Abstract
Background
Childhood abuse and intimate partner violence have each been associated with migraine headaches in previous studies, but these associations have not been explored among pregnant women.
Objective
To examine the independent and joint associations of childhood abuse and intimate partner violence with migraine among pregnant women.
Methods
A cross-sectional study was conducted among a cohort of 2,970 pregnant women attending prenatal clinics in Lima, Peru. History of childhood abuse (i.e., physical or sexual abuse) was assessed using the Childhood Physical and Sexual Abuse Questionnaire. Intimate partner violence (IPV) was assessed using the World Health Organization questionnaire. Migraine classification (including migraine and probable migraine) was based on International Classification of Headache Disorders (ICHD)-III beta criteria. Multivariable logistic regression analyses were performed to estimate odd ratios (OR) and 95% confidence intervals (95% CI).
Results
The prevalence of any migraine was 33.5% while approximately 70% of participants reported a history of childhood abuse and 36.7% a history of IPV. Women with a history of any childhood abuse had a 38% increased odds of any migraine compared to women with no history of childhood abuse (OR=1.38; 95% CI 1.15-1.64). The odds of migraine increased with increasing numbers of experienced childhood abuse events (Ptrend <0.001). Additionally, after adjusting for confounders women with a history of IPV had a 43% increased odds of any migraine as compared to women without intimate partner violence (OR=1.43; 95%CI 1.02-2.02). Women with a joint positive history of childhood abuse and IPV as compared with the reference group, had a 88% increased odds of migraine (aOR=1.88, 95%CI: 1.51-2.35).
Conclusion
Childhood abuse and IPV are associated with increased odds of migraine in pregnant women. Our findings highlight the importance of screening for abuse among pregnant migraineurs to help guide treatment strategies.
Introduction
Childhood abuse is a major global public health problem with serious adverse health and economic consequences across the life course [1-5]. According to a recent report by the Centers for Disease Control and Prevention (CDC), the cost of childhood abuse and other childhood adversities in the United States was estimated to be greater than $120 billion each year [6]. Epidemiologic studies have shown that exposure to childhood abuse is associated with a myriad of health outcomes including asthma [7], early age at menarche [8-10], sleep disturbances [10,11], chronic systemic inflammation [12], substance abuse [13], mood and anxiety disorders [14-16], suicidal behaviors [2], adulthood revictimization [16], preterm delivery [17] and premature mortality [18]. There is also a growing body of literature documenting associations of childhood abuse with migraine headaches [5,19-22]. Moreover, epidemiologic studies have shown that migraine, often debilitating neurologic disorder, is associated with an increased risk of adverse perinatal outcomes including preeclampsia [23], placental abruption [24], and preterm delivery [25]. However, to our knowledge, no prior published study has examined the relationship between childhood abuse and migraine in pregnancy.
Intimate partner violence (IPV), abuse directed towards women by their male partners, is another significant global public health problem with estimated prevalence ranging from 15% to 71%. [26-28] There is a well-established body of literature that documents the relationship between exposure to IPV and subsequent risk of women's physical and mental health, including preeclampsia, abnormal vaginal bleeding, spontaneous abortion or miscarriage [29-33], mood and anxiety disorders [34], hyperarousal and chronic stress [35]. While there is extensive research pertaining to risks of adverse mental, physical and reproductive health outcomes among pregnant women exposed to IPV, few investigators have explored the risk of migraine among victims of IPV [36,37]. Given (1) the existing gap in the literature; (2) the long-term consequences of childhood abuse; (3) the high prevalence of IPV; and (4) the adverse perinatal outcomes associated with migraine, we examined the independent and joint associations of history of childhood abuse and intimate partner violence with migraine among a well-characterized cohort of pregnant women in Peru. We reasoned that evaluation of associations of childhood abuse and IPV with migraine may add greater specificity to an existing literature focused on assessing risks of adverse perinatal risks among migraineurs with a history of childhood abuse and IPV. An understanding of these relationships is of particular interest among Peruvian women given the high burden of gender-based violence and associated adverse mental and physical health outcomes in this population.[10,38]
Methods
Study Population
The population for the present study was drawn from participants of Pregnancy Outcomes, Maternal and Infant Study (PrOMIS) Cohort. The PrOMIS study was designed to examine maternal social and behavioral risk factors of preterm birth and other adverse pregnancy outcomes among Peruvian women [10,11,16]. The study population consisted of women attending prenatal care clinics at the Instituto Nacional Materno Perinatal (INMP) in Lima, Peru. INMP is the primary reference establishment for maternal and perinatal care operated by the Ministry of Health of the Peruvian government. Women were eligible to be included in this study if they initiated prenatal care prior to 16 weeks gestation, were at least 18 years of age, and could read and speak Spanish. All participants provided written informed consent. Women were ineligible if they were younger than 18 years of age, did not speak and read Spanish, or had completed more than 16 weeks gestation. The eligibility criteria threshold of initiating prenatal care prior to the completion of 16 weeks gestation was set so as to mitigate concerns about reverse causality and recall bias while enrolling as study population that is sufficiently generalizable to the source population of women seeking care at the study site. Study procedures were approved by the institutional review boards of the INMP, Lima, Peru, and the Harvard T. H. Chan School of Public Health Boston, MA.
