Abstract
Objective
Accumulating evidence has linked elevated parathyroid hormone (PTH) with insulin resistance, beta cell dysfunction, and dysglycemia, however, its role in the development of diabetes is largely unclear, particularly among nonwhites. We sought to examine the association of PTH with the incidence of diabetes.
Methods
We studied 8,066 white and 2,034 black adults aged 46–70 years at baseline (1990–92) from the ARIC Study with follow-up for incident diabetes ascertained during study visits conducted in 1993–95 and 1996–98. Hazard ratios (HR) and their 95% CIs for diabetes adjusted for demographics, lifestyle, and 25-hydroxyvitamin D were estimated according to PTH measured at baseline.
Results
PTH was higher among blacks than whites [median (IQR), 43.8 (35.0 – 55.8) vs. 37.9 (30.4 – 47.3) pg/mL; p < 0.001]. During a median follow-up of 6 years, 498 white and 167 black participants developed diabetes. The association of PTH with diabetes varied significantly by race (p-interaction 0.02). PTH was not associated with risk for diabetes among black adults. Among whites, HRs according to quintiles of PTH were 1 (referent), 0.95 (0.71, 1.29), 0.95 (0.70, 1.28), 1.12 (0.84, 1.51), and 1.31 (0.98, 1.76) (p-trend 0.03). When a clinical cut-point for PTH was applied (≥65 pg/mL; 5.7% of whites), the HR for diabetes among whites was 1.38 (1.01, 1.88). Results were similar when restricted to participants with normal baseline kidney function.
Conclusion
In this large, population-based study, elevated PTH was independently associated with risk for diabetes among white, but not black adults. Further studies are needed to elucidate the mechanisms that may underlie this differential association of PTH with diabetes across race groups.
Keywords: diabetes, parathyroid hormone, prospective study, race
1. Introduction
Parathyroid hormone (PTH) helps to regulate circulating calcium concentrations by promoting bone resorption, suppressing urinary calcium loss, and enhancing the formation of calcitriol, the active metabolite of vitamin D. PTH levels are elevated in primary hyperparathyroidism and secondarily in vitamin D deficiency, chronic kidney disease, and other conditions.
Recent evidence has linked elevated PTH concentrations with insulin resistance, beta cell dysfunction, and dysglycemia [1–5], which may eventually lead to the development of diabetes. Indeed, studies of patients with primary hyperparathyroidism have shown a higher prevalence of diabetes compared to control populations [6–9]. While this evidence has suggested a role for PTH in the development of diabetes, these studies have primarily included small numbers of patients recruited from medical clinics. In addition, these studies have almost exclusively included only white adults. Blacks are known to have a higher prevalence and incidence of diabetes [10, 11], higher concentrations of PTH [12], and differences in PTH-calcium metabolism compared to whites [13–15].
The objective of the current study was to examine the association of PTH with the incidence of diabetes in the Atherosclerosis Risk in Communities (ARIC) study, a population-based cohort of white and black adults. We hypothesized that elevated PTH would be associated with greater risk of incident diabetes and that this association would vary significantly according to race group (black vs. white).
2. Materials and Methods
2.1 Participants
The ARIC Study is a prospective cohort of 15,792 middle-aged adults from four U.S. communities: Forsyth County, NC; Jackson MS; Minneapolis, MN, and Washington County, MD. Only blacks were recruited in Jackson, MS, while participants in the other centers reflected the source population (mostly white). The first examination of participants (visit 1) took place from 1987 to 1989, with the first three follow-up visits (visits 2–4), each occurring approximately every 3 years. All participants provided written informed consent at each examination, and institutional review boards from each center approved the study annually.
Serum PTH levels were measured in samples collected at visit 2 (1990–1992; baseline for this analysis), which was attended by 14,348 participants. Excluded from the analysis were participants who self-identified as neither black nor white (n = 42) and blacks from the Minnesota and Maryland centers (n = 49), due to small numbers; those who had at visit 2 fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, a self-report of physician diagnosed diabetes or use of diabetes medications (n = 2,146), a glycated hemoglobin (HbA1c) ≥6.5% (n = 178); an unknown diabetes status at visit 2 or during follow-up (n = 217); those who did not attend visits 3 or 4 (n = 982); those missing specimens for measurement of PTH at visit 2 (n = 629), and those with extreme PTH values (> 200 pg/mL, n = 5). For the primary analysis, our final analytic sample included 10,100 participants (8,066 whites; 2,034 blacks).
2.2 Clinical measurements
Standard protocols for data collection were used across study centers and examinations. Participants were asked to fast for at least 12 h before each examination and to avoid smoking or engaging in heavy physical activity for at least 2 h.
2.3 Serum PTH levels
Intact PTH was measured in previously unthawed serum on the Roche Elecsys 2010 analyzer using a second generation electrochemiluminescence immunoassay that uses a biotinylated monoclonal antibody (Roche Diagnostics, Indianapolis, Indiana, USA) in 2012–2013 at the Advanced Research and Diagnostic Laboratory, University of Minnesota, Minneapolis, Minnesota. Serum PTH by the Elecsys method has shown excellent long-term stability at −80°C [16]. Using split samples collected at visit 2 and stored, we estimated the coefficient of variation to be 9.7% for PTH. In a sample of participants (n = 1,330), we had a second measurement of PTH taken 3 years later and measured in the same laboratory with the same method. The 3-year Spearman correlation was 0.64.
2.4 Incident diabetes
Among those without prevalent diabetes at baseline (visit 2), we classified individuals as having incident diabetes if they met any of the following criteria: fasting glucose level of at least 126 mg/dL; nonfasting glucose level of at least 200 mg/dL; reported current use of glucose lowering medication; or a positive response to the question, “Has a doctor ever told you that you had diabetes (sugar in the blood)?” Serum glucose was available for all participants at visit 2 and nearly all participants at visits 3 and 4 (97% and 89%, respectively). Among whites, 99% fasted for at least 8 hours at each visit, and for blacks, 97%, 96%, and 95% were fasted at visits 2–4, respectively.
