Abstract
Objective
To determine if cartilage T1ρ and T2 relaxation time measures after ACL injury and prior to reconstruction (baseline) are associated with patient-reported outcomes at baseline, 6-months, and 1-year after surgery.
Design
Fifty-four ACL-injured participants were scanned in both knees at baseline using 3T MR T1ρ and T2 mapping. Participants also completed Knee-injury and Osteoarthritis Outcome Score (KOOS) and Marx activity level questionnaires at baseline, 6-months, and 1-year after reconstruction. The difference between cartilage T1ρ or T2 of the injured and contralateral knee (side-to-side difference, SSD) was calculated to account for physiological variations among patients. Linear regression models were built to evaluate the association between the baseline SSD T1ρ or T2 and KOOS or Marx at all time points.
Results
Higher baseline SSD T1ρ posterolateral tibia (pLT) was associated with worse KOOS in all subscales except symptoms at baseline, worse KOOS pain at 6-months, and worse KOOS in all subscales except sports function at 1-year. Higher baseline SSD T2 femoral trochlea was associated with worse KOOS activities of daily living at 1-year. Higher baseline SSD T1ρ pLT was associated with lower Marx activity level at 1-year. More severe cartilage lesions, as assessed by Whole-Organ MRI Scoring (WORMS), was significantly associated with worse KOOS pain at 6-months and 1-year.
Conclusion
T1ρ and T2 of cartilage after ACL injury were associated with KOOS after injury and both KOOS and Marx after reconstruction. Such associations may help clinicians stratify outcomes post-injury, and thus, improve patient management.
Keywords: T1ρ, T2, cartilage, ACL reconstruction, KOOS, Marx activity rating scale
Introduction
Anterior cruciate ligament (ACL) tears are prevalent and serious knee injuries that often involve concomitant damage to the cartilage1. In acute injuries, the most severe chondral damage is observed in the lateral compartment, where the pivot shift and transchondral impaction occurs2–6. The reported incidence of cartilage lesions ranges from 16% to 88% in ACL-injured knees, and such lesions have been shown to be a risk factor for osteoarthritis (OA) development 5 to 15 years after ACL injury7–11.
In the current literature, the reported effects of cartilage injury and patient-reported outcomes after ACL reconstruction (ACLR) are inconsistent. Several recent cohort studies have shown that full-thickness cartilage lesions result in worse patient-reported outcome measures two- and six-year after ACLR, whereas other studies did not find such significant associations12–16. Since the short-term success of ACLR has been largely predicated on a patient’s time to return to activity, level of pain, and quality of life, it has become increasingly important to identify sensitive measures of cartilage damage that can potentially predict patient outcomes17.
Standard magnetic resonance imaging (MRI) is an accurate, noninvasive means to detect morphological changes associated with cartilage breakdown, but is limited from evaluating early degenerative changes of the cartilage matrix18–20. Recent advances in quantitative MRI, such as T1ρ and T2, have been used to assess the biochemical matrix depletion of the cartilage in ACL-deficient and reconstructed knees8, 21, 22. However, to date, there has been little to no investigation on determining the relationship between these cartilage imaging techniques and patient-reported outcomes after ACLR23. Determining such a relationship may help clinicians provide more accurate functional expectations to patients prior to surgery.
The objective of this study was to determine if MR T1ρ and T2 measures in knee cartilage after ACL injury are associated with patient-reported outcome measures at baseline, 6-months, and 1-year after reconstruction. We hypothesize that increased cartilage T1ρ and T2 of the lateral compartment after ACL injury would associate with worse post-surgical outcomes and activity levels.
Materials and Methods
Study Participants
This prospective study was conducted after obtaining approval from our Institutional Review Board. Fifty-four participants with unilateral ACL injuries were consented and enrolled. Patients with concomitant ligamentous injuries, history of inflammatory or primary osteoarthritis, or previous knee surgery were excluded from the baseline cohort. Patients were excluded from follow-up if they chose to decline ACLR. All ACLRs were performed by one of three board-certified, fellowship-trained orthopaedic surgeons. All patients underwent the standard postoperative rehabilitation protocol.
Of the 54 participants who had bilateral knee MR scans at baseline (after injury but before reconstruction), 51 completed the validated patient-reported outcomes surveys [Knee Injury and Osteoarthritis Outcome Score (KOOS) and Marx activity rating scale]24, 25. One MR scan of the contralateral uninjured knee was confounded by excessive motion artifact and was omitted, as it was not possible to obtain accurate T1ρ and T2 measurements. Forty-six patients completed only KOOS at the 6-month follow-up, while 42 patients completed both questionnaires at the 1-year follow-up.
Patient-Reported Outcome Questionnaires
The KOOS survey assesses 5 categories: pain, symptoms, activities of daily living (ADL), sport and recreation function, and knee-related quality of life (QOL). The scale ranges from 0 to 100, with 0 being the worst and 100 being the best. The Marx activity rating scale surveys subjects regarding their level of physical activity, specifically inquiring about the frequency of various physical actions (running, cutting, decelerating, and pivoting) during the subject’s healthiest and most active state in the past year. The scale ranges from 0 to 16, with 0 and 16 being the least and most active, respectively.
Magnetic Resonance Image Acquisition
All images were acquired using a 3T MRI scanner (GE Milwaukee, WI) with an eight-channel knee coil (Invivo Inc, Gainesville, FL). High-resolution, 3D fast spin-echo (CUBE) images were used to evaluate cartilage, ligamentous, and meniscal morphology. The imaging parameters included: repetition time (TR), 1500 ms; echo time (TE), 25 ms; echo train length, 32; matrix, 384 × 384; field of view (FOV), 16 cm; slice thickness, 1 mm; and acquisition time, 8 minutes 13 seconds. Sagittal T1ρ- and T2-weighted sequences were obtained using a previously developed method based on combined T1ρ and T2 acquisition techniques26. The imaging parameters included: TR/TE, 9 ms/3 ms; FOV, 14 cm; matrix, 256 × 128; slice thickness, 4 mm; views per segment, 64; spin-lock frequency, 500 Hz; T1ρ time of spin-lock: 0, 10, 40, 80 ms; T2 preparation TE: 0, 13.7, 27.3, 54.7 ms; and acquisition time, 9 minutes 37 seconds. Although the typical slice thickness of knee MRs range from 2.5 to 3 mm, the use of 4 mm was to keep the MRI examination within clinically acceptable time constraints while still being able to cover the entire knee.
Image Post-Processing
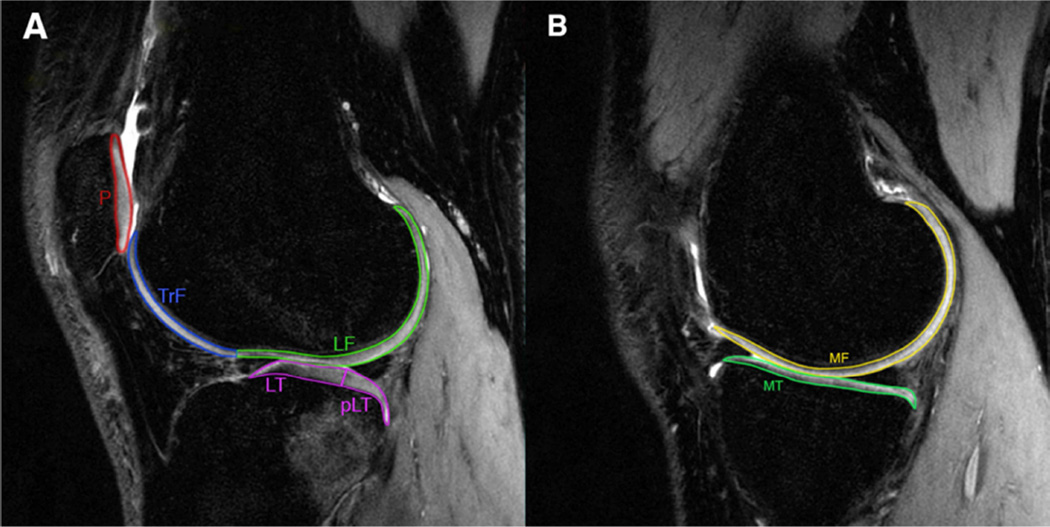
After image acquisition, the CUBE images of the injured knee were registered and down-sampled in the sagittal direction to match the images of the first T1ρ image. Cartilage was segmented semi-automatically on CUBE into six compartments [lateral femoral condyle (LF), lateral tibia (LT), medial femoral condyle (MF), medial tibia (MT), femoral trochlea (TrF), and patella (P)] using an in-house program developed with MATLAB (Mathworks, Natick, MA)27, 28. Based on previous literature and the clinical assumption that the posterolateral tibia (pLT) is often injured during ACL disruption, the LT was further subdivided to include this region using the posterior horn of the lateral meniscus as an anatomical landmark (Figure 1). Care was taken not to include the subchondral plate and synovial fluid in the segmentations.