Analytical Population
Information was collected from participants enrolled in the PrOMIS Cohort Study between February 2012 and March 2014. During this period, 3,775 eligible women were approached, and 3,045 (80.7%) women completed the interview. A total of 89 women were excluded because of missing information for childhood abuse (69) or migraine status (20). A total of 2,970 women were included in the analysis. The excluded participants did not differ from the rest of the cohort with respect to sociodemographic or lifestyle characteristics.
Childhood Abuse Assessment
We used the Childhood Physical and Sexual Abuse Questionnaire to collect information regarding the participants' experiences with physical and sexual abuse in childhood [16,39]. We classified participants as having experienced childhood abuse before the age of 18 years if they reported that an older person touched them, or were made to touch someone else in a sexual way, or intercourse was attempted or completed (sexual abuse); or that they were hit, kicked, or beaten often and/or their life was seriously threatened (physical abuse). Participants who responded ‘no’ to all questions regarding sexual and physical abuse were categorized as ‘no abuse’. Those responding ‘yes’ to only physical abuse questions were categorized as ‘physical abuse only’ and those responding ‘yes’ to only sexual abuse questions were categorized as ‘sexual abuse only’. Those responding ‘yes’ to any physical abuse questions and ‘yes’ to any sexual abuse questions were categorized as having experienced ‘both physical and sexual abuse’. Participants who responded ‘yes’ to any questions of physical abuse or ‘yes’ to any questions of sexual abuse or yes to both abuse types were categorized as having experienced ‘any abuse’.
Intimate Partner Violence (IPV) Assessment
We assessed IPV exposures using questions adapted from the protocol of Demographic Health Survey Questionnaires and Modules: Domestic Violence Module [40] and the WHO Multi-Country Study on Violence Against Women [27]. Women were asked for a range of physical and/or sexual coercive acts used against them by a current or former spouse or intimate partner without their consent during their life. We classified participants as having experienced physical violence if they endorsed any of the following acts: being slapped, having her arms twisted or something thrown at her, being pushed or shoved, being hit, kicked, dragged or beaten up, being choked or burnt on purpose, or being threatened or hurt with a weapon (such as, gun, knife, or other object). Sexual violence was defined if participants endorsed any of the following: being physically forced to have sexual intercourse, having had unwanted sexual intercourse because of fear of what the partner might do, and being forced to perform other sexual acts that the respondent found degrading or humiliating. In this study, participants were categorized as having experienced any IPV if they experienced one or more acts of physical or sexual violence at any time from a current or former male partner.
Migraine Assessment
Migraine and probable migraine was classified by trained interviewers using a Spanish-language questionnaire, administered during early pregnancy, and based on the International Classification of Headache Disorders (ICHD)-III beta criteria [41]. In addition the combined group of women fulfilling either migraine or probable migraine were categorized as “any migraine”.
Other Covariates
Participants completed a structured questionnaire to collect information on sociodemographic characteristics and lifetime intimate partner violence (IPV). Participants' age was categorized as 18-20, 20-29, 30-24, or ≥35 years. Other sociodemographic variables were categorized as follows: maternal ethnicity (mestizo vs. other); educational attainment (≤6, 7–12, and >12 years of education); marital status (married or living with partner vs. others); employment status (employed vs. not employed); access to basic foods (hard, not very hard); parity (nulliparous vs. multiparous); planned pregnancy (yes vs. no); gestational age at interview; and early pregnancy BMI (≤18.5, 18.5-24.9, 25- 29.9, ≥30 kg/m2).
Statistical Analysis
Distributions of socio-demographic characteristics and reproductive history were examined according to history of childhood abuse. Characteristics were summarized using means (± standard deviation (SD)) for continuous variables and number (percent, %) for categorical variables. Chi-squared tests were used to evaluate distribution differences for categorical variables, and the one-way analysis of variance (ANOVA) was used to evaluate differences in means for continuous variables. Multivariable logistic regression procedures were used to estimate odds ratios (OR) and 95% confidence intervals (95% CI) of migraine in relation to childhood abuse. Potential confounders were considered a priori on the basis of the hypothesized relationship with childhood abuse and migraine. Multivariable logistic regression models were adjusted for covariates including maternal age, ethnicity, and history of IPV. We examined the association between migraine and type of childhood abuse (i.e., no abuse, physical abuse only, sexual abuse only, physical and sexual abuse). Participants reporting no abuse served as the reference group. In multivariable logistic regression models, significance for monotonic trends in risk between odds of migraine and number of childhood abuse events were assessed by treating the number of childhood abuse events as a continuous variable. In addition, given evidence of high risks of IPV among women with a history of childhood abuse [16,42,43], we examined the independent and joint effects of childhood abuse and IPV exposure on the risk of migraine. For these analyses we categorized participants into four groups based on combinations of childhood abuse and IPV. The four resulting categories were as follows: (1) no childhood abuse and no IPV, (2) childhood abuse only, (3) IPV only, and (4) childhood abuse and IPV. Participants with no childhood abuse and no IPV comprised the reference group, against which participants in the other three categories were compared. All reported p-values are two-sided and have a statistical significance set at 0.05. All analyses were computed using SAS 9.4 (SAS Institute, Cary, NC, USA) and Stata version 13.0 (StataCorp, College Station, TX, USA).
