Abstract
BACKGROUND
Variation in physician adoption of new medications is poorly understood. Traditional approaches (e.g., measuring time to first prescription) may mask substantial heterogeneity in technology adoption.
OBJECTIVE
Apply group-based trajectory models to examine the physician adoption of dabigratran, a novel anticoagulant.
METHODS
A retrospective cohort study using prescribing data from IMS Xponent™ on all Pennsylvania physicians regularly prescribing anticoagulants (n=3,911) and data on their characteristics from the American Medical Association Masterfile. We examined time to first dabigatran prescription as well as group-based trajectory models to identify adoption trajectories in the first 15 months. Factors associated with rapid adoption were examined using multivariate logistic regressions.
OUTCOMES
Trajectories of monthly share of oral anticoagulant prescriptions for dabigatran
RESULTS
We identified five distinct adoption trajectories: 3.7% rapidly and extensively adopted dabigatran (adopting in ≤3 months with 45% of prescriptions) and 13.4% were rapid and moderate adopters (≤3 months with 20% share). Two groups accounting for 21.6% and 16.1% of physicians, respectively, were slower to adopt (6 to 10 months post-introduction) and dabigatran accounted for <10% share. Nearly half (45.2%) of anticoagulant prescribers did not adopt dabigatran. Cardiologists were much more likely than primary care physicians to rapidly adopt (odds ratio [OR] 12.2, 95%CI: 9.27–16.1) as were younger prescribers (age 36–45 years: OR 1.49, 95%CI: 1.13–1.95, age 46–55: OR 1.34, 95%CI 1.07–1.69 vs. >55 years).
CONCLUSIONS
Trajectories of physician adoption of dabigatran were highly variable with significant differences across specialties. Heterogeneity in physician adoption has potential implications for the cost and effectiveness of treatment.
Keywords: anticoagulants, direct thrombin inhibitor, physician prescribing behavior, dabigatran, group-based trajectory models
INTRODUCTION
Atrial fibrillation is associated with substantial mortality and morbidity from stroke and thromboembolism.1 Warfarin and other vitamin K antagonists significantly reduce the risk of stroke and death in patients with non-valvular atrial fibrillation and have been the cornerstone of therapy for this condition for 60 years.2 Though these agents are inexpensive, they require monitoring and have a narrow therapeutic window, and patients frequently discontinue use.3 Dabigatran, a direct thrombin inhibitor, was the first in the class of novel oral anticoagulants to be introduced in October 2010.
Randomized trials have demonstrated similar or superior efficacy and safety of the new oral anticoagulants relative to warfarin.4–6 In addition, novel anticoagulants have fewer food and drug interactions, and do not require laboratory monitoring.7 However, major concerns about the new anticoagulants include poor adherence in the absence of monitoring, cost (15 times more costly than warfarin), bleeding risks, and absence of antidotes.7, 8 Therefore, there may be variation across physicians in prescribing dabigatran, depending on how they assess the benefits and risks of dabigatran.7, 8
Studies showing significant uptake of dabigatran (12%–32.8%) have thus far relied on patient-level data.9–11 Little is known about how physicians have adopted dabigatran or the physician-level factors associated with rapid adoption. Studies in other medication classes point to widespread variation in physician prescribing of new drugs based on specialty, practice setting, age, sex, and training.12–17 We used an all-payer dataset on the prescribing behavior of all physicians in Pennsylvania who regularly prescribed oral anticoagulants in the 12 months prior to dabigatran’s introduction to examine adoption patterns at the physician-level. We measured time to first prescription of dabigatran, a frequently-used adoption measure. To fully characterize the dynamics of both the speed and the extent of dabigatran adoption among physicians, we used group-based trajectory models to account for the dynamic nature of medication prescribing and identify differential patterns over time.18, 19
METHODS
Data Source and Population
We used IMS Health’s Xponent™ prescription database to characterize physician prescribing of oral anticoagulants, the American Medical Association (AMA) Physician Masterfile to obtain information on physician characteristics (e.g., age), and IMS Healthcare Organization Services™ (HCOS) database for physician specialty and organizational affiliations. Xponent™ directly captures >70% of all US prescriptions filled in retail pharmacies and utilizes a patented proprietary projection method to represent 100% of prescriptions filled in these outlets.9, 15, 20–22 We obtained monthly physician-level data on all oral anticoagulant prescriptions dispensed in Pennsylvania between October 1, 2009 and December 31, 2011. Xponent™ contains limited patient-level information relevant to the prescriptions including the source of payment (Medicare, Medicaid fee-for-service (FFS), commercial insurance, cash or uninsured), and patient age, with no patient identifiers. Physician demographic characteristics and education profiles were obtained from the AMA Physician Masterfile data linked to Xponent™ by physician name and National Provider Identifier. The AMA Physician Masterfile provides data on nearly all allopathic physicians, residents, and medical students as well as 93% of osteopathic physicians practicing in the US.23 We obtained information on physician specialty and organizational affiliations (e.g., medical group) from IMS Health’s HCOS database.
Study Cohort
We identified 7,821 physicians who prescribed any oral anticoagulants during the year prior to dabigatran’s introduction in October, 2010. We then limited our study sample to those who prescribed anticoagulants regularly because they were more likely to actively choose to prescribe anticoagulants and therefore were eligible to adopt dabigatran as opposed to those simply renewing prescriptions written by other physicians. We defined regular prescribers as those prescribing ≥1 anticoagulant prescriptions each quarter and at least 9 anticoagulant prescriptions (the median among the 7,821 anticoagulant prescribers) during the year before dabigatran was introduced (October 1, 2009 to September 30, 2010). To ensure that physicians were still actively seeing patients after dabigatran was introduced without conditioning on our outcome of interest (anticoagulant prescribing), we excluded 121 physicians who did not prescribe at least one drug from the following widely used medication classes in the 15 months after dabigatran’s introduction (i.e., October 1, 2010 to December 31, 2011): oral hypoglycemic, anti-hypertensives or statins. The final sample had 3,911 physicians, which accounted for 78% of total anticoagulant prescribing volume in Pennsylvania during the study period (eFigure 1). eTable 1 compares the characteristics between 3,911 regular prescribers and 3,910 non-regular prescribers. Non-regular anticoagulants prescribers were more likely to be primary care providers (PCP) and significantly lower volume prescribers compared to regular prescribers.