2.5 Other variables
Information on age, race, educational level, usual alcohol intake, and parental history of diabetes was based on self-report. Participants were asked to bring to each visit all medications taken in the 2 weeks before the examination; all medication names were transcribed and coded. Physical activity was measured with the Baecke questionnaire at visit 1, but not at visit 2, so values from visit 1 were carried forward [17]. Height and weight were measured, and body mass index (BMI) at visit 2 was calculated as weight in kilograms divided by height in meters squared. Waist circumference was measured in centimeters at the level of the umbilicus. Sitting blood pressure was measured in triplicate with a random-zero sphygmomanometer; the mean of the last two measurements was used.
Serum 25-hydroxyvitamin D [25(OH)D], calcium, and phosphorous were also measured in 2012–13 in stored specimens from visit 2. 25(OH)D was measured using LC/MS/MS instrumentation (coefficient of variation 10.9%). Calcium, albumin, and phosphorous were measured on the Roche Modular P Chemistry Analyzer (Roche Diagnostics) using colorimetric methods. The coefficient of variation was 2.4% for calcium and 3.0% for phosphorous. Calcium levels corrected for albumin were calculated. Serum glucose level was measured by a modified hexokinase-glucose-6-phosphate dehydrogenase procedure at each visit. Insulin was measured by radioimmunoassay at visit 2. HbA1c was measured in frozen whole-blood samples from visit 2 using high performance liquid chromatography (Tosoh 2.2 Plus in 2002–04 and the Tosoh G7 in 2007–08; Tosoh Corporation, Tokyo, Japan) [18]. Serum creatinine was measured at visit 2 using a modified Jaffe reaction. Cystatin C was measured in 2012–2013 from stored samples collected at visit 2 using the Gentian cystatin C assay on the Roche Modular P Chemistry analyzer. Plasma triglycerides were determined by enzymatic methods. HDL-cholesterol was measured after dextran-magnesium precipitation. Estimated glomerular filtration rate (eGFR) was estimated using the 2012 CKD EPI equation, which incorporates both cystatin C and creatinine [19]. Coronary heart disease was defined by self-reported prior physician diagnosis of myocardial infarction or coronary revascularization, prevalent myocardial infarction by 12 lead ECG at visit 1, or an incident adjudicated coronary heart disease event between ARIC visits 1 and 2. Vitamin D binding protein single nucleotide polymorphism (SNP) genotypes for rs4588 and rs7041 were obtained from the ITMAT-Broad-CARe Chip, a custom 50K SNP genotyping array, with genotyping performed at the Broad Institute of Massachusetts Institute of Technology and Harvard.
2.6 Data analysis
Participant characteristics overall and according to PTH quintiles were described using means or proportions as appropriate. Pearson’s correlation coefficients between PTH and other markers of mineral metabolism were also performed. We calculated the incidence rate of diabetes per 1,000 person-years according to quintiles and a clinical cut-point for PTH (≥65 pg/mL). For participants without diabetes, we calculated person-years from baseline to the last clinic date attended (visit 3 or 4). For participants with incident diabetes, we assigned the date of occurrence according to the method described by Duncan and colleagues [20] as the date when the glucose value crossed the diagnostic threshold, estimated by linear interpolation. We used multivariable Cox proportional hazards regression models to estimate hazard ratios (HR) and their corresponding 95% confidence intervals (CI) according to baseline PTH. In multivariable model 1, we adjusted for age, sex, race, season of blood draw, education, and family history of diabetes. In a second model, we adjusted for model 1 covariates in addition to BMI, physical activity, smoking status, alcohol use, and eGFR. A third model adjusted for model 2 covariates in addition to serum 25(OH)D. Tests for a linear trend across PTH quintiles were performed by modeling the median of the PTH quintiles in the multivariable models as a continuous term. We used restricted cubic splines to explore the dose-response association. Baseline PTH was modeled using restricted cubic splines with knots at the 5th, 25th, 75th, and 95th percentiles (22.1, 31.1, 48.8, and 69.8 pg/mL, respectively). A quadratic PTH term revealed no evidence of a non-linear association. Potential effect modification of the association between PTH and incident diabetes by race, sex, eGFR, and 25(OH)D was evaluated by testing the statistical significance of a multiplicative interaction term in models that also included lower order terms. eGFR and 25(OH)D were entered as categorical variables. We also assessed whether key vitamin D binding protein SNPs (i.e., rs4588 and rs7041), which together explain nearly 80% of the variability in vitamin D binding protein levels, modified the association between PTH and diabetes.
Sensitivity analyses were conducted restricting our analysis to participants with normal baseline kidney function (eGFR ≥90 ml/min/1.73 m2), or excluding those with suspected primary hyperparathyroidism (PTH ≥65 pg/mL and calcium >10.2 mg/dL) [21]. The assumption of proportional hazards was evaluated qualitatively by visual inspection of ln(-ln) survival plots and with the inclusion of cross-product terms between PTH quintiles and ln(time).
Tests of statistical significance were two-tailed, with an alpha level of 0.05. SAS version 9.3 (SAS Institute, Cary, NC) was used to perform all analyses.
3. Results
3.1 Participant characteristics
The 10,100 participants had a mean age of 56.6 years at baseline, 20.1% were black, and 57.0% were women. PTH was higher among blacks than whites [median (IQR), 43.8 (35.0 – 55.8) vs. 37.9 (30.4 – 47.3) pg/mL; p < 0.001]. Likewise, the prevalence of PTH ≥65 pg/mL was also higher among blacks compared to whites (15.0% vs. 5.7%). Levels of PTH were correlated inversely with serum 25(OH)D (r = −0.27), phosphorous (r = −0.15), and positively with calcium (r = 0.09). Using similar quintile cut-points in whites and blacks, those with higher PTH levels among both races were older; female; less likely to smoke; consumed less alcohol; had higher BMI, calcium, systolic and diastolic blood pressure levels; were more likely to use antihypertensive medications; and had lower eGFR and 25(OH)D levels (Table 1). White adults with higher PTH levels also had higher glucose levels. No association of insulin with PTH was observed in whites or blacks (p-trend 0.56 and 0.26, respectively).