Results
Sociodemographic and Participant Characteristics
Socio-demographic, lifestyle and reproductive characteristics of the study sample are summarized in Table 1. Pregnant women between the ages of 18 and 35 years were included in the analysis. The majority of the cohort was married or living with a partner (80.7%), unemployed (53.9%), and with less than 12 years of education (54.7%).
Table 1. Socio-demographic and reproductive characteristics of the study population according to types of childhood abuse in Lima, Peru.
| Characteristics | All participants (N = 2970) |
No abuse (N = 839) |
Physical abuse only (N = 1148) |
Sexual abuse only (N = 233) |
Physical and sexual abuse (N = 750) |
P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Age (years) a | 28.1 ± 6.3 | 27.7 ± 6.1 | 28.1 ± 6.4 | 28.2 ± 6.5 | 28.7 ± 6.3 | 0.02 | |||||
| Age (years) | |||||||||||
| 18-20 | 162 | 5.5 | 39 | 4.6 | 67 | 5.8 | 15 | 6.4 | 41 | 5.5 | 0.01 |
| 20-29 | 1662 | 56.0 | 514 | 61.3 | 635 | 55.3 | 129 | 55.4 | 384 | 51.2 | |
| 30-34 | 610 | 20.5 | 155 | 18.5 | 235 | 20.5 | 40 | 17.2 | 180 | 24.0 | |
| ≥35 | 536 | 18.0 | 131 | 15.6 | 211 | 18.4 | 49 | 21.0 | 145 | 19.3 | |
| Education (years) | |||||||||||
| ≤6 | 127 | 4.3 | 37 | 4.4 | 49 | 4.3 | 5 | 2.1 | 36 | 4.8 | 0.66 |
| 7-12 | 1625 | 54.7 | 469 | 55.9 | 624 | 54.4 | 125 | 53.6 | 407 | 54.3 | |
| >12 | 1211 | 40.8 | 331 | 39.5 | 473 | 41.2 | 101 | 43.3 | 306 | 40.8 | |
| Mestizo ethnicity | 2228 | 75.0 | 641 | 76.4 | 854 | 74.4 | 180 | 77.3 | 553 | 73.7 | 0.45 |
| Married/living with a partner | 2396 | 80.7 | 691 | 82.4 | 930 | 81.0 | 179 | 76.8 | 596 | 79.5 | 0.21 |
| Employed | 1370 | 46.1 | 383 | 45.6 | 515 | 44.9 | 113 | 48.5 | 359 | 47.9 | 0.53 |
| Access to basic foods | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||||||
| Hard | 1475 | 49.7 | 350 | 41.7 | 560 | 48.8 | 127 | 54.5 | 438 | 58.4 | <0.0001 |
| Not very hard | 1493 | 50.3 | 489 | 58.3 | 586 | 51.0 | 106 | 45.5 | 312 | 41.6 | |
| Nulliparous | 1446 | 48.7 | 452 | 53.9 | 547 | 47.6 | 116 | 49.8 | 331 | 44.1 | 0.001 |
| Planned pregnancy | 1225 | 41.2 | 368 | 43.9 | 482 | 42.0 | 92 | 39.5 | 283 | 37.7 | 0.09 |
| Gestational age at interview a | 9.2 ± 3.5 | 9.3 ± 3.4 | 9.3 ± 3.5 | 9.3 ± 3.3 | 9.2 ± 3.6 | 0.94 | |||||
| Early pregnancy body mass index (kg/m2) | |||||||||||
| <18.5 | 59 | 2.0 | 21 | 2.5 | 23 | 2.0 | 4 | 1.7 | 11 | 1.5 | 0.49 |
| 18.5-24.9 | 1425 | 48.0 | 385 | 45.9 | 563 | 49.0 | 121 | 51.9 | 356 | 47.5 | |
| 25-29.9 | 1087 | 36.6 | 323 | 38.5 | 414 | 36.1 | 77 | 33.0 | 273 | 36.4 | |
| ≥30 | 366 | 12.3 | 93 | 11.1 | 143 | 12.5 | 26 | 11.2 | 104 | 13.9 | |
| Intimate partner violence b | 1090 | 36.7 | 202 | 24.1 | 383 | 33.4 | 101 | 43.3 | 404 | 53.9 | <0.0001 |
Due to missing data, percentages may not add up to 100%.
mean ± SD (standard deviation)
Lifetime intimate partner violence
For continuous variables, P-value was calculated using the ANOVA; for categorical variables, P-value was calculated using the Chi-square test.
The prevalence of any migraine was 33.5% (migraine 12.5%; probable migraine 21%). A total of 71.8% of the cohort experienced childhood abuse and 36.8% IPV. Women with a positive history of childhood abuse reported a mean of 2 abuse events (SD=1.8). Of the participants with childhood abuse, 38.7% reported childhood physical abuse only, 7.8% reported sexual childhood abuse only; and 25.3% reported both physical and sexual abuse. Age, access to basic foods, parity, and exposure to IPV were statistically significantly associated with childhood abuse (Table 1).
