Abstract
Thyroid hormones are essential for proper neurodevelopment in early life. There is evidence that exposure to polybrominated diphenyl ethers (PBDEs) affects thyroid function, but previous studies have been inconsistent, and no studies among children have been conducted in the United States where PBDE levels are particularly high. Serum levels of seven PBDE congeners and thyroid hormones and other thyroid parameters were measured in 80 children aged 1-5 years from the southeastern United States between 2011-2012. Parents of the children completed questionnaires with details on demographics and behaviors. Multivariate linear regression models were used to estimate the associations between serum PBDE levels, expressed as quartiles and as log-transformed continuous variables, and markers of thyroid function. BDE-47, 99, 100 and 153 were detected in >60% of samples, and were summed (ΣPBDE). PBDE congeners and ΣPBDE were positively associated with thyroid-stimulating hormone (TSH). A log-unit increase in ΣPBDE was associated with a 22.1% increase in TSH (95% CI: 2.0%, 47.7%). Compared with children in the lowest quartile of ΣPBDE exposure, children in higher quartiles had greater TSH concentrations as modelled on the log-scale (second quartile: β=0.32, 95% confidence interval (CI): -0.09, 0.74; third quartile: β=0.44, 95% CI: 0.04, 0.85; and fourth quartile: β=0.49, 95% CI: 0.09, 0.89). There was also a tendency toward lower total T4 and higher free T3 with increasing PBDE exposure. Results suggest that exposure to PBDEs during childhood subclinically disrupts thyroid hormone function, with impacts in the direction of hypothyroidism.
Keywords: brominated flame retardants, PBDEs, thyroid hormones, thyroid function, endocrine disruption
1. Introduction
Polybrominated diphenyl ethers (PBDEs) are persistent chemicals that are used as flame retardants in the manufacturing of various consumer products such as furniture, polyurethane foams, and textiles (Abbasi et al., 2015; Betts, 2015). These compounds are not chemically bound to the products they are used in (Birnbaum and Staskal, 2004), so they are released into the environment where they eventually become incorporated into house dust. This leads to human uptake primarily through oral routes, and they are stored long-term in lipid-rich tissues (Johnson-Restrepo and Kannan, 2009). Half-lives in the human body range from 2-7 years with lower brominated congeners with 6 bromines or less being the most persistent (Geyer HJ et al., 2004).
Data from the National Health and Nutrition Examination Survey (NHANES) have shown that 100% of people in the U.S. have detectable levels of at least one PBDE congener in their blood (Sjodin et al., 2014a). Although these compounds are ubiquitous in the environment, certain populations are disproportionately exposed. Studies have consistently shown that children have the highest serum levels of PBDEs compared with other age groups (Lunder et al., 2010), peaking between 2-6 years (Sjodin et al., 2014b). This is thought to be due primarily to increased dust ingestion in the first years of life, because of time spent on the floor and frequent hand-to-mouth behavior (Jones-Otazo et al., 2005). Recent breastfeeding may also contribute to these high levels (Stapleton et al., 2012).
Thyroid function regulates basic metabolism and growth, but it is particularly essential for healthy brain development in young children (Braverman and Cooper, 2012). Specifically, severe hypothyroidism (e.g. increased thyroid-stimulating hormone (TSH), decreased thyroxine (T4)) in early childhood can lead to intellectual disability, language and memory deficits, and poor fine motor, auditory and executive processing skills (Porterfield and Hendrich, 1993; Williams, 2008; Zoeller and Rovet, 2004). Due to the structural similarities between PBDEs and the two major thyroid hormones, T4 and triiodothyronine (T3) (Ibhazehiebo et al., 2011), much research has focused on the potential for PBDEs and their metabolites to interfere with thyroid function. Thyroid hormone concentrations are regulated by the hypothalamic-pituitary-thyroid axis, a complex system that functions as a negative feedback loop (Jameson and De Groot, 2010). TSH is produced by the pituitary gland in response to low T4 levels which stimulates the thyroid to secrete more T4; conversely TSH production is down-regulated when circulating T4 levels are high. PBDEs may disrupt this system at various points (Zoeller et al., 2007).
Many experimental studies in rats and mice have shown that PBDEs induce decreases in total T4 and its metabolically active unbound form, free T4 (Darnerud and Thuvander, 1998; Hallgren and Darnerud, 2002; Stoker et al., 2004; Zhou et al., 2002). Epidemiologic studies have also shown that PBDE exposure in humans is associated with thyroid hormone concentrations, but results have been inconsistent among adults (Dallaire et al., 2009; Turyk et al., 2008) and pregnant women (Abdelouahab et al., 2013; Chevrier et al., 2010). However, despite the higher burden of exposure among young children, few studies have been conducted among this age group (Gascon et al., 2011; Xu et al., 2014), and none have been conducted in the United States, where PBDE levels are particularly high due to historically strict flammability standards (Birnbaum and Staskal, 2004). However, multiple studies among young children have found that PBDEs are associated with neurological and cognitive deficits (Herbstman and Mall, 2014), and thyroid disruption could be a mediating pathway.
Given the potential for neurodevelopmental impacts of thyroid disruption among young children, it is important to quantify the risks associated with this elevated and chronic exposure specifically among young children. The purpose of this study was to evaluate the associations between specific and summed PBDE congeners and thyroid function in a cohort of young children from the southeastern United States.
2. Materials and Methods
2.1. Study Population
Study participants were recruited between 2011-2012 from the population of pediatric anesthesia patients, aged 1 to 5 years, at Children’s Healthcare of Atlanta who were undergoing general anesthesia for myringotomy, adenoidectomy, tonsillectomy and/or bronchoscopy. Children were healthy at the time of surgery and were not taking any medications with known endocrine impacts. Informed consent was obtained from a parent on the day of surgery.
A parent of each child completed a questionnaire which included information on the child as well as the parent(s) such as age, race and ethnicity, family history of thyroid or other endocrine or auto-immune diagnoses, breastfeeding history and duration, birth order, hours per day spent inside the home, residence time at the current residential address, medications, and parental occupations and smoking. A research nurse recorded the child’s height, weight, and insurance status.
2.2. Data Collection
After the surgical procedure(s) and while the child was still under general anesthesia, the research nurse collected up to 15 mL of blood in two red-top Vacutainer tubes. Tubes were inverted several times, stored in a cooler, and transferred the same day to the lab. The samples were centrifuged and serum was then aliquoted into two storage vials, one for PBDE analysis and the other for thyroid hormone analysis, and stored at -20°C prior to analysis.
Prepared serum samples were analyzed for seven PBDE congeners (BDE-47, BDE-85, BDE-99, BDE-100, BDE-153, BDE-154, and BDE-209) using gas chromatography-tandem mass spectrometry (GS-MS/MS) or GC-MS (only for BDE-209) at the Laboratory for Exposure Assessment and Method Development in Environmental Research (LEADER) at Emory University’s Rollins School of Public Health (Agilent Technologies, Santa Clara, CA; 7000 GC/MS Triple Quad). The method limits of detection (LODs) for each congener (shown in Table 1) were defined as three times the standard deviation of the amount measured in blanks plus the blank value or the lowest standard measured above that calculated value that had a signal: noise ratio of greater than 3. Details on the laboratory methods are presented in the Supplemental Material (See Supplemental Material, p. 1-2).
Table 1.
