Summary
Background
The effect of many contemporary chemotherapeutic drugs on pregnancy and livebirth is not well established. We aimed to establish the effects of these drugs on pregnancy in male and female survivors of childhood cancer not exposed to pelvic or cranial radiotherapy.
Methods
We used data from a subset of the Childhood Cancer Survivor Study cohort, which followed 5-year survivors of the most common types of childhood cancer who were diagnosed before age 21 years and treated at 27 institutions in the USA and Canada between 1970 and 1999. We extracted doses of 14 alkylating and similar DNA interstrand crosslinking drugs from medical records. We used sex-specific Cox models to establish the independent effects of each drug and the cumulative cyclophosphamide equivalent dose of all drugs in relation to pregnancies and livebirths occurring between ages 15 years and 44 years. We included siblings of survivors as a comparison group.
Findings
We included 10 938 survivors and 3949 siblings. After a median follow-up of 8 years (IQR 4–12) from cohort entry or at age 15 years, whichever was later, 4149 (38%) survivors reported having or siring a pregnancy, of whom 3453 (83%) individuals reported at least one livebirth. After a median follow-up of 10 years (IQR 6–15), 2445 (62%) siblings reported having or siring a pregnancy, of whom 2201 (90%) individuals reported at least one livebirth. In multivariable analysis, survivors had a decreased likelihood of siring or having a pregnancy versus siblings (male survivors: hazard ratio [HR] 0·63, 95% CI 0·58–0·68; p<0·0001; female survivors: 0·87, 0·81–0·94; p<0·0001) or of having a livebirth (male survivors: 0·63, 0·58–0·69; p<0·0001; female survivors: 0·82, 0·76–0·89; p<0·0001). In male survivors, reduced likelihood of pregnancy was associated with upper tertile doses of cyclophosphamide (HR 0·60, 95% CI 0·51–0·71; p<0·0001), ifosfamide (0·42, 0·23–0·79; p=0·0069), procarbazine (0·30, 0·20–0·46; p<0·0001) and cisplatin (0·56, 0·39–0·82; p=0·0023). Cyclophosphamide equivalent dose in male survivors was significantly associated with a decreased likelihood of siring a pregnancy (per 5000 mg/m2 increments: HR 0·82, 95% CI 0·79–0·86; p<0·0001). However, in female survivors, only busulfan (<450 mg/m2 HR 0·22, 95% CI 0·06–0·79; p=0·020; ≥450 mg/m2 0·14, 0·03–0·55; p=0·0051) and doses of lomustine equal to or greater than 411 mg/m2 (0·41, 0·17–0·98; p=0·046) were significantly associated with reduced pregnancy; cyclophosphamide equivalent dose was associated with risk only at the highest doses in analyses categorised by quartile (upper quartile vs no exposure: HR 0·85, 95% CI 0·74–0·98; p=0·023). Results for livebirth were similar to those for pregnancy.
Interpretation
Greater doses of contemporary alkylating drugs and cisplatin were associated with a decreased likelihood of siring a pregnancy in male survivors of childhood cancer. However, our findings should provide reassurance to most female survivors treated with chemotherapy without radiotherapy to the pelvis or brain, given that chemotherapy-specific effects on pregnancy were generally few. Nevertheless, consideration of fertility preservation before cancer treatment remains important to maximise the reproductive potential of all adolescents newly diagnosed with cancer.
Funding
National Cancer Institute, National Institutes of Health, and the American Lebanese–Syrian Associated Charities.
Introduction
Nowadays, more than 80% of children with cancer become long-term survivors, and reproductive health is a leading concern in young adult survivors.1,2 As such, there is a growing emphasis on reducing the burden of long-term effects—including adverse effects on fertility—partly by reducing exposure to radiation and increasing reliance on chemotherapy.3,4 Previous studies5,6 have identified some chemotherapeutic drugs, mainly alkylating drugs, as being associated with reduced fertility in both sexes. However, little is known about the dose effects on reproductive outcomes from newer drugs, such as ifosfamide and similar DNA interstrand crosslinking drugs (ie, cisplatin and carboplatin), in survivors of childhood cancer. For example, guidelines from the Children's Oncology Group (COG) rate the evidence for effects of DNA interstrand crosslinking drugs on gonadal function as “uniform but lower-level supporting evidence” for both sexes, and more detailed dose-threshold information for conventional alkylating drugs is absent for female survivors.7
Beginning in 2008, the Childhood Cancer Survivor Study (CCSS) expanded to include more than 10 000 5-year survivors treated from 1987 to 1999. This expansion provided an opportunity to examine the reproductive effects of these newer drugs in detail. Combined with the original CCSS cohort treated from 1970 to 1986, we examined the effects of alkylator and DNA interstrand crosslinking drugs on pregnancy and livebirth in a subset of the cohort not exposed to pelvic or cranial radiotherapy.
Methods
Study design and participants
Study methods and participant accrual in the CCSS have been reported previously.8 We included individuals in the CCSS who were diagnosed before age 21 years with the most common types of childhood cancer (all leukaemia types, CNS tumour, lymphoma, kidney tumour, neuroblastoma, soft-tissue sarcoma, and bone tumour) and treated at 27 institutions in the USA and Canada between 1970 and 1999, and who survived at least 5 years after diagnosis. To be consistent with national birth data and previous CCSS reports,9–11 we restricted the present analysis to pregnancies and livebirths occurring between ages 15 years and 44 years in individuals who had not received radiotherapy to the pelvis or the brain. We also excluded individuals exposed to high-dose scatter radiation to the pelvis and brain. We included a random sample of siblings of survivors treated from 1970 to 1986 as a comparison group (in which a randomly selected subset of survivors were asked to identify all their living siblings, from which the sibling closest in age to the survivor was selected and asked to participate); reproductive outcomes for siblings of survivors treated from 1987 to 1999 were not available. Procedures were approved by the ethics board into the investigation of human participants at each institution. Participants provided written, oral (via telephone), or online informed consent.