Childhood Abuse and Migraine
A history of childhood abuse was statistically significantly associated with the migraine in this study cohort (Table 2). After adjusting for confounders, women who experienced any childhood abuse had 1.38-fold increased odds of migraine (aOR=1.38; 95% CI 1.16-1.64) as compared to women with no history of childhood abuse. Women who experienced both childhood physical and sexual abuse had 1.71-fold increased odds of migraine (aOR=1.71; 95% CI 1.34-2.10) compared to women with no abuse history. These associations remained statistically significant even after adjusting for lifetime IPV experiences. Women who experienced childhood sexual abuse only (aOR=1.24; 95% CI 0.98-1.68) or childhood physical abuse only (aOR=1.22; 95% CI 1.01-1.48) had increased odds of migraine, although statistical significance was not achieved for sexual abuse only.
Table 2. Association between childhood abuse and migraine during pregnancy.
| Childhood abuse | No migraine (N = 1973) | Migraine (N = 997) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| n | % | n | % | Unadjusted OR (95% CI) |
Adjusted OR (95% CI) a |
Adjusted OR (95% CI) b |
|
| No abuse | 599 | 30.4 | 240 | 24.1 | Reference | Reference | Reference |
| Any abuse | 1374 | 69.6 | 757 | 75.9 | 1.38 (1.15, 1.64) | 1.38 (1.16, 1.64) | 1.28 (1.08, 1.53) |
| Types of abuse | |||||||
| No abuse | 599 | 30.4 | 240 | 25.9 | Reference | Reference | Reference |
| Physical abuse only | 771 | 39.1 | 377 | 37.8 | 1.22 (1.00, 1.48) | 1.22 (1.01, 1.48) | 1.17 (0.97, 1.43) |
| Sexual abuse only | 156 | 7.9 | 77 | 7.7 | 1.23 (0.90, 1.68) | 1.24 (0.90, 1.68) | 1.14 (0.84, 1.57) |
| Physical & sexual abuse | 447 | 22.7 | 303 | 30.4 | 1.69 (1.37, 2.08) | 1.71 (1.34, 2.10) | 1.55 (1.24, 1.92) |
| Number of childhood abuse events | |||||||
| 0 | 599 | 30.4 | 240 | 24.1 | Reference | Reference | Reference |
| 1 | 407 | 20.6 | 165 | 16.5 | 1.01 (0.80, 1.28) | 1.01 (0.79, 1.27) | 0.99 (0.78, 1.26) |
| 2 | 240 | 12.2 | 125 | 12.5 | 1.30 (1.00, 1.69) | 1.30 (1.00, 1.69) | 1.26 (0.97, 1.64) |
| 3 | 387 | 19.6 | 277 | 27.8 | 1.46 (1.17, 1.83) | 1.47 (1.18, 1.84) | 1.37 (1.10, 1.72) |
| 4 | 159 | 8.1 | 90 | 9.0 | 1.41 (1.05, 1.90) | 1.42 (1.05, 1.91) | 1.29 (0.96, 1.76) |
| 5 | 101 | 5.1 | 75 | 7.5 | 1.85 (1.32, 2.58) | 1.89 (1.35, 2.64) | 1.70 (1.21, 2.39) |
| 6-8 | 80 | 4.1 | 75 | 7.5 | 2.34 (1.65, 3.31) | 2.38 (1.68, 3.43) | 2.14 (1.50, 3.06) |
| P-value for linear trend | <0.0001 | 0.0006 | <0.0001 | ||||
| Intimate Partner Violence (IPV) | |||||||
| No | 1,305 | 66.3 | 565 | 57.0 | Reference | Reference | Reference |
| Yes | 663 | 33.7 | 427 | 43.0 | 1.48 (1.27, 1.74) | 1.52 (1.29, 1.78) | 1.46 (1.25, 1.72)c |
Odds ratios were calculated using logistic regression. Abbreviations: OR, odds ratio; CI, confidence interval
Adjusted for maternal age, maternal ethnicity
Adjusted for maternal age, maternal ethnicity, and lifetime intimate partner violence
Adjusted for maternal age, maternal ethnicity, and childhood abuse
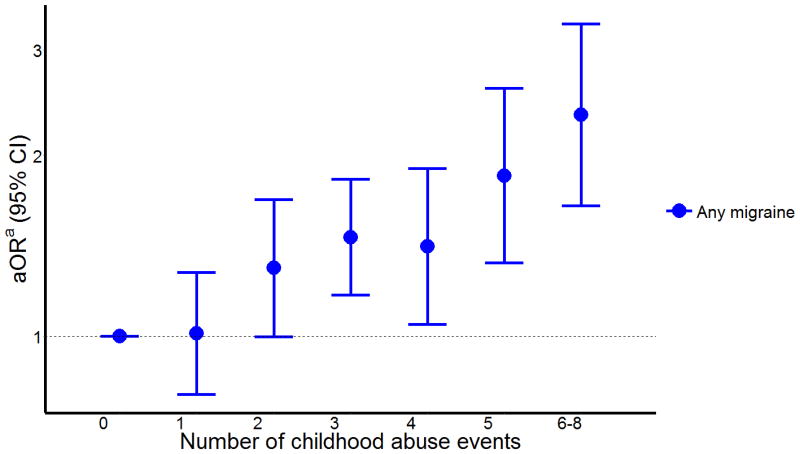
There was a strong positive linear relationship between odds of migraine with the number of childhood abuse events (Ptrend<0.001) (Table 2 and Figure 1). Compared with women who experienced no abuse during childhood, those who experienced six or more childhood abuses had a 2.1-fold increased odds of migraine (aOR=2.14; 95%CI 1.50-3.06).
