Abstract
We recently demonstrated, in a rat model of chemotherapy-induced peripheral neuropathy (CIPN), that there is a significant decrease in the duration of the depolarization-evoked Ca2+ transient in isolated somata of putative nociceptive afferents innervating the glabrous skin of the hindpaw, but no change in transient magnitude or the resting concentration of intracellular Ca2+ ([Ca2+]i). Because the Na+-Ca2+ exchanger (NCX) only contributes to the regulation of the duration of the evoked Ca2+ transient, in putative nociceptive dorsal root ganglion (DRG) neurons, we hypothesized that an increase in NCX activity underlies the CIPN-induced change in this subpopulation of neurons. Acutely dissociated retrogradely labeled sensory neurons from naïve, vehicle-, and paclitaxel-treated rats were studied with fura-2 based Ca2+ imaging. There was no difference in the relative level of NCX activity between glabrous neurons from paclitaxel-treated or control rats. However, in contrast to the relatively large and long lasting Ca2+ transients needed to evoke NCX activity in neurons from naïve rats, there was evidence of resting NCX activity in glabrous neurons from both vehicle- and paclitaxel-treated rats. More interestingly, there was a paclitaxel-induced increase in NCX activity in putative nociceptive neurons innervating the thigh, neurons in which there is no evidence of a change in the depolarization-induced Ca2+ transient, or a body site in which there was a change in nociceptive threshold. Furthermore, while the majority of NCX activity in glabrous neurons is sensitive to the NCX3-preferring blocker KB-R7943, the increase in NCX activity in thigh neurons was resistant to KB-R7943 but sensitive to the NCX1-preferring blocker SEA0400. These results suggest that a mechanism(s) other than NCX underlies the paclitaxel-induced decrease in the duration of the evoked Ca2+ transient in putative nociceptive glabrous skin neurons. However, the compensatory response to paclitaxel observed may also explain why only subpopulations of sensory neurons are impacted by paclitaxel, raising the intriguing possibility that CIPN is due to the failure of injured neurons to appropriately compensate for the deleterious consequences of this compound.
Keywords: capsaicin sensitive, isolectin B4, neuropathic pain, nociceptor, retrograde tracer
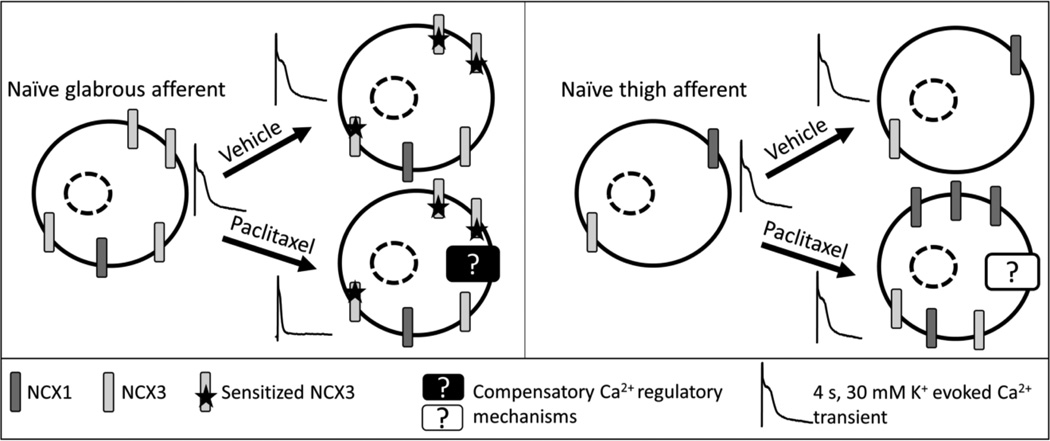
Graphical abstract
1. INTRODUCTION
Chemotherapeutic-induced peripheral neuropathy (CIPN) is a painful condition mainly restricted to the hands and feet [1]. We have recently shown that a rat model of CIPN is associated with a significant decrease in the duration of the depolarization-evoked Ca2+ transients in isolated cell bodies of putative nociceptive afferents, but no changes in the magnitude of the transient, or in resting levels of Ca2+ [2]. Moreover, the degree of this change in duration was significantly larger in neurons innervating the glabrous skin of the hindpaw, than those targeting the hindpaw hairy skin or the inner thigh where no change in transient duration was detected. Interestingly, paclitaxel-induced mechanical hypersensitivity was only detected in the glabrous skin [2]. These data indicate the presence of a subpopulation-specific dysregulation of Ca2+ caused by paclitaxel treatment in this model.
The purpose of the present study was to test the hypothesis that increased activity of the Na+-Ca2+ exchanger (NCX) is the underlying mechanism for the paclitaxel-induced changes in the evoked Ca2+ duration. This hypothesis was based on several observations: 1) NCX is a major Ca2+ extrusion mechanism, but with a low affinity for Ca2+, it is only activated with relatively high [Ca2+]i such as during depolarization-induced Ca2+ transients [3, 4]. In the sensory neuron cell soma, NCX plays a major role in the regulation of the duration of the evoked Ca2+ with no influence on transient magnitude [5, 6]. Consequently, NCX has the biophysical properties in DRG neurons to account for the selective paclitaxel-induced changes in the evoked Ca2+ transient. 2) Among sensory neurons, NCX is active only in putative nociceptive neurons [5, 6], the same subpopulation in which we observed the paclitaxel-induced decrease in the evoked Ca2+ transient duration [2]. 3) There is evidence that a change in NCX activity is associated with inflammatory hypersensitivity, albeit, a decrease in NCX activity [5]. Thus, given the often opposing cellular response to inflammation and nerve injury [7], it is possible that paclitaxel-induced neuropathy is associated with an increase in NCX activity.