Procedures
We extracted information about chemotherapy, radiotherapy, and surgery from medical records. We examined the cumulative doses of 14 drugs: busulfan, carboplatin, carmustine, chlorambucil, chlormethine, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, lomustine, melphalan, procarbazine, temozolomide, and thiotepa. Cumulative dose information for dacarbazine was only available for survivors treated from 1987 to 1999. Doses of these drugs (exclusive of carboplatin, cisplatin, dacarbazine, and temozolomide) were also converted into a cyclo phosphamide equivalent dose.12 Radiotherapy records were centrally reviewed and field-specific maximum total doses were calculated for each body region separately (eg, brain, abdomen, and pelvis). In defining exposures for each body region, regions directly adjacent to the primary treatment field were classified as exposed to high-dose scatter radiation (eg, high scatter to pelvis occurred if the primary radiotherapy treatment field included the abdomen, the proximal half of the legs, or the lower spine). Finally, study participants were screened for the presence of any sterilising procedures on the basis of International Classification of Diseases codes derived from medical record abstraction, supplemented with self-reported surgical procedures including vasectomy, tubal ligation, bilateral orchiectomies or oophorectomies, and hysterectomy from the CCSS questionnaires.
In addition to surgical procedures, the CCSS questionnaires cover a broad range of demographic characteristics, health conditions, and health-related behaviours, and have been administered to the cohort serially over time beginning in 1994. Proxy responses from family members were used for 5-year survivors who had subsequently died, were younger than 18 years, or were unable to complete the questionnaires. For pregnancy and livebirth, the primary outcomes for this analysis, participants were asked about their history of pregnancy and whether they had borne or sired children at the baseline questionnaire, and were then asked to update that information on subsequent questionnaires. Female participants were also periodically surveyed about their menstrual history. Participants diagnosed with cancer before 1987 answered questions about menstrual history in surveys done in 1992, 2000, and 2007. More recently enrolled participants diagnosed with cancer after 1986 were first surveyed in 2008. Notably, information about whether attempts to conceive lasted more than 1 year and whether assisted reproductive techniques were used were not routinely asked on most CCSS surveys.
Statistical analysis
We used time-to-event methods with age as the timescale to evaluate the incidence of, and factors associated with, pregnancy and livebirth outcomes. Participants entered the analysis at cohort entry (ie, 5 years after initial cancer diagnosis) or at age 15 years, whichever was later, and were followed up until the age of the analysis-specific outcome (first pregnancy or first livebirth), death, age 45 years, or last contact, whichever came first. Occurrence of relapse or secondary malignancy after cohort entry (since additional cancer therapy exposures for those events were not systematically recorded), sterilising procedure, or death was deemed a competing risk event. As described previously,10,11 age at first pregnancy or livebirth was not available for some survivors and was imputed.13 Finally, all analyses used appropriate inverse probability weighting to account for undersampling of survivors of acute lymphoblastic leukaemia in the expanded CCSS cohort (ie, diagnosis years 1987–99).
We calculated the cumulative incidence of first pregnancy and livebirth for survivors and siblings by sex, with staggered entry for survivors and siblings starting at age 15 years, or at 5 years after cancer diagnosis, whichever was later. To examine first pregnancy and livebirth among older individuals, we also examined the cumulative incidence in survivors and siblings without these events before age 30 years. We used sex-specific Cox proportional hazards models to compare the likelihood of reporting any pregnancy and livebirth in survivors versus siblings, using robust standard-error estimates to appropriately account for within-family correlation.14–16 Models were adjusted for self-reported race or ethnic origin (white non-Hispanic vs other) and participant birth-year (<1965, 1965–84, 1985–99).
We used survivor-only models to examine the individual effects of the 14 alkylating and similar DNA interstrand crosslinking drugs of interest, with adjustment for age at cancer diagnosis (5-year increments) and self-reported race or ethnic origin. Before proceeding with regression modelling, we first assessed numbers of participants exposed to each of the 14 chemotherapy drugs of interest. For drugs with adequate numbers (≥20 survivors within each sex exposed; although all but two drugs assessed in models had >50 survivors exposed), initial univariate models examined each as a dichotomous exposure (any vs no exposure) in relation to pregnancy and livebirth. For the subset of drugs with doses captured, subsequent models tested dose categories on the basis of tertile or median cutoff points (tertile if exposed groups had ≥100 survivors per sex; median if exposed groups had 50–99 survivors per sex). The high prevalence of the fertility outcomes allowed for simultaneous assessment of a large number of covariates in multivariable models. With inclusion of all 14 drugs of interest, plus age at diagnosis and self-reported race or ethnic origin as a-priori covariates of interest, the minimum ratio of events per variable was 89, sufficiently high (ie, ten or more events) to avoid bias.17 We developed final multivariable models in a stepwise fashion, with the final model including drugs significant at a two-sided p value of less than 0·05 for at least one sex, with non-significant drugs grouped together as other alkylating and similar drugs. We did analyses with SAS (version 9.3) and Stata (version 14.0).
Role of the funding source
The funders of the study had a role in study design, data collection, data management, data analysis, data interpretation, and writing of the report, but were not involved in the review or approval of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Figure 1 shows the cohort profile. We included 10 938 survivors and 3949 siblings. 8631 (79%) survivors were treated with chemotherapy, with 5922 (54%) receiving at least one alkylating or similar DNA interstrand crosslinking drug (table 1, appendix). Among survivors who were treated with one of the 14 drugs of interest, the median number of drugs received was one (IQR one to two), with 2335 (21%) survivors receiving two or more drugs. The maximum number of drugs received was six. The distribution of doses, including median and tertile cutoff points, was similar between male and female participants (appendix). Only 1856 (17%) survivors received radiotherapy, excluding pelvis or brain (both in-field and high-dose scatter). After median follow-up of 8 years (IQR 4–12) from cohort entry or at age 15 years, whichever was later, 4149 (38%) survivors reported having or siring a pregnancy, of whom 3453 (83%) individuals reported at least one livebirth (table 2). After a median follow-up of 10 years (IQR 6–15), 2445 (62%) of 3949 siblings reported having or siring a pregnancy, of whom 2201 (90%) individuals reported at least one livebirth (table 2).
Figure 1.
Flow diagram for selection of study participants
Table 1.