Figure 1.
The CUBE image demonstrates the delineation of the cartilage overlying the (A) lateral and (B) medial compartments of the knee. LF, lateral femoral condyle; LT, lateral tibia; pLT, posterolateral tibia; P, patella; TrF, femoral trochlea; MF, medial femoral condyle; MT, medial tibia.
Piecewise rigid registration was applied along both T1ρ and T2 echoes to account for non-rigid movement of the femur, tibia, and patella with respect to one another. An image mask for each bone was defined by the cartilage segmentations and used to constrain the registration. The T1ρ and T2 maps of each bone were subsequently reconstructed on a pixel-by-pixel basis using a two-parameter, monoexponential fitting algorithm. Additionally, all T1ρ and T2 echoes of the contralateral knee were registered to first T1ρ echo of the injured knee to assure that the same anatomical regions of cartilage were being compared in the analysis. The registration was accomplished using an intensity-based multi-resolution pyramidal approach29, 30. Mean T1ρ and T2 values were calculated for each cartilage compartment after transferring the segmentations from CUBE onto the maps.
After recruitment of 23 participants, the 3T HDx Long Bore MR scanner was replaced with a 3T MR750 Wide Bore unit. In order to account for potential differences in T1ρ and T2 values from using different MR systems, phantoms and human subjects were scanned on both units within a 4-month period: 9 individuals for T1ρ [average time between scans, 49.5 (range, 9–114) days] and 5 individuals for T2 [average time between scans, 13.6 (range, 9–18) days]. A decrease in T1ρ and T2 was observed between the old Long Bore system and the new Wide Bore system, with the measurements being highly correlated (R2 = 0.95 and R2 = 0.92 for T1ρ and T2, respectively) (see Supplemental Figure S1). A linear regression model was established to adjust T1ρ and T2 values as follows:
where the subscripts old and new signify the values of the old and new systems, respectively.
Clinical MR Assessment
All images were evaluated by two board-certified, fellowship-trained musculoskeletal radiologists each with over 10 years of experience. A modified Whole-Organ MRI Scoring (WORMS) system was used to assess the lateral and medial menisci as follows: 0, intact menisci; 1, intact menisci with at least one region with intra-substance abnormalities; 2, only one non-displaced tear in one region; 3, more than one non-displaced tear or one complex tear in the meniscus; 4, more than one displaced or complex tear with deformity in the meniscus; 5, maceration of only one region; and 6, maceration of more than one region. An unmodified eight-point WORMS scale was used to evaluate the cartilage overlying the medial and lateral femoral condyles and tibial plateaus, as well as the cartilage overlying the patella and trochlea31. Bone marrow edema-like lesions (BMEL) was assessed and quantified as absent (Grade 0), mild (Grade 1: diameter, d < 5 mm), moderate (Grade 2: 5 mm < d < 20 mm), or severe (Grade 3: d > 20 mm) over both the femoral condyles and the tibial plateaus.
Statistical Analysis
Paired t-tests were used to compare T1ρ and T2 values between the injured and contralateral knees for each cartilage compartment. Linear regression models were built to determine the relationship between cartilage T1ρ and T2 values at baseline and KOOS and Marx at baseline, 6-months, and 1-year. A side-to-side difference (SSD) in T1ρ or T2, defined as the difference between the relaxation time values in the injured and contralateral knee, was calculated to account for physiological variations among patients and used in all regression analyses. To reduce the number of included predictors and the degree of multiple testing, we first screened variables by testing if their relaxation times were significantly different between sides. Only SSDs that were statistically significant were included as independent variables in the regression models for predicting KOOS and Marx scores. The dependent variables consisted of the 5 subscales of KOOS and the Marx activity rating score at each time point. The regression models were adjusted for age, gender, BMI, WORMS for medial and lateral menisci, total BMEL, and total cartilage lesions. For the 6-month and 1-year follow-up, lateral meniscectomy at the time of ACLR (categorized as yes or no) and baseline KOOS and Marx were also included in the adjustments. Medial meniscectomy was not included in the follow-up analyses since only two participants had undergone surgical treatment. All statistical analyses were performed using SPSS Statistics version 22.0.0 (IBM, Armonk, NY). To account for multiple comparisons made between baseline T1ρ and T2 of ACL-injured and contralateral knees in seven compartments, Bonferroni correction was applied and the significance level was set to 0.007. For the regression models, the significance level was 0.05.
Results
Baseline Patient and Clinical Characteristics
Fifty-four patients were enrolled (31 men, 23 women), with a mean age of 29.6 years (range, 15– 50 years) and average BMI of 24.4 ± 3.5 kg/m2 [Table 1(a)]. The average time between injury and MRI was 61.5 ± 49.5 days. Of the initial cohort, 52 patients underwent ACLR using hamstring autograft (n = 36) or soft tissue allograft (n = 16). The clinical characteristics of the analyzed cohort are provided in Table 1(b). Based on MR evaluation, lateral meniscal injury (WORMS ≥ 2) was noted in the ACL-deficient knee of 24 (44%) subjects, with 10 undergoing debridement and 3 undergoing repairs. Medial meniscal injury (WORMS ≥ 2) was observed in 20 (37%) subjects, with 2 undergoing partial meniscectomy and 1 undergoing repair. Thirty-five (65%) patients also sustained a MRI-detectable cartilage injury in their ACL-ruptured knee, most frequently observed over the LT (n = 20). Forty-two (78%) patients had BMEL in at least one compartment, with the LF and LT being most affected (n = 27 and n = 42, respectively).
Table 1.
