Abstract
Background
Right ventricular (RV) functional reserve affects functional capacity and prognosis in patients with pulmonary arterial hypertension (PAH). PAH associated with systemic sclerosis (SSc-PAH) has a substantially worse prognosis as compared to idiopathic PAH (IPAH), even though many measures of resting RV function and pulmonary vascular load are similar. We therefore tested the hypothesis that RV functional reserve is depressed in SSc-PAH patients.
Methods and Results
RV pressure-volume relations were prospectively measured in IPAH (n=9) and SSc-PAH (n=15) patients at rest and during incremental atrial pacing or supine bicycle ergometry. Systolic and lusitropic function increased at faster heart rates in IPAH patients, but were markedly blunted in SSc-PAH. The recirculation fraction, which indexes intracellular calcium recycling, was also depressed in SSc-PAH (0.32±0.05 versus 0.50±0.05; p=0.039). At matched exercise (25 Watts), SSc-PAH patients failed to augment contractility (end-systolic elastance) whereas IPAH did (p<0.001). RV afterload assessed by effective arterial elastance rose similarly in both groups; thus, ventricular-vascular coupling declined in SSc-PAH. Both end-systolic and end-diastolic RV volumes increased in SSc-PAH patients to offset contractile deficits, whereas chamber dilation was absent in IPAH (+37±10% versus +1±8%, p=0.004, and +19±4% versus −1±6%, p<0.001, respectively). Exercise-associated RV dilation also strongly correlated with resting ventricular-vascular coupling in a larger cohort.
Conclusions
RV contractile reserve is depressed in SSc-PAH versus IPAH subjects, associated with reduced calcium recycling. During exercise, this results in ventricular-pulmonary vascular uncoupling and acute RV dilation. RV dilation during exercise can predict adverse ventricular-vascular coupling in PAH patients.
Keywords: hypertension, pulmonary, right ventricle, exercise physiology, force-frequency response, pulmonary heart disease, systemic sclerosis
INTRODUCTION
Pulmonary arterial hypertension (PAH) results from progressive adverse remodeling of the distal pulmonary arteries. Although the right ventricle (RV) initially compensates in part by developing muscle wall hypertrophy, a significant proportion of patients develop progressive RV failure and ultimately death.1 This highlights a key link between RV functional status and survival.2 Systemic sclerosis-associated PAH (SSc-PAH) heralds a particularly poor prognosis among PAH subtypes, with a median survival of only 4 years3 and a two-fold higher rate of death compared to individuals with idiopathic PAH (IPAH).4 A few recent studies have identified resting RV dysfunction in SSc-PAH that may underlie poor outcomes.5,6 RV functional reserve in SSc-PAH remains unknown, yet this may be a more relevant factor, as recent studies have proposed such reserve as well as RV-pulmonary artery coupling during exercise may better predict clinical status and survival beyond resting RV performance alone.7–9
Thus far, characterization of RV functional reserve in patients with PAH has been based on non-invasive imaging, right heart catheterization (RHC) data, and in some cases, mathematically-derived estimates of end-systolic elastance.9–12 The latter has yet to be validated in humans at rest or under stress. While direct measurement of pressure-volume (PV) relations remains the gold standard for cardiac hemodynamics,13 such data are scant for the RV, and not yet reported for stress conditions. Accordingly, this study prospectively assessed RV functional reserve in subjects with IPAH and SSc-PAH referred for RHC. We measured hemodynamic responses to two stressors—incremental atrial pacing and supine bicycle cardiopulmonary exercise testing (CPET)—while concurrently measuring continuous RV PV-relationships. We hypothesized that unlike IPAH, SSc-PAH has depressed RV systolic and diastolic reserve, forcing maladaptive compensatory mechanisms during physiological stress that could contribute to its poor prognosis.