Demographic and clinical characteristics
| Male survivors (n=5640) | Female survivors (n=5298) | |
|---|---|---|
| Age at diagnosis (years) | ||
| <5 | 2085 (37%) | 2048 (39%) |
| 5-9 | 1254 (22%) | 1012 (19%) |
| 10-14 | 1287 (23%) | 1225 (23%) |
| 15-20 | 1014 (18%) | 1013 (19%) |
| Year of diagnosis | ||
| 1970-79 | 1001 (18%) | 976 (18%) |
| 1980-89 | 2185 (39%) | 2087 (39%) |
| 1990-99 | 2454 (44%) | 2235 (42%) |
| Original cancer diagnosis | ||
| Acute lymphoblastic leukaemia | 1123 (20%) | 1120 (21%) |
| Other leukaemias | 300 (5%) | 297 (6%) |
| CNS tumour | 799 (14%) | 772 (15%) |
| Hodgkin's lymphoma | 634 (11%) | 653 (12%) |
| Non-Hodgkin lymphoma | 800 (14%) | 402 (8%) |
| Kidney tumour | 385 (7%) | 453 (9%) |
| Neuroblastoma | 493 (9%) | 525 (10%) |
| Soft-tissue sarcoma | 352 (6%) | 338 (6%) |
| Bone tumour | 754 (13%) | 738 (14%) |
| Alkylating or DNA interstrand crosslinking drug | ||
| None | 2427 (43%) | 2461 (46%) |
| Busulfan | 62 (1%) | 54 (1%) |
| Carmustine | 144 (3%) | 112 (2%) |
| Carboplatin | 158 (3%) | 120 (2%) |
| Cisplatin | 455 (8%) | 468 (9%) |
| Chlorambucil | 5 (<1%) | 11 (<1%) |
| Chlormethine | 244 (4%) | 255 (5%) |
| Cyclophosphamide | 2549 (45%) | 2216 (42%) |
| Dacarbazine | 225 (4%) | 228 (4%) |
| Ifosfamide | 320 (6%) | 275 (5%) |
| Lomustine | 69 (1%) | 62 (1%) |
| Melphalan | 40 (1%) | 44 (1%) |
| Procarbazine | 432 (8%) | 496 (9%) |
| Temozolomide | 1 (<1%) | 2 (<1%) |
| Thiotepa | 21 (<1%) | 24 (<1%) |
| Unknown | 64 (1%) | 64 (1%) |
| Radiotherapy* | ||
| None | 4713 (84%) | 4369 (82%) |
| Neck | 135 (2%) | 59 (1%) |
| Chest | 644 (11%) | 736 (14%) |
| Arms or legs | 148 (3%) | 134 (3%) |
| Sterilising procedure | 144 (3%) | 464 (9%) |
| Pregnancy | 1694 (30%) | 2455 (46%) |
| Livebirth | 1425 (25%) | 2028 (38%) |
Data are n (%).
The study population excluded individuals treated with cranial or pelvic radiotherapy, including those exposed to high-dose scatter radiation to the pelvis (primary treatment fields were adjacent to the pelvis).
Table 2.
Likelihood of reporting first pregnancy and livebirth in survivors of childhood cancer compared with siblings
| Male survivors |
Female survivors |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy |
Livebirth |
Pregnancy |
Livebirth |
|||||||||
| n | HR (95% CI) | p value | n | HR (95% CI) | p value | n | HR (95% CI) | p value | n | HR (95% CI) | p value | |
| Siblings | 1066 | 1 | 961 | 1 | 1379 | 1 | 1240 | 1 | ||||
| Survivors, all | 1694 | 0·63 (0·58-0·68) | <0·0001 | 1425 | 0·63 (0·58-0·69) | <0·0001 | 2455 | 0·87 (0·81-0·94) | 0·00015 | 2028 | 0·82 (0·76-0·89) | <0·0001 |
| Ages 15-29 years during follow-up | 527 | 0·65 (0·59-0·71) | <0·0001 | 383 | 0·64 (0·58-0·71) | <0·0001 | 901 | 0·93 (0·86-1·01) | 0·069 | 681 | 0·87 (0·80-0·95) | 0·0025 |
| Ages 30-44 years during follow-up | 1167 | 0·56 (0·48-0·66) | <0·0001 | 1042 | 0·60 (0·51-0·70) | <0·0001 | 1554 | 0·60 (0·50-0·71) | <0·0001* | 1347 | 0·63 (0·53-0·76) | <0·0001* |
| Not exposed to any alkylating or similar drugs | 762 | 0·72 (0·66-0·80) | <0·0001 | 640 | 0·72 (0·65-0·80) | <0·0001 | 1083 | 0·90 (0·83-0·98) | 0·018 | 885 | 0·86 (0·79-0·94) | 0·0015 |
| Exposed to alkylating or similar drugs | 911 | 0·57 (0·52-0·63) | <0·0001† | 767 | 0·58 (0·52-0·64) | <0·0001* | 1345 | 0·86 (0·79-0·94) | 0·00040 | 1122 | 0·82 (0·75-0·90) | <0·0001 |
| Unknown alkylating drug status | 21 | .. | 18 | .. | 27 | .. | 21 | .. | ||||
Sex-specific models adjusted for race and ethnic origin, and year of birth. Age at first pregnancy or livebirth was not available for some survivors and was imputed (pregnancy, n=127; livebirth, n=84).
HR=hazard ratio.
For interaction with age, 30-44 years versus 15-29 years, p<0·0001 (pregnancy) and p=0·0016 (livebirth).
For exposure versus no exposure to any alkylating or similar drugs, difference in HRs, p=0·00058 (pregnancy) and p=0·0034 (livebirth).