To test this hypothesis, retrograde tracer-labeled, small-diameter, IB4+, capsaicin responsive DRG neurons from naïve, vehicle-treated, and paclitaxel-treated rats were studied with ratiometric Ca2+ imaging in combination with a variety of pharmacological manipulations. Our results suggest that the paclitaxel-induced decrease in the duration of the evoked Ca2+ transient is not due to an increase in NCX activity. However, both vehicle and paclitaxel treatments were associated with NCX sensitization. A compensatory Ca2+ regulatory mechanism was also present in afferents innervating target areas where there was no detectable evidence of a chemotherapy-induced change in mechanical sensitivity. Furthermore, paclitaxel treatment affects NCX subtypes differentially based on target of innervation.
2. EXPERIMENTAL PROCEDURES
2.1. Animals
Adult (250–320g) male Sprague-Dawley rats (Harlan, Indianapolis, IN)) were used for all experiments. Rats were housed two per cage in a temperature and humidity controlled, Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited animal housing facility on a 12h:12h light:dark schedule with food and water available ad libitum. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines for the use of laboratory animals in research.
2.2. Tissue labeling
1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbo-cyanine perchlorate (DiI) was injected intradermally at three different sites, one location per animal, so as to label subpopulations of cutaneous afferents identified based on the target of innervation. These sites included the glabrous skin of the hind paw, the hairy skin on the dorsal side of the hind paw, and the hairy skin of the upper inner thigh. The hair covering the thigh was removed with an electrical shaver before retrograde labeling. DiI was injected with a 30 g needle under isoflurane (Abbott Laboratories, North Chicago, IL) anesthesia at 3–5 sites per target for a total volume of 10 µL in the dorsal and ventral hindpaw and 20 µL in the thigh.
2.3. Paclitaxel treatment
One week following the DiI injection, rats were anesthetized with isofluorane and received 2 mg/kg paclitaxel or its vehicle (1:1:23, cremophor EL:ethanol:0.9% saline) via the tail vein. The tail vein injection was repeated three more times every other day for a total of four injections.
2.4. Sensory Neuron Isolation
Rats were deeply anesthetized with an intraperitoneal injection (1 ml/kg) of an anesthetic cocktail containing ketamine (55 mg/kg), xylazine (5.5 mg/kg) and acepromazine (1.1 mg/kg). L4 and L5 DRGs were removed bilaterally, enzymatically treated, and mechanically dissociated. DRG neurons were plated on laminin (Invitrogen, 1mg/ml) and poly-L-ornithine (Sigma-Aldrich, 1 mg/ml) coated glass cover slips as previously described [6]. All subsequent experiments were performed within 8 h of tissue harvest. Only neurons containing the retrograde label DiI were included for further analysis.
2.5. Ca2+ Imaging
Neurons were first incubated with 2.5 µM Ca2+ indicator fura-2 AM ester with 0.01 % Pluronic F-127 for 20 min at room temperature. Neurons were then incubated with FITC-conjugated IB4 (5 µg/ml) for 10 min at room temperature. Following labeling, neurons were placed in a recording chamber and continuously superfused with a HEPES-buffered bath solution (HBS) consisting of (in mM): 130 NaCl, 3 KCl, 2.5 CaCl2, 0.6 MgCl2, 10 HEPES, 10 glucose, pH 7.4, osmolality 325 mOsm. Fluorescence data were acquired on a PC running Metafluor software (Molecular Devices, Sunnyvale, CA) via an EMCCD camera (Photometrics, Tucson, AZ; model QuantEM 512SC). The ratio (R) of fluorescence emission (510 nm) in response to 340/380nm excitation (controlled by a DG-4 (Sutter Instrument, Novato, CA)) was acquired at 1 Hz during application of KCl or capsaicin, which were applied through a computer-controlled, piezo-driven perfusion system (switching time <20 ms; Warner Instruments, Hamden, CT, USA, Fast-Step Model SF-77B). The concentration of intracellular Ca2+ ([Ca2+]i) was determined from fura-2 ratio according to the equation [Ca2+]i (nM) = Kd (Sf2/Sb2) ((R-Rmin)/(Rmax-R)) following in situ calibration as described previously [8], where Kd is the dissociation constant for fura-2 for Ca2+ at room temperature (224 nM); Sf2/Sb2 is the fluorescence ratio of the emission intensity excited with the 380 nm wavelength in the absence of Ca2+ to that in the presence of saturating Ca2+; Rmin and Rmax are the minimal and maximal fluorescence ratios, respectively. Sf2/Sb2, Rmin and Rmax were determined empirically with calibration experiments as described previously [9], run periodically throughout the data collection period.
2.6. Chemicals
The retrograde tracer, DiI (Invitrogen, Carlsbad, CA, USA), was dissolved at 170 mg/mL in dimethylsufoxide (DMSO, Sigma-Aldrich, St Louis, MO, USA) and diluted 1:10 in 0.9% sterile saline. Paclitaxel (Sigma-Aldrich), was dissolved at 25 mg/mL in 1:1 Cremophor EL (Sigma-Aldrich): ethanol and freshly diluted 1:12.5 in 0.9% sterile saline prior to injections. FITC-conjugated Isolectin B4 (IB4, Sigma-Aldrich) was dissolved in dH20 as a stock solution of 1 mg/ml, and then diluted to a final concentration of 10 µg/ml in HBS the day of use. Fura-2 acetoxymethyl (AM) ester (TEF Laboratories, Austin, TX, USA) was dissolved in DMSO as a 2.5 mM stock solution and diluted to a final concentration of 2.5 µM in HBS. Pluronic F-127 (TEF Laboratories) was dissolved in DMSO as a 20% stock solution and diluted to 0.01% in HBS. Capsaicin (Sigma-Aldrich) was dissolved in ethanol as a 10 mM stock solution and diluted to 500 nM in HBS. LiCl (Sigma-Aldrich) was used to replace NaCl in HBS. KB-R7943 mesylate (Tocris, Bristol, UK) was dissolved in DMSO as a 100 mM stock solution and diluted to 100 nM in HBS. SEA0400 (ChemScene, Monmouth Junction, NJ, USA) was dissolved in DMSO as a 100 mM stock solution and diluted to 1 µM in HBS.