Table 1.
Baseline characteristicsa of participants according to race and quintiles of parathyroid hormoneb, the ARIC Study, 1990–92
| Whites | Blacks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n=1,758) |
Q2 (n=1,732) |
Q3 (n=1,640) |
Q4 (n=1,592) |
Q5 (n=1,344) |
Q1 (n=259) |
Q2 (n=288) |
Q3 (n=383) |
Q4 (n=430) |
Q5 (n=674) |
|
| Median (pg/mL) | 25.1 | 32.7 | 39.1 | 46.5 | 60.2 | 25.4 | 32.5 | 39.0 | 46.6 | 63.5 |
| Range (pg/mL) | 3.2–29.3 | 29.3–35.7 | 35.7–42.4 | 42.4–51.7 | 51.7–153.1 | 2.1–29.3 | 29.3–35.7 | 35.7–42.4 | 42.4–51.7 | 51.7–179.8 |
| Selected characteristics | ||||||||||
| Age (years) | 56.4 (5.6) | 56.6 (5.6) | 56.9 (5.6) | 57.1 (5.7) | 57.6 (5.7) | 54.7 (5.6) | 54.9 (5.6) | 55.3 (5.6) | 55.5 (5.5) | 56.2 (5.8) |
| Women, % (n) | 53.4 (939) | 53.2 (922) | 53.8 (883) | 58.0 (923) | 59.4 (798) | 47.5 (123) | 54.5 (157) | 59.8 (229) | 66.3 (285) | 74.5 (502) |
| > HS education, % (n) | 36.9 (648) | 40.8 (705) | 39.7 (651) | 43.7 (695) | 41.4 (555) | 36.4 (94) | 35.7 (102) | 37.6 (144) | 37.2 (160) | 36.6 (246) |
| Current smoker, % (n) | 33.8 (594) | 22.2 (384) | 17.7 (291) | 12.2 (194) | 10.4 (140) | 42.8 (110) | 30.7 (88) | 25.9 (99) | 21.7 (93) | 18.5 (124) |
| Alcohol (g/wk) | 44.8 (97.4) | 45.0 (95.7) | 41.6 (91.7) | 35.7 (76.5) | 32.9 (80.6) | 46.5 (102.0) | 36.9 (145.9) | 22.6 (70.1) | 20.9 (91.8) | 20.4 (77.7) |
| Sport index | 2.6 (0.8) | 2.6 (0.8) | 2.6 (0.8) | 2.5 (0.8) | 2.5 (0.8) | 2.2 (0.7) | 2.2 (0.7) | 2.2 (0.7) | 2.1 (0.7) | 2.1 (0.6) |
| Fam history diabetes, % (n) | 22.5 (396) | 22.6 (391) | 20.9 (343) | 20.5 (327) | 21.5 (289) | 19.3 (50) | 26.4 (76) | 23.2 (89) | 24.9 (107) | 24.5 (165) |
| Clinical characteristics | ||||||||||
| Body mass index (kg/m2) | 26.1 (4.2) | 26.3 (4.1) | 26.9 (4.5) | 27.3 (4.7) | 28.4 (5.5) | 26.9 (5.2) | 28.5 (5.4) | 28.4 (5.0) | 29.3 (5.5) | 30.9 (6.8) |
| Waist circumference (cm) | 93.6 (12.6) | 94.0 (12.3) | 95.5 (12.9) | 96.6 (13.1) | 99.4 (14.9) | 93.0 (12.9) | 96.6 (13.8) | 96.8 (12.9) | 98.8 (13.6) | 101.6 (16.2) |
| eGFR (ml/min/1.73m2) | 94.3 (14.1) | 94.9 (14.4) | 94.6 (14.3) | 94.5 (15.4) | 92.4 (16.2) | 103.7 (17.4) | 102.8 (15.0) | 101.8 (17.3) | 102.1 (18.0) | 100.8 (18.4) |
| Systolic BP (mm Hg) | 116.2 (16.5) | 117.0 (16.8) | 118.4 (16.9) | 120.0 (16.7) | 121.2 (18.2) | 122.4 (20.8) | 121.8 (18.2) | 122.0 (17.9) | 124.2 (19.2) | 128.8 (21.0) |
| Diastolic BP (mm Hg) | 69.5 (9.4) | 70.2 (9.6) | 71.1 (9.8) | 71.9 (9.4) | 72.5 (9.8) | 74.9 (11.7) | 75.1 (10.1) | 74.4 (10.2) | 75.1 (10.1) | 77.1 (11.3) |
| BP lowering meds, % (n) | 22.8 (401) | 22.1 (383) | 23.5 (386) | 25.1 (399) | 30.7 (412) | 30.5 (79) | 37.2 (107) | 42.6 (163) | 41.3 (177) | 44.5 (300) |
| Lipid lowering meds, % (n) | 8.0 (141) | 5.7 (98) | 6.7 (110) | 6.0 (96) | 6.0 (80) | 2.8 (7) | 2.4 (7) | 4.5 (17) | 3.0 (13) | 2.2 (15) |
| Heart disease, % (n) | 4.6 (78) | 5.2 (88) | 4.7 (75) | 4.4 (69) | 5.2 (68) | 2.0 (5) | 2.8 (8) | 2.9 (11) | 3.1 (13) | 3.0 (20) |
| Blood concentration | ||||||||||
| Glucose (mg/dL)c | 100.0 (9.3) | 100.7 (9.2) | 101.1 (9.2) | 101.2 (9.4) | 102.3 (9.2) | 103.8 (17.6) | 101.5 (10.1) | 103.1 (9.1) | 103.3 (10.2) | 103.7 (9.8) |
| Insulin (mU/L)c | 19.9 (13.2) | 16.0 (5.2) | 15.6 (4.7) | 18.0 (8.6) | 19.0 (8.2) | 20.2 (6.4) | 18.7 (5.5) | 20.5 (13.9) | 17.6 (5.9) | 32.0 (28.9) |
| HbA1c (%) | 5.4 (0.3) | 5.4 (0.3) | 5.3 (0.3) | 5.3 (0.3) | 5.4 (0.3) | 5.5 (0.5) | 5.6 (0.4) | 5.6 (0.4) | 5.6 (0.4) | 5.6 (0.4) |
| HbA1c (mmol/mol) | 35.3 (3.6) | 35.1 (3.7) | 35.0 (3.5) | 34.8 (3.7) | 35.1 (3.8) | 37.1 (5.1) | 37.5 (4.5) | 37.7 (4.6) | 37.5 (4.4) | 37.9 (4.5) |
| Calcium (mg/dL) | 7.5 (2.1) | 7.6 (2.2) | 7.6 (2.1) | 7.7 (2.0) | 7.8 (2.1) | 8.2 (2.3) | 8.4 (2.2) | 8.2 (2.2) | 8.5 (2.3) | 8.7 (2.2) |
| Phosphorous (mg/dL) | 3.6 (0.5) | 3.5 (0.5) | 3.5 (0.5) | 3.5 (0.4) | 3.4 (0.5) | 3.7 (0.5) | 3.7 (0.5) | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) |
| HDL-cholesterol (mg/dL) | 49.2 (16.6) | 49.8 (16.1) | 50.4 (16.8) | 50.2 (16.5) | 50.4 (16.9) | 55.7 (18.1) | 56.1 (19.2) | 55.0 (17.9) | 54.7 (16.4) | 55.3 (17.0) |