Distribution of PBDE congener concentrations and lipids in serum samples of 80 young children recruited from Children’s Healthcare of Atlanta
| PBDE (ng/mL) | LOD (ng/mL) | Percent detection | Meana,b | Minimum | 25th percentile | Median | 75th percentile | Maximum |
|---|---|---|---|---|---|---|---|---|
| PBDE-47 | 0.002 | 100.0% | 0.15 | 0.02 | 0.09 | 0.14 | 0.26 | 2.47 |
| PBDE-85 | 0.005 | 11.3% | — | <LOD | <LOD | <LOD | <LOD | 0.06 |
| PBDE-99 | 0.002 | 100.0% | 0.04 | 0.01 | 0.02 | 0.04 | 0.07 | 0.74 |
| PBDE-100 | 0.002 | 83.8% | 0.02 | <LOD | 0.01 | 0.02 | 0.04 | 0.48 |
| PBDE-153 | 0.016 | 63.8% | 0.02 | <LOD | <LOD | 0.03 | 0.05 | 0.30 |
| PBDE-154 | 0.007 | 15.0% | — | <LOD | <LOD | <LOD | <LOD | 0.09 |
| PBDE-209 | 0.100 | 0.0% | — | <LOD | <LOD | <LOD | <LOD | <LOD |
| ΣPBDEc | 0.25 | 0.05 | 0.14 | 0.23 | 0.44 | 4.00 | ||
| ΣPBDE (pmol/mL)c,d | 0.48 | 0.09 | 0.27 | 0.44 | 0.84 | 7.73 | ||
| Lipids (mg/dL) | ||||||||
| Cholesterol | 102.8 | 10.2 | 77.7 | 101.1 | 127.9 | 262.2 | ||
| Triglycerides | 127.8 | 37.2 | 100.8 | 127.4 | 140.4 | 275.3 | ||
| Total lipidse | 423.4 | 200.0 | 353.4 | 403.8 | 478.1 | 788.0 |
Abbreviations: LOD: limit of detection
Geometric means reported for PBDE congeners and arithmetic means reported for lipids.
Geometric means not calculated for congeners with detection frequencies less than 50%. BDE-100 and -153 were imputed based on a lognormal probability distribution whose parameters were determined by maximum likelihood estimation.
Sum of congeners with detection frequencies greater than 60% (BDE-47, -99, -100, and -153). Including congeners -85 and -154 minimally impacted the sum.
Molar sum calculated by dividing each congener by its molecular weight, multiplying by 1,000, then summing congeners.
Total lipids = (2.27×Cholesterol)+Triglycerides+62.3 (Phillips et al. 1989)
Total serum triglyceride content was measured using BioVision Triglyceride Quantification Assay Kit (BioVision Research Products; Mountain View, CA), and total cholesterol content was measured using Cayman Cholesterol Assay Kit (Cayman Chemical Company; Ann Arbor, MI) according to manufacturer instructions. Total lipids were calculated using conventional methods based on these individual lipid components (Phillips et al., 1989).
2.3. Hormone Analyses
Thyroid hormones and other thyroid markers were analyzed at the Biomarkers Core Laboratory at Emory University’s Yerkes National Primate Research Center. TSH, free T4, and T3 uptake, a marker of binding protein saturation by T4, were analyzed by ELISA (IBL International; Minneapolis, MN). Total T4, total T3, and free T3 were measured by radioimmunoassay (Siemens; Los Angeles, CA) and reverse T3 (rT3), the inactive T3 isomer resulting from deiodination of T4, and thyroid antibodies (thyroglobulin autoantibody (TgAb) and antibodies to thyroid peroxidase (anti-TPO)) were analyzed by radioimmunoassay (Alpco; Salem, NH). All assays were conducted in duplicate. Inter and intra-assay coefficients of variation are reported in Supplemental Table 2. We refer to this complete set of measures as thyroid parameters.
2.4. Statistical Analyses
PBDE measurements below the LOD were imputed using a distribution-based maximum likelihood technique (Chen et al., 2011; Lubin et al., 2004). For each congener that had values below the LOD, we assumed a lognormal probability distribution based on quantile-quantile plots that compared the observed and expected quantiles of the exposure that corresponded to data above the LOD and truncated log-normal distributions, respectively (Lyles et al., 2001b). We then generated maximum likelihood estimates of the log-scale mean and variance (μ, σ2) assuming lognormal probability distributions. In order to incorporate uncertainty in the maximum likelihood estimates, we generated ten sets of distribution parameters and imputed values below the LOD based on each set of parameters, creating ten complete datasets (Chen et al., 2011; Lyles et al., 2001a). Based on congeners with detection frequencies > 60% (BDE-47, 99, 100 and 153), we created a sum (ΣPBDE). We also considered this sum on the molar basis, which takes into account the different molecular weights of each homolog.
We explored the distribution of exposure in our study population by computing geometric means (GM) of ΣPBDE for each covariate stratum. We fit separate multivariable linear regression models for each PBDE congener with a detection frequency > 60% (BDE-47, 99, 100 and 153) and the sums of these congeners, both as natural log-transformed continuous variables and as quartiles. Modeling exposure using quantiles allows for the examination patterns of association across the range of exposure and the potential for non-linear dose response. Due to the debate in the literature regarding how to adjust for serum lipids, we performed this in two ways. In our primary analysis, we expressed PBDEs on the wet weight basis (ng/mL serum) and controlled for lipids as a covariate, and we repeated all analyses with PBDEs on the lipid basis (ng/g lipid) in supplemental analyses. In analyses with each congener that was subject to left-censoring by an LOD as well as ΣPBDE, we used the SAS procedure PROC MIANALYZE to generate summary regression coefficients, standard errors, and 95% confidence intervals (SAS, Cary, NC) (Little and Rubin, 2002).
TSH was natural log-transformed because it was right skewed, and because there is evidence to suggest that TSH concentrations change on the logarithmic scale as opposed to the additive scale (Demmers and Spencer, 2002). We fit separate linear regression models for each thyroid parameter. All regression models controlled for covariates that were identified a priori based on the literature and our conceptualized directed acyclic graph: lipids, sex, age (1; 2; 3; 4; 5 years old), body mass index z-score (<0; ≥0-≤1; >1) breastfeeding history (0 months; <6 months; ≥6 months), time of blood collection (7am-9am; 9am-12pm; 12pm-3pm), race/ethnicity (non-Hispanic black; non-Hispanic white; Hispanic/mixed race/other), and insurance status (Medicaid; private insurance). We evaluated the influence of each covariate on the estimated PBDE β-coefficients and standard errors. In models for T3 uptake, we additionally controlled for total T4 given that the interpretation of this marker depends on T4 concentrations (Dunlap, 1990). Our study population included two sets of siblings, so we used generalized estimating equations with robust standard errors to account for the potential correlated nature of their outcomes.
We fit logistic regression models in order to estimate the association between PBDE congeners and detection of thyroid antibodies. Because TgAb was detected in less than <50% of samples, we dichotomized it as detected vs. undetected. However, because only two children had an anti-TPO result greater than the laboratory sensitivity cut-off of 30 U/mL, we did not analyze it further.
In sensitivity analyses, we evaluated the influence of extreme thyroid parameter values by excluding observations that were outside of kit reference ranges. All statistical analyses were performed using SAS Version 9.4. Study protocols were approved by the Institutional Review Board at Emory University.