2.7. Data Analysis
Neurons from a single field were studied on each coverslip. Resting Ca2+ was determined prior to stimulation as the average of measurements taken over the 30 second prior to evoking a Ca2+ transient with high K+ (30 mM, 4 seconds). The magnitude of the evoked Ca2+ transient was determined as the difference between resting and the peak of the evoked Ca2+ transient. The duration of the evoked Ca2+ transient was determined as the time for the transient to decay to 50% of the peak (T50). For experiments involving the application of test compounds, a vehicle control group was always included. Neurons with a small cell body diameter (<30 µm) [10], responsive the capsaicin (500 nM) [11], and labeled with the lectin IB4 [12], were studied and are referred to as putative nociceptors. While there are other subpopulations of putative nociceptors, IB4-negative nociceptors were not included in this study both because we previously failed to detect an influence of paclitaxel on the evoked Ca2+ transient in this subpopulation of neurons [2], and because NCX is restricted to the subpopulation of small diameter, capsaicin responsive, IB4+ neurons [5]. Cell body diameter was determined with a calibrated eye-piece reticle. Capsaicin sensitivity was assessed at the end of every experiment and neurons were considered capsaicin sensitive if application of capsaicin (500 nM, 1 second) resulted in an increase in [Ca2+]i greater than 20% above baseline. IB4 binding was determined under epifluorescence illumination prior to the start of each experiment. Neurons in which the plasma membrane was clearly defined by epifluorescence were considered IB4+. Data are expressed as mean ± s.e.m. One and two-way ANOVA were used for analysis of more than two groups with the Holm-Sidak test used for post-hoc analysis. Statistical significance was assessed at p < 0.05. The Chi-Square or Fisher exact tests were used to assess the presence of significant differences between groups with respect to the proportion or percentage of neurons with any evidence of NCX activity, or evidence of KB-R7943-sensitive or SEA0400-sensitive NCX activity.
3. RESULTS
3.1. NCX does not mediate the paclitaxel-induced attenuation of the depolarization-evoked Ca2+ transient duration in putative glabrous nociceptors
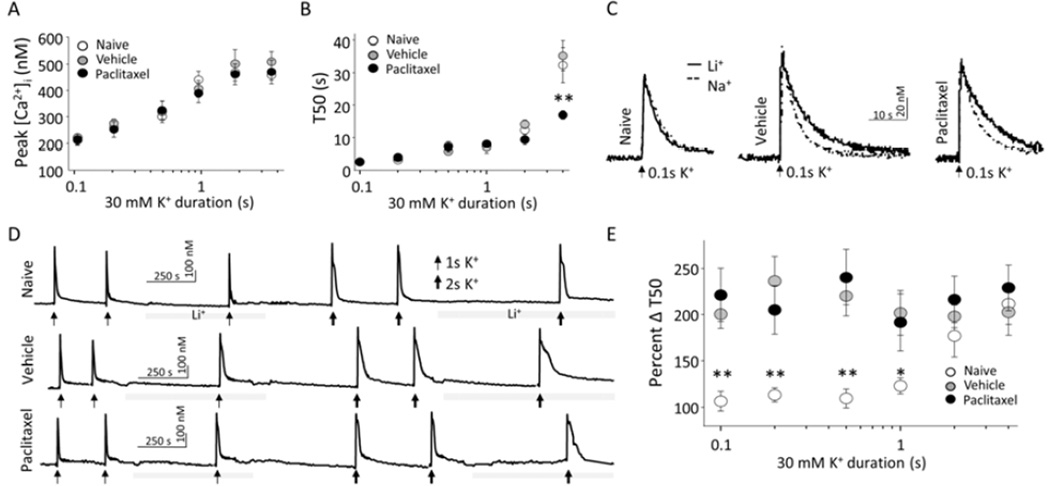
To determine whether paclitaxel-induced attenuation of the evoked Ca2+ transients was due to increased NCX activity, we assessed NCX activity in glabrous skin neurons from naïve, vehicle- and paclitaxel-treated rats. We focused on IB4-binding, capsaicin-responsive, small diameter (<30 µm) lumbar DRG neurons that project to the glabrous skin of the hindpaw, because of our previous results indicating that the paclitaxel-induced attenuation of the depolarization-evoked Ca2+ transient was manifest in this subpopulation [2]. We blocked NCX activity by replacing Na+ in HBS with Li+ taking advantage of the fact that NCX activity requires presence of Na+ ions in the extracellular environment [13], and minimal impact that replacing Na+ with Li+ has on the functioning of voltage-gated Na+ channels [14]. The relative increase in the duration of depolarizationevoked Ca2+ transient in the presence of Li+ was assumed to reflect the loss NCX activity, and was therefore used as an indirect measure of NCX activity. Consequently, in neurons from paclitaxel-treated rats, an increase in NCX activity should be associated with a relatively larger increase in the duration of the evoked Ca2+ transient following NCX block. Consistent with our previous results [2], the duration of the high K+ (30 mM, 4 sec)-evoked Ca2+ transient in neurons from paclitaxel-treated rats was shorter than that neurons from naïve or vehicle treated rats (Figure1A and 1B). Further, as expected, in the presence of Li+, the duration of the evoked Ca2+ transient was increased in neurons from naïve, vehicle- and paclitaxel-treated rats (Figure 1A, solid lines). However, the relative increase in the duration of evoked Ca2+ transients was comparable (p > 0.05) in neurons from all three groups of rats as illustrated when these data were analyzed as a percent change in duration (Figure 1C).
Figure 1.
