, anterior cingulate cortex and superomedial prefrontal cortices during facial emotion processing has been reported (Azuma et al., 2015), suggesting abnormal maturation of these regions. Therefore, it remains unclear how cultural differences may ultimately influence the development of brain regions for social cognition. Future work can employ functional neuroimaging to examine the potential cultural effects on social neural networks in the two cohorts. Additionally, as social cognition has been consistently implicated in psychopathology and functioning in 22q11DS (Jalbrzikowski et al., 2012; Shashi et al., 2012), the difference in Emotion Identification is possibly due to differences in the level of psychopathology between the two samples. We intend to investigate the effect of psychopathology on PCNB performance between the two cohorts in a future study. In particular, a study examining neurocognitive deficits in 22q11DS individuals with and without psychotic features will be of importance as it is unclear whether specific deficits are associated with psychosis in 22q11DS.
The Spatial Processing test presents two lines at an angle, and asks participants to click on a button to make one line rotate until the two lines are parallel to each other. The relative location of the lines and their sizes vary across trials (Gur et al., 2012). In our experience working with 22q11DS individuals, we found that many participants did not understand the concept of ‘parallel’ even when it was expected based on their education level. In the PHL cohort, we have routinely opted to provide more explicit example of parallel lines (e.g., demonstrating parallel line using two fingers) until the participant understood the concept. This adaptation was not routinely used in the TLV cohort and may explain the differences observed. A closer coordination of the administration process may control for such differences in a future study. Additionally, cultural differences have been implicated in DMN activity during visuo-spatial task (Goh et al., 2013) and cultural differences may account for some observed difference. Abnormal maturation of connectivity in DMN in 22q11DS (Debbane et al., 2012; Padula et al., 2015; Schreiner et al., 2014) likely also contribute to the deficits observed in the Spatial Processing task and this would be an important area for future study as DMN disconnectivity have been associated with prodromal symptoms in 22q11DS (Debbane et al., 2012).
Our confirmatory bifactor model demonstrated good fit. In a bifactor model, as each test loads onto two factors, one general (“Efficiency”) and one specific (e.g. “Memory”), the factors compete for variance. Consequently, it is a truly orthogonal model where modeling of the natural covariance among the specific factors is not necessary due to the presence of the general factor. Therefore, if other predictors or outcomes were to be added to the model, their relationships with the specific or general factors could be considered independent of all other factors. We found non-significance in Social Cognition and Memory factors, suggesting that most of the covariance among those tests is accounted for by the general factor. This is consistent with findings that the most dominant general factor loadings (Emotion Differentiation, Emotion Identification, Non-verbal Reasoning, and Spatial Processing) are for tests on these two factors. These results mostly replicate findings using the same model of the PCNB in a large community sample, suggesting that the PCNB can safely be used in 22q11DS samples (Moore et al., 2015). It is not unusual for tests or batteries to demonstrate different psychometric properties when used in population versus unique clinical samples. For example, one might find that a particular test is an excellent measure of nonverbal reasoning in a community sample but is less valuable in a clinical sample (e.g. because it is too hard). Such problems do not appear to be the case here.
While there are some site differences in PCNB performance, we demonstrate that PCNB can be effectively translated into Hebrew and employed in 22q11DS cohort in Israel. Moving forward, for effective implementation of PCNB, several factors need to be considered. As discussed with Emotion Identification and Spatial Processing, there are known cultural effects on certain cognitive tasks, so it will be important to establish local neurotypical controls in TLV for direct comparison with 22q11DS participants. To this aim, additional cross-cultural testing should be conducted with sites in Europe, Australia and South America that are part of International 22q11.2 Brain Behavior Consortium. Furthermore, to more stringently harmonize the administration process, it will be necessary to conduct a cross-site validation of administration process, for example by recording and reviewing PCNB administrations to identify and reduce any adaptations or modifications during administration. Furthermore, potentially confounding administration variables (such as laptop computer quality, testing setting, time of day, lighting level) can be standardized across sites to minimize any differences that may affect PCNB performance.
In conclusion, we demonstrate that the PCNB can be used in an international 22q11DS cohort and the neurocognitive profiles between the PHL and TLV samples are similar with marked deficits in Executive Function, Episodic Memory and Social Cognition domains. Furthermore, we have confirmed a factor structure of PCNB previously found in the PHL sample, suggesting the PCNB is suitable for use in 22q11DS studies.