3. Results
The parents of 95 children were approached and asked to participate in the study, and 94% consented (n=89). However, nine children were subsequently excluded due to difficulty obtaining sufficient blood volumes, failing to meet eligibility criteria, or not returning questionnaires, yielding the final sample size of 80.
Detection frequencies varied by PBDE congener (Table 1 and Supplemental Table 1). BDE-85, 154 and 209 were detected in less than 20% of samples and were excluded from further analyses. BDE-47 was the main contributor to ΣPBDE, followed by BDE-99, 153, and 100. Congeners BDE-47, 99, and 100 were highly correlated (r=0.7-0.9; p<0.0001), and correlations with BDE-153 were slightly lower (r=0.4-0.5; p<0.0001) (data not shown).
Table 2 shows the characteristics of the study population and the GM ΣPBDE levels and corresponding geometric standard deviations for each covariate stratum. The study population was racially diverse, with approximately equal numbers of black (n=33) and white children (n=31), and the majority on Medicaid (63.8%). Children had their blood drawn throughout the day, but samples drawn between 7-9 am had lower serum ΣPBDE concentrations, which may have been due to fasting, but this was not explained by lipid levels alone because the GM lipid-standardized ΣPBDE was low as well (ΣPBDE= 0.20 ng/mL; 49 ng/g lipid).
Table 2.
Total polybrominated diphenyl ether (ΣPBDE)a serum concentrations (ng/mL serum and ng/g lipid) by demographic characteristics in a population of young children recruited from Children’s Healthcare of Atlanta
| Total Cohort (n=80)
|
ΣPBDE (ng/mL serum)
|
ΣPBDE (ng/glipid)
|
|
|---|---|---|---|
| n (%b) | GM (GSD) | GM (GSD) | |
| Sex | |||
| Female | 35 (43.8) | 0.32 (0.05) | 77 (11) |
| Male | 45 (56.3) | 0.20 (0.02) | 51(6) |
| Age (years) | |||
| 1 | 11 (13.8) | 0.36 (0.13) | 89 (31) |
| 2 | 23 (28.8) | 0.23 (0.03) | 54 (8) |
| 3 | 20 (25.0) | 0.29 (0.05) | 68 (11) |
| 4 | 13 (16.3) | 0.17 (0.03) | 45 (10) |
| 5 | 13 (16.3) | 0.26 (0.06) | 60 (16) |
| Race/Ethnicity | |||
| Black | 33 (41.3) | 0.30 (0.04) | 74 (8) |
| White | 31 (38.8) | 0.23 (0.04) | 57 (10) |
| Other | 16 (20.0) | 0.20 (0.04) | 45 (8) |
| Breastfeeding history | |||
| Not breastfed | 33 (41.3) | 0.29 (0.05) | 67 (11) |
| <6 months | 21 (26.3) | 0.21 (0.03) | 54 (7) |
| ≥6 months | 26 (32.5) | 0.23 (0.04) | 58 (10) |
| Time of blood collection | |||
| 7-9 am | 23 (28.8) | 0.20 (0.03) | 49 (8) |
| 9-12 pm | 33 (41.3) | 0.26 (0.04) | 63 (8) |
| 12-3 pm | 24 (30.0) | 0.29 (0.05) | 71 (13) |
| BMI z-score for agec | |||
| < 0 | 27 (33.8) | 0.31 (0.05) | 75 (12) |
| ≥0 - ≤1 | 24 (30.0) | 0.23 (0.05) | 54 (11) |
| > 1 | 29 (36.3) | 0.22 (0.03) | 56 (6) |
| Insurance type | |||
| Medicaid | 51 (63.8) | 0.26 (0.03) | 63 (7) |
| Private | 29 (36.3) | 0.22 (0.04) | 57 (9) |
| Parental smoking | |||
| Yes | 14 (17.5) | 0.26 (0.07) | 68 (19) |
| No | 56 (70.0) | 0.23 (0.03) | 56 (6) |
| Missing | 10 (12.5) | 0.35 (0.09) | 86 (17) |
Abbreviations: GM: geometric mean; GSD: geometric standard deviation
Sum of congeners with detection frequencies greater than 60% (BDE-47, -99, -100, and -153). BDE-100 and -153 were imputed based on a lognormal probability distribution whose parameters were determined by maximum likelihood estimation. ΣPBDEs in this table are based on a single imputation.
Percentages may not sum to 100% due to rounding.
BMI z-score based on the CDC 2000 standards for children 24 months or greater, and for children less than 24 months (n=10), BMI z-score was imputed based on the difference between CDC standards and WHO standards at age 24 months.
PBDE congeners and ΣPBDE were positively associated with TSH concentrations (Table 3). On average, for a log-ng/mL increase in a given PBDE congener, estimates ranged from increases of 5.1% (95% CI: -11.6%, 24.6%) for BDE-153 to 22.1% for BDE-47 (95% CI: 2.0%, 44.8%) in TSH concentrations (computed from Table 3). For an interquartile range increase in ΣPBDE (i.e., to go from the 25th percentile (0.14 ng/mL) to the 75th percentile (0.44 ng/mL)), we estimated a 25.6% increase in TSH (95% CI: 2.3%, 56.0%). Results were nearly identical when we considered the molar sum (Table 3). Standardizing PBDE concentrations to the lipid basis did not meaningfully change our results (Supplemental Table 3). Although adjusted estimates are presented, most covariates in our models did not affect β-coefficient estimates by more than 10%. The most influential covariate was time of blood collection. Blood collections between 7-9am were associated with higher TSH concentrations compared with later collections (p<0.0001; data not shown).
Table 3.
β-Coefficients and 95% confidence intervals from regression modelsa for associations of ln-transformed PBDE concentrations (ng/mL serum) with TSHb and other thyroid function parametersc
| PBDE | ln TSH (mIU/L) | Total T4 (μg/dL) | Total T3 (ng/dL) | Free T4 (ng/dL) | Free T3 (ng/dL) | Reverse T3 (ng/mL) | T3 Uptaked (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
|
| |||||||
| PBDE-47 | 0.20 (0.02, 0.37) | -0.18 (-0.64, 0.29) | 3.0 (-4.4, 10.5) | -0.02 (-0.08, 0.04) | 0.02 (0.00, 0.04) | -0.01 (-0.02, 0.01) | 0.28 (-0.52, 1.10) |
| PBDE-99 | 0.20 (0.04, 0.36) | -0.20 (-0.67, 0.28) | 1.4 (-6.0, 8.8) | -0.03 (-0.09, 0.03) | 0.01 (-0.01, 0.03) | -0.01 (-0.02, 0.01) | 0.26 (-0.54, 1.07) |
| PBDE-100 | 0.10 (0.02, 0.18) | -0.03 (-0.33, 0.27) | -0.7 (-5.7, 4.3) | 0.00 (-0.03, 0.03) | 0.01 (-0.01, 0.02) | 0.00 (-0.01, 0.00) | 0.41 (-0.07, 0.89) |
| PBDE-153 | 0.05 (-0.11, 0.22) | 0.09 (-0.35, 0.52) | 5.6 (-1.7, 12.8) | 0.01 (-0.04, 0.06) | 0.01 (-0.01, 0.03) | 0.00 (-0.01, 0.01) | 0.64 (-0.17, 1.45) |
| ΣPBDEe | 0.20 (0.02, 0.39) | -0.15 (-0.62, 0.33) | 3.5 (-4.4, 11.3) | -0.02 (-0.08, 0.04) | 0.02 (0.00, 0.04) | -0.01 (-0.02, 0.01) | 0.43 (-0.46, 1.31) |
| ΣPBDE (pmol/mL)e,f | 0.20 (0.02, 0.39) | -0.15 (-0.63, 0.33) | 3.3 (-4.5, 11.1) | -0.02 (-0.08, 0.04) | 0.02 (0.00, 0.04) | -0.01 (-0.02, 0.01) | 0.41 (-0.47, 1.28) |
Abbreviations: TSH: thyroid-stimulating hormone; T4: thyroxine; T3: triiodothyronine; 95% CI: 95% Confidence Interval.