| Triglycerides (mg/dL) | 137.3 (76.0) | 130.1 (73.8) | 133.2 (70.7) | 131.1 (73.1) | 135.8 (80.9) | 97.2 (48.1) | 102.8 (56.5) | 101.6 (50.3) | 105.8 (86.2) | 104.5 (69.7) |
| 25(OH)D (ng/mL) | 28.7 (9.0) | 27.5 (8.9) | 26.5 (8.3) | 25.0 (7.9) | 23.3 (8.4) | 21.9 (7.3) | 20.8 (7.4) | 19.6 (6.7) | 19.0 (7.0) | 16.9 (6.5) |
Presented as the mean (SD) unless otherwise noted.
Quintile categories of parathyroid hormone are based on the total population (white and black adults).
Glucose and insulin concentrations are presented irrespective of fasting time.
Q, quintile; HS, high school; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin
3.2 PTH and incident diabetes
During a median follow-up of 6 years, there were 665 incident cases of diabetes from visit 2 (1990–1992) to visit 4 (1996–1998) (498 among whites and 167 among blacks). The association of PTH with diabetes adjusted for model 3 covariates varied by race (p-interaction 0.02), but not sex, eGFR, or 25(OH)D (p-interaction >0.2, for all). Table 2 shows the number of cases of diabetes and their associations with quintiles of PTH in the overall cohort and also stratified by race. Among whites, in multivariable analyses, there was a positive association of PTH with risk for incident diabetes (Table 2, model 1). Further adjustment for lifestyle factors and eGFR (model 2) attenuated the association somewhat. This attenuation was primarily due to adjustment for BMI. Further adjustment for 25(OH)D had minimal influence on the association (model 3). Additional adjustment for waist circumference also had little influence on the association (data not shown). However, further adjustment for calcium levels modestly strengthened the positive association at the highest levels of PTH. HRs (95% CIs) for diabetes according to higher quintiles of PTH were 1 (referent), 0.99 (0.74, 1.34), 0.97 (0.72, 1.31), 1.14 (0.85, 1.53), and 1.36 (1.01, 1.83), respectively (p-trend 0.02).
Table 2.
Adjusted hazard ratios (95% confidence intervals) for incident diabetes according to quintiles of parathyroid hormone in the overall cohort and by race, the ARIC Study 1990–98
| Quintiles (Q) | ||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p-trend | |
| Median (pg/mL) | 25.2 | 32.6 | 39.1 | 46.5 | 60.8 | |
| Range (pg/mL) | 2.1–29.3 | 29.3–35.7 | 35.7–42.4 | 42.4–51.7 | 51.7–179.8 | |
| Overall cohort | ||||||
| N events | 112 | 116 | 118 | 131 | 188 | |
| Event ratea | 10.0 | 10.3 | 10.4 | 11.6 | 17.2 | |
| Model 1b | 1 (ref) | 1.03 (0.79, 1.33) | 1.04 (0.80, 1.35) | 1.20 (0.93, 1.55) | 1.74 (1.37, 2.21) | < 0.001 |
| Model 2c | 1 (ref) | 1.04 (0.80, 1.35) | 1.03 (0.79, 1.34) | 1.10 (0.85, 1.43) | 1.31 (1.02, 1.69) | 0.03 |
| Model 3d | 1 (ref) | 0.98 (0.75, 1.28) | 1.00 (0.76, 1.30) | 1.07 (0.82, 1.39) | 1.26 (0.97, 1.64) | 0.05 |
| Whites | ||||||
| N events | 95 | 88 | 87 | 105 | 123 | |
| Event ratea | 9.7 | 9.0 | 9.4 | 11.8 | 16.7 | |
| Model 1b | 1 (ref) | 1.00 (0.75, 1.34) | 1.04 (0.77, 1.39) | 1.36 (1.02, 1.80) | 1.82 (1.38, 2.39) | < 0.001 |
| Model 2c | 1 (ref) | 0.98 (0.73, 1.32) | 0.96 (0.71, 1.29) | 1.17 (0.88, 1.56) | 1.38 (1.03, 1.84) | 0.01 |
| Model 3d | 1 (ref) | 0.95 (0.71, 1.29) | 0.95 (0.70, 1.28) | 1.12 (0.84, 1.51) | 1.31 (0.98, 1.76) | 0.03 |
| Blacks | ||||||
| N events | 17 | 28 | 31 | 26 | 65 | |
| Event ratea | 12.3 | 18.1 | 14.9 | 11.1 | 18.1 | |
| Model 1b | 1 (ref) | 1.44 (0.78, 2.64) | 1.23 (0.68, 2.23) | 0.94 (0.51, 1.74) | 1.57 (0.91, 2.72) | 0.25 |
| Model 2c | 1 (ref) | 1.33 (0.72, 2.46) | 1.22 (0.67, 2.22) | 0.85 (0.45, 1.58) | 1.20 (0.68, 2.10) | 0.94 |
| Model 3d | 1 (ref) | 1.12 (0.60, 2.11) | 1.11 (0.60, 2.04) | 0.84 (0.45, 1.57) | 1.19 (0.67, 2.11) | 0.77 |
Event rate per 1,000 person-years
Model 1 adjusts for age, sex, race (except for race stratified analyses), season of blood draw, education (less than high school, high school, more than high school), and family history of diabetes (yes/no).