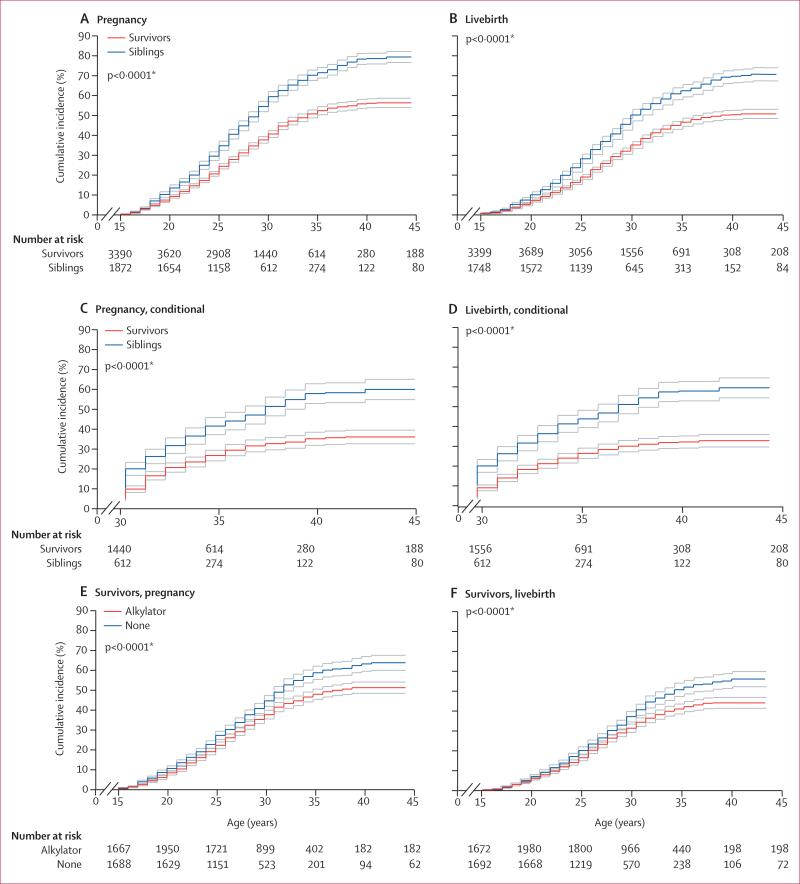
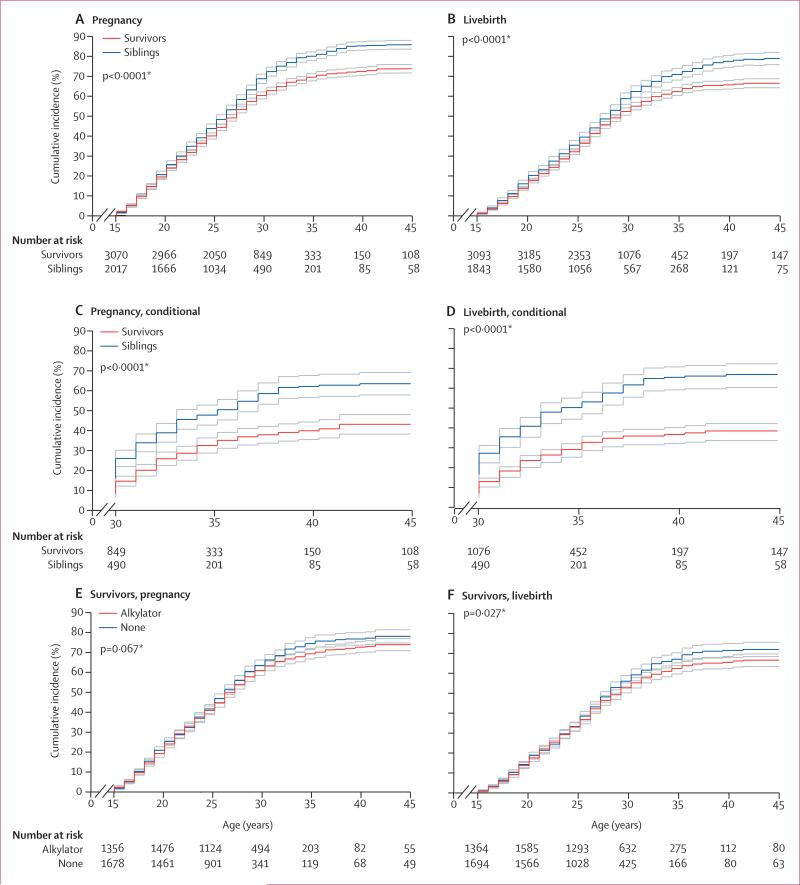
The cumulative incidence at age 44 years of having or siring a pregnancy or livebirth was significantly lower for both male (figure 2) and female survivors (figure 3) than for same-sex siblings. In multivariable analyses, both male and female survivors were less likely than siblings to have ever sired or had a pregnancy, or to have had a livebirth (table 2). When we examined risk estimates by age strata, female (but not male) survivors who did not report a pregnancy or livebirth before age 30 years had a further reduced likelihood, versus siblings, of subsequently reporting either outcome before age 45 years (table 2). Estimates were slightly attenuated (and overall remained significantly different) when analyses were limited to the ages during which female survivors and siblings were menstruating (appendix). Attenuation of estimates was also shown when analyses were limited to survivors who did not receive any of 14 alkylating or similar drugs of interest (n=4888), with outcomes again remaining significantly decreased (appendix). When this group was examined according to original cancer diagnosis, only survivors who had had CNS tumours (both sexes) and leukaemias (male survivors only) had a reduced likelihood of pregnancy and livebirth versus siblings (appendix).
Figure 2. Cumulative incidence of pregnancies and livebirths in male cancer survivors and siblings.
Incidence curves are shown with upper and lower 95% CIs. First ever pregnancy sired (A). First ever livebirth sired (B). Restricted to individuals without pregnancy (C) or livebirth (D) before age 30 years. Pregnancy (E) or livebirths (F) sired among survivors, stratified by exposure to any alkylating or similar drugs. *For difference in cumulative incidence at age 44 years; data were censored at age 45 years.
Figure 3. Cumulative incidence of pregnancies and livebirths in female cancer survivors and siblings.
Incidence curves are shown with upper and lower 95% CIs. First ever pregnancy (A). First ever livebirth (B). Restricted to individuals without pregnancy (C) or livebirth (D) before age 30 years. Pregnancy (E) or livebirths (F) among survivors, stratified by exposure to any alkylating or similar drugs. *For difference in cumulative incidence at age 44 years; data were censored at age 45 years.