METHODS
Study Subjects
We prospectively enrolled 43 patients from November 2012 to October 2015 at the Johns Hopkins Hospital referred for RHC for the diagnosis or management of PAH. The research protocol was approved by the Johns Hopkins Medical Institutions Institutional Review Board, and informed consent obtained in all patients. Systemic sclerosis (SSc) patients met recently updated 2013 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) diagnostic criteria.14 PAH was diagnosed by a mean pulmonary artery pressure (mPAP) ≥ 25 mm Hg and pulmonary vascular resistance (PVR) greater than 3 Wood units with pulmonary artery wedge pressure (PAWP) ≤ 15 mm Hg during RHC.15 IPAH patients had all known causes of PAH excluded.15 Patients were diagnosed with SSc-PAH if they met criteria for SSc and PAH and had secondary causes of pulmonary hypertension (PH) such as interstitial lung disease (based on radiographic evidence and forced vital capacity less than 70%) or left-sided heart failure (based on echocardiography and RHC) excluded. Comparisons of RV functional reserve were made between IPAH and SSc-PAH subjects (n=24); additional regression analyses were made in an expanded cohort that also included a normal and several SSc subjects without PAH as well as SSc patients with secondary PH (n=33).
Study Design
Baseline clinical characteristics were obtained from standardized clinic data obtained by PH physicians. All reported laboratory data were obtained at the time of this visit or within six months prior. Patients underwent cardiac magnetic resonance imaging (CMR) within five hours prior to RHC. After standard RHC, the internal jugular introducer sheath was exchanged for a dual-entry 9F sheath (#406333, St. Jude’s Medical, St. Paul, MN) to enable simultaneous placement of a 5F PV catheter (SPC-570-2, Millar Instruments, Houston, TX), and either a bipolar pacing wire (2.8F, D98500H, Edwards, Irvine, CA, or 4F, #401994, St. Jude’s Medical, St. Paul, MN) or 4F balloon-tipped pulmonary artery (PA) wedge catheter (AI-07122, Teleflex, Morrisville, NC).
The PV catheter was advanced under fluoroscopic guidance until its pigtail tip reached the RV apex. Signals generated from individual electrode pairs on the catheter were examined and those in phase and consistent with an intra-cavitary position were summed to generate total volume. Steady state data were measured at end-expiration and during a multi-beat decline in RV end-diastolic filling during a Valsalva maneuver, as previously described and validated.6
After basal PV data were obtained, a bipolar pacing wire was positioned in the right atrium and pacing capture confirmed. Incremental atrial pacing starting at ~80–90 min−1 (slightly above normal sinus rate) was then increased in steps of 20 min−1 to a peak of 140–150 min−1 or rate when 2nd degree atrioventricular block (Mobitz I) was observed. Data were recorded at end-expiration after 1 minute of pacing, and during the first 15 seconds upon acute cessation of pacing, to assess contractility potentiation and its rate of decay.16
The pacing catheter was then replaced by the balloon-tipped PA wedge catheter, and subjects positioned into a supine bicycle ergometer. A nose clip and mouthpiece (Innocor, Innovision, Denmark) were placed to measure continuous oxygen consumption. Subjects then underwent bicycle exercise using a modified protocol beginning at 15 Watts during stage 1, increasing in 10-Watt increments per 2-minute stage until symptom-limited maximum effort was achieved. Pulmonary artery pressures, gas exchange, and PV data were continuously recorded.
Data Analysis
The RV volume signal was calibrated to resting RV volume measurements obtained by same day CMR. End-systolic PV points were determined from a set of loops with varying preload volumes during phase 2 of the Valsalva maneuver, and fit by perpendicular regression using an iterative algorithm to derive the slope (end-systolic elastance, Ees) of the end-systolic pressure-volume relationship (ESPVR). This slope was then applied to the resting PV loop to determine the x-intercept (V0) of the ESPVR, as described.6 Effective arterial elastance (Ea) was determined by dividing end-systolic pressure (ESP) by stroke volume (SV). Right ventricular-pulmonary artery coupling was assessed by the ratio of Ees/Ea.