Paclitaxel-induced attenuation of the duration of the depolarization-evoked Ca2+ transient in putative nociceptive glabrous skin neurons is not mediated by NCX. A) Ca2+ transients evoked with 4s application of 30 mM K+ in putative nociceptive neurons labeled from the glabrous skin from naïve (left) vehicle-treated (middle) and paclitaxel-treated (right) rats before (dotted traces) and after (solid traces) block of NCX by exchanging Na+ for Li+ in the bath solution. The duration of the evoked Ca2+ transient was quantified as the time to decay to 50 percent of the magnitude of the evoked Ca2+ transient (T50). B) Pooled T50 data from naïve (n = 8), vehicle-treated (n = 17 neurons) and paclitaxel-treated (n = 16 neurons) glabrous skin neurons in Na+ bath. C) Relative increase in the duration of evoked Ca2+ transients from the same group of neurons in (B) analyzed as a percent increase in T50 following block of NCX with Li+. * is p < 0.05.
3.2. NCX is sensitized by the vehicle of paclitaxel
NCX activity not only depends on the extracellular Na+ concentration but also on [Ca2+]i. Because NCX has a low affinity for Ca2+ [3], the magnitude and the duration of the evoked Ca2+ transient required to reach the threshold for engaging NCX activity are relatively large [5]. However, the duration of the evoked Ca2+ transient in putative nociceptors from paclitaxel-treated rats was shorter than that previously demonstrated to be required for NCX activation in glabrous skin neurons [5]. This raised the possibility that NCX was sensitized in glabrous skin neurons from paclitaxel treated rats. An increase in NCX activity in response to smaller and shorter duration increases in [Ca2+]i could contribute to an overall decrease in the duration of the evoked transient, even if there was no difference in peak NCX activity in neurons from paclitaxel- and vehicle-treated rats. We tested this possibility with a stimulation-duration analysis of the activation of NCX, by application of 30 mM KCl for durations ranging between 0.1 to 2 s in the presence and absence of Li+ to neurons from vehicle- and paclitaxel-treated rats. Consistent with our previous findings [5], both the amplitude (Figure 2A) and duration (Figure 2B) of the depolarization-evoked transient depends on the duration of KCl application. The amplitude saturates with a KCl application of ~2 seconds while the duration of the evoked transient appears to be bi-phasic, and has not fully saturated with even a 4 second application of KCl. A neuron was considered Li+-responsive, if the duration of the evoked Ca2+ transient increased ≥ 20% in the presence of Li+. There was no difference in the proportion of Li+-responsive neurons from paclitaxel- (12/15 and 13/14 for 1 and 2 sec applications of 30 mM KCl, respectively) and vehicle- (12/12 and 10/10 for 1 and 2 sec applications of 30 mM KCl, respectively) treated rats. Similarly, in neurons stimulated with 4 sec of high K+ (i.e., in figure 1), 1 out of 17 and 4 out of 21 neurons from paclitaxel and vehicle-treated rats, respectively, were unresponsive to Li+. Furthermore, there were no detectable differences between the Li+-responsive and non-responsive neurons from paclitaxel-treated rats with respect to the properties of the evoked Ca2+ transient. However, in contrast to our previous results with glabrous neurons from naïve rats, where [Ca2+]i above 325 nM for at least 12 seconds was required for NCX activation [5], in the presence of Li+ there was a ~2-fold increase in the duration of the evoked Ca2+ transient in response to any stimulus duration that was sufficient to drive an increase in [Ca2+]i (Figure 2C, 2D, and 2E). This apparent sensitization of NCX was observed in neurons from both vehicle- and paclitaxel-treated rats.
Figure 2.
Paclitaxel vehicle sensitizes NCX. A) Peak evoked Ca2+ transients from naïve, vehicle-, and paclitaxel-treated rat putative nociceptor neurons as a function of 30 mM K+ application duration (n for each 30 mM K+ duration: 0.1s n = 4, 4, and 5; 0.2s n = 6, 7, and 10; 0.5s n = 6, 7, and 10; 1s n = 7, 12, and 15; 2s n = 7, 10, and 14; 4s n = 9, 17, and 21 neurons for naïve, vehicle, and paclitaxel, respectively). B) The T50 duration of the evoked Ca2+ transients of the same neurons plotted in (A). C) Ca2+ transients evoked with 0.1s application of 30 mM K+ in putative nociceptive neurons labeled from the glabrous skin from naïve (left), vehicle-treated (middle), and paclitaxel-treated (right) rats in the before (dotted traces) and after (solid traces) block of NCX with Li+ bath solution. D) Ca2+ transients evoked in the same neurons as in A, with 1 and 2 s applications of 30 mM K+, before and after block of NCX with Li+ bath. E) Pooled data from neurons stimulated with 30 mM K+ as illustrated in A and B, analyzed as a percent increase in T50 following NCX block with Li+ (Percent Δ T50), are plotted as a function of 30 mM K+ application duration.
Evidence of NCX activity induced with relatively small and brief Ca2+ transients in putatively nociceptive glabrous skin neurons suggested the possibility that the paclitaxel vehicle (Cremaphor EL and ethanol) was responsible for the sensitization of NCX. To test this possibility, we repeated the stimulation-duration experiment in glabrous neurons from naïve rats. The results of this experiment were comparable to our previous findings [5], where an increase in NCX activity was only detected in neurons that had an increase in [Ca2+]i above 325 nM that lasted longer than 11 seconds.
3.3. The impact of paclitaxel on NCX activity is dependent on target of innervation
The observation that NCX was sensitized in neurons from vehicle-treated rats, in the absence of any detectable influence of the paclitaxel vehicle on the duration of the depolarization-evoked Ca2+ transient in these neurons (as compared to neurons from naïve rats (Figure 1 and [2]) suggests that there are at least two Ca2+ regulatory mechanisms that are altered in neurons from vehicle treated rats: 1) the apparent increase in NCX activity and 2) another mechanism that compensates for the increase in NCX activity. Because we also previously failed to detect an influence of paclitaxel or its vehicle on depolarization-evoked Ca2+ transients in putative nociceptive neurons innervating the inner thigh [2], the presence of compensatory changes in glabrous skin neurons that were undetected with a depolarizing stimulus alone raised the possibility that compensatory mechanisms contributed to the negative results in thigh neurons as well. To test this possibility, we repeated the Li+ application experiments with neurons labeled from the hairy skin of the hindpaw and the inner thigh.