4.1. Limitations
There were several limitations of the current study. As the study sample excluded those with more severe intellectual disability (e.g., IQ ≤60), the findings are generalizable only to those in the higher functioning spectrum. The standardization (z-transformation) of PCNB efficiency scores in both samples was done using a subset of the Philadelphia Neurodevelopmental Cohort (PNC). Although we have no reason to believe that typically-developing healthy individuals in Israel would perform differently from their counterparts in the United States, it is nevertheless possible that standardization using PNC may have introduced unintended bias in the TLV data. Such bias could be due to differential effect of culture on typically-developing versus 22q11DS individuals in Israel, which would have been masked by using PNC for standardization. A future study should replicate our findings using typically-developing healthy individuals from Israel. Finally, the current study did not account for other potential confounding variables including level of psychopathology, psychiatric care, socioeconomic status and functioning that may affect PCNB performance. Future studies underway which do account for these variables can explore their relationships to PCNB performance.
Supplementary Material
Highlights.
We characterized cognitive profile of two cohorts with 22q11.2 deletion syndrome.
US and Israeli cohorts performed alike on Penn Computerized Neurocognitive Battery.
Deficits were present in executive function, episodic memory and social cognition.
Fit of structural model of performance based on a community sample was excellent.
The Computerized Neurocognitive Battery can be applied in an international cohort.
Acknowledgments
The authors are grateful to participants and their families. We thank Sean Gallagher, Arielle Swenson, Kelly Peters, Amy Cassidy, Allison Mott, Chad Jackson, Adam Savitt and Kosha Ruparel of the Neuropsychiatry Section in the Department of Psychiatry at the University of Pennsylvania for data acquisition and coordination. We thank Margaret Souders, Lauren Dicairano, Allison Krajewski and Harold Salmons of the “22q and You” Center for recruitment and genotyping of PHL sample. Study was funded by T32 MH019112 (J.Y.), MH087626, MH087636, U01MH101722 and Binational Science Foundation (2011378).
Abbreviations
- 22q11DS
22q11.2 deletion syndrome
- PCNB
Penn Computerized Neurocognitive Battery
- PHL
Philadelphia
- TLV
Tel Aviv
- CHOP
Children’s Hospital of Philadelphia
- PDD
pervasive developmental disorder
- PNC
Philadelphia Neurodevelopmental Cohort
- DMN
Default mode network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest statement
Dr. RE Gur participated in an advisory board for Otsuka Pharmaceuticals unrelated to the study. Dr. RC Gur receives royalties from the Brain Resource Centre. The other authors have no competing interests to declare.
Contributor Information
James J. Yi, Email: yij@email.chop.edu.
Ronnie Weinberger, Email: ronnie.wein@gmail.com.
Tyler M. Moore, Email: tymoore@mail.med.upenn.edu.
Monica E. Calkins, Email: mcalkins@mail.med.upenn.edu.
Yael Guri, Email: yaelig65@gmail.com.
Donna M. McDonald-McGinn, Email: mcginn@email.chop.edu.
Elaine H. Zackai, Email: zackai@email.chop.edu.
Beverly S. Emanuel, Email: emanuel@email.chop.edu.
Raquel E. Gur, Email: raquel@mail.med.upenn.edu.
Doron Gothelf, Email: gothelf@post.tau.ac.il.
Ruben C. Gur, Email: gur@mail.med.upenn.edu.