Outcome measures
Studies of physician adoption typically measure the time to first prescription, dividing physicians into ‘rapid’ or ‘slow’ adopters.15, 24–29 However, the decision to adopt a new drug is multifaceted. A physician needs to decide whether to adopt a new drug, the speed with which he/she will do so, and the volume of prescribing he/she will do for the new drug. Therefore, we constructed two measures of adoption in the first 15 months post-FDA approval of dabigatran: 1) number of months to first dabigatran prescription, and 2) the trajectory of adoption defined by monthly share of dabigatran prescriptions (i.e., number of dabigatran prescriptions/total oral anticoagulant prescriptions). Group-based trajectory models account for both the timing and extent of adoption and can therefore identify more heterogeneity in adoption behavior than traditional time-to-event models. Using share as an outcome as opposed to number of prescriptions enables us to distinguish true adoption among physicians with high prescribing volume.30–31
Predictors
Our analyses were guided by the conceptual framework for physician adoption of new drugs depicted in Figure 1 and was informed by prior studies.15–16, 24–38 A physician’s decision to adopt a new drug is influenced by his/her own characteristics/preferences; his/her patient case mix; training, health care and payer institutions; and other environmental factors (e.g., pharmaceutical firms). In addition, physician adoption decisions are influenced by peers in local (e.g., regional or organizational) social networks.29, 36 While we are not able to directly measure the influences of all of these factors on physician adoption we describe below the physician, patient, institutional and environmental variables available in our data.
Figure 1.
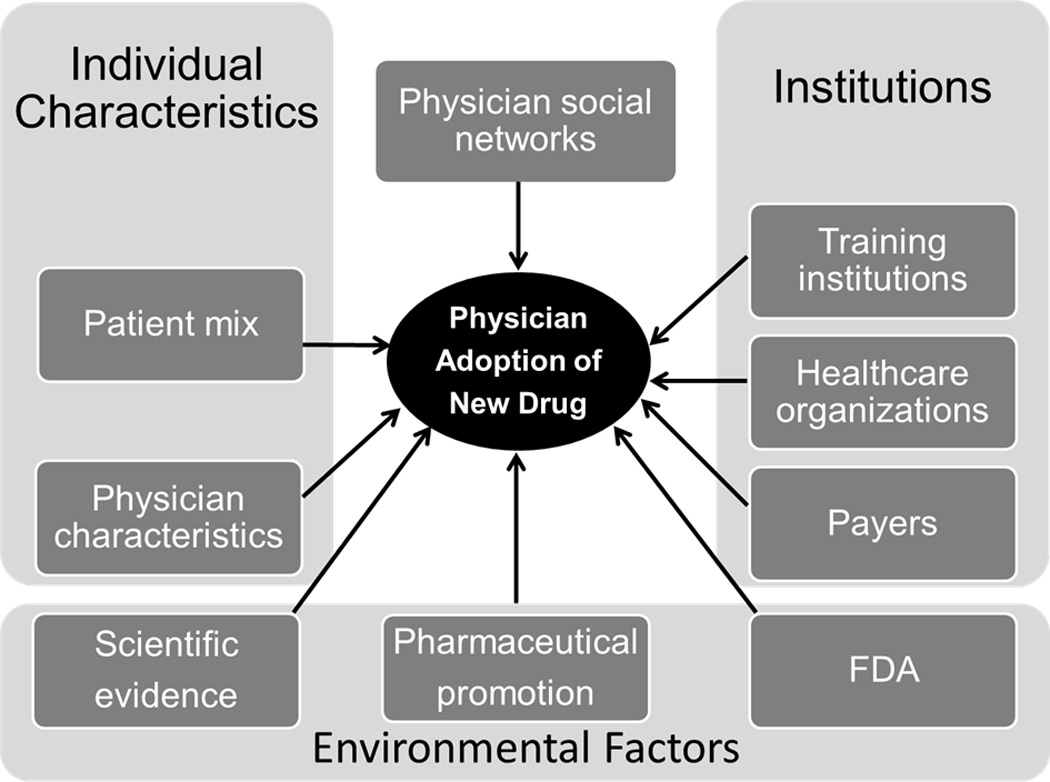
Conceptual Framework of Physician Adoption of New Drugs
We include several physician characteristics including demographics (sex and age), specialty, and prescribing volume. We include an indicator for quartile of total anticoagulant prescribing volume in the year before dabigatran’s introduction. Our data are at the prescription- not patient-level but we expect prescribing volume to be correlated with patient volume and we expect high-volume prescribers to have a greater opportunity to adopt. Regarding specialty, 95% of the physicians in our cohort were either PCP (including internal medicine, family medicine, and family practice) or cardiologists. Therefore, we created a categorical variable for specialty (PCP, cardiologists, or others). We then combined information on physician specialty with information on organizational affiliation assuming that prescribing behavior would be influenced by the degree of interaction with others in the same specialty or organizations and whether these interactions were with physicians of the same or other specialties.28 We created five mutually exclusive categories: 1) PCPs practicing in a primary care medical group, 2) PCP practicing in other settings (e.g., multispecialty group), 3) cardiologists practicing in a cardiology medical group, 4) cardiologists practicing in other settings, and 5) physicians trained in other specialties (e.g., surgery) practicing in any setting.