All models adjusted for serum lipids, sex, age, race/ethnicity, breastfeeding history, time of blood collection, BMI z-score, and insurance type.
TSH is ln-transformed and thus β-coefficients should be interpreted as follows: a log-unit increase in a given PBDE congener is associated with a multiplicative change in TSH of eβ. In terms of changes in PBDE levels (not logged), a p% change in a given PBDE congener is associated with a multiplicative change in TSH of eβ [log([100+p]/100)].
Other thyroid hormones and parameters are not transformed and thus β-coefficients should be interpreted as follows: a log-unit increase in a given PBDE congener is associated with an additive change in each hormone of β. In terms of changes in PBDE levels (not logged), a p% change in a given PBDE congener is associated with an additive change in each hormone of β· [log([100+p]/100)].
Model additionally controlled for total T4.
Sum of congeners with detection frequencies greater than 60% (PBDE-47, -99, -100, and -153). PBDE-100 and -153 were multiply imputed (n=10) based on a lognormal probability distribution whose parameters were determined by maximum likelihood estimation. Including congeners -85 and -154 minimally impacted the sum.
Molar sum calculated by dividing each congener by its molecular weight, multiplying by 1,000, then summing congeners.
In addition, children with higher serum concentrations of PBDE congeners and ΣPBDE showed a tendency toward lower total T4 and higher free T3 (Table 3 and Supplemental Table 3). Estimated differences in total T4 were smaller than differences in TSH: a log-unit increase in ΣPBDE predicted a decrease of 0.15 μg/dL (95% CI: -0.62, 0.33). Similarly, a log-unit increase in ΣPBDE was associated with an increase of 0.02 ng/dL (95% CI: 0.0, 0.04) in free T3. Relative to the average total T4 of 8.97 μg/dL and average free T3 of 0.34 ng/dL (Supplemental Table 2), these changes correspond to approximately a 1.7% decrease and a 5.9% increase in concentrations, respectively. Finally, a log-unit increase in ΣPBDE was suggestive of an increased odds of TgAb detection (adjusted odds ratio (aOR)=1.93, 95% CI: 0.81, 4.62) (data not shown). We did not find significant or consistently suggestive associations between PBDEs and other thyroid parameters. We note that β-coefficients cannot be directly compared between thyroid parameters due to the dramatic differences in distributions across measures (Supplemental Table 2). For example, the interquartile range for total T3 is 41.8 ng/dL and for free T3 is 0.10 ng/dL.
Analyses of PBDE congeners and ΣPBDE as quartiles yielded similar conclusions as the continuous log-transformed analyses (Table 4 and Figure 1). Compared with the first quartile of exposure for BDE-47, 99, and 100, exposure in higher quartiles was associated with greater TSH concentrations (Table 4). Modeling PBDEs on the lipid basis did not substantially change our results; the most notable difference was slightly stronger associations for BDE-100 (Supplemental Table 4). ΣPBDE concentrations were also associated with greater TSH (Figure 1 and Supplemental Table 5). Compared with children in the lowest quartile of ΣPBDE exposure, children in higher quartiles had greater TSH concentrations (β (for change in log TSH)=0.32, 95% CI: -0.09, 0.74, β=0.44, 95% CI: 0.04, 0.85, and β=0.49, 95% CI: 0.09, 0.89 for the second, third, and fourth quartiles, respectively). In addition, quartile analyses showed a suggestion of lower total T4 and higher free T3 with increasing ΣPBDE exposure (Figure 1). Overall, associations between BDE-153 quartiles and most hormones were usually closer to the null than estimates for the other congeners (Table 4 and Supplemental Table 5).
Table 4.
β-Coefficients and 95% confidence intervals (95% CI) from regression modelsa for associations of polybrominated diphenyl ether (PBDE) congener quartiles (ng/mL serum) with TSHb and other thyroid function parametersc
| PBDE | Comparison | ln TSH (mIU/L) | Total T4 (μg/dL) | Total T3 (ng/dL) | Free T4 (ng/dL) | Free T3 (ng/dL) | Reverse T3 (ng/mL) | T3 Uptaked (%) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | ||
|
| ||||||||
| PBDE-47 | Quartile 2 vs. 1 | 0.45 (0.07, 0.82) | -0.25 (-1.42, 0.93) | 2.9 (-17.2, 22.9) | 0.11 (-0.03, 0.25) | 0.03 (-0.01, 0.08) | 0.02 (-0.01, 0.05) | -2.03 (-4.00, -0.10) |
| Quartile 3 vs. 1 | 0.38 (0.01, 0.76) | -0.70 (-2.01, 0.60) | 3.8 (-16.9, 24.5) | -0.04 (-0.19, 0.10) | 0.03 (-0.02, 0.08) | -0.01 (-0.04, 0.02) | -0.61 (-2.70, 1.50) | |
| Quartile 4 vs. 1 | 0.56 (0.19, 0.93) | -0.86 (-2.08, 0.36) | 8.8 (-9.8, 27.5) | -0.07 (-0.22, 0.07) | 0.07 (0.02, 0.12) | -0.02 (-0.06, 0.01) | -0.73 (-2.60, 1.20) | |
| PBDE-99 | Quartile 2 vs. 1 | 0.36 (-0.04, 0.76) | 0.70 (-0.50, 1.90) | 2.7 (-20.2, 25.6) | 0.13 (-0.01, 0.26) | 0.03 (-0.02, 0.08) | 0.02 (-0.01, 0.05) | -0.38 (-2.36, 1.61) |
| Quartile 3 vs. 1 | 0.37 (-0.01, 0.76) | -0.25 (1.32, 0.81) | 6.9 (-12.0, 25.8) | 0.03 (-0.09, 0.16) | 0.02 (-0.03, 0.07) | 0.01 (-0.02, 0.04) | -1.49 (-3.52, 0.54) | |
| Quartile 4 vs. 1 | 0.49 (0.09, 0.88) | -0.29 (0.59, -1.44) | 2.7 (-15.9, 21.2) | -0.11 (-0.26, 0.03) | 0.04 (-0.01, 0.09) | -0.02 (-0.05, 0.01) | 0.40 (-1.64, 2.45) | |
| PBDE-100 | Quartile 2 vs. 1 | 0.28 (-0.09, 0.64) | -0.24 (-1.57, 1.08) | -20.7 (-40.2, -1.3) | 0.15 (0.01, 0.28) | -0.03 (-0.08, 0.02) | -0.01 (-0.04, 0.02) | 0.65 (-2.26, 3.55) |
| Quartile 3 vs. 1 | 0.62 (0.16, 1.07) | -0.23 (-1.32, 0.86) | -3.4 (-21.8, 15.1) | 0.02 (-0.10, 0.13) | 0.04 (-0.01, 0.08) | -0.02 (-0.05, 0.01) | 0.01 (-2.58, 2.60) | |
| Quartile 4 vs. 1 | 0.38 (-0.05, 0.81) | -0.43 (-1.77, 0.91) | 0.7 (-22.0, 23.3) | -0.03 (-0.21, 0.14) | 0.04 (-0.01, 0.09) | -0.04 (-0.07, 0.00) | 1.43 (-1.59, 4.45) | |
| PBDE-153 | Quartile 2 vs. 1 | -0.16 (-0.61, 0.30) | -0.21 (-2.41, 1.99) | -0.3 (-25.3, 24.8) | 0.03 (-0.20, 0.25) | 0.02 (-0.05, 0.08) | 0.00 (-0.05, 0.06) | -0.23 (-2.51, 2.05) |
| Quartile 3 vs. 1 | 0.11 (-0.31, 0.53) | 0.24 (-1.34, 1.81) | 8.9 (-14.7, 32.5) | 0.00 (-0.17, 0.17) | 0.01 (-0.04, 0.05) | -0.01 (-0.05, 0.03) | 0.02 (-2.25, 2.29) | |
| Quartile 4 vs. 1 | -0.03 (-0.48, 0.41) | 0.02 (-1.62, 1.65) | 16.0 (-6.1, 38.0) | 0.05 (-0.12, 0.22) | 0.05 (-0.01, 0.10) | 0.00 (-0.04, 0.05) | 1.48 (-0.88, 3.83) | |
Abbreviations: TSH: thyroid-stimulating hormone; T4: thyroxine; T3: triiodothyronine; 95% CI: 95% Confidence Interval.