| (a) Baseline Patient Characteristics | |
|---|---|
| Characteristic | |
| Sex (n = 54)a | |
| Male | 31 (57%) |
| Female | 23 (43%) |
| Age (years)b | 29.6 ± 8.4 |
| BMI (kg/m2)b | 24.4 ± 3.5 |
| Time from Injury to MRI (days)b | 61.5 ± 49.5 |
| Time from Injury to Surgery (days)b | 76.3 ± 54.5 |
| ACL Graft (n = 52)a | |
| Hamstring Autograft | 36 (69%) |
| Posterior Tibialis Allograft | 14 (27%) |
| Hamstring Allograft | 2 (4%) |
| (b) Baseline Clinical Characteristics as Assessed by WORMSa | |||
|---|---|---|---|
| Characteristic | Characteristic | ||
| Medial Meniscus | Lateral Meniscus | ||
| Normal | 29 (54%) | Normal | 21 (39%) |
| Grade 1 | 5 (9%) | Grade 1 | 9 (17%) |
| Grade 2 | 10 (18%) | Grade 2 | 20 (37%) |
| Grade 3 | 2 (4%) | Grade 3 | 1 (2%) |
| Grade 4 | 7 (13%) | Grade 4 | 3 (5%) |
| Grade 5 | 1 (2%) | Grade 5 | 0 (0%) |
| Grade 6 | 0 (0%) | Grade 6 | 0 (0%) |
| MF Cartilage Lesion | LF Cartilage Lesion | ||
| Normal | 49 (90%) | Normal | 46 (85%) |
| Grade 1 | 1 (2%) | Grade 1 | 3 (6%) |
| Grade 2 | 2 (4%) | Grade 2 | 4 (7%) |
| Grade 2.5 | 0 (0%) | Grade 2.5 | 1 (2%) |
| Grade 3 | 2 (4%) | Grade 3 | 0 (0%) |
| Grade ≥ 4 | 0 (0%) | Grade ≥ 4 | 0 (0%) |
| MT Cartilage Lesion | LT Cartilage Lesion | ||
| Normal | 46 (85%) | Normal | 34 (62%) |
| Grade 1 | 5 (9%) | Grade 1 | 10 (19%) |
| Grade 2 | 3 (6%) | Grade 2 | 10 (19%) |
| Grade 2.5 | 0 (0%) | Grade 2.5 | 0 (0%) |
| Grade ≥ 3 | 0 (0%) | Grade ≥ 3 | 0 (0%) |
| Patellar Cartilage Lesion | Trochlear Cartilage Lesion | ||
| Normal | 41 (75%) | Normal | 44 (81%) |
| Grade 1 | 3 (6%) | Grade 1 | 2 (4%) |
| Grade 2 | 3 (6%) | Grade 2 | 4 (7%) |
| Grade 2.5 | 0 (0%) | Grade 2.5 | 0 (0%) |
| Grade 3 | 7 (13%) | Grade 3 | 3 (6%) |
| Grade 4 | 0 (0%) | Grade 4 | 0 (0%) |
| Grade 5 | 0 (0%) | Grade 5 | 1 (2%) |
| Grade 6 | 0 (0%) | Grade 6 | 0 (0%) |
| MF Bone Marrow Edema | LF Bone Marrow Edema | ||
| Normal | 50 (92%) | Normal | 27 (50%) |
| Grade 1 | 1 (2%) | Grade 1 | 1 (2%) |
| Grade 2 | 3 (6%) | Grade 2 | 13 (24%) |
| Grade 3 | 0 (0%) | Grade 3 | 13 (24%) |
| MT Bone Marrow Edema | LT Bone Marrow Edema | ||
| Normal | 46 (85%) | Normal | 12 (22%) |
| Grade 1 | 5 (9%) | Grade 1 | 0 (0%) |
| Grade 2 | 3 (6%) | Grade 2 | 17 (31%) |
| Grade 3 | 0 (0%) | Grade 3 | 25 (47%) |
Data expressed as Count (Percentage %).
Data expressed as Mean ± Standard Deviation.
Data expressed as Count (Percentage %). WORMS, Whole-Organ MRI Scoring; MF, medial femoral condyle; LF, lateral femoral condyle; MT, medial tibial plateau; LT, lateral tibial plateau.
Patient-Reported Outcome Scores
The baseline and follow-up outcome scores for KOOS and Marx are presented in Table 2. From baseline to 6-months following reconstruction, KOOS in the pain, ADL, and sports subscales significantly improved (p = 0.005, < 0.001, and 0.006, respectively). At the 1-year follow-up, patients had reported significantly higher KOOS scores in all categories than at 6-months (all p < 0.001). The Marx activity level of patients at 1-year post-reconstruction was less than that prior to injury, but this finding was not significant (p = 0.21).
Table 2.
Patient-Reported Outcome Scores Over Timea
| Outcome | Baseline (n = 51) |
6-month Follow-up (n = 46) |
1 -year Follow-up (n = 42) |
|---|---|---|---|
| KOOS | |||
| Pain | 74.4 ± 18.0 | 83.5 ± 12.4 | 86.4 ± 11.1 |
| Symptoms | 68.6 ± 19.4 | 74.4 ± 15.4 | 79.9 ± 13.1 |
| ADL | 81.9 ± 18.4 | 92.0 ± 9.4 | 94.6 ± 6.7 |
| Sports | 55.1 ± 27.7 | 68.9 ± 20.1 | 78.0 ± 17.8 |
| QOL | 43.4 ± 24.5 | 52.3 ± 19.3 | 62.4 ± 19.3 |
| Marx Activity | 11.2 ± 3.9 | - | 10.2 ± 3.8 |
Data expressed as Mean ± Standard Deviation. KOOS, Knee-injury and Osteoarthritis Outcome Score; ADL, activity of daily living; QOL, knee-related quality of life.
Cartilage T1ρ and T2 after ACL Injury
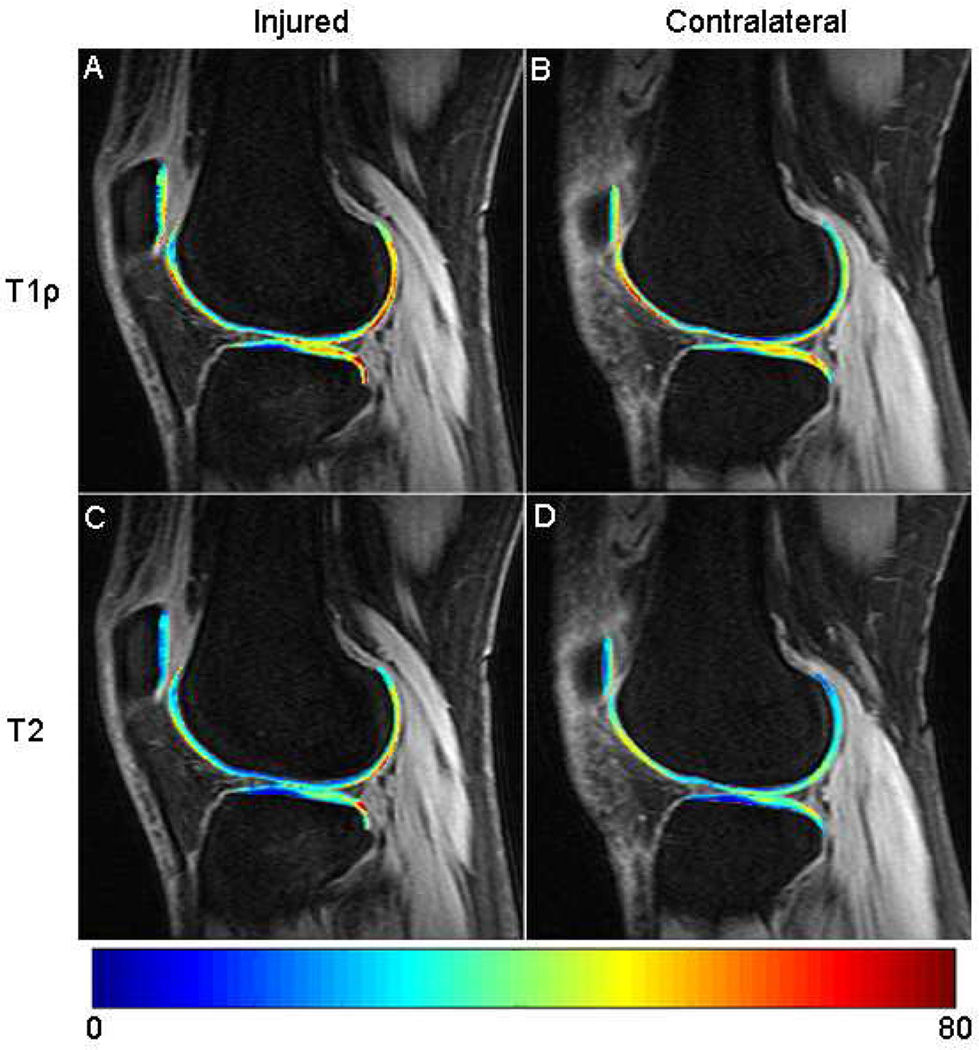
At baseline, mean T1ρ and T2 values were significantly elevated in the cartilage of the injured knee overlying the posterolateral tibia (pLT) with respect to the contralateral knee (both p < 0.0001) (Figure 2) (Table 3). The T2 cartilage value of both the lateral tibia (LT) and femoral trochlea (TrF) were also significantly higher in ACL-deficient knees compared to that of the uninjured knees (p = 0.002 and p < 0.0001, respectively).