We include the only patient-level information available in our data (patient age categories) specified as the proportion of a physician’s prescriptions filled by patients aged ≤64, 65–74, 75–84, and ≥85 years. Institutional factors we used as predictors included a payer mix variable to account for potential differences in pharmacy benefit design. Specifically, we included a measure of the percent of a physician’s patients whose prescriptions were paid by Medicare (both fee-for-service and managed care), fee-for-service Medicaid, commercial insurers (including Medicaid managed care plans) and cash payment. In addition, we included measures of whether the physician attended a top-20 medical school based on 2013 U.S News and World Report, or graduation from a foreign medical school.
Finally, we included hospital referral regions (HRRs) where physicians primarily practiced as a measure representing regional health care markets for tertiary medical care that generally requires the services of a major referral center.39 Including an HRR indicator may account for differences in the structure of health care markets, physician social networks, or socioeconomic differences by region.29, 36 Pennsylvania consists of 14 HRRs and Pittsburgh was used as reference group in our analysis. The small number of Pennsylvania physicians in non-Pennsylvania HRRs were included in an ‘other’ HRR group.
Statistical Analysis
Our analysis followed three steps. First, we described baseline characteristics as mean (standard deviation) or median (range) for continuous variables, and as a frequency (percentage) for categorical variables. We plotted a scatterplot to examine market share of anticoagulants from 2007–2011. Second, we estimated time to first dabigatran prescription using the Kaplan-Meier method.40 Third, we fit group-based trajectory models using the SAS procedure PROC TRAJ18, 19 to identify differential adoption patterns of dabigatran (SAS Institute, Cary, NC, USA). We then identified factors associated with rapid adoption using multivariable logistic regression.
Time to first adoption
To assess time to first prescription, we used the Kaplan-Meier method to compute the proportion of physicians who had not adopted dabigatran for each month during the first post-FDA approval 15 months (i.e., October 2010 to December 2011). For ease of visual presentation and interpretation, we provided probabilities of dabigatran adoption over the first 15 months.
Trajectories of Physician Adoption of Dabigatran
Group-based trajectory models were used to identify differential trajectory patterns of individual physician change in the average monthly share for dabigatran prescriptions over time and to characterize subgroups more likely to follow certain trajectories.18, 19 We first transformed the average monthly share for dabigatran prescriptions with a log function, and then modeled the average transformed monthly share for dabigatran prescriptions as a longitudinal, continuous outcome for each month, and the time variable was months since dabigatran’s introduction (1–15). The transformed share data were modeled using a censored normal distribution with a minimum of 0 and a maximum of 1. The purpose of the transformation of the data is to meet the assumption for the finite mixture trajectory model with a censored normal distribution for each distinct group. We used the most flexible functional form of time using up to a fifth order polynomial to allow the trajectories to emerge from the data. The output of group-based trajectory models includes estimated probabilities of group membership for each physician and each group, and an estimated trajectory curve over time for each group.21 Plot values were transformed back to the original scale with the exponential function. The final model was selected based on the Bayesian information criterion (BIC); wherein the largest value indicates the best-fitting model, and an estimated proportion of each trajectory group that was sufficiently large (>0.05).18, 19 We assessed the final model adequacy based on Nagin’s criteria: (1) average posterior probability of assignment for all groups > 0.7; (2) odds of correct classification >5; (3) estimated probability of membership in each group close to proportion of sample assigned to each group; and (4) confidence intervals for estimated probability of the membership that were reasonably narrow.18
Factors Associated with Rapid Adoption of Dabigatran
We used multivariable logistic regression to estimate the odds ratio (OR) and 95% confidence intervals (CI) for each variable and identify factors associated with rapid adopters (i.e., the top two groups identified by trajectory model that adopted dabigatran rapidly and substantially) compared with all others. An odds ratio (OR) >1 indicated that physicians with a particular characteristic adopted dabigatran faster than the reference group. An OR <1 indicated that physicians with that characteristic adopted dabigatran slower than the reference group. Statistical significance was determined using 2-tailed p-values <0.05.
RESULTS
Characteristics of the sample
Among 3,911 physicians in Pennsylvania who prescribed anticoagulants regularly, 17% were women, 75% were aged ≥45 years, and 10% attended a top-20 medical school. Half of the prescribers were PCP practicing in a primary care medical group, 25% were PCP in other settings (e.g., multi-specialty group), 18% were cardiologists, and 5% were physicians in other specialties (Table 1).
Table 1.