References
- Azuma R, Deeley Q, Campbell LE, Daly EM, Giampietro V, Brammer MJ, et al. An fMRI study of facial emotion processing in children and adolescents with 22q11.2 deletion syndrome. J Neurodev Disord. 2015;7(1):1. doi: 10.1186/1866-1955-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW. Schizophrenia and 22q11.2 deletion syndrome. Curr Psychiatry Rep. 2008;10(2):148–157. doi: 10.1007/s11920-008-0026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD, et al. Clinical features of 78 adults with 22q11 Deletion Syndrome. Am J Med Genet A. 2005;138(4):307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham PM, Lynch D, McDonald-McGinn D, Zackai E. Polymicrogyria in chromosome 22 delection syndrome. Neurology. 1998;51(5):1500–1502. doi: 10.1212/wnl.51.5.1500. [DOI] [PubMed] [Google Scholar]
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112(1 Pt 1):101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13(3):296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26(2):379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Gur R, Gur R, Pinkham A, Ochsner K. CNTRICS final task selection: social cognitive and affective neuroscience-based measures. Schizophr Bull. 2009;35(1):153–162. doi: 10.1093/schbul/sbn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. Statistical power analysis for the behavioral sciences. New Your, NY: Academic Press; 1977. [Google Scholar]
- Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, et al. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol Med. 2000;30(5):1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- Debbane M, Lazouret M, Lagioia A, Schneider M, Van De Ville D, Eliez S. Resting-state networks in adolescents with 22q11.2 deletion syndrome: associations with prodromal symptoms and executive functions. Schizophr Res. 2012;139:1–3. 33–39. doi: 10.1016/j.schres.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Derntl B, Habel U, Robinson S, Windischberger C, Kryspin-Exner I, Gur RC, et al. Culture but not gender modulates amygdala activation during explicit emotion recognition. BMC Neurosci. 2012;13:54. doi: 10.1186/1471-2202-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, et al. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci. 2011;29(3):259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet. 1999;64(4):1076–1086. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg ML, Ousley OY, Cubells JF, Walker EF. Prodromal and autistic symptoms in schizotypal personality disorder and 22q11.2 deletion syndrome. J Abnorm Psychol. 2013;122(1):238–249. doi: 10.1037/a0028373. [DOI] [PubMed] [Google Scholar]
- Fenelon K, Xu B, Lai CS, Mukai J, Markx S, Stark KL, et al. The pattern of cortical dysfunction in a mouse model of a schizophrenia-related microdeletion. J Neurosci. 2013;33(37):14825–14839. doi: 10.1523/JNEUROSCI.1611-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes M, Solot C, Wang PP, Moss E, LaRossa D, Randall P, et al. Cognitive and behavior profile of preschool children with chromosome 22q11.2 deletion. Am J Med Genet. 1999;85(2):127–133. [PubMed] [Google Scholar]
- Goh JO, Hebrank AC, Sutton BP, Chee MW, Sim SK, Park DC. Culture-related differences in default network activity during visuo-spatial judgments. Soc Cogn Affect Neurosci. 2013;8(2):134–142. doi: 10.1093/scan/nsr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodship J, Cross I, LiLing J, Wren C. A population study of chromosome 22q11 deletions in infancy. Arch Dis Child. 1998;79(4):348–351. doi: 10.1136/adc.79.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Gur RC, Braff DL, Calkins ME, Dobie DJ, Freedman R, Green MF, et al. Neurocognitive performance in family-based and case-control studies of schizophrenia. Schizophr Res. 2015;163:1–3. 17–23. doi: 10.1016/j.schres.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, et al. Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry. 2014;71(4):366–374. doi: 10.1001/jamapsychiatry.2013.4190. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, Marom O, Hughett P, Macy L, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115(2):137–143. doi: 10.1016/s0165-0270(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D, et al. Neurocognitive development in 22q11.2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Mol Psychiatry. 2014;19(11):1205–1211. doi: 10.1038/mp.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Ma Y. Cultural differences in human brain activity: a quantitative meta-analysis. Neuroimage. 2014;99:293–300. doi: 10.1016/j.neuroimage.2014.05.062. [DOI] [PubMed] [Google Scholar]
- Hedman AM, van Haren NE, van Baal CG, Kahn RS, Hulshoff Pol HE. IQ change over time in schizophrenia and healthy individuals: a meta-analysis. Schizophr Res. 2013;146:1–3. 201–208. doi: 10.1016/j.schres.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry. 2013;18(11):1153–1165. doi: 10.1038/mp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger KJ, Swineford F. The bi-factor method. Psychometrika. 1937;2(1):41–54. [Google Scholar]