All models adjusted for serum lipids, sex, age, race/ethnicity, breastfeeding history, time of blood collection, BMI z-score, and insurance type.
TSH is ln-transformed and thus β-coefficients should be interpreted as follows: compared with exposure in quartile 1, exposure in other quartiles are associated with multiplicative changes in TSH of eβ
Other thyroid hormones and parameters are not transformed and thus β-coefficients should be interpreted as follows: compared with exposure in quartile 1, exposure in other quartiles are associated with additive changes in each hormone of β.
Model additionally controlled for total T4.
Figure 1.
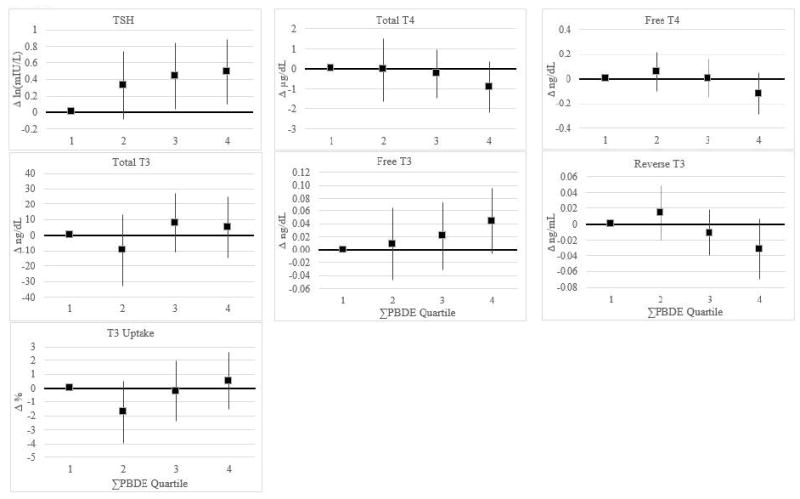
β-Coefficients and 95% confidence intervals (95% CI) from regression modelsa for associations of total polybrominated diphenyl ether (ΣPBDE)b (ng/mL serum) with TSHc and other thyroid function parametersc
Abbreviations: TSH: thyroid-stimulating hormone; T4: thyroxine; T3: triiodothyronine.
aAll models adjusted for serum lipids, sex, age, race/ethnicity, breastfeeding history, time of blood collection, BMI z-score, and insurance type. β-coefficients and 95% CIs reported in Supplemental Table 4.
bSum of congeners with detection frequencies greater than 60% (PBDE-47, -99, -100, and -153). PBDE-100 and -153 were multiply imputed (n=10) based on a lognormal probability distribution whose parameters were determined by maximum likelihood estimation.
cInterpretations of β-coefficients can be found in the footnotes of Supplemental Table 4.
Our results were robust to the exclusion of observations that had thyroid parameter concentrations outside of kit reference ranges, which are thresholds that would motivate further investigation into thyroid function in a clinical setting. Two children were outside the reference ranges for TSH (reference range=0.3-4 mIU/L; minimum=0.12 mIU/L and maximum=6.46 mIU/L). When excluded, the β-coefficients in models for TSH did not substantially change, although they attenuated slightly (data not shown).
4. Discussion
In this cross-sectional study of 80 young US children, serum concentrations of PBDE congeners (BDE-47, BDE-99, BDE-100) were associated with subclinical thyroid hormone changes, including higher TSH concentrations, and a tendency toward lower total T4 and greater free T3. BDE-47 and BDE-99 had the strongest associations with TSH, which also primarily drove the associations with ΣPBDE. These findings were consistent when we modeled PBDEs on the lipid basis, adjusted for potentially confounding variables, and excluded children who had TSH concentrations outside the clinically normal range.
Our results raise concerns about the public health impact of exposure to PBDEs. Although our findings suggest modest subclinical effects on thyroid hormone levels, increased TSH and decreased total T4 represent a physiologic pattern in the direction of hypothyroidism, which among infants and children can adversely affect brain development. Furthermore, although we did not observe an inverse association with free T4, which would also be expected in the case of hypothyroidism, our suggestive finding of increased free T3 may be physiologically consistent. In the hypothyroid state, the thyroid may attempt to compensate and convert more thyroid hormone to the most biologically active form, T3 (Bursell and Warner, 2007; Jameson and De Groot, 2010). Furthermore, although we found that PBDEs were associated with TSH, and potentially total T4 and free T3, it is possible that these associations were not independent due to the thyroid’s negative feedback loop which results in functional relationships between hormones. For example, it may be that PBDEs affect total T4 directly, and that the increases in TSH may be secondary to this. Although the effect sizes for TSH were larger than those for total T4, this is also biologically plausible due to the extreme sensitivity of the pituitary thyrotroph, which leads to large changes in TSH in response to smaller changes in T4. Given our small sample size, it is possible we only had power to detect these changes in TSH and not the smaller changes in T4. The associations we observed were modest, particularly for total T4 and free T3, and may not be clinically significant. Despite this, it is important to interpret β-coefficients in the context of each parameter’s distribution because thyroid parameters are measured in different units and circulate in concentrations that vary by many orders of magnitude.