In survivors, male participants who received cumulative doses of cyclophosphamide, ifosfamide, and procarbazine in the upper tertiles (≥7412 mg/m2, ≥53 000 mg/m2, and ≥5060 mg/m2, respectively) reported a significantly decreased likelihood of siring a pregnancy compared with those not exposed to each drug (table 3). Cyclophosphamide doses of 5567 mg/m2 or higher (median cutoff point) were associated with a reduced likelihood of pregnancy (data not shown). High cisplatin doses (upper tertile ≥488 mg/m2) were also significantly associated with a decreased likelihood of siring a pregnancy in male survivors (ptrend=0·00079 across tertile dose categories; table 3). Overall, the magnitude of effects noted with cisplatin, cyclophosphamide, ifosfamide, and procarbazine did not differ when all 14 chemotherapy drugs were adjusted concurrently as individual drugs (appendix). In female survivors, only busulfan (any dose category) and lomustine (≥411 mg/m2) were associated with significantly decreased pregnancy (table 3).
Table 3.
Likelihood of reporting first pregnancy and livebirth, by individual chemotherapy exposures and cyclophosphamide equivalent dose
| Male survivors |
Female survivors |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy |
Livebirth |
Pregnancy |
Livebirth |
|||||||||
| n | HR (95% CI) | p value | n | HR (95% CI) | p value | n | HR (95% CI) | p value | n | HR (95% CI) | p value | |
| Model one* | ||||||||||||
| Busulfan dose level† | ||||||||||||
| Lower (<450 mg/m2) | 3 | 0·46 (0·15-1·42) | 0·17 | 3 | 0·58 (0·19-1·80) | 0·34 | 3 | 0·22 (0·06-0·79) | 0·020 | 2 | 0·20 (0·05-0·82) | 0·025 |
| Upper (≥450 mg/m2) | 12 | 1·39 (0·76-2·52) | 0·28 | 12 | 1·58 (0·87-2·88) | 0·13 | 2 | 0·14 (0·03-0·55) | 0·0051 | 2 | 0·18 (0·04-0·71) | 0·015 |
| Cisplatin dose level† | ||||||||||||
| Lower (<355 mg/m2) | 29 | 0·85 (0·58-1·27) | 0·44 | 26 | 0·95 (0·63-1·44) | 0·82 | 62 | 1·03 (0·77-1·38) | 0·83 | 51 | 1·06 (0·77-1·45) | 0·72 |
| Middle (355-487 mg/m2) | 35 | 0·74 (0·52-1·07) | 0·11 | 27 | 0·64 (0·43-0·97) | 0·038 | 82 | 1·18 (0·91-1·53) | 0·21 | 69 | 1·26 (0·96-1·66) | 0·094 |
| Upper (≥488 mg/m2) | 33 | 0·56 (0·39-0·82) | 0·0023 | 26 | 0·53 (0·36-0·79) | 0·0019 | 72 | 0·95 (0·73-1·22) | 0·68 | 57 | 0·86 (0·65-1·14) | 0·28 |
| Cyclophosphamide dose level† | ||||||||||||
| Lower (<3625 mg/m2) | 229 | 1·22 (1·07-1·40) | 0·0038 | 186 | 1·15 (0·99-1·34) | 0·065 | 319 | 0·92 (0·82-1·04) | 0·20 | 264 | 0·93 (0·81-1·06) | 0·26 |
| Middle (3625-7411 mg/m2) | 266 | 0·89 (0·77-1·03) | 0·13 | 230 | 0·90 (0·77-1·05) | 0·17 | 316 | 1·04 (0·91-1·19) | 0·57 | 268 | 1·06 (0·92-1·22) | 0·44 |
| Upper (≥7412 mg/m2) | 190 | 0·60 (0·51-0·71) | <0·0001 | 157 | 0·58 (0·48-0·69) | <0·0001 | 335 | 0·99 (0·87-1·12) | 0·86 | 279 | 0·99 (0·87-1·13) | 0·88 |
| Ifosfamide dose level† | ||||||||||||
| Lower (<26 853 mg/m2) | 22 | 0·90 (0·56-1·45) | 0·67 | 18 | 0·90 (0·54-1·51) | 0·70 | 38 | 0·92 (0·64-1·30) | 0·62 | 29 | 0·86 (0·58-1·27) | 0·45 |
| Middle (26 853-52 999 mg/m2) | 22 | 0·61 (0·36-1·01) | 0·054 | 18 | 0·61 (0·36-1·04) | 0·071 | 40 | 0·82 (0·58-1·18) | 0·28 | 34 | 0·84 (0·57-1·24) | 0·38 |
| Upper (≥53 000 mg/m2) | 11 | 0·42 (0·23-0·79) | 0·0069 | 10 | 0·46 (0·24-0·89) | 0·020 | 38 | 1·05 (0·74-1·48) | 0·80 | 31 | 1·03 (0·70-1·50) | 0·89 |
| Lomustine dose level† | ||||||||||||
| Lower (<411 mg/m2) | 13 | 1·13 (0·58-2·20) | 0·72 | 10 | 0·82 (0·36-1·85) | 0·63 | 13 | 0·87 (0·46-1·65) | 0·67 | 13 | 1·12 (0·59-2·13) | 0·72 |
| Upper (≥411 mg/m2) | 3 | 0·82 (0·26-2·60) | 0·74 | 3 | 0·94 (0·28-3·14) | 0·91 | 7 | 0·41 (0·17-0·98) | 0·046 | 6 | 0·60 (0·27-1·34) | 0·21 |
| Procarbazine dose level† | ||||||||||||
| Lower (<3352 mg/m2) | 36 | 0·63 (0·44-0·91) | 0·014 | 30 | 0·61 (0·40-0·91) | 0·015 | 74 | 0·99 (0·74-1·32) | 0·94 | 58 | 0·87 (0·64-1·20) | 0·40 |
| Middle (3352-5059 mg/m2) | 23 | 0·38 (0·24-0·60) | <0·0001 | 22 | 0·45 (0·29-0·71) | 0·00064 | 89 | 0·97 (0·74-1·26) | 0·81 | 80 | 1·03 (0·79-1·35) | 0·82 |
| Upper (≥5060 mg/m2) | 30 | 0·30 (0·20-0·46) | <0·0001 | 25 | 0·30 (0·20-0·46) | <0·0001 | 76 | 0·93 (0·70-1·22) | 0·59 | 63 | 0·78 (0·58-1·05) | 0·11 |
| Other alkylating or similar drugs†‡ | 228 | 1·19 (1·01-1·41) | 0·038 | 197 | 1·24 (1·04-1·48) | 0·019 | 362 | 0·93 (0·80-1·08) | 0·34 | 310 | 1·00 (0·85-1·16) | 0·96 |
| Model two§ | ||||||||||||
| Cyclophosphamide equivalent dose level† | ||||||||||||
| Lower (<4897 mg/m2) | 284 | 1·14 (1·00-1·30) | 0·045 | 232 | 1·08 (0·94-1·25) | 0·28 | 368 | 0·97 (0·86-1·08) | 0·55 | 301 | 0·95 (0·84-1·08) | 0·41 |
| Middle (4897-9638 mg/m2) | 272 | 0·79 (0·68-0·91) | 0·0010 | 241 | 0·84 (0·72-0·97) | 0·021 | 366 | 0·98 (0·87-1·11) | 0·76 | 310 | 1·01 (0·89-1·16) | 0·86 |
| Upper (≥9639 mg/m2) | 215 | 0·55 (0·47-0·64) | <0·0001 | 177 | 0·53 (0·44-0·62) | <0·0001 | 401 | 0·90 (0·79-1·01) | 0·07 | 338 | 0·91 (0·80-1·03) | 0·14 |
| Model three§ | ||||||||||||
| Cyclophosphamide equivalent linear dose per 5000 mg/m2 | 771 | 0·82 (0·79-0·86) | <0·0001 | 650 | 0·82 (0·78-0·86) | <0·0001 | 1135 | 0·97 (0·94-1·00) | 0·10 | 949 | 0·97 (0·94-1·00) | 0·049 |
HR=hazard ratio.