- Jalali GR, Vorstman JA, Errami A, Vijzelaar R, Biegel J, Shaikh T, et al. Detailed analysis of 22q11.2 with a high density MLPA probe set. Hum Mutat. 2008;29(3):433–440. doi: 10.1002/humu.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Carter C, Senturk D, Chow C, Hopkins JM, Green MF, et al. Social cognition in 22q11.2 microdeletion syndrome: relevance to psychosis? Schizophr Res. 2012;142:1–3. 99–107. doi: 10.1016/j.schres.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF, et al. Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: Relationship with psychotic symptoms. Neuroimage Clin. 2013;3:405–415. doi: 10.1016/j.nicl.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas RK, Montojo CA, Bearden CE. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry. 2014;75(5):351–360. doi: 10.1016/j.biopsych.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70(10):1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11(6):402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res. 2006;88:1–3. 26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kos MZ, Carless MA, Peralta J, Blackburn A, Almeida M, Roalf D, et al. Exome Sequence Data From Multigenerational Families Implicate AMPA Receptor Trafficking in Neurocognitive Impairment and Schizophrenia Risk. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DR, McDonald-McGinn DM, Zackai EH, Emanuel BS, Driscoll DA, Whitaker LA, et al. Cerebellar atrophy in a patient with velocardiofacial syndrome. J Med Genet. 1995;32(7):561–563. doi: 10.1136/jmg.32.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Kirschner R, Goldmuntz E, Sullivan K, Eicher P, Gerdes M, et al. The Philadelphia story: the 22q11.2 deletion: report on 250 patients. Genet Couns. 1999;10(1):11–24. [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci U S A. 2009;106(38):16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelovsky E, Frisch A, Carmel M, Patya M, Zarchi O, Green T, et al. Genotype-phenotype correlation in 22q11.2 deletion syndrome. BMC Med Genet. 2012;13:122. doi: 10.1186/1471-2350-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montojo CA, Ibrahim A, Karlsgodt KH, Chow C, Hilton AE, Jonas RK, et al. Disrupted working memory circuitry and psychotic symptoms in 22q11.2 deletion syndrome. Neuroimage Clin. 2014;4:392–402. doi: 10.1016/j.nicl.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montojo CA, Jalbrzikowski M, Congdon E, Domicoli S, Chow C, Dawson C, et al. Neural substrates of inhibitory control deficits in 22q11.2 deletion syndrome. Cereb Cortex. 2015;25(4):1069–1079. doi: 10.1093/cercor/bht304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 2015;29(2):235–246. doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, McDonald-McGinn DM, Driscoll DA, et al. Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. J Pediatr. 1999;134(2):193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh. Los Angeles, CA: Muthén & Muthén; 2013. [Google Scholar]
- Padula MC, Schaer M, Scariati E, Schneider M, Van De Ville D, Debbane M, et al. Structural and functional connectivity in the default mode network in 22q11.2 deletion syndrome. J Neurodev Disord. 2015;7(1):23. doi: 10.1186/s11689-015-9120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Sasson NJ, Calkins ME, Richard J, Hughett P, Gur RE, et al. The other-race effect in face processing among African American and Caucasian individuals with schizophrenia. Am J Psychiatry. 2008;165(5):639–645. doi: 10.1176/appi.ajp.2007.07101604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees E, Walters JT, Georgieva L, Isles AR, Chambert KD, Richards AL, et al. Analysis of copy number variations at 15 schizophrenia-associated loci. Br J Psychiatry. 2014;204(2):108–114. doi: 10.1192/bjp.bp.113.131052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reise SP, Moore TM, Haviland MG. Bifactor models and rotations: exploring the extent to which multidimensional data yield univocal scale scores. J Pers Assess. 2010;92(6):544–559. doi: 10.1080/00223891.2010.496477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf DR, Ruparel K, Gur RE, Bilker W, Gerraty R, Elliott MA, et al. Neuroimaging predictors of cognitive performance across a standardized neurocognitive battery. Neuropsychology. 2014;28(2):161–176. doi: 10.1037/neu0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Kirby A, Ruparel K, Yang J, McGrath L, Anttila V, et al. The genetic architecture of pediatric cognitive abilities in the Philadelphia Neurodevelopmental Cohort. Mol Psychiatry. 2015;20(4):454–458. doi: 10.1038/mp.