Thyroid hormone insufficiency in early childhood may lead to neurodevelopmental problems such as language and memory deficits (Zoeller and Rovet, 2004). Although maternal thyroid function during pregnancy is vital to fetal brain development, the postnatal period is also a critical time. Brain development after birth continues to rely on thyroid hormones until age 2, and is particularly related to cerebellar proliferation and myelination (Williams, 2008). However, it is not known whether subclinical variation in thyroid hormones has an impact on neurodevelopment, and further studies are needed in order to elucidate this potential relationship. Still, studies have found that PBDEs are associated with neurodevelopmental deficits in children (Eskenazi et al., 2013; Herbstman et al., 2010), and postnatal thyroid function alterations may be a mediating pathway. The high exposures among young children combined with their particular vulnerability to the deleterious effects of overt thyroid dysfunction highlights the importance of additional studies among this age group.
The distribution of serum PBDE concentrations in this cohort were similar to those found in other toddler cohorts in the United States (Sjodin et al., 2014b; Stapleton et al., 2012; Wu et al., 2015), which is one to two orders of magnitude higher than in other countries worldwide (Birnbaum and Cohen Hubal, 2006; Rose et al., 2010; Sjodin et al., 2008). Despite the high exposure burden among this age group, few studies assessing impacts on thyroid function have been conducted among children and adolescents (Gascon et al., 2011; Han et al., 2011; Kicinski et al., 2012; Leijs et al., 2012; Xu et al., 2014), and to our knowledge, this is the first conducted in the United States. However, despite the small number of published studies among this age group, there is some consistency with four of the five studies indicating a positive association between PBDEs and TSH.
Our findings of a tendency toward lower total T4 is supported by experimental studies in animals. Studies in rats, mice, fish, and birds have shown that PBDEs and specifically, BDE-47, induce decreases in total T4 (Darnerud and Thuvander, 1998; Fernie et al., 2005; Hallgren and Darnerud, 2002; Richardson et al., 2008; Stoker et al., 2004; Tomy et al., 2004; Zhou et al., 2002); and one study additionally noted increases in TSH (Stoker et al., 2004), which is specifically consistent with our results. These decreases in total T4 have been found to be associated with decreases in T4 binding to transthyretin, the main thyroid transport protein in rats (Hallgren and Darnerud, 2002). PBDEs and their metabolites have been shown to competitively bind to and replace T4 on these and other transport proteins (Marchesini et al., 2008; Meerts et al., 2000). Decreases in total T4 were also associated with induction of hepatic microsomal enzymes, which increase metabolism and elimination of thyroid hormones (Hallgren and Darnerud, 2002; Richardson et al., 2008; Zhou et al., 2002). One study that exposed pregnant rats to PBDEs found that dams and their offspring had lower serum T4 concentrations compared with controls, and that these effects were most pronounced among offspring at postnatal day 14 (Zhou et al., 2002). These findings highlight the biologic plausibility for PBDE-induced thyroid hormone disruption in humans as well as the potential for early life as a critical window of exposure to PBDEs.
Although studies on PBDEs and thyroid function among adults have been inconsistent, one recent longitudinal study among office workers in Boston, MA found that PBDEs were associated with decreases in total T4 (Makey et al., 2015). Based on the toxicological literature, Makey et al. hypothesized that this observed decrease in total T4 could be due to either competitive replacement of T4 on transport proteins or a decrease in transport proteins. Although our results were modest and not statistically significant, we also observed a tendency toward lower total T4 levels. However, this hypothesis would have been additionally supported by increases in T3 uptake, which is a proxy measure of serum thyroid hormone binding capacity that quantifies the relative amount of hormone binding receptors on transport proteins that are unoccupied (Dunlap, 1990). If increased, this could signify low binding availability, which could occur if transport proteins were occupied by PBDE metabolites. In this study, we did not observe evidence that PBDE serum concentrations were related to T3 uptake.
Another potential mechanism of PBDE-induced thyroid disruption could be through interference with deiodinase activity (Noyes et al., 2010; Szabo et al., 2009). Deiodinases are enzymes that activate or deactivate thyroid hormones by removing an iodine atom from different locations on thyroid hormones, which alters their bioavailability to target tissues (Greer, 1990; Jameson and De Groot, 2010). Type 2 deiodinase is an activating enzyme that primarily converts T4 to T3, type 3 is deactivating and converts T4 to rT3, whereas type 1 can catalyze both activities. PBDEs and their metabolites have been found to alter deiodinase activity both in vitro and in vivo (Noyes et al., 2010; Szabo et al., 2009). One in vitro study exposed human hepatocytes to BDE-99 and found an association with up-regulation of type 1 deiodinase, which results in increased conversion of T4 to T3 and/or rT3 (Stapleton et al., 2009); thus potentially consistent with lower circulating T4 levels. However, a recent in vitro study with human astrocytes found that BDE-99 and BDE metabolites (3-OH-BDE-47 and 5’-OH-BDE-99) were associated with decreased type 2 deiodinase activity (Roberts et al., 2015), which would lead to decreased conversion of T4 to T3, and thus would not be expected to lead to decreased T4, although deiodinase activity in the brain, or in local tissue, may not affect circulating thyroid hormone levels (Bianco and Kim, 2006; Dentice and Salvatore, 2011; Kohrle, 1999).
In studies involving lipophilic chemicals, there has been considerable debate about how to statistically handle serum lipids (Chevrier, 2013; O’Brien et al., 2015; Schisterman et al., 2005). Serum PBDEs expressed on the lipid basis (ng/g lipid) are correlated with the amount stored in body fat (Brown and Lawton, 1984; Hirai et al., 2012) and because PBDEs accumulate in lipids, this may be the most biologically relevant parameter for adjusting for lipids; although it constrains the form of lipids into the denominator of the PBDE term (Schisterman et al., 2005). In contrast, expressing PBDEs on the wet weight basis (ng/mL serum) and controlling for lipids as a covariate allows for more flexible modeling and still accounts for serum lipids (Schisterman et al., 2005). Schisterman et al. considered different possible underlying causal scenarios of the relationship between polychlorinated biphenyls (PCBs), serum lipids, and a health outcome and showed through statistical simulations that expressing PCBs on the lipid basis induced more bias than controlling for lipids as a covariate (Schisterman et al., 2005). Based on these findings, in our primary analysis, we considered PBDEs on the volume basis and controlled for lipids as a covariate, although we also performed all analyses on the lipid basis, as well (Supplemental Tables 3-5). More recently, O’Brien et al. published a study similar to that of Schisterman et al. that concluded that modeling PBDEs on the lipid basis incurred less bias than other methods (O’Brien et al., 2015). In our study, results did not meaningfully change when we considered both of these options.
This issue is further complicated by the complex relationship between lipids and thyroid function. Thyroid hormones regulate lipid metabolism and it has been shown that overt hypothyroidism is associated with increases in blood lipids and dyslipidemia (Duntas and Brenta, 2012; Pucci et al., 2000; Reinehr, 2010). However, it is unclear whether subclinical variation in thyroid hormones affect lipid levels, especially in children (Reinehr et al., 2006; Tagliaferri et al., 2001). If thyroid hormones do influence blood lipid levels, the potential for reverse causality must be considered (Chevrier, 2013). Greater TSH levels would be expected to be related to increased lipids, and thus greater PBDE concentrations on the wet weight basis, but lower levels on the lipid basis. However, if this scenario was operating, we might expect to observe different results when comparing the two analytical techniques for adjusting for lipids, which we did not. Furthermore, TSH and lipids were not positively correlated in our study (data not shown).