Figure 1. Odds ratio (aOR) and 95% confidence intervals (95%) of any migraine in relation to number of childhood abuse events.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
a Adjusted for age, ethnicity, and IPV history.
Ptrend= <0.0001
Intimate Partner Violence (IPV) and Migraine
A history of IPV was also statistically significantly associated with migraine in this cohort (Table 2). After adjusting for confounders, women who experienced any childhood abuse had 1.52-fold increased odds of migraine (aOR=1.52; 95% CI 1.29-1.78) as compared to women with no history of IPV; and this association remained significant even after adjusting for history of childhood abuse (OR=1.46; 95% CI 1.25-1.72).
Independent and Joint Effects of Childhood Abuse and IPV
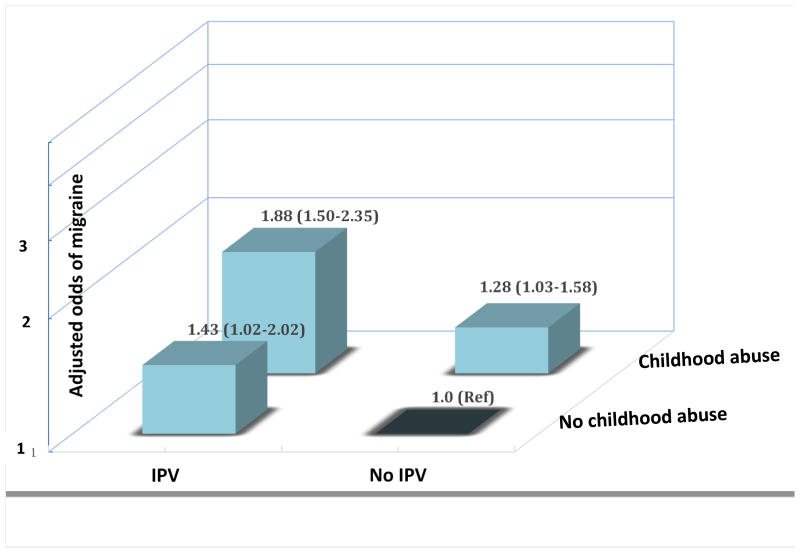
Finally, we explored the independent and joint effect of history of childhood abuse and experiences of IPV with the odds of migraine. As compared to the reference group (women with no history of childhood abuse and IPV)), women who experienced childhood abuse alone (i.e., no IPV) had 1.28-fold elevated odds of migraine (aOR=1.28, 95% CI 1.03-1.58). The corresponding odds of migraine for women who reported IPV exposure alone (i.e., no childhood abuse) was 1.43 (aOR=1.43, 95% CI 1.02-2.02). Women with a joint positive history of childhood abuse and IPV, as compared with the reference group, had 1.88-fold increased odds of migraine (aOR=1.88, 95%CI: 1.51-2.35).
Discussion
To our knowledge, this is the first study to examine the relationship between history of childhood abuse and migraine among pregnant women. Childhood abuse was reported by a majority of participants (71.7%), and among those with a history of child abuse. Women who reported any childhood abuse had a 38% increased odds of migraine compared to women with no history of childhood abuse. Furthermore, the odds of migraine also increased with increased number of childhood abuse events experienced. Likewise, after adjusting for confounders women with a history of IPV had a 43% increased odds of any migraine as compared to women without history of IPV. Furthermore, compared with the reference group, women with a joint positive history of childhood abuse and IPV had 88% increased odds of migraine.
Our results are consistent with prior studies conducted among non-pregnant women and men [37,44,45]. In a recent study by Brennenstuhl et al. [45], women who reported childhood physical abuse (OR=1.61; 95% CI 1.42-1.83), childhood sexual abuse (OR=1.32; 95% CI 1.11-1.57) and parental domestic violence (OR=1.64; 95% CI 1.39-1.93) had increased odds of migraine compared to those without a history of abuse [45]. Other investigators have reported similar findings [21]. We found that women who experienced six or more childhood abuse events had 2.14-fold increased odds (aOR=2.14; 95% CI 1.50-3.06) of migraine compared with women who experienced no childhood abuse. Others have reported similar increased migraine risk with increasing numbers of childhood abuse events studies of men and non-pregnant women [44-46]. For example, Brennenstuhl and Fuller-Thomson, in their study of Canadians, found that experiences of three or more adverse childhood experiences was associated with increased odds of migraine in women (OR=2.82;95%CI: 2.25-3.60) and men (OR=3.26;95%CI: 2.09-5.07) [45]. Our study results showing women who experienced IPV had increased odds of migraine are also consistent with prior studies. For instance, Domino and Haber [47] in their study of community-based survey found that women who experienced adult physical violence had increased odds (OR=1.62, 95% CI 1.07–2.70) of headaches/migraine. Similarly Romans et al. [48] in New Zealand examined physical and/or sexual violence history and chronic headache, reported headache pain developed in all the abused women after trauma began. Finally, Cripe et al. [36] in a Peruvian study found that compared with pregnant women without a history of IPV, women with experiences of IPV were associated with 1.44-fold increased odds (aOR=1.44, 95% CI 1.19–1.75) of any migraine.