Characteristics of 3,911 physicians who prescribed anticoagulants regularly during October 2009–September 2010
| Characteristics | N= 3,911 |
|---|---|
| Female, n (%) | 671 (17.2) |
| Mean age (SD), years | 52.4 (9.0) |
| Age group, n (%) | |
| ≤ 35 | 93 (2.4) |
| 36–45 | 866 (22.1) |
| 46–55 | 1441 (36.8) |
| > 55 | 1511 (38.6) |
| Top-20 medical school, n (%) | 386 (9.9) |
| Foreign medical graduate, n (%) | 775 (19.8) |
| Type of specialty and affiliation, n (%) | |
| Primary care physicians practicing in a primary care medical group | 2,001 (51.2) |
| Primary care physicians practicing in other settings | 1,002 (25.6) |
| Cardiologists affiliated with a cardiology medical group | 452 (11.6) |
| Cardiologists practicing in other settings | 265 (6.8) |
| Physicians with other specialties | 191(4.9) |
| Average monthly anticoagulant volume quartile (prescriptions/month), n (%) | |
| Quartile 1 (< 13.3) | 978 (25.0) |
| Quartile 2 (13.3–18.9) | 978 (25.0) |
| Quartile 3 (18.9–28.0) | 977 (25.0) |
| Quartile 4 (≥ 28.0) | 978 (25.0) |
| Hospital referral regions of primary physician’s practice location, n (%) | |
| Pittsburgh | 928 (23.7) |
| Allentown | 313 (8.0) |
| Altoona | 99 (2.5) |
| Danville | 171 (4.4) |
| Erie | 207 (5.3) |
| Harrisburg | 342 (8.7) |
| Johnstown | 97 (2.5) |
| Lancaster | 197 (5.0) |
| Philadelphia | 918 (23.5) |
| Reading | 161 (4.1) |
| Sayre | 38 (1.0) |
| Scranton | 139 (3.6) |
| Wilkes-Barre | 105(2.7) |
| York | 120 (3.1) |
| Other | 76 (1.9) |
| Physician’s patient payer mix | |
| Medicare, % (SD) | 43.7 (14.8) |
| Medicaid, % (SD)a | 3.8 (7.9) |
| Commercial, % (SD)a | 48.2 (16.1) |
| Cash, % (SD) | 4.3 (4.6) |
| Physician’s patient age mix | |
| ≤ 64, % (SD) | 30.8 (16.3) |
| 65–74, % (SD) | 22.5 (10.3) |
| 75–84, % (SD) | 30.6 (12.5) |
| ≥ 85, % (SD) | 16.1 (10.7) |
Prescriptions paid for by Medicaid according to Xponent™ only captures those paid by the Medicaid fee-for-service program. Medicaid managed care plans are designated as commercial insurers
Market share and time to first adoption of dabigatran
Total anticoagulant prescribing volume was relatively stable over time (eFigure 2). Warfarin accounted for approximately 90% of monthly anticoagulant prescription volume at the end of 2011. The prescription volume for dabigatran increased from 0.6% in November 2010 to 9.0% in December 2011. Almost two-thirds (65.3%) of the physicians had prescribed dabigatran at least once during its first 15 months on the market (Figure 2). The median time to first adoption among the cohort prescribers was 9 months (range: 1–14).
Figure 2.
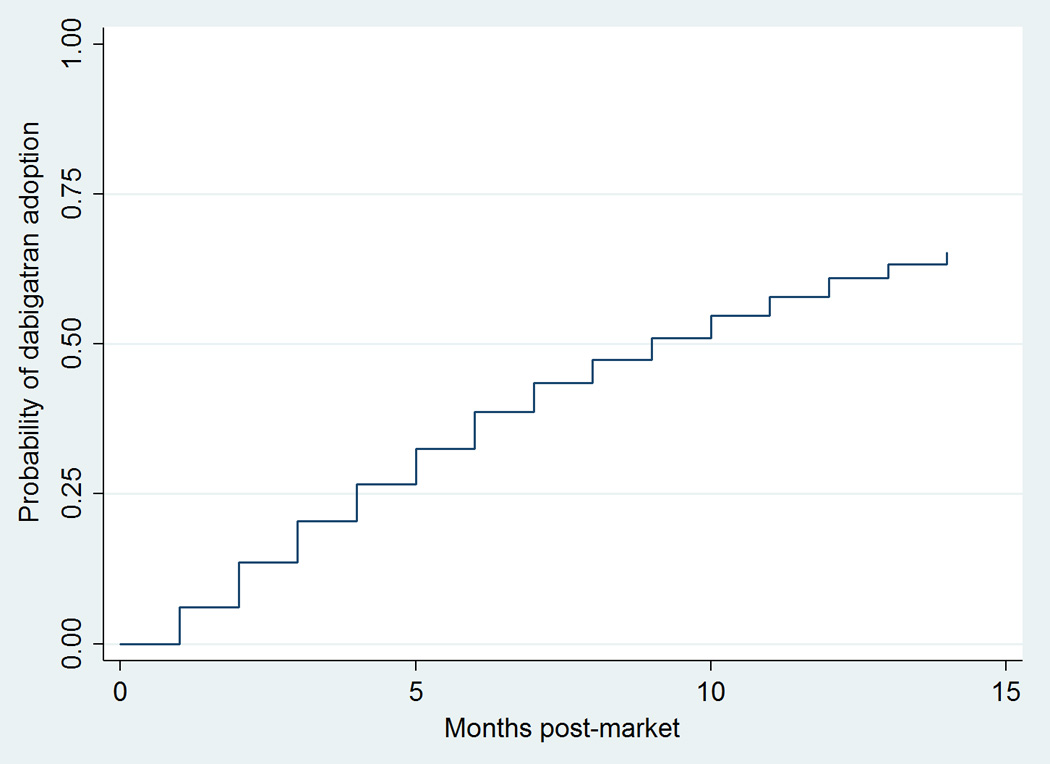
Kaplan-Meier Curves of Probability of Physician Adoption of Dabigatran in the First 15 Months Postmarket (N=3,911)
Trajectories of adoption of dabigatran
Figure 3 illustrates the estimated monthly share of dabigatran prescriptions for physicians in each trajectory group. In the analysis of 30 trajectory models considered, a 5-group model had the best test characteristics based on BIC values (3704.28) and Nagin’s criteria (eTable 1). We identified five distinct groups based on physician adoption behavior. Two groups rapidly adopted dabigatran in the first 3 months post-introduction but varied in the extent of take-up, with 3.7% adopting early and extensively writing up to 45% of anticoagulant prescriptions for dabigatran and 13.4% adopting early and writing a moderate share of prescriptions (20%) for dabigatran. Notably, the rapid adopters accounted for two-thirds of all dabigatran prescriptions by physicians in our sample during the study period. A third group (21.6%) adopted between 3 and 5 months and writing <10% of prescriptions for dabigatran. A fourth group (16.1%) did not adopt dabigatran until 10 months post-introduction and wrote a small share of prescriptions for dabigatran (<5%). Nearly half (45.2%) were non-adopters who did not adopt dabigatran at all (n=1,357) or had <1% share for dabigatran (n=409).
Figure 3.