One major strength of this study was that we were able to measure an expanded panel of thyroid hormones and other markers such as T3 uptake and thyroid antibodies. We detected an association between time of day and TSH, consistent with its known diurnal pattern (Braverman and Cooper, 2012). In addition, the physiologically consistent results we observed with multiple hormones reduce the likelihood that our results are driven entirely by chance.
We suspect that the paucity of studies on this topic is due to the challenges in obtaining voluntary blood samples from individual young children for research. By obtaining consent from parents of children who were receiving general anesthesia, we were able to obtain blood samples from healthy young children with a 94% participation rate. We have no reason to believe that selection of this population threatened internal validity given that both PBDEs and thyroid hormones would have to be related to indications for myringotomy and related procedures in order to induce bias (Hernán et al., 2004). Furthermore, although we have no a priori reason to believe that associations between PBDEs and thyroid hormones are different among children receiving myringotomy compared to the general population of healthy children in the United States, it is possible that our results are only generalizable to those children with similar indications for these procedures. However, an estimated 500,000 children per year receive ear tube surgeries, usually as treatment for recurrent otitis media, making it the most common surgical procedure performed on children (Ah-Tye et al., 2001). Thus, even if results are only generalizable to these children, the public health impact could be considerable. Lastly, although the cross-sectional design of this study does not allow for causal inference due to lack of temporal sequence, PBDEs are extremely persistent in the environment (de Wit, 2002; de Wit et al., 2006; Ikonomou et al., 2002) and in human biota (Hites, 2004). Therefore, serum PBDE levels remain relatively constant over time and can reflect long-term exposure (Birnbaum and Cohen Hubal, 2006). Given that the children in this study were aged 1-5 years old, serum PBDE levels may reflect an integrated exposure metric over their lifetimes. Furthermore, because the outcomes of interest were subclinical as opposed to a disease state, this research question is well suited for the cross-sectional design.
Our study was limited by small sample size, and for certain hormones, this made it difficult to discern between potentially important subclinical changes and the influence of random error or multiple testing. This also limited our ability to evaluate potential effect modification, such as by antibody detection status. Another limitation was the relatively high analytical LOD for BDE-153 which led to lower detection of this congener (64%) in our study when compared with other studies among US toddlers (Sjodin et al., 2014b; Stapleton et al., 2012). However, our treatment of left-censored values due to the LOD using distribution-based multiple imputation was robust, and incorporated variability in the estimation of concentrations, avoiding underestimation of variance that can occur when using methods such as imputing the LOD/√2 (Chen et al., 2011; Lubin et al., 2004). Nonetheless, because a substantial proportion of samples was below the LOD for this congener, this could have been a reason that the associations with thyroid hormones were often not as strong as for other congeners.
Although PentaBDE and OctaBDE have been voluntarily phased out in the U.S. since 2004 (Birnbaum and Cohen Hubal, 2006; Dodson et al., 2012), exposure to these compounds is still of concern. Because of their chemical stability in the environment and their typical use in durable goods such as furniture and electronics, PBDEs will likely remain in the environment for a long time (Sjodin et al., 2014a). Furthermore, other brominated flame retardants with similar chemical structures, such as tetrabromopisphenol A and hexabromo-cyclododecane, are still being produced and are used as replacements for PBDEs (Birnbaum and Staskal, 2004). Therefore, this work may be relevant outside of exposure to these particular compounds, as PBDEs may serve as a model for exposure to these other flame retardants.
5. Conclusions
We found that PBDEs were associated with greater TSH concentrations in a cohort of young children in the southeastern U.S. There was also a suggestion of lower total T4, consistent with a physiologic pattern in the direction of hypothyroidism. This is the first study on this topic conducted among toddlers in the United States. Given that this age group is highly exposed to PBDEs and at a vulnerable life stage for neurodevelopment, these findings highlight the importance of considering early life exposures in the context of critical stages of development.
Supplementary Material
Highlights.
First study in US children on polybrominated diphenyl ethers and thyroid function.
PBDE serum levels were positively associated with thyroid-stimulating hormone.
Suggestion of lower total thyroxine and higher free triiodothyronine.
Subclinical disruption of thyroid hormones in the direction of hypothyroidism.
Acknowledgments
Funding provided by NIEHS grant R21ES019697, NICHD Reproductive, Perinatal, & Pediatric Training Grant T32HD052460, and NIOSH Environmental and Occupational Epidemiology Training Grant 5T03OH008609-10.
Assay services were provided by the Biomarkers Core Laboratory at the Yerkes National Primate Research Center. This facility is supported by the Yerkes National Primate Research Center Base Grant 2P51RR000165-51.
The authors would like to thank Dr. Mitch Klein for his assistance with multiple imputation procedures, as well as Drs. Jonathan Chevrier and Andreas Sjodin for their insightful comments and helpful critique of this work. We also acknowledge Christina Ryan, Elizabeth Marder, Parinya Panuwet, Ronald Hunter, Priya D’Souza, P. Barry Ryan, Emma Preston, and Grace Lee.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dana B. Barr, Email: dbbarr@emory.edu.
Michele Marcus, Email: mmarcus@emory.edu.
Andrew B. Muir, Email: abmuir@emory.edu.
Robert H. Lyles, Email: rlyles@emory.edu.
Penelope P. Howards, Email: phoward@emory.edu.
Larissa Pardo, Email: larissapardo2@gmail.com.
Lyndsey A. Darrow, Email: ldarrow@emory.edu.