Figure 2.
Sagittal (A) T1ρ and (C) T2 maps of the ACL-ruptured knee show prolonged T1ρ and T2 relaxation times over the posterolateral tibial plateau and posterolateral femoral condyle compared to the (B, D) contralateral knee.
Table 3.
Baseline T1ρ and T2 (ms) of ACL-injured and Contralateral Kneesa
| MF | LF | MT | LT | P | TrF | pLT | |
|---|---|---|---|---|---|---|---|
| T1ρ | |||||||
| Injured | 38.9 ± 2.7 | 39.1 ± 2.5 | 35.5 ± 3.0 | 34.8 ± 2.8 | 38.9 ± 3.3 | 40.5 ± 2.7 | 41.9 ± 4.25 |
| Contralateral | 38.9 ± 3.1 | 38.4 ± 2.5 | 35.8 ± 3.0 | 34.3 ± 3.1 | 39.3 ± 3.6 | 40.0 ± 2.7 | 39.1 ± 3.9 |
| p-value | 0.89 | 0.012 | 0.48 | 0.14 | 0.28 | 0.093 | < 0.0001 |
| SSDb | 0.048 (−1.3, 1.0) |
0.72 (−0.3, 2.3) |
−0.34 (−2.6, 1.7) |
0.529 (−1.3, 1.9) |
−0.38 (−2.2, 1.0) |
0.52 (−0.6, 1.5) |
2.85 (0.9, 5.6) |
| T2 | |||||||
| Injured | 30.1 ± 2.2 | 29.7 ± 2.3 | 27.0 ± 2.8 | 25.5 ± 2.3 | 28.6 ± 3.0 | 31.3 ± 2.4 | 31.9 ± 3.5 |
| Contralateral | 29.9 ± 2.6 | 29.4 ± 2.0 | 26.9 ± 2.6 | 24.7 ± 2.5 | 28.5 ± 2.6 | 29.8 ± 2.1 | 29.3 ± 3.3 |
| p-value | 0.42 | 0.27 | 0.62 | 0.002 | 0.72 | < 0.0001 | < 0.0001 |
| SSDb | 0.19 (−0.8, 1.0) |
0.30 (−0.9, 1.2) |
0.17 (−1.0, 2.1) |
0.84 (−0.5, 2.0) |
0.10 (−1.1, 1.1) |
1.5 (0.5, 2.2) |
2.5 (0.8, 4.8) |
Data is expressed as Mean ± Standard Deviation. MF, medial femoral condyle; LF, lateral femoral condyle; MT, medial tibia; LT, lateral tibia; P, patella; TrF, femoral trochlea; pLT, posterolateral tibia. Bold denotes significance.
Data is expressed as Mean (Interquartile Range).
Summary of Significant Predictors of KOOS and Marx at each Time Point
Table 4 displays the significant associations identified for each individual outcome after linear regression. Baseline SSD T2 LT and pLT were not included in the model, as they are highly correlated with baseline SSD T1ρ pLT (p < 0.001). At baseline, higher SSD T1ρ pLT was significantly associated with lower KOOS in all subscales except symptoms (p = 0.073).
Table 4.
Significant Predictors of each Outcome at Baseline, 6-month, and 1-year Follow-upa
| Baseline SSD T1r pLT |
Baseline SSD T2 TrF |
WORMS Medial Meniscus |
WORMS Lateral Meniscus |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| Baseline (n = 51) | ||||||||||||
| KOOS | ||||||||||||
| Pain | −0.38 | 0.14 | 0.008 | −0.04 | 0.14 | 0.773 | −0.12 | 0.15 | 0.426 | −0.02 | 0.14 | 0.882 |
| Symptoms | −0.26 | 0.14 | 0.073 | 0.07 | 0.14 | 0.635 | 0.15 | 0.16 | 0.333 | −0.22 | 0.15 | 0.136 |
| ADL | −0.38 | 0.13 | 0.006 | −0.06 | 0.13 | 0.668 | −0.04 | 0.15 | 0.776 | −0.10 | 0.14 | 0.488 |
| Sports | −0.37 | 0.16 | 0.022 | 0.03 | 0.16 | 0.833 | 0.04 | 0.18 | 0.825 | −0.15 | 0.17 | 0.379 |
| QOL | −0.33 | 0.15 | 0.034 | 0.17 | 0.15 | 0.274 | 0.09 | 0.17 | 0.583 | −0.05 | 0.16 | 0.747 |
| 6-month Follow-up (n = 46) | ||||||||||||
| KOOS | ||||||||||||
| Pain | −0.34 | 0.16 | 0.050 | 0.04 | 0.16 | 0.816 | −0.04 | 0.21 | 0.837 | −0.15 | 0.21 | 0.495 |
| Symptoms | −0.27 | 0.16 | 0.105 | 0.06 | 0.17 | 0.728 | 0.09 | 0.21 | 0.667 | −0.05 | 0.22 | 0.827 |
| ADL | −0.04 | 0.16 | 0.820 | −0.14 | 0.15 | 0.362 | −0.11 | 0.19 | 0.568 | −0.17 | 0.20 | 0.415 |
| Sports | −0.14 | 0.18 | 0.435 | 0.16 | 0.18 | 0.371 | 0.06 | 0.22 | 0.805 | −0.22 | 0.23 | 0.352 |
| QOL | −0.10 | 0.15 | 0.538 | 0.28 | 0.17 | 0.114 | −0.05 | 0.20 | 0.806 | −0.01 | 0.20 | 0.979 |
| 1-year Follow-up (n = 42) | ||||||||||||
| KOOS | ||||||||||||
| Pain | −0.45 | 0.15 | 0.006 | −0.07 | 0.15 | 0.639 | −0.04 | 0.18 | 0.831 | −0.15 | 0.17 | 0.384 |
| Symptoms | −0.36 | 0.16 | 0.027 | −0.09 | 0.17 | 0.606 | 0.08 | 0.20 | 0.698 | −0.11 | 0.19 | 0.559 |
| ADL | −0.30 | 0.14 | 0.046 | −0.33 | 0.15 | 0.032 | −0.23 | 0.18 | 0.210 | −0.07 | 0.17 | 0.680 |
| Sports | −0.28 | 0.16 | 0.098 | −0.07 | 0.17 | 0.697 | 0.12 | 0.20 | 0.534 | 0.09 | 0.19 | 0.648 |
| QOL | −0.50 | 0.17 | 0.006 | 0.06 | 0.19 | 0.737 | −0.01 | 0.22 | 0.983 | 0.10 | 0.20 | 0.620 |
| Marx | −0.47 | 0.17 | 0.013 | 0.07 | 0.22 | 0.717 | 0.21 | 0.21 | 0.341 | 0.06 | 0.23 | 0.807 |
| WORMS Total BMEL |
WORMS Total Cartilage Lesion |
Lateral Meniscectomy |
Baseline KOOS or Marx Score |