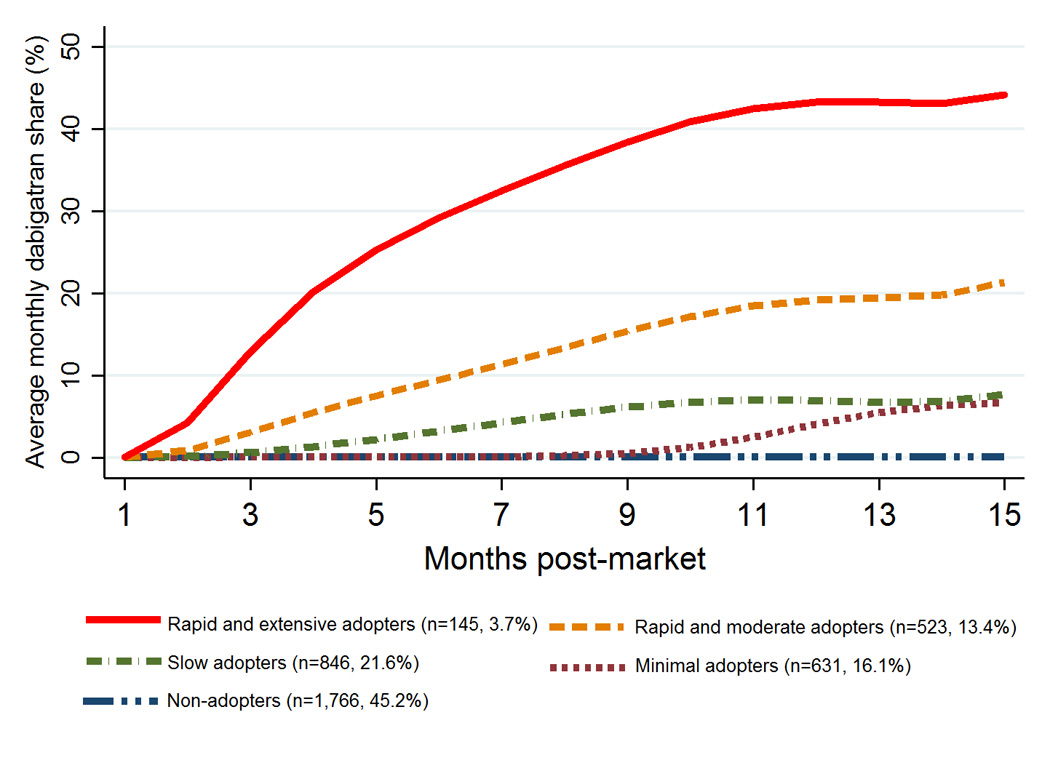
Trajectories of Physician Adoption of Dabigatran in the First 15 Months Postmarket (N=3,911)
Note: In the legend, the values in parenthesis are the number and proportion of physicians in each trajectory group. The output represents predicated average monthly share for dabigatran prescriptions.
Predictors of time to first adoption of dabigatran
In the multivariate logistic regressions (Table 2 and eTable 3), younger physicians were more likely to adopt dabigatran than those aged >55 years (aged ≤ 35 years: OR 1.69, 95% CI: 0.85–3.36; 36–45 years: 1.49, 95% CI: 1.13–1.95; 46–55 years: OR: 1.34, 95% CI: 1.07–1.69). Cardiologists were significantly more likely than primary care or other physicians to be rapid adopters even after adjusting for other covariates (in cardiology medical groups: OR 12.2, 95% CI: 9.27–16.1, and in other settings: OR 11.3, 95% CI: 8.27–15.5). Physicians who primarily practiced in four HRRs including Allentown (OR 0.35, 95% CI: 0.23–0.54), Danville (OR 0.43, 95% CI: 0.24, 0.78), Harrisburg (OR 0.39, 95% CI: 0.25–0.59), and Lancaster (OR 0.35, 95% CI: 0.20–0.61) were less likely to adopt dabigatran compared to those in Pittsburgh. Further, physicians with a greater share of their anticoagulant prescriptions paid by Medicaid FFS (OR 0.14, 95% CI: 0.06–0.34) and Medicare (OR 0.03, 95% CI: 0.003–0.29)) were less likely to be rapid adopters.
Table 2.