Model 2 adjusts for Model 1 + body mass index (kg/m2), physical activity (Baecke sport activity index), smoking status (current, former, never), alcohol use (g/wk), and eGFR (ml/min/1.73 m2).
Model 3 adjusts for Model 2 + 25(OH)D (ng/mL).
Q, quintile
The cubic spline regression analyses adjusted for model 3 covariates among whites confirmed a positive association of PTH with diabetes risk that became statistically significant at approximately 60 pg/mL (Figure 1A). When PTH was defined by a clinical cut-point of 65 pg/mL, the multivariable-adjusted HR for diabetes among whites with PTH ≥65 pg/mL was 1.38 (95% CI: 1.01, 1.88) (Table 3, model 3). This HR was 1.38 (95% CI: 1.02, 1.88) following further adjustment for calcium levels.
Figure 1.
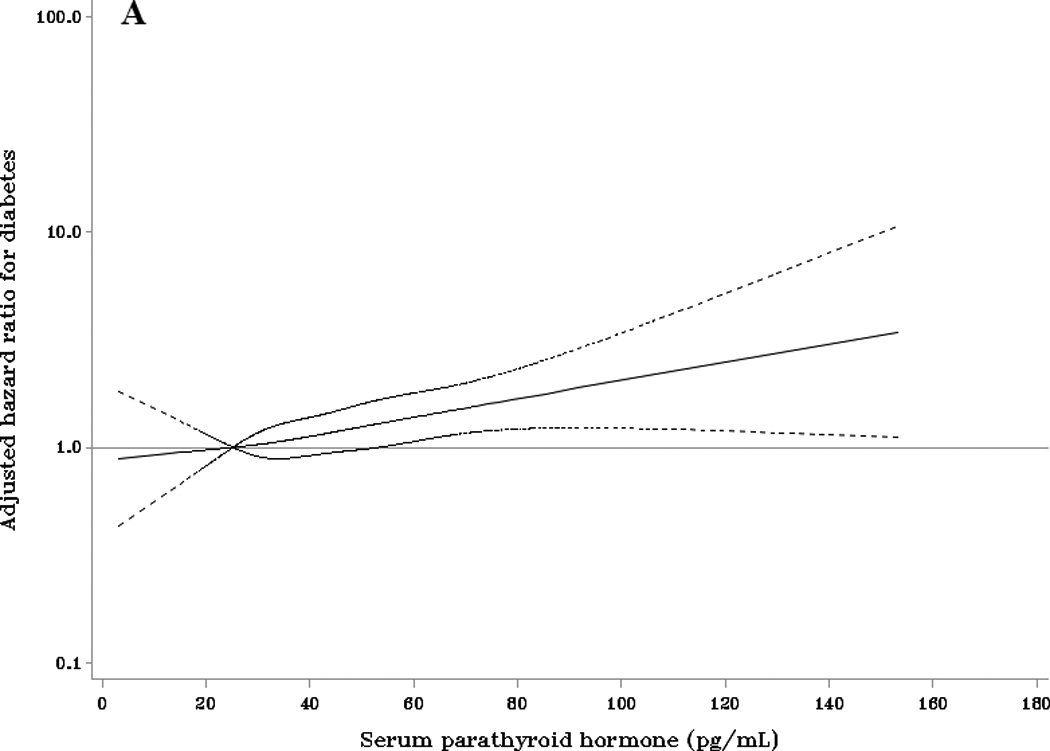
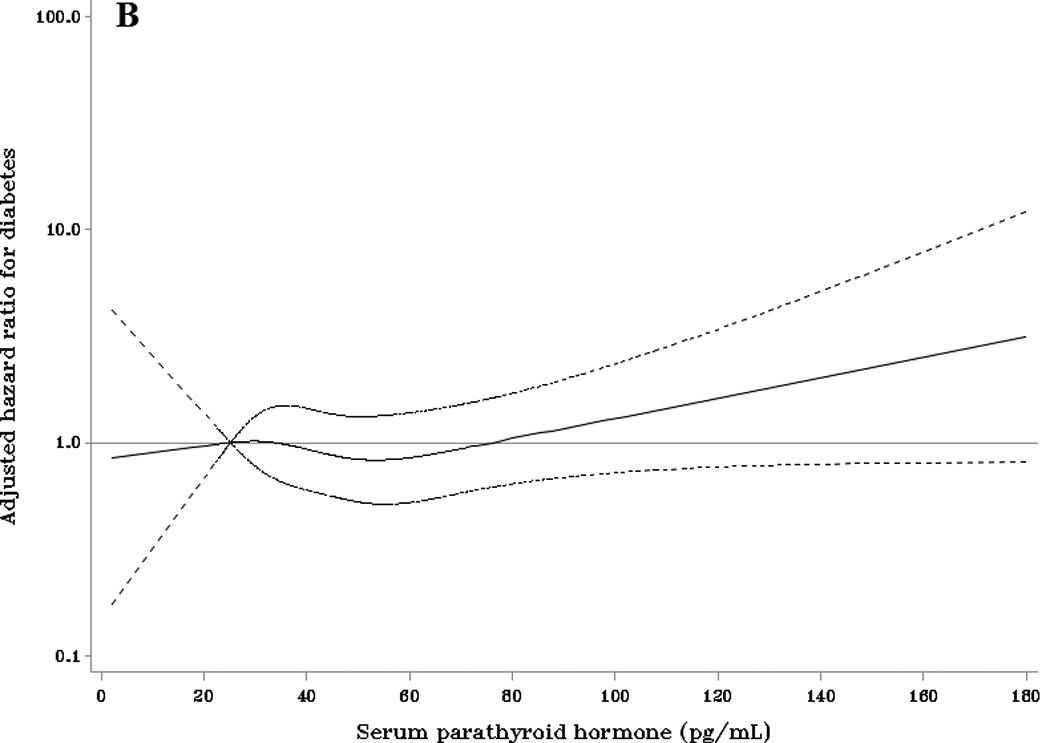
Adjusted HRs (95% CI) for incident diabetes according to baseline parathyroid hormone compared with the referent of 25.2 pg/mL (the 10th percentile) among (A) whites and (B) blacks. Adjusted HRs are from Cox proportional hazards models with adjustment for age, sex, race, season, education, family history of diabetes, body mass index, physical activity, smoking status, alcohol use, eGFR, and 25(OH)D.