Biological mechanisms underlying consistently observed statistical associations of childhood abuse and IPV with migraine have not been fully examined. However, neurodevelopmental and neurobiological changes secondary to abuse experiences have been posited [5]. For example, Teicher et al have hypothesized that early life trauma leads to reduced size of the mid-portions of the corpus callosum and attenuated development of the left neocortex, hippocampus, and amygdala [49]. Furthermore, investigators have noted that early life traumatic exposures such as sexual and physical abuse are associated with long-term changes in neural circuits involved in emotional learning [46]. Early life traumatic experiences have been shown to activate the hypothalamic-pituitary-adrenal (HPA) axis and lead to increased synthesis and release of corticotrophin releasing hormone (CRH) from the hippocampus. For example, authors have noted that childhood abuse may cause increased expression of CRH receptors in the amygdala, known to be involved in threat detection and response [46], thus may contribute to migraine. Another potential explanation is that childhood abuse predisposes to conditions such as obesity, depression and anxiety that in turn influence migraine [22].
The present study has several strengths, namely the large sample size and the high prevalence of childhood abuse and IPV, giving us ample statistical power to study the associations of interest. We employed a rigorous analytic approach that accounted for potential confounding socio-demographic factors as well as experiences of adulthood abuse. However, some important limitations should be considered when interpreting our findings. The first primary limitation is experience of childhood abuse and IPV were assessed based on self-report in this cross-sectional study. Therefore, these measures may be subjected to non-systematic errors in recall, as well as systematic non-disclosure leading to misclassification. Investigators have noted that individuals are likely to minimize experiences of past abuse rather than suggest that they had experienced abuse in their lifetime [50]. Such errors in recall may have led to an underestimation of reported odds ratios. To help mitigate the likelihood of systematic reporting errors, well-trained interviewers used a standard questionnaire to collect information from all study participants. [39] In addition, neither the interviewers nor study participants were aware of any of the specific study hypotheses. Second, migraine diagnosis was made based on information collected using a well-established structured questionnaire based on ICHD-III criteria [41]. Use of structured interviews is the most feasible method of data collection for large-scale epidemiologic studies. Studies that systematically use screening and confirmatory diagnostic evaluations will attenuate greatly concerns about misclassification of migraine diagnoses in epidemiological studies. We were also unable to differentiate migraineurs on the basis of features such as migraine with aura, migraine frequency and timing of most recent attacks. Finally although we used multivariable logistic regression procedures to adjust for putative confounders, we cannot exclude the possibility of residual confounding.
In conclusion, our results indicate that childhood abuse and IPV are highly prevalent and important risk factors for migraine. These findings have important clinical and public health implications. First, clinicians may consider inquiring women about history of abuse during prenatal visits and appropriately refer them to intervention programs. Second, evidence-based public health efforts aimed at preventing childhood and adulthood abuse, identifying women with a history of abuse and providing assistance with management and ongoing care are urgently needed. Finally, development and rigorous evaluation of targeted and cross-culturally acceptable trauma-informed care for pregnant migraineurs who have been victimized by physical and sexual violence is warranted.
Supplementary Material
Supplemental Figure 1. Odds ratio (aOR) and 95% confidence intervals (95%) of migraine (red) and probable migraine (blue) in relation to number of childhood abuse events
Supplemental Table 1. Association between childhood abuse and migraine during pregnancy
Figure 2. Adjusted odds ratios and 95% confidence intervals of migraine in relation to childhood abuse and intimate partner violence exposure histories.
Odds ratios and 95% confidence intervals are adjusted for age, and ethnicity
Acknowledgments
This research was supported by awards from the National Institutes of Health (NIH), National Institute of Minority Health and Health Disparities (T37-MD-001449) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD-059835). The NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The authors wish to thank the dedicated staff members of Asociacion Civil Proyectos en Salud (PROESA), Peru, and Instituto Especializado Materno Perinatal, Peru, for their expert technical assistance with this research.