Odds of rapid adoption of dabigatran based on a multivariate logistic regression modela
| OR (95% CI) | P-value | |
|---|---|---|
| Gender (ref =male) | 0.79 (0.59, 1.06) | 0.11 |
| Age group (ref= >55 years) | ||
| ≤ 35 | 1.69 (0.85, 3.36) | 0.14 |
| 36–45 | 1.49 (1.13, 1.95) | 0.004 |
| 46–55 | 1.34 (1.07, 1.69) | 0.010 |
| Top-20 medical school (ref=non-top 20 medical school) | 1.07 (0.79, 1.45) | 0.64 |
| Foreign medical graudate (ref=US medical graduate) | 1.00 (0.77, 1.29) | 0.99 |
| Type of specialty and affiliation (ref= Primary care physicians practicing in a primary care medical group) | ||
| Cardiologists affiliated with a cardiology medical group | 12.2 (9.27, 16.1) | <0.0001 |
| Cardiology practicing in other settings | 11.3 (8.27, 15.5) | <0.0001 |
| Primary care physicians practicing in other settings | 1.20 (0.92, 1.56) | 0.18 |
| Physicians with other specialties | 0.36 (0.15, 0.83) | 0.02 |
| Average monthly prescribing volume quartile (ref <13.3 prescriptions) | ||
| 13.3–18.8 | 0.98 (0.73, 1.30) | 0.87 |
| 18.9–27.9 | 1.09 (0.82, 1.46) | 0.54 |
| ≥ 28.0 | 1.07 (0.80, 1.43) | 0.65 |
| HRR of primary practice location: (ref=Pittsburgh) | ||
| Allentown | 0.35 (0.23, 0.54) | <0.0001 |
| Altoona | 0.83 (0.43, 1.58) | 0.57 |
| Danville | 0.43 (0.24, 0.78) | 0.0051 |
| Erie | 0.91 (0.57, 1.46) | 0.71 |
| Harrisburg | 0.39 (0.25, 0.59) | <0.0001 |
| Johnstown | 1.00 (0.53, 1.89) | 0.99 |
| Lancaster | 0.35 (0.20, 0.62) | 0.0003 |
| Philadelphia | 0.77 (0.58, 1.02) | 0.070 |
| Reading | 0.89 (0.54, 1.45) | 0.63 |
| Sayre | 1.19 (0.45, 3.16) | 0.73 |
| Scranton | 0.67 (0.38, 1.17) | 0.16 |
| Wilkes-Barre | 0.50 (0.25, 1.00) | 0.05 |
| York | 1.27 (0.73, 2.20) | 0.40 |
| Other | 1.25 (0.64, 2.45) | 0.51 |
| % Medicaid payer mix (ref=cash and commercial) | 0.14 (0.06–0.34) | <0.0001 |
| % Medicare payer mix (ref=cash and commercial) | 0.03 (0.003–0.29) | 0.003 |
| % Patient age mix: age ≤64 years (ref= age ≥85) | 0.48 (0.13–1.67) | 0.25 |
| % Patient age mix: age 65–74 years (ref= age ≥85) | 0.61 (0.15–2.55) | 0.50 |
| % Patient age mix: age 75–84 (ref= age ≥85) | 1.28 (0.30–5.43) | 0.73 |
Data were obtained from IMS Xponent™, 2007–2011 and results were from multivariate logistic regression analyses. An odds ratio (OR) >1 indicated that on average, physicians with a particular characteristic adopted dabigatran faster than the reference group. An OR <1 indicated that a physician with that characteristic was slower than the reference group to adopt dabigatran.
Abbreviations: HRR: hospital referral regions; OR: odds ratio
DISCUSSION
Our study yielded three key findings regarding physician adoption of the first novel oral anticoagulant to be introduced in decades. First, while dabigatran accounted for 10% of anticoagulant prescriptions during its first 15 months on the market, almost two-thirds of physicians in Pennsylvania regularly prescribing anticoagulants prescribed at least one dabigatran prescription. Second, physicians varied markedly in their speed and extent of dabigatran adoption. In fact, we identified several distinct trajectories of physician adoption of dabigatran, only 17.1% adopted it rapidly into practice with at least 20% share for dabigatran prescriptions 15 months post-introduction. Third, we found that cardiologists, regardless of practice setting, were much more likely to be rapid adopters of dabigatran than PCP who represented the largest specialty prescribing anticoagulants.
To our knowledge, this study is the first to examine longitudinal physician adoption of a new drug using group-based trajectory models. Previous studies have used a single value to represent adoption rate or time to first adoption of medications.15–16, 24–29, 31, 33, 35–38 However, using an overall rate or time to first adoption only provides a gross measure of physician adoption that may mask substantial underlying heterogeneity in physician adoption patterns. For example, physicians prescribing dabigatran to one patient at 6 months and then to none and physicians prescribing it to 100 patients starting at 6 months would be categorized similarly according to traditional measures of adoption in spite of obvious differences in the extent of adoption. Therefore, trajectory models are valuable tools to understand heterogeneity in how drugs and other new technologies diffuse among physicians.
The high variability in adoption of dabigatran could be due to a variety of factors, such as lack of awareness of the drug’s introduction, differences in exposure to pharmaceutical promotion, expertise or comfort level, and an intentionally cautious approach to adopting new drugs on the part of some physicians.41 For example, some physicians might be reluctant to prescribe dabigatran due to poor adherence without laboratory monitoring of anticoagulation effect, no proven method of rapidly reversing its effects and increased risk of major bleeding (e.g., gastrointestinal).4, 7, 8, 42–44 However, we were unable to measure these risk perceptions in our study.
One factor that stood out as a strong predictor of rapid and extensive adoption was specialty. Cardiologists made up 18% of our sample but over half (56.7%) of rapid adopters. A recent study also showed that most dabigatran visits were accounted for by cardiologists (53%) followed by internal medicine (28%), family practice (10%), and others (9%).9 One possible explanation is that cardiologists are more likely to see patients with atrial fibrillation having difficulties in maintaining adequate anticoagulation and therefore may be more aware of and likely to adopt new treatments for atrial fibrillation.41 Alternatively, cardiologists may be more likely to be targeted by marketing efforts by pharmaceutical manufacturers.45, 46 It is also possible that cardiologists initiated dabigatran prescribing and patients’ primary care physicians continued to prescribe refills for the medication. However, as we lacked patient identifiers, we were not able to differentiate between prescribers who were initiating vs. continuing treatment. Consistent with other studies showing that specialist physicians adopted new treatment rapidly,41 our findings showed that patients would have a very different likelihood of receiving dabigatran if they sought care in specialty settings vs. primary care settings.