Table 3.
Adjusted hazard ratios (95% confidence intervals) for incident diabetes according to a clinical cut-point in the overall cohort and by race, the ARIC Study 1990–98
| Normal | Elevated | |
|---|---|---|
| Median (pg/mL) | 37.8 | 74.1 |
| Range (pg/mL) | 2.1–64.9 | 65.0–179.8 |
| Overall cohort | ||
| N participants | 9340 | 760 |
| N events | 585 | 80 |
| Event ratea | 11.3 | 19.6 |
| Model 1b | 1 (ref) | 1.72 (1.36, 2.19) |
| Model 2c | 1 (ref) | 1.26 (0.98, 1.62) |
| Model 3d | 1 (ref) | 1.24 (0.97, 1.60) |
| Whites | ||
| N participants | 7610 | 456 |
| N events | 448 | 50 |
| Event ratea | 10.5 | 20.5 |
| Model 1b | 1 (ref) | 1.86 (1.38, 2.51) |
| Model 2c | 1 (ref) | 1.43 (1.05, 1.94) |
| Model 3d | 1 (ref) | 1.38 (1.01, 1.88) |
| Blacks | ||
| N participants | 1730 | 304 |
| N events | 137 | 30 |
| Event ratea | 14.8 | 18.4 |
| Model 1b | 1 (ref) | 1.35 (0.90, 2.03) |
| Model 2c | 1 (ref) | 1.09 (0.72, 1.65) |
| Model 3d | 1 (ref) | 1.15 (0.75, 1.77) |
Event rate per 1,000 person-years
Model 1 adjusts for age, sex, race (except for race stratified analyses), season of blood draw, education (less than high school, high school, more than high school), and family history of diabetes (yes/no).
Model 2 adjusts for Model 1 + body mass index (kg/m2), physical activity (Baecke sport activity index), smoking status (current, former, never), alcohol use (g/wk), and eGFR (ml/min/1.73 m2).
Model 3 adjusts for Model 2 + 25(OH)D (ng/mL).
Among blacks, there was no evidence of a dose-response association between PTH and incident diabetes regardless of the level of adjustment (Table 2). The cubic spline regression model (Figure 1B) showed some evidence for a positive association of PTH with diabetes at concentrations >80 pg/mL, however, the confidence intervals were wide and included the null value of 1. The multivariable adjusted HR for diabetes among black adults with PTH ≥65 pg/mL was 1.15 (95% CI: 0.75, 1.77) (Table 3, model 3). This HR was 1.16 (95% CI: 0.76, 1.77) following further adjustment for calcium levels.
There was no evidence that the association between PTH and diabetes varied by 25(OH)D level among white or black adults (Supplemental Table 1). Similarly, the associations did not vary by vitamin D binding SNPs (Supplemental Table 2).
3.3 Sensitivity analyses
When restricted to the 5,106 white and 1,561 black participants with normal kidney function at baseline (eGFR ≥90 ml/min per 1.73 m2), there remained a higher risk for diabetes among white participants with PTH levels ≥65 pg/mL following adjustment for model 3 covariates (n cases: 269; HR: 1.65, 95% CI: 1.10, 2.47) and no association among blacks (n cases: 123; HR: 1.32, 95% CI: 0.81, 2.15). Results were also similar when white and black participants with suspected primary hyperparathyroidism (n = 24 and 26, respectively) were excluded from analyses. The HR for PTH ≥65 pg/mL for model 3 was 1.41 (95% CI: 1.03, 1.93) among whites (n cases: 497) and 1.05 (95% CI: 0.67, 1.65) among blacks (n cases: 163).
4. Discussion
In this large, population-based, prospective ARIC study of white and black adults without diabetes at baseline, we found that the association between elevated PTH and incident diabetes varied significantly by race. Among whites, we observed a positive association of PTH with diabetes that was primarily limited to the highest levels of PTH. This association with diabetes was independent of a number of potential confounding factors, including level of adiposity and 25(OH)D. In contrast, PTH levels were not associated with risk for diabetes among black adults. These findings were robust to sensitivity analyses by baseline kidney function as well as the removal of suspected cases of primary hyperparathyroidism. These results suggest that there are important differences in the association of elevated PTH with diabetes between white and black adults.