References
- 1.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, et al. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA. 2001;286:3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- 3.Fang X, Brown DS, Florence CS, Mercy JA. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse Negl. 2012;36:156–165. doi: 10.1016/j.chiabu.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachs-Ericsson N, Blazer D, Plant EA, Arnow B. Childhood sexual and physical abuse and the 1-year prevalence of medical problems in the National Comorbidity Survey. Health Psychol. 2005;24:32–40. doi: 10.1037/0278-6133.24.1.32. [DOI] [PubMed] [Google Scholar]
- 5.Tietjen GE, Peterlin BL. Childhood abuse and migraine: epidemiology, sex differences, and potential mechanisms. Headache. 2011;51:869–879. doi: 10.1111/j.1526-4610.2011.01906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Brown DS, Florence CS, Mercy JA. The economic burden of child maltreatment in the United States and implications for prevention. Child Abuse & Neglect. 2012;36:156–165. doi: 10.1016/j.chiabu.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coogan PF, Wise LA, O'Connor GT, Brown TA, Palmer JR, et al. Abuse during childhood and adolescence and risk of adult-onset asthma in African American women. J Allergy Clin Immunol. 2013;131:1058–1063. doi: 10.1016/j.jaci.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendall-Tackett KA, Simon AF. Molestation and the onset of puberty: data from 365 adults molested as children. Child Abuse Negl. 1988;12:73–81. doi: 10.1016/0145-2134(88)90009-9. [DOI] [PubMed] [Google Scholar]
- 9.Wise LA, Palmer JR, Rothman EF, Rosenberg L. Childhood abuse and early menarche: findings from the black women's health study. Am J Public Health. 2009;99(Suppl 2):S460–466. doi: 10.2105/AJPH.2008.149005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrios YV, Sanchez SE, Nicolaidis C, Garcia PJ, Gelaye B, et al. Childhood abuse and early menarche among Peruvian women. J Adolesc Health. 2015;56:197–202. doi: 10.1016/j.jadohealth.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelaye B, Kajeepeta S, Zhong QY, Borba CP, Rondon MB, et al. Childhood abuse is associated with stress-related sleep disturbance and poor sleep quality in pregnancy. Sleep Med. 2015;16:1274–1280. doi: 10.1016/j.sleep.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med. 2012;43:611–620. doi: 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banducci AN, Hoffman EM, Lejuez CW, Koenen KC. The impact of childhood abuse on inpatient substance users: specific links with risky sex, aggression, and emotion dysregulation. Child Abuse Negl. 2014;38:928–938. doi: 10.1016/j.chiabu.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wosu AC, Gelaye B, Williams MA. History of childhood sexual abuse and risk of prenatal and postpartum depression or depressive symptoms: an epidemiologic review. Arch Womens Ment Health. 2015;18:659–671. doi: 10.1007/s00737-015-0533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornor G. Child sexual abuse: consequences and implications. J Pediatr Health Care. 2010;24:358–364. doi: 10.1016/j.pedhc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Barrios YV, Gelaye B, Zhong Q, Nicolaidis C, Rondon MB, et al. Association of childhood physical and sexual abuse with intimate partner violence, poor general health and depressive symptoms among pregnant women. PLoS One. 2015;10:e0116609. doi: 10.1371/journal.pone.0116609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wosu AC, Gelaye B, Williams MA. Maternal history of childhood sexual abuse and preterm birth: an epidemiologic review. BMC Pregnancy Childbirth. 2015;15:174. doi: 10.1186/s12884-015-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, et al. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37:389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Kucukgoncu S, Yildirim Ornek F, Cabalar M, Bestepe E, Yayla V. Childhood trauma and dissociation in tertiary care patients with migraine and tension type headache: a controlled study. J Psychosom Res. 2014;77:40–44. doi: 10.1016/j.jpsychores.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Straube A, Heinen F, Ebinger F, von Kries R. Headache in school children: prevalence and risk factors. Dtsch Arztebl Int. 2013;110:811–818. doi: 10.3238/arztebl.2013.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tietjen GE, Buse DC, Fanning KM, Serrano D, Reed ML, et al. Recalled maltreatment, migraine, and tension-type headache: results of the AMPP study. Neurology. 2015;84:132–140. doi: 10.1212/WNL.0000000000001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tietjen GE, Brandes JL, Peterlin BL, Eloff A, Dafer RM, et al. Childhood maltreatment and migraine (part I). Prevalence and adult revictimization: a multicenter headache clinic survey. Headache. 2010;50:20–31. doi: 10.1111/j.1526-4610.2009.01556.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez SE, Qiu C, Williams MA, Lam N, Sorensen TK. Headaches and migraines are associated with an increased risk of preeclampsia in Peruvian women. Am J Hypertens. 2008;21:360–364. doi: 10.1038/ajh.2007.46. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez SE, Williams MA, Pacora PN, Ananth CV, Qiu C, et al. Risk of placental abruption in relation to migraines and headaches. BMC Womens Health. 2010;10:30. doi: 10.1186/1472-6874-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cripe SM, Frederick IO, Qiu C, Williams MA. Risk of preterm delivery and hypertensive disorders of pregnancy in relation to maternal co-morbid mood and migraine disorders during pregnancy. Paediatr Perinat Epidemiol. 2011;25:116–123. doi: 10.1111/j.1365-3016.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Moreno C, Heise L, Jansen HA, Ellsberg M, Watts C. Public health. Violence against women. Science. 2005;310:1282–1283. doi: 10.1126/science.1121400. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Moreno C, Jansen HA, Ellsberg M, Heise L, Watts CH. Prevalence of intimate partner violence: findings from the WHO multi-country study on women's health and domestic violence. Lancet. 2006;368:1260–1269. doi: 10.1016/S0140-6736(06)69523-8. [DOI] [PubMed] [Google Scholar]