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet. 1997;34(10):798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M, Debbane M, Bach Cuadra M, Ottet MC, Glaser B, Thiran JP, et al. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross-sectional and longitudinal study. Schizophr Res. 2009;115:2–3. 182–190. doi: 10.1016/j.schres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Vandekar S, Yi J, Calkins ME, Ruparel K, Roalf DR, et al. Aberrant Cortical Morphometry in the 22q11.2 Deletion Syndrome. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Yi JJ, Roalf DR, Loevner LA, Ruparel K, Whinna D, et al. Incidental radiologic findings in the 22q11.2 deletion syndrome. AJNR Am J Neuroradiol. 2014;35(11):2186–2191. doi: 10.3174/ajnr.A4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Debbane M, Bassett AS, Chow EW, Fung WL, van den Bree M, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171(6):627–639. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Van der Linden M, Glaser B, Rizzi E, Dahoun SP, Hinard C, et al. Preliminary structure and predictive value of attenuated negative symptoms in 22q11.2 deletion syndrome. Psychiatry Res. 2012;196:2–3. 277–284. doi: 10.1016/j.psychres.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Schreiner MJ, Karlsgodt KH, Uddin LQ, Chow C, Congdon E, Jalbrzikowski M, et al. Default mode network connectivity and reciprocal social behavior in 22q11.2 deletion syndrome. Soc Cogn Affect Neurosci. 2014;9(9):1261–1267. doi: 10.1093/scan/nst114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Saitta SC, O’Hare AM, Hu P, Roe BA, et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9(4):489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- Shashi V, Keshavan M, Kaczorowski J, Schoch K, Lewandowski KE, McConkie-Rosell A, et al. Socioeconomic status and psychological function in children with chromosome 22q11.2 deletion syndrome: implications for genetic counseling. J Genet Couns. 2010;19(5):535–544. doi: 10.1007/s10897-010-9309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashi V, Veerapandiyan A, Schoch K, Kwapil T, Keshavan M, Ip E, et al. Social skills and associated psychopathology in children with chromosome 22q11.2 deletion syndrome: implications for interventions. J Intellect Disabil Res. 2012;56(9):865–878. doi: 10.1111/j.1365-2788.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Khuri J, Taylor L, Blundell M, et al. Neuropsychological characteristics of children with the 22q11 Deletion Syndrome: a descriptive analysis. Child Neuropsychol. 2005;11(1):39–53. doi: 10.1080/09297040590911167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J, Niendam T, Hendren R, Carter C, Simon TJ. Attenuated positive symptoms of psychosis in adolescents with chromosome 22q11.2 deletion syndrome. Schizophr Res. 2010;118:1–3. 118–121. doi: 10.1016/j.schres.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, McDonald-McGinn D. Developmental trajectories in 22q11.2 deletion. Am J Med Genet C Semin Med Genet. 2015;169(2):172–181. doi: 10.1002/ajmg.c.31435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Vandeputte L, Cracco J, Maes B, Ghesquiere P, Devriendt K, et al. Neuropsychological, learning and psychosocial profile of primary school aged children with the velo-cardio-facial syndrome (22q11 deletion): evidence for a nonverbal learning disability? Child Neuropsychol. 1999;5(4):230–241. doi: 10.1076/0929-7049(199912)05:04;1-R;FT230. [DOI] [PubMed] [Google Scholar]
- Tan GM, Arnone D, McIntosh AM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies in chromosome 22q11.2 deletion syndrome (velocardiofacial syndrome) Schizophr Res. 2009;115:2–3. 173–181. doi: 10.1016/j.schres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Tang SX, Yi JJ, Moore TM, Calkins ME, Kohler CG, Whinna DA, et al. Subthreshold psychotic symptoms in 22q11.2 deletion syndrome. J Am Acad Child Adolesc Psychiatry. 2014;53(9):991–1000e1002. doi: 10.1016/j.jaac.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JA, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, et al. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. 2015;72(4):377–385. doi: 10.1001/jamapsychiatry.2014.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test Fourth Edition. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Woodin M, Wang PP, Aleman D, McDonald-McGinn D, Zackai E, Moss E. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet Med. 2001;3(1):34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40(7):880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Yi JJ, Calkins ME, Tang SX, Kohler CG, McDonald-McGinn DM, Zackai EH, et al. Impact of psychiatric comorbidity and cognitive deficit on function in 22q11.2 deletion syndrome. Journal of Clinical Psychiatry. 2015 doi: 10.4088/JCP.14m09197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