References
- Abbasi G, et al. Stocks and flows of PBDEs in products from use to waste in the US and Canada from 1970 to 2020. Environmental science & technology. 2015;49:1521–1528. doi: 10.1021/es504007v. [DOI] [PubMed] [Google Scholar]
- Abdelouahab N, et al. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol. 2013;178:701–13. doi: 10.1093/aje/kwt141. [DOI] [PubMed] [Google Scholar]
- Ah-Tye C, et al. Otorrhea in young children after tympanostomy-tube placement for persistent middle-ear effusion: prevalence, incidence, and duration. Pediatrics. 2001;107:1251–8. doi: 10.1542/peds.107.6.1251. [DOI] [PubMed] [Google Scholar]
- Betts KS. Hand-me-down hazard: flame retardants in discarded foam products. Environ Health Perspect. 2015;123:A56–63. doi: 10.1289/ehp.123-A56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–9. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Cohen Hubal EA. Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ Health Perspect. 2006;114:1770–5. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman LE, Cooper D. Werner & Ingbar’s the thyroid: a fundamental and clinical text. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- Brown JF, Jr, Lawton RW. Polychlorinated biphenyl (PCB) partitioning between adipose tissue and serum. Bull Environ Contam Toxicol. 1984;33:277–80. doi: 10.1007/BF01625543. [DOI] [PubMed] [Google Scholar]
- Bursell JD, Warner JT. Interpretation of thyroid function in children. Paediatrics and Child Health. 2007;17:361–366. [Google Scholar]
- Chen H, et al. A distribution-based multiple imputation method for handling bivariate pesticide data with values below the limit of detection. Environ Health Perspect. 2011;119:351–6. doi: 10.1289/ehp.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J. Invited commentary: Maternal plasma polybrominated diphenyl ethers and thyroid hormones--challenges and opportunities. Am J Epidemiol. 2013;178:714–9. doi: 10.1093/aje/kwt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, et al. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118:1444–9. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire R, et al. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect. 2009;117:1380–6. doi: 10.1289/ehp.0900633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud P, Thuvander A. Studies on immunological effects of polybrominated diphenyl ether (PBDE) and polychlorinated biphenyl (PCB) exposure in rats and mice. Organohalogen Compounds. 1998;35:415–418. [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- de Wit CA, et al. Levels and trends of brominated flame retardants in the Arctic. Chemosphere. 2006;64:209–33. doi: 10.1016/j.chemosphere.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Demmers L, Spencer C. Laboratory support for the diagnosis and monitoring of thyroid disease. National Academy of Clinical Biochemistry; 2002. [Google Scholar]
- Dentice M, Salvatore D. Deiodinases: the balance of thyroid hormone: local impact of thyroid hormone inactivation. J Endocrinol. 2011;209:273–82. doi: 10.1530/JOE-11-0002. [DOI] [PubMed] [Google Scholar]
- Dodson RE, et al. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environmental Science & Technology. 2012;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap D. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: 1990. Thyroid Function Tests. [PubMed] [Google Scholar]
- Duntas LH, Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Med Clin North Am. 2012;96:269–81. doi: 10.1016/j.mcna.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121:257–62. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie KJ, et al. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius) Toxicol Sci. 2005;88:375–83. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- Gascon M, et al. Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age. Environ Int. 2011;37:605–11. doi: 10.1016/j.envint.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, et al. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds. 2004;66:3867–3872. [Google Scholar]
- Greer MA. The Thyroid gland. Raven Press; 1990. [Google Scholar]
- Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–43. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Han G, et al. Correlations of PCBs, DIOXIN, and PBDE with TSH in children’s blood in areas of computer E-waste recycling. Biomed Environ Sci. 2011;24:112–6. doi: 10.3967/0895-3988.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK. Developmental Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment. Curr Environ Health Rep. 2014;1:101–112. doi: 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, et al. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hirai T, et al. Distribution of polybrominated diphenyl ethers in Japanese autopsy tissue and body fluid samples. Environ Sci Pollut Res Int. 2012;19:3538–46. doi: 10.1007/s11356-012-0915-z. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Ibhazehiebo K, et al. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect. 2011;119:168–75. doi: 10.1289/ehp.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomou MG, et al. Exponential increases of the brominated flame retardants, polybrominated diphenyl ethers, in the Canadian Arctic from 1981 to 2000. Environ Sci Technol. 2002;36:1886–92. doi: 10.1021/es011401x. [DOI] [PubMed] [Google Scholar]
- Jameson JL, De Groot LJ. Endocrinology: adult and pediatric Elsevier Health Sciences 2010 [Google Scholar]
- Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009;76:542–8. doi: 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- Jones-Otazo HA, et al. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol. 2005;39:5121–30. doi: 10.1021/es048267b. [DOI] [PubMed] [Google Scholar]
- Kicinski M, et al. Neurobehavioral function and low-level exposure to brominated flame retardants in adolescents: a cross-sectional study. Environ Health. 2012;11:86. doi: 10.1186/1476-069X-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrle J. Local activation and inactivation of thyroid hormones: the deiodinase family. Mol Cell Endocrinol. 1999;151:103–19. doi: 10.1016/s0303-7207(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Leijs MM, et al. Thyroid hormone metabolism and environmental chemical exposure. Environ Health. 2012;11(Suppl 1):S10. doi: 10.1186/1476-069X-11-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis and missing data. Wiley; Hoboken, NJ: 2002. [Google Scholar]
- Lubin JH, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunder S, et al. Significantly higher polybrominated diphenyl ether levels in young US children than in their mothers. Environmental science & technology. 2010;44:5256–5262. doi: 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- Lyles RH, et al. Correlation coefficient estimation involving a left censored laboratory assay variable. Stat Med. 2001a;20:2921–33. doi: 10.1002/sim.901. [DOI] [PubMed] [Google Scholar]
- Lyles RH, et al. Correlating two viral load assays with known detection limits. Biometrics. 2001b;57:1238–44. doi: 10.1111/j.0006-341x.2001.01238.x. [DOI] [PubMed] [Google Scholar]
- Makey CM, et al. Polybrominated Diphenyl Ether Exposure and Thyroid Function Tests in North American Adults. Environ Health Perspect. 2015 doi: 10.1289/ehp.1509755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini GR, et al. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol. 2008;232:150–60. doi: 10.1016/j.taap.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Meerts IA, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Noyes PD, et al. Characterizing the in vitro hepatic biotransformation of the flame retardant BDE 99 by common carp. Aquat Toxicol. 2010;97:142–50. doi: 10.1016/j.aquatox.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, et al. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ Health Perspect. 2015 doi: 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ. Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development--current perspectives. Endocr Rev. 1993;14:94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- Pucci E, et al. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S109–12. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–71. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Reinehr T, et al. Hyperthyrotropinemia in obese children is reversible after weight loss and is not related to lipids. J Clin Endocrinol Metab. 2006;91:3088–91. doi: 10.1210/jc.2006-0095. [DOI] [PubMed] [Google Scholar]
- Richardson VM, et al. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol. 2008;226:244–50. doi: 10.1016/j.taap.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Roberts SC, et al. Disruption of type 2 iodothyronine deiodinase activity in cultured human glial cells by polybrominated diphenyl ethers. Chem Res Toxicol. 2015;28:1265–74. doi: 10.1021/acs.chemrestox.5b00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M, et al. PBDEs in 2-5 year-old children from California and associations with diet and indoor environment. Environ Sci Technol. 2010;44:2648–53. doi: 10.1021/es903240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, et al. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113:853–7. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, et al. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the national health and nutrition examination survey: 2003-2008. Environ Sci Technol. 2014a;48:753–60. doi: 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, et al. Polybrominated diphenyl ethers, 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB-153), and p,p’-dichlorodiphenyldichloroethylene (p,p’-DDE) concentrations in sera collected in 2009 from Texas children. Environ Sci Technol. 2014b;48:8196–202. doi: 10.1021/es5016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003-2004. Environ Sci Technol. 2008;42:1377–84. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, et al. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120:1049–54. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, et al. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ Health Perspect. 2009;117:197–202. doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, et al. Assessment of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture, in the EDSP male and female pubertal protocols. Toxicol Sci. 2004;78:144–55. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- Szabo DT, et al. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferri M, et al. Subclinical hypothyroidism in obese patients: relation to resting energy expenditure, serum leptin, body composition, and lipid profile. Obes Res. 2001;9:196–201. doi: 10.1038/oby.2001.21. [DOI] [PubMed] [Google Scholar]
- Tomy GT, et al. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush) Environ Sci Technol. 2004;38:1496–504. doi: 10.1021/es035070v. [DOI] [PubMed] [Google Scholar]
- Turyk ME, et al. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect. 2008;116:1635–41. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–94. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- Wu XM, et al. Polybrominated diphenyl ether serum concentrations in a Californian population of children, their parents, and older adults: an exposure assessment study. Environ Health. 2015;14:23. doi: 10.1186/s12940-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, et al. Elevated serum polybrominated diphenyl ethers and alteration of thyroid hormones in children from guiyu, china. PLoS One. 2014;9:e113699. doi: 10.1371/journal.pone.0113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, et al. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicological Sciences. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–18. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, et al. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37:11–53. doi: 10.1080/10408440601123446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