- 28.Perales MT, Cripe SM, Lam N, Sanchez SE, Sanchez E, et al. Prevalence, types, and pattern of intimate partner violence among pregnant women in Lima, Peru. Violence Against Women. 2009;15:224–250. doi: 10.1177/1077801208329387. [DOI] [PubMed] [Google Scholar]
- 29.Han A, Stewart DE. Maternal and fetal outcomes of intimate partner violence associated with pregnancy in the Latin American and Caribbean region. Int J Gynaecol Obstet. 2014;124:6–11. doi: 10.1016/j.ijgo.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Johri M, Morales RE, Boivin JF, Samayoa BE, Hoch JS, et al. Increased risk of miscarriage among women experiencing physical or sexual intimate partner violence during pregnancy in Guatemala City, Guatemala: cross-sectional study. BMC Pregnancy Childbirth. 2011;11:49. doi: 10.1186/1471-2393-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moraes CL, Reichenheim M, Nunes AP. Severe physical violence among intimate partners: a risk factor for vaginal bleeding during gestation in less privileged women? Acta Obstet Gynecol Scand. 2009;88:1041–1048. doi: 10.1080/00016340903128439. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez SE, Qiu C, Perales MT, Lam N, Garcia P, et al. Intimate partner violence (IPV) and preeclampsia among Peruvian women. Eur J Obstet Gynecol Reprod Biol. 2008;137:50–55. doi: 10.1016/j.ejogrb.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar NN. The impact of intimate partner violence on women's reproductive health and pregnancy outcome. J Obstet Gynaecol. 2008;28:266–271. doi: 10.1080/01443610802042415. [DOI] [PubMed] [Google Scholar]
- 34.Rallis S, Skouteris H, McCabe M, Milgrom J. A prospective examination of depression, anxiety and stress throughout pregnancy. Women Birth. 2014;27:e36–42. doi: 10.1016/j.wombi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Walker R, Shannon L, Logan TK. Sleep loss and partner violence victimization. J Interpers Violence. 2011;26:2004–2024. doi: 10.1177/0886260510372932. [DOI] [PubMed] [Google Scholar]
- 36.Cripe SM, Sanchez SE, Gelaye B, Sanchez E, Williams MA. Association between intimate partner violence, migraine and probable migraine. Headache. 2011;51:208–219. doi: 10.1111/j.1526-4610.2010.01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterlin BL, Ward T, Lidicker J, Levin M. A retrospective, comparative study on the frequency of abuse in migraine and chronic daily headache. Headache. 2007;47:397–401. doi: 10.1111/j.1526-4610.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Beloz A, Williams MA, Sanchez SE, Lam N. Intimate partner violence and risk for depression among postpartum women in Lima, Peru. Violence Vict. 2009;24:380–398. doi: 10.1891/0886-6708.24.3.380. [DOI] [PubMed] [Google Scholar]
- 39.Felitti V, Anda R, Nordenberg D, Williamson D, Spitz A, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 40.DHS. Demographic Health Survey questionnaires and modules: Domestic violence module. [September 19, 2014];2005 Available at : http://www.measuredhs.com/aboutsurveys/dhs/modules_archive.cfm.
- 41.Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders. Cephalalgia. (3rd) 2013;33:629–808. (beta version) [Google Scholar]
- 42.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. Jama. 1997;277:1362–1368. [PubMed] [Google Scholar]
- 43.Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27:1247–1258. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Anda R, Tietjen G, Schulman E, Felitti V, Croft J. Adverse childhood experiences and frequent headaches in adults. Headache. 2010;50:1473–1481. doi: 10.1111/j.1526-4610.2010.01756.x. [DOI] [PubMed] [Google Scholar]
- 45.Brennenstuhl S, Fuller-Thomson E. The Painful Legacy of Childhood Violence: Migraine Headaches Among Adult Survivors of Adverse Childhood Experiences. Headache. 2015;55:973–983. doi: 10.1111/head.12614. [DOI] [PubMed] [Google Scholar]
- 46.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domino JV, Haber JD. Prior physical and sexual abuse in women with chronic headache: clinical correlates. Headache. 1987;27:310–314. doi: 10.1111/j.1526-4610.1987.hed2706310.x. [DOI] [PubMed] [Google Scholar]
- 48.Romans S, Belaise C, Martin J, Morris E, Raffi A. Childhood abuse and later medical disorders in women. An epidemiological study. Psychother Psychosom. 2002;71:141–150. doi: 10.1159/000056281. [DOI] [PubMed] [Google Scholar]
- 49.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 50.Ellsberg MC, Winkvist A, Pena R, Stenlund H. Women's strategic responses to violence in Nicaragua. J Epidemiol Community Health. 2001;55:547–555. doi: 10.1136/jech.55.8.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Odds ratio (aOR) and 95% confidence intervals (95%) of migraine (red) and probable migraine (blue) in relation to number of childhood abuse events
Supplemental Table 1. Association between childhood abuse and migraine during pregnancy