Physicians who had more prescriptions paid for by Medicaid FFS were less likely to adopt dabigatran, which may reflect more restrictions on prescribing these medications for Medicaid patients or differences in the health status or primary indication for treatment among Medicaid enrollees compared to others. In addition, similar to other studies of regional variation in prescribing of brand name vs. generic medications,47 we find significant variation in adoption behavior across the physicians in our sample depending on hospital referral region. This regional variation could be due to variation in the structure of physician social networks, the structure of health care organizations, and many other factors.29, 32, 36
Our study has several limitations. First, we lacked clinical information on the patients filling prescriptions including the indication for which the drug was prescribed, the severity of illness, and comorbidities that may have driven treatment decisions. In addition, we lacked information on patient preferences for newer medications. Physician adoption decisions may be influenced by their patient case mix although other studies suggest this explains little of the variation in adoption.13, 14 Patient preferences or health plan coverage could also influence adoption of dabigatran.48 Second, to the extent that we did not have data on prescriptions written, only those dispensed, our results were confounded by factors affecting patients’ decisions to fill prescriptions.49 Third, we lacked data on the number of free samples distributed by each physician and on use of patient assistance programs, although use of such programs is quite low.50 Forth, we were unable to identify a physician’s residency training program, which may have more influence on prescribing than medical school attended. In addition, because of a lack of data, we were unable to adjust for some of the external influences on prescribing behavior, such as manufacturer promotional efforts directed at physicians, characteristics of the specific organizations in which physician practice, physician’s time spent in inpatient versus outpatient settings, and health plan coverage of different anticoagulants. Fifth, we were unable to explore the take-up of rivaroxaban, the second novel entrant in the class that was introduced in July 2011. With fewer than 100 prescribers in our dataset having one or more prescriptions for rivaroxaban we lacked sufficient power to examine trajectories or predicators of its adoption. Finally, while Pennsylvania is the 6th most populous state in the US and resembles national averages closely in terms of age, gender, education attainment, income, and most measures of health care utilization, our findings of prescribers in Pennsylvania may not be generalizable to physicians in other states.
CONCLUSION
Physician adoption of dabigatran and other new drugs into practice may have implications of quality of care, patient safety, and for health care spending. Our findings point to widespread adoption of this novel therapy but with substantial heterogeneity in prescribing patterns. The majority of prescriptions for dabigatran in our sample were prescribed by only 14% of physicians. The patients of these rapidly adopting physicians have a very different likelihood of being prescribed new therapies than patients seeing providers who take a more cautious approach to adopting new drugs. Improving our understanding of the diffusion of new drugs can inform interventions to improve the uptake of evidence-based care and ultimately the efficiency of our health care system.
Supplementary Material
Acknowledgments
Funding/Support: This work was funded by National Heart, Lung, and Blood Institute (R01HL119246). Dr. Lo-Ciganic was funded by a post-doctoral fellowship through the University of Pittsburgh Health Policy Institute, Center for Pharmaceutical Policy and Prescribing.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERNCES
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 3.Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Juurlink DN. Persistence with therapy among patients treated with warfarin for atrial fibrillation. Arch Intern Med. 2012;172:1687–1689. doi: 10.1001/archinternmed.2012.4485. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L Committee R-LS and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L Committees A and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM Investigators RA. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 7.Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program. 2013;2013:464–470. doi: 10.1182/asheducation-2013.1.464. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175:18–24. doi: 10.1001/jamainternmed.2014.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander GC. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5:615–621. doi: 10.1161/CIRCOUTCOMES.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinberg BA, Holmes DN, Piccini JP, Ansell J, Chang P, Fonarow GC, Gersh B, Mahaffey KW, Kowey PR, Ezekowitz MD, Singer DE, Thomas L, Peterson ED, Hylek EM. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation I and Patients. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation. J Am Heart Assoc. 2013;2:e000535. doi: 10.1161/JAHA.113.000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, Spettell CM, Brennan TA, Matlin OS, Avorn J, Choudhry NK. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation- quality and cost implications. Am J Med. 2014;127:1075–1082. e1. doi: 10.1016/j.amjmed.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Hellerstein JK. The importance of the physician in the generic versus trade-name prescription decision. Rand J Econ. 1998;29:108–136. [PubMed] [Google Scholar]
- 13.Schneeweiss S, Glynn RJ, Avorn J, Solomon DH. A Medicare database review found that physician preferences increasingly outweighed patient characteristics as determinants of first-time prescriptions for COX-2 inhibitors. J Clin Epidemiol. 2005;58:98–102. doi: 10.1016/j.jclinepi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Solomon DH, Schneeweiss S, Glynn RJ, Levin R, Avorn J. Determinants of selective cyclooxygenase-2 inhibitor prescribing: are patient or physician characteristics more important? Am J Med. 2003;115:715–720. doi: 10.1016/j.amjmed.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Huskamp HA, O'Malley AJ, Horvitz-Lennon M, Taub AL, Berndt ER, Donohue JM. How quickly do physicians adopt new drugs? The case of second-generation antipsychotics. Psychiatr Serv. 2013;64:324–330. doi: 10.1176/appi.ps.201200186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steffensen FH, Sorensen HT, Olesen F. Diffusion of new drugs in Danish general practice. Fam Pract. 1999;16:407–413. doi: 10.1093/fampra/16.4.407. [DOI] [PubMed] [Google Scholar]