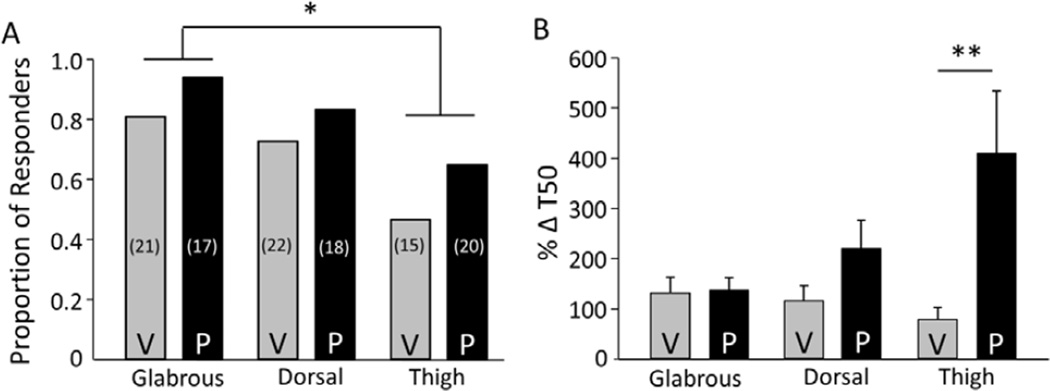
The fraction of Li+-responsive neurons was significantly (p < 0.05, Chi-square test) lower in thigh skin neurons (7/15 neurons from vehicle-treated rats and 13/20 neurons from paclitaxel-treated rats) than that in glabrous skin neurons. However, the fraction of Li+-responders in hairy hindpaw neurons (16/22 neurons from vehicle-treated rats and 15/18 neurons from paclitaxel-treated rats) was not significantly (p > 0.05, Chi-square test) different from that in either thigh or glabrous skin neurons (Figure 3A). Moreover, of the Li+-responders, there was a significant interaction (p < 0.05) between treatment (paclitaxel vs vehicle) and target of innervation (thigh, hairy hindpaw and glabrous skin), where post-hoc analysis confirmed that the paclitaxel-induced increase in NCX activity in thigh neurons was significantly greater than that in neurons innervating either the hairy hindpaw (p < 0.05) or glabrous skin (p < 0.01). To determine whether the relative increase in evoked transient duration observed in thigh neurons was different than that in the hairy hindpaw or glabrous skin neurons, duration data for the paclitaxel groups from each target of innervation were analyzed as a percent increase over the mean change in duration in the respective vehicle groups. Statistical analysis of these data confirmed that this difference based on target of innervation was significant (p < 0.01, one-way ANOVA), where post-hoc analysis confirmed that the increase in duration in thigh neurons was significantly greater than that in either hairy hindpaw (p<0.05) or glabrous skin neurons (p<0.01) (Figure 3B). This indicated that in contrast to glabrous skin neurons, paclitaxel was associated with an increase in NCX activity in thigh neurons.
Figure 3.
Effect of paclitaxel on NCX depends of target of innervation. A) Putative nociceptive neurons from vehicle (V) and paclitaxel (P) treated rats retrogradely labeled from the glabrous skin (Glabrous), the dorsal skin of the hindpaw (Dorsal), and the inner thigh skin (Thigh), were analyzed as a function of whether or not Li+ was associated with an increase in the duration of the evoked Ca2+. The proportion of Li+ responsive neurons in each group is plotted. The total number of neurons studied in each group is indicated in parenthesis. B) Li+-induced increase in the duration of the Ca2+ transient evoked with a 4 s application of 30 mM K+ was analyzed as a percent increase T50. Pooled data are from the Li+-responsive neurons plotted in A. Data from the glabrous skin neurons from Figure 1C have been replotted here to facilitate comparisons between groups. * is p < 0.05
3.4. Paclitaxel-induced increase in NCX activity in thigh neurons is not due to NCX3 activity
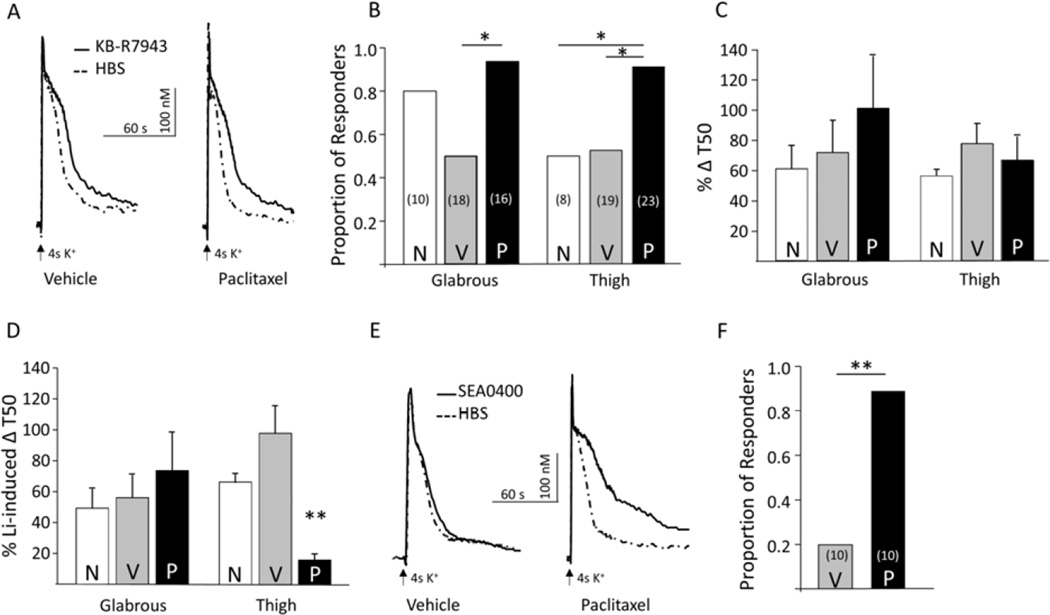
We sought to confirm our Li+ results with another NCX blocker both because Li+ influences a number of cellular processes in addition to blocking NCX and because of previous evidence that all three NCX isoforms are expressed in sensory neurons [5]. Based on previous evidence that NCX3 accounts for the majority of depolarization-evoked NCX activity in glabrous skin neurons, we used the NCX3 preferring blocker, KB-R7943 [5, 15]. In this set of experiments, we focused on glabrous skin neurons and thigh neurons, in which there was no effect of paclitaxel on the Li+-induced increase in the evoked Ca2+ transient duration and the largest paclitaxel-induced change in the response to Li+ was observed, respectively. As with Li+, neurons were again subgrouped based on the impact of KB-R7943 on the duration of the depolarization (4 seconds of 30 mM K+) evoked Ca2+ transient, where those in which the duration of the evoked Ca2+ transient was ≥20% were considered KB-R7943 responders (Figure 4A). Of the 10 glabrous skin neurons from naïve rats tested, 8 were KB-R7943 responders. A similar proportion of glabrous skin neurons from paclitaxel-treated rats (15/16) were KB-R7943 responders. However, the proportion of KB-R7943 responders from vehicle-treated rats (9/18) was significantly (p < 0.01) lower than that in the paclitaxel group (Figure 4B). In thigh neurons, paclitaxel treatment was associated with a significant (p < 0.01, Chi Square test) increase in the proportion of KB-R7943 responders (21/23) relative to that observed in neurons from either naïve (4/8) or vehicle-treated (10/19) rats (Figure 4B). This suggested at least some of the paclitaxel-induced increase in NCX activity in thigh neurons was due to an increase in a KB-R7943 sensitive NCX isoform (i.e., NCX3).