Adjusted for all drugs listed, and for age at diagnosis and race and ethnic origin.
Dose levels in individuals with exposure are based on tertiles or, if sample size is limited, on the median.
No dose level served as a reference group.
Exposure to any carmustine, carboplatin, chlorambucil, dacarbazine, melphalan, chlormethamine, temozolomide, or thiotepa.
Adjusted for cyclophosphamide equivalent dose and for age at diagnosis, cisplatin dose levels, and any exposure to carboplatin, dacarbazine, or temozolomide.
When individual doses of alkylating drugs were converted into cyclophosphamide equivalent doses, greater cyclophosphamide equivalent doses were significantly associated with a decreased likelihood of male survivors siring a pregnancy, either by tertile of equivalent dose (both middle and upper tertiles) or in a linear model (per 5000 mg/m2 increments: table 3). There was no association with cyclophosphamide equivalent dose in female survivors, either by tertile of equivalent dose (upper tertile vs no exposure) or by dose linear model (table 3). In subanalyses, when cyclophosphamide equivalent dose was categorised by quartile, female survivors exposed to the upper quartile (≥11 295 mg/m2) had a lower likelihood of pregnancy than did those not exposed (hazard ratio [HR] 0·85, 95% CI 0·74–0·98; p=0·023).
For both sexes, estimates in relation to livebirth were similar to the corresponding estimates for pregnancy, including an association between cyclophosphamide equivalent dose and female survivors (per 5000 mg/m2 dose linear model: HR 0·97, 95% CI 0·94–1·00, p=0·049; upper quartile ≥11 295 mg/m2 vs none: HR 0·85, 95% CI 0·73–0·98, p=0·026). For both sexes, there was no consistent association between age at diagnosis and reduced pregnancy or livebirth (appendix).
Discussion
On the basis of the gradual elimination of radiotherapy from many paediatric treatment regimens over time, replaced, in many instances, by more intensive chemotherapy,3,4 we sought to identify the effect of chemotherapy alone on pregnancy and livebirth. Few well powered analyses have examined the dose–response association of individual chemotherapeutic drugs without radiotherapy across a broad range of cancer diagnoses and chemotherapeutic drugs in this population, particularly in regard to the effects of newer and increasingly widely used drugs, such as ifosfamide and cisplatin, and to multiple drugs in combination. To address these gaps in knowledge, we examined more than 10 000 survivors in the CCSS who were not exposed to gonadal or cranial radiotherapy, and found that male survivors were still less likely than siblings to ever sire a pregnancy or a livebirth, particularly those exposed to high cumulative doses of alkylating drugs (as measured by cyclophosphamide equivalent dose) and cisplatin. The association with cisplatin has not been clearly documented previously in survivors of childhood cancer. By contrast, in female survivors, aside from busulfan and possibly high-dose lomustine, other alkylating and similar DNA interstrand crosslinking drugs were not associated with reduced pregnancy or livebirth except at very high cumulative doses. Nevertheless, female survivors still had a reduced likelihood of these outcomes versus siblings and, in those who had not reported a pregnancy by age 30 years, the likelihood of ever becoming pregnant by age 45 years was even more reduced versus siblings.
Although a general association between fertility and alkylating drugs is well established for male survivors of childhood cancer, specific data for ifosfamide and DNA interstrand crosslinking drugs such as cisplatin are scarce.5 Findings from an Italian study18 showed that 15 (94%) of 16 patients with osteosarcoma who had received 24 000–60 000 mg/m2 of ifosfamide were oligospermic or azoospermic. These patients also received concurrent cisplatin (range 360–690 mg/m2). A separate study19 of 33 male survivors of childhood cancer who were exposed to either cyclophosphamide (n=8; median dose 19 000 mg/m2) or ifosfamide (n=25; median dose 54 000 mg/m2) showed significantly lower sperm counts and smaller testicular volumes in men exposed to cyclophosphamide. A British study20 reported that five (45%) of 11 male survivors who received ifosfamide at a dose of more than 60 000 mg/m2 and cyclophosphamide at a dose of less than 2500 mg/m2 were oligospermic or azoospermic after minimum 3 years’ follow-up.