- 17.Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142:260–273. doi: 10.7326/0003-4819-142-4-200502150-00008. [DOI] [PubMed] [Google Scholar]
- 18.Nagin DS. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 19.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 20.Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH, Jr, Schrag SJ. US Outpatient Antibiotic Prescribing Variation According to Geography, Patient Population, and Provider Specialty in 2011. Clin Infect Dis. 2015;60:1308–1316. doi: 10.1093/cid/civ076. [DOI] [PubMed] [Google Scholar]
- 21.Hicks LA, Chien YW, Taylor TH, Jr, Haber M, Klugman KP. Active Bacterial Core Surveillance T Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53:631–639. doi: 10.1093/cid/cir443. [DOI] [PubMed] [Google Scholar]
- 22.Donohue J, O'Malley AJ, Horvitz-Lennon M, Taub AL, Berndt ER, Huskamp HA. Changes in physician antipsychotic prescribing preferences, 2002–2007. Psychiatr Serv. 2014;65:315–322. doi: 10.1176/appi.ps.201200536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AMA Physician Masterfile. Chicago: American Medical Association; [Google Scholar]
- 24.Williamson PM. The adoption of new drugs by doctors practising in group and solo practice. Soc Sci Med. 1975;9:233–236. doi: 10.1016/0037-7856(75)90027-x. [DOI] [PubMed] [Google Scholar]
- 25.Peay MY, Peay ER. The role of commercial sources in the adoption of a new drug. Soc. Sci. Med. 1988;26:1183–1189. doi: 10.1016/0277-9536(88)90149-9. [DOI] [PubMed] [Google Scholar]
- 26.Van den Bulte C, Lilien GL. Medical Innovation Revisited: Social Contagion versus Marketing Effort. Am J Soc. 2001;106:1409–1435. [Google Scholar]
- 27.Kozyrskyj A, Raymond C, Racher A. Characterizing early prescribers of newly marketed drugs in Canada: a population-based study. Eur. J. Clin. Pharmacol. 2007;63(6):597–604. doi: 10.1007/s00228-007-0277-5. [DOI] [PubMed] [Google Scholar]
- 28.Manchanda P. The Role of Targeted Communication and Contagion in Product Adoption. Marketing Science. 2008;27:961–976. [Google Scholar]
- 29.Iyengar R, Bulte CVd, Eichert J, West B, Valente TW. How Social Networks and Opinion Leaders Affect the Adoption of New Products. GfK MIR. 2011;3:16–25. [Google Scholar]
- 30.Dybdahl T, Andersen M, Sondergaard J, Kragstrup J, Kristiansen IS. Does the early adopter of drugs exist? A population-based study of general practitioners' prescribing of new drugs. Eur J Clin Pharmacol. 2004;60:667–672. doi: 10.1007/s00228-004-0797-1. [DOI] [PubMed] [Google Scholar]
- 31.Dybdahl T, Andersen M, Kragstrup J, Kristiansen IS, Sondergaard J. General practitioners' adoption of new drugs and previous prescribing of drugs belonging to the same therapeutic class: a pharmacoepidemiological study. Br J Clin Pharmacol. 2005;60:526–533. doi: 10.1111/j.1365-2125.2005.02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82:581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menchik DA, Meltzer DO. The cultivation of esteem and retrieval of scientific knowledge in physician networks. J Health Soc Behav. 2010;51:137–152. doi: 10.1177/0022146510372231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chressanthis GA, Khedkar P, Jain N, Poddar P, Seiders MG. Can access limits on sales representatives to physicians affect clinical prescription decisions? A study of recent events with diabetes and lipid drugs. J Clin Hypertens. 2012;14:435–446. doi: 10.1111/j.1751-7176.2012.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freiman MP. The rate of adoption of new procedures among physicians. The impact of specialty and practice characteristics. Med Care. 1985;23:939–945. doi: 10.1097/00005650-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Iyengar R, Bulte CVd, Valente TW. Opinion Leardership and Social Contagion in New Product Diffusion. Marketing Science. 2011;30:195–212. [Google Scholar]
- 37.Cutler D, Skinner J, Stern AD, Wennberg D. Physician Beliefs and Patient Preferences: A New Look at Supplier-Induced Demand. 2012 doi: 10.1257/pol.20150421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamblyn R, McLeod P, Hanley JA, Girard N, Hurley J. Physician and practice characteristics associated with the early utilization of new prescription drugs. Med Care. 2003;41:895–908. doi: 10.1097/00005650-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 39.The Dartmouth Atlas of Health Care: Data by Region. [Accessed Feb 14, 2016]; http://www.dartmouthatlas.org/data/region/ [Google Scholar]
- 40.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 41.Schiff GD, Galanter WL. Promoting more conservative prescribing. JAMA. 2009;301:865–867. doi: 10.1001/jama.2009.195. [DOI] [PubMed] [Google Scholar]
- 42.Schulman S. Advantages and limitations of the new anticoagulants. J Intern Med. 2014;275:1–11. doi: 10.1111/joim.12138. [DOI] [PubMed] [Google Scholar]
- 43.Kopecky S. New anticoagulants for stroke prophylaxis in atrial fibrillation: assessing the impact on medication adherence. Am J Cardiovasc Drugs. 2012;12:287–294. doi: 10.1007/BF03261837. [DOI] [PubMed] [Google Scholar]
- 44.Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 45.Grande D, Frosch DL, Perkins AW, Kahn BE. Effect of exposure to small pharmaceutical promotional items on treatment preferences. Arch Intern Med. 2009;169:887–893. doi: 10.1001/archinternmed.2009.64. [DOI] [PubMed] [Google Scholar]
- 46.Greving JP, Denig P, van der Veen WJ, Beltman FW, Sturkenboom MC, Haaijer-Ruskamp FM. Determinants for the adoption of angiotensin II receptor blockers by general practitioners. Soc Sci Med. 2006;63:2890–2898. doi: 10.1016/j.socscimed.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Donohue JM, Morden NE, Gellad WF, et al. Sources of regional variation in Medicare Part D drug spending. N Engl J Med. 2012;366:530–538. doi: 10.1056/NEJMsa1104816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon CH, Park YK, Kim SJ, Lee MJ, Ryoo S, Kim GM, Chung CS, Lee KH, Kim JS, Bang OY. Eligibility and preference of new oral anticoagulants in patients with atrial fibrillation: comparison between patients with versus without stroke. Stroke. 2014;45:2983–2988. doi: 10.1161/STROKEAHA.114.005599. [DOI] [PubMed] [Google Scholar]
- 49.Fischer MA, Choudhry NK, Brill G, Avorn J, Schneeweiss S, Hutchins D, Liberman JN, Brennan TA, Shrank WH. Trouble getting started: predictors of primary medication nonadherence. Am J Med. 2011;124:1081 e9–1081 e22. doi: 10.1016/j.amjmed.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 50.Gellad WF, Huskamp HA, Li A, Zhang Y, Safran DG, Donohue JM. Use of prescription drug samples and patient assistance programs, and the role of doctor-patient communication. J Gen Intern Med. 2011;26:1458–1464. doi: 10.1007/s11606-011-1801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