|||||||||
| Outcome | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value |
| Baseline (n = 51) | ||||||||||||
| KOOS | ||||||||||||
| Pain | 0.17 | 0.14 | 0.224 | −0.22 | 0.14 | 0.133 | - | - | - | - | - | - |
| Symptoms | 0.00 | 0.14 | 0.983 | −0.30 | 0.15 | 0.052 | - | - | - | - | - | - |
| ADL | 0.22 | 0.13 | 0.107 | −0.27 | 0.14 | 0.060 | - | - | - | - | - | - |
| Sports | 0.18 | 0.16 | 0.255 | 0.06 | 0.17 | 0.714 | - | - | - | - | - | - |
| QOL | 0.00 | 0.15 | 0.999 | −0.18 | 0.16 | 0.271 | - | - | - | - | - | - |
| 6-month Follow-up (n = 46) | ||||||||||||
| KOOS | ||||||||||||
| Pain | 0.10 | 0.19 | 0.596 | −0.41 | 0.18 | 0.030 | −0.03 | 0.24 | 0.892 | 0.15 | 0.16 | 0.379 |
| Symptoms | 0.24 | 0.19 | 0.215 | −0.21 | 0.19 | 0.280 | −0.10 | 0.24 | 0.672 | 0.26 | 0.18 | 0.160 |
| ADL | −0.04 | 0.18 | 0.841 | −0.49 | 0.17 | 0.009 | −0.10 | 0.23 | 0.660 | 0.38 | 0.16 | 0.027 |
| Sports | 0.25 | 0.20 | 0.231 | −0.36 | 0.19 | 0.070 | 0.09 | 0.26 | 0.743 | 0.36 | 0.17 | 0.046 |
| QOL | 0.07 | 0.18 | 0.712 | −0.33 | 0.17 | 0.062 | −0.24 | 0.23 | 0.310 | 0.21 | 0.16 | 0.190 |
| 1-year Follow-up (n = 42) | ||||||||||||
| KOOS | ||||||||||||
| Pain | 0.10 | 0.17 | 0.541 | −0.36 | 0.16 | 0.033 | 0.12 | 0.20 | 0.543 | 0.08 | 0.15 | 0.614 |
| Symptoms | 0.28 | 0.18 | 0.121 | −0.24 | 0.18 | 0.185 | 0.11 | 0.21 | 0.590 | 0.27 | 0.16 | 0.094 |
| ADL | 0.08 | 0.16 | 0.641 | −0.18 | 0.15 | 0.251 | −0.03 | 0.19 | 0.861 | 0.25 | 0.16 | 0.125 |
| Sports | 0.09 | 0.18 | 0.603 | −0.08 | 0.17 | 0.644 | −0.17 | 0.21 | 0.424 | 0.27 | 0.15 | 0.077 |
| QOL | 0.02 | 0.20 | 0.930 | −0.09 | 0.19 | 0.643 | −0.06 | 0.23 | 0.795 | 0.04 | 0.16 | 0.820 |
| Marx | −0.21 | 0.21 | 0.337 | 0.30 | 0.19 | 0.123 | 0.07 | 0.23 | 0.807 | 0.18 | 0.21 | 0.430 |
Regression analyses adjusted for age, gender, and BMI. SSD, side-to-side difference pLT, posterolateral tibia; TrF, femoral trochlea; WORMS, Whole-Organ MRI Scoring; BMEL, bone marrow edema-like lesions; β, standardized regression coefficient; SE, standard error; KOOS, Knee-injury and Osteoarthritis Outcome Score; ADL, activities of daily living; QOL, knee-related quality of life.
Bold denotes statistical significance.
At 6-months post-reconstruction, higher baseline SSD T1ρ pLT was associated with worse KOOS pain (p = 0.050). Regarding WORMS, more severe cartilage lesions in the entire knee were significantly associated with worse KOOS outcomes in pain and ADL subscales (p = 0.030 and p = 0.008, respectively). The baseline outcome score for KOOS ADL was significantly associated with the 6-month score.
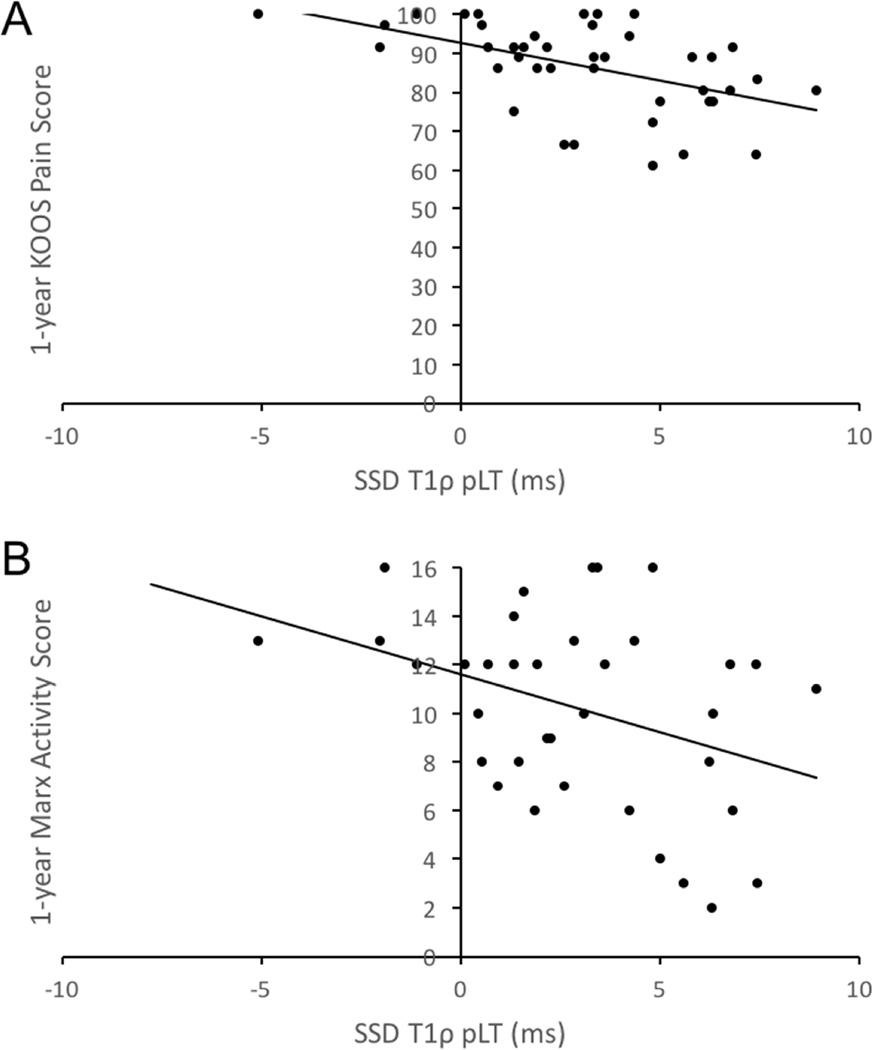
At 1-year follow-up, higher baseline SSD T1ρ pLT was significantly associated with worse KOOS in all subscales except sports (p = 0.098). Higher baseline SSD T2 TrF was significantly associated with worse 1-year KOOS ADL scores (p = 0.032). More severe articular cartilage injuries, as assessed by WORMS at baseline, were significantly associated with worse 1-year KOOS in the pain subscale (p = 0.030). For Marx at 1-year following surgery, only higher baseline SSD T1ρ pLT was associated with lower activity levels (p = 0.013) (Figure 3).
Figure 3.
Relationship between SSD T1 ρ pLT and (A) 1-year KOOS Pain Score and (B) 1-year Marx Activity Score. SSD, side-to-side difference; pLT, posterolateral tibia; KOOS, Knee-Injury and Osteoarthritis Outcome Score.
Table 5 shows the change in patient-reported outcomes at all time points due to increases in the significant predictors from the lower to upper quartile. Clinically meaningful changes in a KOOS subscale and Marx activity level were estimated to be 8 and 2 points, respectively. At baseline, the effect of increasing the SSD T1ρ pLT was associated with clinically meaningful decreases in KOOS scores. At the 6-month follow-up, the effect of increasing the WORMS for total cartilage lesions was associated with clinically worse outcomes in KOOS pain. At 1-year follow-up, the effect of increasing the baseline SSD T1ρ pLT was associated with clinically relevant decreases in the KOOS QOL subscale and Marx activity level.
Table 5.