Evidence linking cisplatin to reduced fertility in survivors of childhood cancer has been sparse.21 Additional data have come from adult survivors of testicular cancer. Although testicular cancer itself, separate from its treatment, is associated with decreased spermatogenesis,5,22 high doses of cisplatin (without radiotherapy) are associated with further increased risks of both hypogonadism and reduced fertility.23 Surgery for testicular cancer also often involves retro peritoneal lymph-node dissection, which can lead to retrograde ejaculation in some survivors, further compromising fertility.23 However, many adult patients with testicular cancer seem to recover some degree of sperma togenesis, although this recovery can sometimes take years.23
With our large sample size (which did not include survivors of germ-cell tumours), we were able to examine the independent effects of these drugs across a broad range of doses, with much greater precision than most previous studies. Our results suggest that male survivors who received ifosfamide doses of more than 25 000 mg/m2, procarbazine doses of more than 3000 mg/m2, and cisplatin doses of more than 475 mg/m2 had a significantly reduced likelihood of siring pregnancies and livebirths compared with those with no exposure. The COG guidelines classify ifosfamide doses of 60 000 mg/m2 or more as high risk; no specific dose cutoff points are currently recommended for procarbazine or cisplatin in relation to gonadal dysfunction.7 Similar to the COG guidelines, we recorded that cyclophosphamide at a dose of 7500 mg/m2 of more remains an important threshold for male survivors of childhood cancer, although even survivors who received doses of more than 5000 mg/m2 seemed to be at risk. Because many patients receive more than one alkylating drug, identification of overall risk in survivors who received multiple drugs at low doses can be difficult. In this situation, application of cyclophosphamide equivalent dose might be particularly useful, given that we found a cyclophosphamide equivalent dose of around 5000 mg/m2, and particularly doses exceeding 10 000 mg/m2, to be strongly associated with a reduced likelihood of siring pregnancies. The St Jude Lifetime Cohort Study24 recently reported that cyclophosphamide equivalent dose was inversely correlated with sperm concentration in 214 adult male survivors of childhood cancer treated without radiotherapy, with impaired spermatogenesis being less common if the cyclophosphamide equivalent dose was less than 4000 mg/m2.
Previous CCSS analyses featuring the original cohort of female survivors diagnosed between 1970 and 1986, reported a decreased likelihood (relative risk of roughly 0·8) of pregnancy versus siblings.10 Although some chemotherapeutic drugs were associated with reduced pregnancy (eg, lomustine, cyclophosphamide), up to two-thirds of the original cohort also received pelvic or cranial radiotherapy, and increased doses of ovarian–uterine and hypothalamic–pituitary radiotherapy were strongly associated with reduced pregnancy.10 A German cohort study25 of female survivors who had survived for 5 years or longer since diagnosis of childhood Hodgkin's lymphoma (n=467; 8% pelvic radiation) reported that the likelihood of livebirth did not differ versus national norms. Parenthood was reduced only in participants who received pelvic radiation and those who delayed childbirth (age 40–44 years); no chemotherapy exposure was associated with reduced livebirths (borderline associations with increased cycles of procarbazine and increased cumulative alkylating drug dose; p=0·05–0·06).
Our study supports the finding that most individual chemotherapy exposures were not associated with a strong independent effect on pregnancy or livebirth in female survivors. Similar to Bramswig and colleagues’ findings,25 we also showed that older maternal age could be associated with a reduced likelihood of pregnancy and livebirth. Because women are born with a finite number of oocytes, chemotherapy exposure might accelerate this natural depletion and hasten menopause.26,27 Previous CCSS analyses have shown strong associations between cumulative alkylator dose and premature menopause.12,28
However, by contrast with the German report,25 female survivors in our study were still less likely to become pregnant or bear children than were siblings. Only a subset of women not exposed to any alkylating or related drugs were similar to siblings. Of drugs examined, only busulfan was consistently associated with a differential effect on reproductive outcomes, with cyclophosphamide equivalent dose associated only at the highest quartile. Most busulfan-exposed patients also received cyclophosphamide as part of haemopoietic cell transplantation. This combination has been a recognised risk factor for impaired ovarian function and reduced pregnancy rates.29 Nevertheless, there could be non-additive effects related to multiple exposures that our models did not completely account for.
When interpreting our findings, it is important to note that we relied on self-reported pregnancy and livebirth; up to a quarter of pregnancies can be unrecognised by women.30 We also did not directly assess gonadal function using laboratory methods, although our results are consistent with studies that used such methods.18–20,22,24 Assessment of fertility would ideally also account for other factors, such as marital or cohabitation status, in addition to intention to conceive and duration of time attempting to conceive (usually ≥1 year), which might explain some of the differences between survivors and siblings. A more detailed analysis of intention and duration of attempt to conceive among CCSS participants with available data showed that the relative risk of clinical infertility in male survivors was 2·6 and in female survivors was 1·5.31,32 However, because questions assessing intention and duration of conception attempts were not asked on all questionnaires, we could not incorporate those covariates into this analysis.
In conclusion, our results should provide reassurance to most female survivors treated with chemotherapy without radiotherapy to the pelvis or brain. However, women who delay childbearing until later ages and those exposed to very high cumulative doses of alkylating drugs might want to consider earlier consultation with reproductive specialists. We also identified a strong association between cisplatin and reduced fertility in male survivors, which will need to be studied further. For individuals who are post-pubertal at time of cancer diagnosis, especially those likely to be exposed to high cumulative doses of alkylating drugs, greater consideration of fertility preservation (ie, sperm banking, oocyte or embryo cryopreservation) before cancer treatment is important to maximise reproductive potential.33,34 Fertility preservation for prepubertal children remains investigational.35
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed from 1966 up until June 1, 2015, for English-language publications, with the keywords “childhood cancer”, “survivor”, “fertility”, “pregnancy”, “birth”, “ovary”, and “sperm”. We additionally examined the bibliographies of selected references. Most of the previous literature, including analyses from the Childhood Cancer Survivor Study, included large numbers of individuals exposed to radiotherapy with known effects on gonadal function (ie, radiation to the gonads or the hypothalamic–pituitary axis). We identified only a few analyses (most with sample sizes <50) that specifically examined the effects of newer chemotherapeutic drugs, such as ifosfamide and cisplatin, in survivors of childhood cancer who were not exposed to such radiotherapy.