Figure 4.
The paclitaxel-induced increase in NCX activity in thigh neurons is KB-R7943 resistant and SEA0400 sensitive. A) Ca2+ transients evoked with 4s application of 30 mM K+ in putative nociceptive neurons labeled from the thigh skin from vehicle-treated (left) and paclitaxel-treated (right) rats before (dotted traces) and after (solid traces) block of NCX with KB-R7943 (100 nM). B) Putative nociceptive neurons from vehicle (V) and paclitaxel (P) treated rats retrogradely labeled from the glabrous skin (Glabrous) and the inner thigh skin (Thigh), were analyzed as a function of whether or not KB-R7943 was associated with an increase in the duration of the evoked Ca2+. The proportion of KB-R7943-responsive neurons in each group is plotted. The total number of neurons studied in each group is indicated in parenthesis. C) KB-R7943-induced increase in the duration of the Ca2+ transient evoked with a 4 s application of 30 mM K+ was analyzed as a percent increase T50. Pooled data are from the KB-R7943-responsive neurons plotted in B. D) To estimate the fraction of total NCX activity sensitive to KB-R7943, increase in T50 associated with KB-R7943 was analyzed as a percentage of the average increase in T50 in response to Li+ (%Li+-induced ΔT50). Pooled data from C analyzed in this way are plotted in D). E) Ca2+ transients evoked with 4s application of 30 mM K+ in putative nociceptive neurons labeled from the thigh skin from vehicle-treated (left), and paclitaxel-treated (right) rats before (dotted traces) and after (solid traces) application of the putative NCX1 preferring blocker SEA0400. F) The proportion of SEA0400-responsive thigh neurons from paclitaxel (P) treated rats was significantly greater than that in neurons from vehicle (V) treated rats. The number in parentheses is the total number of neurons studied from each group.* is p < 0.05, ** is p < 0.01.
To further assess the contribution of a KB-R7943-sensitive NCX isoform to the paclitaxel-induced increase in NCX activity in thigh neurons, we analyzed the KB-R7943-induced increase in the Ca2+ transient duration of responders. Strikingly, the KB-R7943-induced increase in transient duration was comparable (p > 0.05) in glabrous and thigh skin neurons from naïve, vehicle-, and paclitaxel-treated rats (Figure4A and 4C) suggesting that the majority of NCX activity in these neurons was KB-R7943-insensitive. To confirm this impression, KB-R7943 data were analyzed as a percentage of the mean response (increase in transient duration) to Li+. Analysis of these data revealed a significant interaction between target of innervation and treatment, where post-hoc analysis confirmed that there was a significant reduction in the KB-R7943 sensitive fraction of the Li+-induced increase in the duration of the evoked Ca2+ transient (Figure 4D). Finally, the NCX1 preferring blocker, SEA0400 was used to determine whether the apparent paclitaxel-induced increase in KB-R7943 insensitive NCX activity in thigh neurons was due to an increase in NCX1 activity. Thigh neurons from vehicle- and paclitaxel-treated rats were studied before and after application of SEA0400 (1 µM) (Figure 4E). Nine out of 10 thigh neurons tested from paclitaxel-treated rats were SEA0400 responders, however only two out of 10 thigh neurons from vehicle-treated rats were SEA0400 responders (Figure 4F, p < 0.01, Fisher Exact test). Analysis of the pooled transient duration data of the responders indicated that the paclitaxel-induced increase in KB-R7943-insensitive NCX activity in thigh neurons is associated, at least partially, with an increase in SEA0400-sensitive NCX activity, which accounted for 42 ± 24 percent of the Li+-induced increase in the duration of the evoked transient.