Few, if any, studies have investigated the association of PTH with incident diabetes, especially in large, diverse, well-characterized population-based samples such as ARIC. To date, evidence on the relationship between PTH and diabetes has come predominately from case-control studies of patients with primary hyperparathyroidism. Cheung et al. [6] reported that the prevalence of diabetes was approximately 3-fold higher among patients with primary hyperparathyroidism compared with the general population. Taylor [8] reported that the frequency of diabetes was 7.8% in a sample of 205 patients with primary hyperparathyroidism and 3.0% among 200 consecutive patients without hyperparathyroidism attending the same medical clinic. Similarly, Werner et al. [9] reported a 4-fold higher prevalence of diabetes in patients with primary hyperparathyroidism. Our findings suggest a positive association of elevated PTH with incident diabetes in a population-based sample of white adults, which remained after exclusion of those with suspected primary hyperparathyroidism and individuals with kidney dysfunction, the latter of which typically results in secondary hyperparathyroidism.
More recent studies have shown that PTH is independently associated with insulin resistance, β-cell dysfunction, and dysglycemia in human and animal models [1–5]. In contrast to findings showing that elevated PTH levels are not associated with insulin sensitivity, β-cell function, or glycemia, if accompanied by 25(OH)D sufficiency (4), we found no evidence to suggest that the PTH-diabetes association varied according to 25(OH)D status. These contrasting findings may be due, at least in part, to a number of reasons, including differences in the sample population, study design, and outcomes of interest. Additional studies are needed to determine whether 25(OH)D sufficiency may moderate the potential influence of elevated PTH levels in the development of diabetes.
Patients with primary hyperparathyroidism are frequently insulin resistant, and insulin resistance has been shown to improve following parathyroidectomy in these patients [22]. PTH interacts with pancreatic islets and can modulate insulin secretion, potentially through the PTH/parathyroid hormone-related protein receptor (PTH1R) [23]. In animal models, insulin-stimulated glucose uptake in adipocytes has been shown to decline secondary to a PTH-induced elevation in intracellular calcium both in vivo and in vitro [24]. In addition, PTH has been shown to promote serine phosphorylation of insulin receptor substrate1 in adipocytes, which subsequently results in reduced insulin-stimulated glucose uptake [25].
In the current study, we found that both PTH levels and the incidence of diabetes were higher among blacks, compared to whites, confirming findings that have been reported previously [10–12]. However, despite higher levels of PTH, there was no significant association observed among blacks. While the association of 25(OH)D, for example, with diabetes has been shown to differ between white and black adults, including in the ARIC Study [26, 27], a limited number of studies of PTH with other outcomes such as hypertension [28] and mortality [29] have generally revealed consistent findings between the races. The reasons for the lack of an association with diabetes in blacks in the current study are unclear, but may be due, at least in part, to systemic and peripheral resistance to the effects of PTH. There is a noted paradox in the area of bone health whereby blacks have greater bone mass and lower fracture risk than do whites, despite higher PTH and lower 25(OH)D levels [12, 30, 31]. Other explanations may include differences in calcium homeostasis between the races, including a greater ability to absorb calcium, preserve skeletal calcium, and limit the excretion of calcium among blacks [14, 32–34]. Further studies are needed to elucidate the mechanisms that may underlie the differential association of PTH with diabetes between the races.
Strengths of the current study include a community-based sampling method; a biracial cohort; extensive data on potential confounders; a large sample size that increased precision and permitted adjustment and stratification by multiple variables; and the standardized data collection protocols and rigorous quality control of the ARIC Study. Nevertheless, at least six limitations deserve mention. First, we measured PTH only a single time at baseline and therefore we were unable to determine whether changes in PTH may have occurred during the follow-up period. PTH was only moderately correlated with measures taken 3 years later. Changes over time or within person variability in PTH would likely lead to an underestimation of the true association between PTH and diabetes. Second, PTH and several other biomarkers were measured on serum stored for 2 decades. Evidence suggests that serum PTH by our method is stable when stored at −80°C [16]. If deterioration occurred, it is likely to again underestimate the true association of PTH with diabetes. Third, we used a second-generation PTH assay, which has cross-reactivity with the inactive 7–84 PTH fragment that is found in higher concentrations in patients with renal failure [21]. However, our sensitivity analysis restricted to participants with normal kidney function revealed similar results. Fourth, despite the large sample size, statistical power was limited for some comparisons, particularly when the analysis was restricted to blacks. Fifth, our study included adults living in the US and may not be representative of white and black individuals living in other parts of the world. Lastly, this is an observational analysis, and residual confounding may be present.
In conclusion, in this large prospective study of mostly middle-aged adults at baseline, the association of elevated PTH with the development of diabetes varied significantly by race. Whereas there was a positive association among whites, there was no evidence for an association among blacks. This study highlights important differences in the association of elevated PTH with diabetes between white and black adults. Further studies are needed to elucidate the mechanisms that may underlie this differential association between the races.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Further support was obtained by grant R01 HL103706 from the NHLBI with a supplement from the NIH Office of Dietary Supplements (to Dr. Lutsey), the National Institute of Neurological Disorders and Stroke (R01NS072243 to Dr. Michos), as well as the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK089174 to Dr. Selvin).
Footnotes
Conflict of interest
The authors have no relevant conflicts of interest to disclose.