Added value of this study
This is one of the largest studies of pregnancy and livebirth in cancer survivors of any age who were not exposed to gonadal or cranial radiation. Importantly, our study features a broad range of commonly used chemotherapy drugs, given at varying doses, which allowed us to establish more precise dose thresholds associated with reduced likelihood of siring a pregnancy or livebirth for male and female survivors of childhood cancer. Our fi ndings show an association between risk and exposure to cisplatin, a finding not consistently reported in survivors of childhood cancer. Female survivors can be reassured by the result that chemotherapy-specifi c effects in women who did not receive any radiotherapy to the pelvis or brain were generally few in relation to these reproductive outcomes, except with exposure to the highest cumulative doses.
Implications of all the available evidence
Counselling of patients and families about fertility preservation before initiation of cancer therapy is important. In particular, sperm banking should be encouraged for all newly diagnosed pubertal men, since this is a proven method of fertility preservation. The association of risk with cisplatin exposure should be investigated further, given the increase in use of that drug in many contemporary paediatric treatment protocols.
Acknowledgments
This study was funded by the by the National Cancer Institute, US National Institutes of Health (grant numbers CA55727, CA151775, and CA21765), and the American Lebanese–Syrian Associated Charities.
Footnotes
Contributors
GTA and LLR provided financial support. EJC, KCO, CAS, SSD, JPG, LBK, LLR, MSt, DMG, and GTA provided study materials and patients. KLS, WML, MSt, LLR, and GTA obtained and assembled data. KLS, WML, and EJC analysed the data. All authors conceived and designed the study, interpreted the data, wrote the manuscript, and approved the final version for publication.
Declaration of interests
We declare no competing interests.
For the CCSS questionnaires see http://ccss.stjude.org
References
- 1.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653–63. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zebrack B, Isaacson S. Psychosocial care of adolescent and young adult patients with cancer and survivors. J Clin Oncol. 2012;30:1221–26. doi: 10.1200/JCO.2011.39.5467. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58:334–43. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60:1083–94. doi: 10.1002/pbc.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenney LB, Cohen LE, Shnorhavorian M, et al. Male reproductive health after childhood, adolescent, and young adult cancers: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:3408–16. doi: 10.1200/JCO.2011.38.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metzger ML, Meacham LR, Patterson B, et al. Female reproductive health after childhood, adolescent, and young adult cancers: guidelines for the assessment and management of female reproductive complications. J Clin Oncol. 2013;31:1239–47. doi: 10.1200/JCO.2012.43.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Children's Oncology Group [Dec 1, 2015];Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, version 4.0. 2013 http://www.survivorshipguidelines.org/
- 8.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–18. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Matthews TJ. Births: final data for 2013. National Center for Health Statistics; Hyattsville, MD: 2015. [PubMed] [Google Scholar]
- 10.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27:2677–85. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:332–39. doi: 10.1200/JCO.2009.24.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley and Sons; New York, NY: 1987. [Google Scholar]
- 14.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer-Verlag; New York, NY: 2000. [Google Scholar]
- 15.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second–primary cancer incidence in long–term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–13. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 16.Hosmer DW, Lemeshow S, May S. Applied survival analysis: regression modeling of time-to-event data. Wiley-Interscience; Hoboken, NJ: 2008. [Google Scholar]
- 17.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–10. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 18.Longhi A, Macchiagodena M, Vitali G, Bacci G. Fertility in male patients treated with neoadjuvant chemotherapy for osteosarcoma. J Pediatr Hematol Oncol. 2003;25:292–96. doi: 10.1097/00043426-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Garolla A, Pizzato C, Ferlin A, Carli MO, Selice R, Foresta C. Progress in the development of childhood cancer therapy. Reprod Toxicol. 2006;22:126–32. doi: 10.1016/j.reprotox.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Williams D, Crofton PM, Levitt G. Does ifosfamide affect gonadal function? Pediatr Blood Cancer. 2008;50:347–51. doi: 10.1002/pbc.21323. [DOI] [PubMed] [Google Scholar]
- 21.Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR, Price DA. Gonadal dysfunction due to cis–platinum. Med Pediatr Oncol. 1989;17:409–13. doi: 10.1002/mpo.2950170510. [DOI] [PubMed] [Google Scholar]
- 22.Eberhard J, Stahl O, Cwikiel M, et al. Risk factors for post–treatment hypogonadism in testicular cancer patients. Eur J Endocrinol. 2008;158:561–70. doi: 10.1530/EJE-07-0684. [DOI] [PubMed] [Google Scholar]
- 23.Haugnes HS, Bosl GJ, Boer H, et al. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J Clin Oncol. 2012;30:3752–63. doi: 10.1200/JCO.2012.43.4431. [DOI] [PubMed] [Google Scholar]
- 24.Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15:1215–23. doi: 10.1016/S1470-2045(14)70408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bramswig JH, Riepenhausen M, Schellong G. Parenthood in adult female survivors treated for Hodgkin's lymphoma during childhood and adolescence: a prospective, longitudinal study. Lancet Oncol. 2015;16:667–75. doi: 10.1016/S1470-2045(15)70140-3. [DOI] [PubMed] [Google Scholar]
- 26.Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14:1903–07. doi: 10.1093/humrep/14.7.1903. [DOI] [PubMed] [Google Scholar]
- 27.Thomas-Teinturier C, Allodji RS, Svetlova E, et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod. 2015;30:1437–46. doi: 10.1093/humrep/dev060. [DOI] [PubMed] [Google Scholar]
- 28.Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–96. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 29.Sanders JE, Hawley J, Levy W, et al. Pregnancies following high–dose cyclophosphamide with or without high–dose busulfan or total–body irradiation and bone marrow transplantation. Blood. 1996;87:3045–52. [PubMed] [Google Scholar]
- 30.Wilcox AJ, Weinberg CR, O'Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 31.Wasilewski–Masker K, Seidel KD, Leisenring W, et al. Male infertility in long-term survivors of pediatric cancer: a report from the childhood cancer survivor study. J Cancer Surviv. 2014;8:437–47. doi: 10.1007/s11764-014-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barton SE, Najita JS, Ginsburg ES, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14:873–81. doi: 10.1016/S1470-2045(13)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9:735–49. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 34.Picton HM, Wyns C, Anderson RA, et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod. 2015;30:2463–75. doi: 10.1093/humrep/dev190. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH. Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol. 2015;3:556–67. doi: 10.1016/S2213-8587(15)00039-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.