4. DISCUSSION
The purpose of this study was to test the hypothesis that the paclitaxel-induced decrease in the duration of the evoked Ca2+ transient in putative nociceptive glabrous skin neurons is due to an increase in activity of NCX. Because we observed the paclitaxel-induced decrease in the duration of the Ca2+ transient evoked with a four second application of high K+, this stimulus duration was used to estimate the total NCX activity. With this stimulus duration, we failed to detect a difference in the relative levels of NCX activity between neurons from paclitaxel or vehicle-treated animals. Furthermore, there was no evidence of a paclitaxel-selective sensitization of NCX that was assessed with shorter duration applications of high K+. In contrast to glabrous skin neurons from naïve rats in which a relatively large and long lasting increase in [Ca2+]i was needed to evoke NCX activity, there was evidence of NCX activity in neurons from both vehicle and paclitaxel-treated rats in response to evoked transients that were significantly smaller and of shorter duration. There were also differences between thigh, hairy hindpaw and glabrous skin with respect to the proportion of neurons with detectable NCX activity. Interestingly, there was a paclitaxel-induced increase in the proportion of thigh neurons with evidence of NCX activity. Even more interestingly, there was also a paclitaxel-induced increase in the impact of Li+ on thigh neurons that was associated with no change in the actions of KB-R7943, but an increase in the impact of the NCX1-preferring blocker, SEA0400. Taken together, while these observations argue against our initial hypothesis, they highlight the potential confounding influence of the chemotherapeutic vehicle on cellular properties that may not only contribute to the manifestation of CIPN, but also potentially mask the underlying mechanism(s). They also suggest the emergence of potential compensatory mechanisms in subpopulations of neurons that serve to protect them from the deleterious consequences of chemotherapeutics.
Our results argue against an increase in NCX activity as the mechanism of the paclitaxel-induced decrease in the duration of the evoked Ca2+ transient in glabrous skin neurons. Nevertheless, this conclusion is made with caution because of limitations associated with assumptions implicit in the indirect assessment of NCX activity. These include the specificity of the reagents used to block NCX activity, that a block of NCX will necessarily increase the duration of the evoked Ca2+ transient, and that it is possible to manipulate a Ca2+ regulatory mechanism, such as NCX, without affecting other mechanisms. While it is clear that these assumptions reflect an over-simplification of a very complex system, we suggest that the limitations associated with these assumptions are unlikely to significantly influence the interpretation of our results. For example, consistent results were obtained with Li+ and the NCX3-prefering blocker KB-R7943, arguing against a contribution of NCX-independent mechanisms contributing to the effects of Li+. Similarly, that it was possible to detect a relative increase in NCX activity in thigh neurons suggests that the failure to detect a change in NCX activity in glabrous skin neurons was not due to a limitation in the sensitivity of this indirect assay. The changes detected in thigh neurons also argue against the possibility that the vehicle effects occluded our ability to detect a change in NCX activity in glabrous skin neurons.
If a change in NCX activity is not the underlying reason for paclitaxel-induced decrease in the Ca2+ transient duration, it is worth considering other Ca2+ regulatory mechanisms that may contribute to this change. It is possible to rule out several regulatory mechanisms, however, because of evidence that they influence resting [Ca2+]i and/or the magnitude of the evoked Ca2+ transient. For example, the high affinity/low efficacy plasma membrane calcium ATPase (PMCA) is the major regulator of resting Ca2+ level in many cells types [16] including putative nociceptive DRG neurons [17], and therefore is unlikely to mediate the selective paclitaxel-induced change in the duration of the evoked transient. Similarly, because voltage-gated Ca2+ channels (VGCCs) and Ca2+-induced Ca2+ release (CICR) machinery contribute to the magnitude of the evoked Ca2+ transient, neither is likely to underlie the paclitaxel-induced decrease of the Ca2+ transient duration. This leaves mitochondria and sarco-endoplasmic reticulum calcium ATPase (SERCA), which have both been shown to contribute to the regulation of the evoked Ca2+ transient duration in sensory neurons [6, 8]. However, because previous results in unlabeled DRG neurons indicate that mitochondria can affect the magnitude of the evoked Ca2+ transients as well as the duration [6], future experiments should consider SERCA as a priority. Alternatively, because of the inter-dependence of Ca2+ regulatory mechanisms [18–20], a more likely explanation for the paclitaxel-induced decrease in the evoked transient duration is that it reflects changes in a combination of regulatory mechanisms. For example, reduced coupling of VGCCs to CICR machinery, thus reduced release of endoplasmic reticulum (ER) Ca2+ after influx through VGCCs, may result in transients that are shorter in duration without an impact on the magnitude.
Evidence of NCX activity in association with small amplitude brief duration Ca2+ transients in both vehicle- and paclitaxel-treated neurons indicates that the paclitaxel vehicle drives the sensitization of NCX. The presence of sensitized NCX in vehicle-treated neurons should have resulted in shorter duration-evoked transients in neurons from both vehicle and paclitaxel-treated rats, relative to that in neurons from naïve rats. However, the observation that evoked transient duration was comparable in neurons from vehicle-treated and naïve rats [2] indicates the presence of a second change in neurons from vehicle-treated rats, needed to compensate for the vehicle-induced increase in NCX activity. This would not be the first evidence of a vehicle effect, as it has been previously reported that cremophor EL is associated with anaphylactoid hypersensitivity reactions, ganglionopathy, axonopathy, and demyelination [21, 22]. Whether an increase in NCX activity contributes to any of these changes has yet to be determined. Furthermore, it is possible that one or both of these changes in Ca2+ regulation, contribute to the side effects associated with at least a taxol-based chemotherapy. In particular, while we failed to detect an influence of vehicle on nociceptive threshold, it is possible that the changes associated with vehicle treatment contribute to the manifestation of hypersensitivity in paclitaxel-treated animals. However, because a variety of chemotherapeutics administered in different vehicles produce a neuropathy with comparable symptoms and manifestation pattern, we suggest that changes associated with the paclitaxel vehicle are unlikely to be an important determinant of CIPN.