Change in Outcome Measures at all Time Points from Increasing the Predictors from Lower to Upper Quartilea
| Baseline SSD T1ρ pLT (0.9, 5.6)b |
Baseline SSD T2 TrF (0.5, 2.2)b |
Total WORMS Cartilage Lesion (0.0, 3.8)b |
||||
|---|---|---|---|---|---|---|
| Outcomes | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| Baseline (n = 51) | ||||||
| KOOS | ||||||
| Pain | −8.92 | −15.4 to −2.5 | −0.57 | −4.5 to 3.4 | −6.58 | −14.8 to 1.6 |
| Symptoms | −6.58 | −13.5 to 0.4 | 1.08 | −3.2 to 5.3 | −9.57 | −19.1 to 0.0 |
| ADL | −9.12 | −15.2 to −3.0 | −0.88 | −4.6 to 2.8 | −8.25 | −16.6 to 0.1 |
| Sports | −13.36 | −24.7 to −2.0 | 0.66 | −6.2 to 7.6 | 2.76 | −12.6 to 18.1 |
| QOL | −10.54 | −19.9 to −1.2 | 3.31 | −2.4 to 9.0 | −7.32 | −20.1 to 5.4 |
| 6-month Follow-up (n = 46) | ||||||
| KOOS | ||||||
| Pain | −5.50 | −10.6 to −0.4 | 0.39 | −2.7 to 3.5 | −8.45 | −15.7 to −1.2 |
| Symptoms | −5.42 | −11.7 to 0.9 | 0.73 | −3.3 to 4.8 | −5.37 | −14.9 to 4.2 |
| ADL | −0.49 | −4.3 to 3.4 | −1.05 | −3.2 to 1.2 | −7.65 | −12.8 to −2.4 |
| Sports | −3.67 | −12.9 to 5.6 | 2.56 | −3.1 to 8.2 | −12.02 | −24.5 to 0.4 |
| QOL | −2.52 | −9.9 to 4.9 | 4.29 | −0.8 to 9.4 | −10.58 | −21.2 to 0.1 |
| 1-year Follow-up (n = 42) | ||||||
| KOOS | ||||||
| Pain | −6.51 | −10.8 to −2.3 | −0.62 | −3.2 to 2.0 | −6.64 | −12.4 to −0.9 |
| Symptoms | −6.15 | −11.5 to −0.8 | −0.94 | −4.4 to 2.5 | −5.22 | −12.9 to 2.5 |
| ADL | −2.62 | −5.0 to −0.2 | −1.76 | −3.3 to −0.2 | −2.00 | −5.3 to 1.3 |
| Sports | −6.50 | −13.8 to 0.8 | −0.99 | −5.7 to 3.7 | −2.37 | −12.2 to 7.5 |
| QOL | −12.6 | −21.0 to −4.2 | 0.92 | −4.8 to 6.6 | −2.88 | −14.8 to 9.1 |
| Marx | −2.33 | −4.0 to −0.7 | 0.21 | −1.1 to 1.5 | 1.89 | −0.5 to 4.2 |
SSD, side-to-side difference pLT, posterolateral tibia; TrF, femoral trochlea; WORMS, Whole-Organ MRI Scoring; CI, confidence interval; KOOS, Knee-Injury and Osteoarthritis Outcome Score; ADL, activities of daily living; QOL, knee-related quality-of-life. Bold denotes clinically meaningful changes.
Interquartile Range.
Discussion
In the present study, quantitative T1ρ and T2 mapping were used to determine the association between cartilage damage at the time of ACL injury and patient-reported outcomes after injury and post-reconstruction. Our results revealed that patients with higher baseline T1ρ in the posterolateral tibia of the ACL-injured knee compared to the contralateral knee reported significantly worse outcomes at the time of injury and at 1-year post-reconstruction. To the best of our knowledge, this the first study to demonstrate that cartilage MR relaxation times can predict patient-reported outcomes after ACL reconstruction.
At baseline, T1ρ and T2 measurements were significantly elevated in the posterolateral tibia of the ACL-deficient knee compared to the contralateral knee. Bone marrow edema-like lesions (BMEL) were also most frequently noted in the lateral compartment of the injured knee. These findings are consistent with our previous studies, which compared ACL-injured knees to knees from a healthy control cohort, and reports from other groups, suggesting that most of the damage is dealt to the lateral compartment during anterior subluxation of the knee8, 21, 22. Thus, the worse baseline KOOS scores reported by patients with higher T1ρ in the posterolateral tibia of the injured knee compared to the contralateral knee may be related to the severity of the cartilage damage experienced during injury. Furthermore, clinical morphological factors presumably related to severity of injury such as BMEL size, depth of cartilage lesions, and meniscal tears were not associated with KOOS at the time of ACL reconstruction. A prior prospective study likewise demonstrated that these factors were not significantly associated with KOOS pain or symptoms at baseline6. These findings suggest that the compositional changes to the cartilage matrix at the time of injury are better indicators of knee pain and function than morphological changes at baseline.
Although our previous quantitative MR studies on ACL-ruptured knees only identified differences in the tibiofemoral joint after injury, the current study establishes that T2 was significantly higher in the femoral trochlea of the ACL-injured knee compared to the contralateral knee at baseline. Frobell et al. previously documented cartilage thinning in the femoral trochlea of the ACL-injured knee within the first year, suggesting that the thinning may be related to development of patellofemoral arthritis32, 33. Furthermore, Potter et al. reported increased risk of cartilage loss in the patellofemoral joint 7 to 11 years after ACL injury8. Additional studies using quantitative MRI will hopefully elucidate the long-term outcomes of the chondral degeneration to the patellofemoral joint after ACL injury.
At 6-month follow-up, higher baseline side-to-side difference T1ρ of the posterolateral tibia predicted worse outcomes in KOOS pain, while more severe cartilage lesions in the entire knee, as assessed by WORMS, predicted worse outcomes in both KOOS pain and ADL subscales. However, at 1-year follow-up, our data demonstrated that higher baseline side-to-side difference T1ρ of the posterolateral tibia predicted worse outcomes for KOOS in most subscales and Marx activity level, while increased severity of cartilage lesions of the entire knee only predicted worse outcomes for KOOS pain. These results suggest that the initial cartilage damage in the posterolateral tibia, as assessed by T1ρ, is superior to the severity of cartilage loss in predicting the patient’s final outcome after postoperative rehabilitation. In addition, neither the severity of meniscal tears nor excision of the lateral meniscus was significantly associated with patient- reported outcomes at 6-months or 1-year follow-up. This finding is supported by Norwegian and Swedish national ACL study that failed to identify significant associations between meniscal lesions and KOOS in any subscale at 2-year follow-up12.
Although these findings were statistically significant, their clinical significance can be debated. Roos et al. previously suggested that a difference of 8 points in a KOOS subscale may represent a clinically significant change following ACL reconstruction24. In regards to Marx activity level, the minimal clinical important difference was previously estimated to be 2 points34. The results of this study show that the effect of increasing the WORMS for cartilage lesions in the entire knee from the lower to upper quartile (3.8 points) decreased 6-month KOOS pain by 8.5 points. The clinical significance of this finding, however, was not observed at 1-year follow-up. For baseline side-to-side difference T1ρ of the posterolateral tibia, an increase from the lower to upper quartile (4.7 ms) decreased 1-year KOOS knee-related quality of life by 12.6 points and Marx activity level by 2.3 points. Thus, increased damage to the posterolateral tibial cartilage during ACL injury may influence clinically meaningful decreases in patient knee-related quality of life and may be a potential factor as to why most patients do not return to pre-injury activity levels 1-year post-reconstruction.