Author Contributions
J.P.R. conceptualized the study, designed the analysis, analyzed the data, interpreted the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. E.S. and E.D.M. obtained funding, interpreted the data, and revised the manuscript for important intellectual content. J.S.P. and C.M.R. interpreted the data and critically revised the manuscript for important intellectual content. P.L.L obtained funding, designed the analysis, interpreted the data, and critically revised the manuscript for important intellectual content. J.P.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
REFERENCES
- 1.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30(6):1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 2.Reis JP, von Muhlen D, Miller ER., 3rd Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol. 2008;159(1):41–48. doi: 10.1530/EJE-08-0072. [DOI] [PubMed] [Google Scholar]
- 3.Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Vitamin D and Parathyroid Hormone Status in Pregnancy: Effect on Insulin Sensitivity, beta-cell Function, and Gestational Diabetes Mellitus. J Clin Endocrinol Metab. 2014;99(12):4506–4513. doi: 10.1210/jc.2014-2341. [DOI] [PubMed] [Google Scholar]
- 4.Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Prospective associations of vitamin D status with beta-cell function, insulin sensitivity, and glycemia: the impact of parathyroid hormone status. Diabetes. 2014;63(11):3868–3879. doi: 10.2337/db14-0489. [DOI] [PubMed] [Google Scholar]
- 5.Chiu KC, Chuang LM, Lee NP, Ryu JM, McGullam JL, Tsai GP, et al. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism. 2000;49(11):1501–1505. doi: 10.1053/meta.2000.17708. [DOI] [PubMed] [Google Scholar]
- 6.Cheung PS, Thompson NW, Brothers TE, Vinik AI. Effect of hyperparathyroidism on the control of diabetes mellitus. Surgery. 1986;100(6):1039–1047. [PubMed] [Google Scholar]
- 7.Ljunghall S, Palmer M, Akerstrom G, Wide L. Diabetes mellitus, glucose tolerance and insulin response to glucose in patients with primary hyperparathyroidism before and after parathyroidectomy. Eur J Clin Invest. 1983;13(5):373–377. doi: 10.1111/j.1365-2362.1983.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 8.Taylor WH. The prevalence of diabetes mellitus in patients with primary hyperparathyroidism and among their relatives. Diabet Med. 1991;8(7):683–687. doi: 10.1111/j.1464-5491.1991.tb01678.x. [DOI] [PubMed] [Google Scholar]
- 9.Werner S, Hjern B, Sjoberg HE. Primary hyperparathyroidism. Analysis of findings in a series of 129 patients. Acta Chir Scand. 1974;140(8):618–625. [PubMed] [Google Scholar]
- 10.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–1753. doi: 10.1007/s00198-010-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, et al. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997;12(6):958–966. doi: 10.1359/jbmr.1997.12.6.958. [DOI] [PubMed] [Google Scholar]
- 15.Kleerekoper M, Nelson DA, Peterson EL, Flynn MJ, Pawluszka AS, Jacobsen G, et al. Reference data for bone mass, calciotropic hormones, and biochemical markers of bone remodeling in older (55–75) postmenopausal white and black women. J Bone Miner Res. 1994;9(8):1267–1276. doi: 10.1002/jbmr.5650090817. [DOI] [PubMed] [Google Scholar]
- 16.Cavalier E, Delanaye P, Hubert P, Krzesinski JM, Chapelle JP, Rozet E. Estimation of the stability of parathyroid hormone when stored at-80 degrees C for a long period. Clin J Am Soc Nephrol. 2009;4(12):1988–1992. doi: 10.2215/CJN.03970609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Coresh J, Jordahl J, Boland L, Steffes MW. Stability of haemoglobin A1c (HbA1c) measurements from frozen whole blood samples stored for over a decade. Diabet Med. 2005;22(12):1726–1730. doi: 10.1111/j.1464-5491.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 19.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 21.Eastell R, Arnold A, Brandi ML, Brown EM, D'Amour P, Hanley DA, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94(2):340–350. doi: 10.1210/jc.2008-1758. [DOI] [PubMed] [Google Scholar]
- 22.Kautzky-Willer A, Pacini G, Niederle B, Schernthaner G, Prager R. Insulin secretion, insulin sensitivity and hepatic insulin extraction in primary hyperparathyroidism before and after surgery. Clin Endocrinol (Oxf) 1992;37(2):147–155. doi: 10.1111/j.1365-2265.1992.tb02299.x. [DOI] [PubMed] [Google Scholar]
- 23.Murray TM, Rao LG, Divieti P, Bringhurst FR. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl- terminal ligands. Endocr Rev. 2005;26(1):78–113. doi: 10.1210/er.2003-0024. [DOI] [PubMed] [Google Scholar]
- 24.Reusch JE, Begum N, Sussman KE, Draznin B. Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology. 1991;129(6):3269–3273. doi: 10.1210/endo-129-6-3269. [DOI] [PubMed] [Google Scholar]
- 25.Chang E, Donkin SS, Teegarden D. Parathyroid hormone suppresses insulin signaling in adipocytes. Mol Cell Endocrinol. 2009;307(1–2):77–82. doi: 10.1016/j.mce.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 27.Reis JP, Michos ED, Selvin E, Pankow J, Lutsey PL. Race, vitamin binding protein gene polymorphisms D, 25-hydroxyvitamin D, and diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.115.107334. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ballegooijen AJ, Kestenbaum B, Sachs MC, de Boer IH, Siscovick DS, Hoofnagle AN, et al. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63(12):1214–1222. doi: 10.1016/j.jacc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kritchevsky SB, Tooze JA, Neiberg RH, Schwartz GG, Hausman DB, Johnson MA, et al. 25-Hydroxyvitamin D, parathyroid hormone, and mortality in black and white older adults: the health ABC study. J Clin Endocrinol Metab. 2012;97(11):4156–4165. doi: 10.1210/jc.2012-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88(2):545S–550S. doi: 10.1093/ajcn/88.2.545S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cauley JA, Danielson ME, Boudreau R, Barbour KE, Horwitz MJ, Bauer DC, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women's Health Initiative (WHI) J Bone Miner Res. 2011;26(10):2378–2388. doi: 10.1002/jbmr.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell NH, Yergey AL, Vieira NE, Oexmann MJ, Shary JR. Demonstration of a difference in urinary calcium, not calcium absorption, in black and white adolescents. J Bone Miner Res. 1993;8(9):1111–1115. doi: 10.1002/jbmr.5650080912. [DOI] [PubMed] [Google Scholar]
- 33.Bryant RJ, Wastney ME, Martin BR, Wood O, McCabe GP, Morshidi M, et al. Racial differences in bone turnover and calcium metabolism in adolescent females. J Clin Endocrinol Metab. 2003;88(3):1043–1047. doi: 10.1210/jc.2002-021367. [DOI] [PubMed] [Google Scholar]
- 34.Cosman F, Nieves J, Dempster D, Lindsay R. Vitamin D economy in blacks. J Bone Miner Res. 2007;22(Suppl 2):V34–V38. doi: 10.1359/jbmr.07s220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