While the heterogeneity among subpopulations of sensory neurons has been well described, our results point to yet another manifestation of this phenomenon among subpopulations of sensory neurons based on target of innervation. First, there are differences between glabrous skin and inner thigh skin putative nociceptive neurons with respect to both the presence of NCX activity, and the NCX isoforms underlying this activity. Second, there is also a difference between these subpopulations with respect to the impact of paclitaxel on NCX activity. More importantly, however, are the implications of the apparently selective increase in NCX activity in thigh neurons. This observation suggests that in addition to the multiple vehicle-associated changes in glabrous skin neurons, there also needs to be (at least) two opposing changes in Ca2+ regulatory processes in putative nociceptors innervating the inner thigh skin to account for the apparent absence of a paclitaxel-induced change in transient duration in these neurons [2]. Furthermore, given the absence of a detectable paclitaxel-induced change in the withdrawal threshold to noxious mechanical stimulation of the inner thigh, one, if not both of the changes in Ca2+ regulation may protect these neurons from the toxic effects of paclitaxel. This suggests an alternative explanation for the stocking glove distribution of CIPN, which is due to the failure of glabrous skin neurons to compensate for the toxic effects of chemotherapeutics, rather than the presence of a unique property of these neurons (such as their axon length), that confers a selective vulnerability. Identification of the protective mechanism(s) in thigh neurons may suggest novel approaches for the treatment, if not prevention of CIPN.
Highlights.
Paclitaxel-induced decreases in Ca2+ transient duration are NCX-independent.
Cremophor EL sensitizes NCX in glabrous skin neurons.
NCX activity in nociceptive afferents varies with target of innervation
NCX activity is increased in neurons resistant to the toxic effects of paclitaxel.
Acknowledgments
We wish to thank Dr. Nicole Scheff for helpful comments on the preparation of this manuscript. This research is supported by the grants from the National Institutes of Health T32 NS073548 (EY) and R01NS083347 (MSG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz E, Gold MS. Sensory neuron subpopulation-specific dysregulation of intracellular calcium in a rat model of chemotherapy-induced peripheral neuropathy. Neuroscience. 2015;300:210–218. doi: 10.1016/j.neuroscience.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiological reviews. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 4.DiPolo R, Beauge L. Sodium/calcium exchanger: influence of metabolic regulation on ion carrier interactions. Physiological reviews. 2006;86:155–203. doi: 10.1152/physrev.00018.2005. [DOI] [PubMed] [Google Scholar]
- 5.Scheff NN, Yilmaz E, Gold MS. The properties, distribution and function of Na(+)-Ca(2+) exchanger isoforms in rat cutaneous sensory neurons. The Journal of physiology. 2014;592:4969–4993. doi: 10.1113/jphysiol.2014.278036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu SG, Zhang X, Gold MS. Intracellular calcium regulation among subpopulations of rat dorsal root ganglion neurons. The Journal of physiology. 2006;577:169–190. doi: 10.1113/jphysiol.2006.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold MS, Gebhart GF. Peripheral Pain Mechanisms and Nociceptor Sensitization. In: Fishman SM, Ballantyne JC, Rathmell JM, editors. Bonica’s Management of Pain. Lippincott, Williams & Wilkins; 2010. pp. 24–34. [Google Scholar]
- 8.Scheff NN, Lu SG, Gold MS. Contribution of endoplasmic reticulum Ca2+ regulatory mechanisms to the inflammation-induced increase in the evoked Ca2+ transient in rat cutaneous dorsal root ganglion neurons. Cell calcium. 2013;54:46–56. doi: 10.1016/j.ceca.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao JP. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods in cell biology. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- 10.Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Experimental physiology. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- 11.Holzer P. Capsaicin as a tool for studying sensory neuron functions. Advances in experimental medicine and biology. 1991;298:3–16. doi: 10.1007/978-1-4899-0744-8_1. [DOI] [PubMed] [Google Scholar]
- 12.Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:7281–7292. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu SP, Choi DW. Na(+)-Ca2+ exchange currents in cortical neurons: concomitant forward and reverse operation and effect of glutamate. The European journal of neuroscience. 1997;9:1273–1281. doi: 10.1111/j.1460-9568.1997.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 14.Gold MS, Thut PD. Lithium increases potency of lidocaine-induced block of voltage-gated Na+ currents in rat sensory neurons in vitro. J Pharmacol Exp Ther. 2001;299:705–711. [PubMed] [Google Scholar]
- 15.Iwamoto T, Shigekawa M. Differential inhibition of Na+/Ca2+ exchanger isoforms by divalent cations and isothiourea derivative. The American journal of physiology. 1998;275:C423–C430. doi: 10.1152/ajpcell.1998.275.2.C423. [DOI] [PubMed] [Google Scholar]
- 16.Carafoli E. The calcium pumping ATPase of the plasma membrane. Annual review of physiology. 1991;53:531–547. doi: 10.1146/annurev.ph.53.030191.002531. [DOI] [PubMed] [Google Scholar]
- 17.Gemes G, Oyster KD, Pan B, Wu HE, Bangaru ML, Tang Q, Hogan QH. Painful nerve injury increases plasma membrane Ca2+-ATPase activity in axotomized sensory neurons. Molecular pain. 2012;8:46. doi: 10.1186/1744-8069-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Sancho J. The coupling of plasma membrane calcium entry to calcium uptake by endoplasmic reticulum and mitochondria. The Journal of physiology. 2014;592:261–268. doi: 10.1113/jphysiol.2013.255661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moccia F, Zuccolo E, Soda T, Tanzi F, Guerra G, Mapelli L, Lodola F, D'Angelo E. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Frontiers in cellular neuroscience. 2015;9:153. doi: 10.3389/fncel.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. European journal of cancer. 2006;42:24–30. doi: 10.1016/j.ejca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. European journal of cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]