In contrast to the results aforementioned, a recent cohort study involving 62 participants showed no significant correlations between cartilage T2 relaxation times and International Knee Documentation Committee and Tegner Lysholm Scoring Scale outcomes after ACL reconstruction23. However, possible differences in the study population, different follow-up periods, and use of different patient-reported outcome measures make it difficult to compare the findings and might explain the discrepancies in the reported results from the previous and the present studies. Moreover, the previous study recruited only male subjects and limited its analyses to the global cartilage compartments and cartilage-on-cartilage weight-bearing regions. Our analysis was more specific in that we included the posterolateral tibia, an area often severely damaged during ACL injury, and the side-to-side difference in relaxation times.
The baseline KOOS scores from the present cohort are comparable to what has been reported by another comprehensive cohort in the United States (Multicenter Orthopaedic Outcomes Network, MOON)34, 35. Similarly, there is no clinically meaningful difference in baseline KOOS between our cohort and patients from the Danish, Swedish, and Norwegian national registries except for sports recreation and function and knee-related quality of life12, 36, 37. The differences in these KOOS subscales between cohorts may be due to the longer times from injury to surgery in the national registries. However, the change in KOOS scores from baseline to 1-year follow-up in this study is comparable to those reported in the Danish ACL Reconstruction Registry37. In regards to activity levels, the results of this study, indicating that most participants (54%) do not return to their pre-injury activity levels after surgery, are corroborated by findings from previous reports35, 38–41.
In this study, the side-to-side difference T1ρ and T2 values of the posterolateral tibia were significantly correlated (r = 0.41, p < 0.001). Consequently, T2 of posterolateral tibia was excluded from the regression models to avoid multicollinearity. In an effort to compare the association between T1ρ and KOOS versus T2 and KOOS, we ran similar regression models using the side-to-side difference T2 of the posterolateral tibia as the only quantitative MR measure. It was observed that T2 of the posterolateral tibia is significantly associated with baseline KOOS except for symptoms and quality of life (p = 0.461 and 0.080, respectively). These findings are similar to that of T1ρ; however, no significant associations are observed between T2 of the posterolateral tibia and KOOS at 6-months and 1-year. These results suggest that although T1ρ and T2 may provide correlated image contrast after acute ACL injury, T1ρ may be more predictive of longitudinal patient-reported outcomes than that of T2. This finding is also corroborated by previous studies that have shown that T1ρ is more sensitive than T2 in detecting changes in proteoglycan concentration, and suggested that the cartilage matrix after acute ACL injury primarily involves loss of proteoglycan rather than significant damage to the collagen network42–45. Furthermore, Zarins et al. identified stronger associations between T1ρ and self-reported outcomes for pain, function, and stiffness in patients with osteoarthritis than with T246. Despite of all this, it should be noted that T1ρ imaging is currently used as a research prototype sequence with limited availability while T2 imaging is a product sequence available on all major vendors. The spin-lock strength of T1ρ imaging is also limited by the amount of energy allowed to be deposited to the tissue (measured by specific absorption rate, SAR). For clinical application at 3T, a spin-lock frequency of 500 Hz is normally used.
The primary limitation of the present study is the relatively small sample size of 42 patients at 1-year follow-up. As such, our models could not provide a more detailed analysis of the injuries involving BMEL, meniscal tears, and cartilage damage by compartment. In the current analysis, the WORMS scores of these potential morphological predictors were summed over the entire knee. Despite using a cumulative score, more severe cartilage lesions in the entire knee after injury, as assessed by WORMS, were almost significantly associated with worse baseline and 6- months KOOS in several subscales. To achieve a power of 80% with a two-sided significance level of 0.05, the sample size required for testing if WORMS total cartilage lesions predict 6-month KOOS in the sports subscale, for example, would be 52 based on the findings of this study. Therefore, cohorts with larger sample sizes are warranted to further investigate these relationships. Another weakness is the use of subjective questionnaires as the only outcome measure. The current assessments for evaluating the success of an ACL reconstruction also include the clinical stability and functional performance of the knee. Furthermore, the methods of this study rely on the use of bilateral knee MRs, which are currently not practical in a clinical setting. Finally, it is unknown how the associations between MR relaxation times and outcomes after ACL reconstruction will change with longer follow-ups. A planned 3-year follow-up will further clarify this.
Despite these limitations, the results from this study suggest that quantitative MRI provides a non-invasive, sensitive measure of cartilage damage that can potentially help clinicians predict the functional outcome of patients after ACL reconstruction. Our models inform us that a more severe injury to the cartilage matrix, especially in the posterolateral tibia, are associated with worse patient-reported outcomes including pain, knee-related quality of life, and activity level 1-year post-reconstruction.
Supplementary Material
Linear regression model of the HDx Long Bore system and MR750 Wide Bore 17 system for both (A) T1ρ and (B) T2. The data points were obtained from measuring the 18 relaxation times of cartilage, ligament (ACL, PCL), gastrocnemius muscle, and meniscus. Nine 19 individuals were able for T1ρ, while only 5 individuals were available for T2.
Acknowledgments
This study was funded by NIH/NIAMS P50 AR060752, AOSSM Cartilage Initiative Grant, and OREF Resident Research Grant A123553. Statistical analysis was supported by CTSA Grant UL1 RR024131. The authors of this study would like to thank Daniel Wu, Musa Zaid, Lauren Tufts, Samuel Wu, and Mike Hoppe for their technical assistance. The authors also appreciate Caroline Currie for her help in recruiting patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s Contributions
- Conception and design: Xiaojuan Li and C. Benjamin Ma
- Obtaining of funding: Xiaojuan Li and C. Benjamin Ma
- Provision of study materials or patients: Xiaojuan Li and C. Benjamin Ma
- Collection and assembly of data: Favian Su
- Analysis and interpretation of data: Favian Su, Valentina Pedoia, Hsiang-Ling Teng, Martin Kretzschmar, Brian C. Lau, and Thomas M. Link
- Statistical expertise: Hsiang-Ling Teng and Charles E. McCulloch
- Drafting the article: Favian Su
- Critical revision of the article for important intellectual content: all authors
- Final approval of the article: all authors
Favian Su (favian.su@pitt.edu) takes responsibility for the integrity of this work as a whole, from inception to finished article.
Competing Interest Statement
The authors involved in this study do not have any competing interests to disclose.
Contributor Information
Valentina Pedoia, Email: valentina.pedoia@ucsf.edu.
Hsiang-Ling Teng, Email: hsiang-ling.teng@ucsf.edu.
Martin Kretzschmar, Email: martin.kretzschmar@ucsf.edu.
Brian C. Lau, Email: brian.lau@ucsf.edu.
Charles E. McCulloch, Email: charles.mcculloch@ucsf.edu.
Thomas M. Link, Email: thomas.link@ucsf.edu.
C. Benjamin Ma, Email: maben@ucsf.edu.
Xiaojuan Li, Email: xiaojuan.li@ucsf.edu.
References
- 1.Brophy RH, Zeltser D, Wright RW, Flanigan D. Anterior cruciate ligament reconstruction and concomitant articular cartilage injury: incidence and treatment. Arthroscopy. 2010;26:112–120. doi: 10.1016/j.arthro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Sanders TG, Medynski MA, Feller JF, Lawhorn KW. Bone contusion patterns of the knee at MR imaging: footprint of the mechanism of injury. Radiographics. 2000;20 Spec No:S135–S151. doi: 10.1148/radiographics.20.suppl_1.g00oc19s135. [DOI] [PubMed] [Google Scholar]
- 3.Hanypsiak BT, Spindler KP, Rothrock CR, Calabrese GJ, Richmond B, Herrenbruck TM, et al. Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med. 2008;36:671–677. doi: 10.1177/0363546508315468. [DOI] [PubMed] [Google Scholar]
- 4.Spindler KP, Schils JP, Bergfeld JA, Andrish JT, Weiker GG, Anderson TE, et al. Prospective study of osseous, articular, and meniscal lesions in recent anterior cruciate ligament tears by magnetic resonance imaging and arthroscopy. Am J Sports Med. 1993;21:551–557. doi: 10.1177/036354659302100412. [DOI] [PubMed] [Google Scholar]
- 5.Piasecki DP, Spindler KP, Warren TA, Andrish JT, Parker RD. Intraarticular injuries associated with anterior cruciate ligament tear: findings at ligament reconstruction in high school and recreational athletes An analysis of sex-based differences. Am J Sports Med. 2003;31:601–605. doi: 10.1177/03635465030310042101. [DOI] [PubMed] [Google Scholar]
- 6.Dunn WR, Spindler KP, Amendola A, Andrish JT, Kaeding CC, Marx RG, et al. Which preoperative factors, including bone bruise, are associated with knee pain/symptoms at index anterior cruciate ligament reconstruction (ACLR)? A Multicenter Orthopaedic Outcomes Network (MOON) ACLR Cohort Study. Am J Sports Med. 2010;38:1778–1787. doi: 10.1177/0363546510370279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph C, Pathak SS, Aravinda M, Rajan D. Is ACL reconstruction only for athletes? A study of the incidence of meniscal and cartilage injuries in an ACL-deficient athlete and non-athlete population: an Indian experience. Int Orthop. 2008;32:57–61. doi: 10.1007/s00264-006-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40:276–285. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 9.Keays SL, Newcombe PA, Bullock-Saxton JE, Bullock MI, Keays AC. Factors involved in the development of osteoarthritis after anterior cruciate ligament surgery. Am J Sports Med. 2010;38:455–463. doi: 10.1177/0363546509350914. [DOI] [PubMed] [Google Scholar]
- 10.Louboutin H, Debarge R, Richou J, Selmi TA, Donell ST, Neyret P, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009;16:239–244. doi: 10.1016/j.knee.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Indelicato PA, Bittar ES. A perspective of lesions associated with ACL insufficiency of the knee. A review of 100 cases. Clin Orthop Relat Res. 1985:77–80. [PubMed] [Google Scholar]
- 12.Rotterud JH, Sivertsen EA, Forssblad M, Engebretsen L, Aroen A. Effect of meniscal and focal cartilage lesions on patient-reported outcome after anterior cruciate ligament reconstruction: a nationwide cohort study from Norway and Sweden of 8476 patients with 2-year follow-up. Am J Sports Med. 2013;41:535–543. doi: 10.1177/0363546512473571. [DOI] [PubMed] [Google Scholar]
- 13.Cox CL, Huston LJ, Dunn WR, Reinke EK, Nwosu SK, Parker RD, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx activity level outcomes after anterior cruciate ligament reconstruction? A 6-year multicenter cohort study. Am J Sports Med. 2014;42:1058–1067. doi: 10.1177/0363546514525910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: a natural history study. J Bone Joint Surg Am. 2003;85-A(Suppl 2):8–16. doi: 10.2106/00004623-200300002-00002. [DOI] [PubMed] [Google Scholar]
- 15.Spindler KP, Warren TA, Callison JC, Jr, Secic M, Fleisch SB, Wright RW. Clinical outcome at a minimum of five years after reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 2005;87:1673–1679. doi: 10.2106/JBJS.D.01842. [DOI] [PubMed] [Google Scholar]
- 16.Widuchowski W, Widuchowski J, Koczy B, Szyluk K. Untreated asymptomatic deep cartilage lesions associated with anterior cruciate ligament injury: results at 10- and 15-year follow-up. Am J Sports Med. 2009;37:688–692. doi: 10.1177/0363546508328104. [DOI] [PubMed] [Google Scholar]
- 17.Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A:1560–1572. doi: 10.2106/00004623-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Dijkgraaf LC, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg. 1995;53:1182–1192. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 19.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, Leigh JS. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med. 1997;38:863–867. doi: 10.1002/mrm.1910380602. [DOI] [PubMed] [Google Scholar]
- 20.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiology. 2011;258:505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage. 2013;21:1058–1067. doi: 10.1016/j.joca.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Chen S, Tao H. Quantitative MRI T2 relaxation time evaluation of knee cartilage: comparison of meniscus-intact and -injured knees after anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43:865–872. doi: 10.1177/0363546514564151. [DOI] [PubMed] [Google Scholar]
- 24.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–218. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Wyatt C, Rivoire J, Han E, Chen W, Schooler J, et al. Simultaneous acquisition of T1rho and T2 quantification in knee cartilage: repeatability and diurnal variation. J Magn Reson Imaging. 2014;39:1287–1293. doi: 10.1002/jmri.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, et al. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthritis Cartilage. 2006;14:974–983. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, Krause S, Link TM, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12:120–135. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedoia V, Li X, Su F, Calixto N, Majumdar S. Fully automatic analysis of the knee articular cartilage T1ρ relaxation time using voxel-based relaxometry. J Magn Reson Imaging. 2015 doi: 10.1002/jmri.25065. 10.1002/jmri.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamonin DP, Bron EE, Lelieveldt BP, Smits M, Klein S, Staring M. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front Neuroinform. 2013;7:50. doi: 10.3389/fninf.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Frobell RB, Le Graverand MP, Buck R, Roos EM, Roos HP, Tamez-Pena J, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17:161–167. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am. 2011;93:1096–1103. doi: 10.2106/JBJS.J.00929. [DOI] [PubMed] [Google Scholar]
- 34.Spindler KP, Huston LJ, Wright RW, Kaeding CC, Marx RG, Amendola A, et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med. 2011;39:348–359. doi: 10.1177/0363546510383481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn WR, Spindler KP. Predictors of activity level 2 years after anterior cruciate ligament reconstruction (ACLR): a Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. Am J Sports Med. 2010;38:2040–2050. doi: 10.1177/0363546510370280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lind M, Menhert F, Pedersen AB. The first results from the Danish ACL reconstruction registry: epidemiologic and 2 year follow-up results from 5,818 knee ligament reconstructions. Knee Surg Sports Traumatol Arthrosc. 2009;17:117–124. doi: 10.1007/s00167-008-0654-3. [DOI] [PubMed] [Google Scholar]
- 37.Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40:1551–1557. doi: 10.1177/0363546512446000. [DOI] [PubMed] [Google Scholar]
- 38.Bjordal JM, Arnly F, Hannestad B, Strand T. Epidemiology of anterior cruciate ligament injuries in soccer. Am J Sports Med. 1997;25:341–345. doi: 10.1177/036354659702500312. [DOI] [PubMed] [Google Scholar]
- 39.Carey JL, Huffman GR, Parekh SG, Sennett BJ. Outcomes of anterior cruciate ligament injuries to running backs and wide receivers in the National Football League. Am J Sports Med. 2006;34:1911–1917. doi: 10.1177/0363546506290186. [DOI] [PubMed] [Google Scholar]
- 40.Deehan DJ, Salmon LJ, Webb VJ, Davies A, Pinczewski LA. Endoscopic reconstruction of the anterior cruciate ligament with an ipsilateral patellar tendon autograft A prospective longitudinal five-year study. J Bone Joint Surg Br. 2000;82:984–991. doi: 10.1302/0301-620x.82b7.10573. [DOI] [PubMed] [Google Scholar]
- 41.Kvist J, Ek A, Sporrstedt K, Good L. Fear of re-injury: a hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2005;13:393–397. doi: 10.1007/s00167-004-0591-8. [DOI] [PubMed] [Google Scholar]
- 42.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23:547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15:789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson DL, Urban WP, Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26:409–414. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 45.Fang C, Johnson D, Leslie MP, Carlson CS, Robbins M, Di Cesare PE. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J Orthop Res. 2001;19:634–641. doi: 10.1016/S0736-0266(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 46.Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage. 2010;18:1408–1416. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linear regression model of the HDx Long Bore system and MR750 Wide Bore 17 system for both (A) T1ρ and (B) T2. The data points were obtained from measuring the 18 relaxation times of cartilage, ligament (ACL, PCL), gastrocnemius muscle, and meniscus. Nine 19 individuals were able for T1ρ, while only 5 individuals were available for T2.