During pacing, high fidelity RV pressure tracings from each paced heart rate (HR) were analyzed for contractile force (maximum of the derivative of pressure with respect to time, or dP/dtmax) and dP/dtmax normalized to the instantaneous pressure developed (dP/dtmax/IP). After cessation of pacing at each HR, the dP/dtmax of the 5–6 subsequent beats were measured while ensuring relatively equivalent volumes between beats. RV dP/dtmax decayed in a geometric fashion similar to that previously described in the left ventricle (LV).16 Each beat was normalized to steady-state dP/dtmax; normalized values were then plotted against that of the subsequent beat to yield a linear decay, with the slope being the recirculation fraction.
For exercise data, signal-averaged representative PV loops were obtained from rest (with subject’s feet elevated in pedals, subsequently referred to as stage 0) as well as each stage of exercise. All subjects exercised through at least stage 2 (25 Watts, 4 minutes), and primary analysis was based on data assessed at this matched workload. Resting V0 was used in order to calculate Ees for subsequent PV loops.17,18 Data were analyzed with custom developed software (WinPVAN-3.5.10).
Statistical Methods
Results are presented as mean ± standard error of the mean (SEM) unless otherwise indicated. Given sample sizes, simple comparisons of continuous variables were performed using the Wilcoxon-Mann-Whitney test, while Fisher’s exact test was used to compare categorical variables. For pacing and exercise analyses, repeated-measures ANOVA was performed using the independent variables of disease group, pacing or exercise stage, and the disease-stage interaction term (heart rates were sorted into 4 pacing bins). The Greenhouse-Geiser correction was applied to repeated variable p-values if sphericity was violated. Pearson correlation coefficients were used to determine correlations between continuous variables. Regression analyses were performed to assess the significance of any correlations or regression coefficient comparisons. Log transformations were done as necessary to normalize distributions for regression. A p-value of < 0.05 (2-sided) was considered significant throughout. Graphics were created and statistical analyses were performed using commercially available software (SigmaPlot-11.0, Systat, San Jose, CA; Stata-11, StataCorp, College Station, TX).
RESULTS
Study Population
Of the 43 patients enrolled, 28 satisfied diagnostic criteria for either IPAH or SSc-PAH, and PV catheter data were obtained from 24 (IPAH, n=9; SSc-PAH, n=15; 2 from each group were excluded for safety or technical reasons). Another 15 had alternate diagnoses; of these, data were obtained from 13 while 2 were excluded for safety or technical reasons (Figure S1). Baseline IPAH and SSc-PAH clinical, hemodynamic, and CMR data are provided in Tables S1–S3. As previously reported,6 there were no significant differences in baseline demographics, PAH-targeted medication use, cardiac output (CO), mean PA pressure (mPAP), or PVR between groups. Also consistent with prior studies,6,19 SSc-PAH patients had increased pro-brain natriuretic peptide levels, reduced resting contractility (end-systolic elastance, Ees, of 0.47±0.04 vs. 1.23±0.15 mmHg/ml, p=0.0002 and preload-recruitable stroke work, MSW, of 22.4±2.1 vs. 26.8±1.5, p=0.058) and reduced resting RV-PA coupling (Ees/Ea of 0.58±0.06 vs. 1.42±0.17, p=0.0004) when compared to IPAH patients. On CMR, SSc-PAH patients had thinner mid-RV free walls than IPAH patients (2.3±0.2 vs. 3.3±0.3 mm, p=0.01), but were otherwise comparable with regards to RV/LV mass, volume, and ejection fraction, indices of RV remodeling and fibrosis, and indices of LV function (Table S3).
Heart-rate dependent change in Contractility, Relaxation, and Calcium handling
Figure 1A shows contractile function assessed by dP/dtmax and the less load-sensitive dP/dtmax/IP (IP = instantaneous pressure), each as a function of incremental atrial pacing rate. Both parameters rose with faster heart rates in both groups (p<0.001), but the force-frequency response was blunted in SSc-PAH subjects (p=0.002 and p=0.03, respectively, for interaction terms). Figure 1B shows individual changes and group differences in RV preload (end-diastolic volume and pressure; EDV, EDP) between lowest and fastest HR. EDV declined in both groups (p<0.01), whereas EDP fell significantly in IPAH only (p<0.001).
Figure 1.
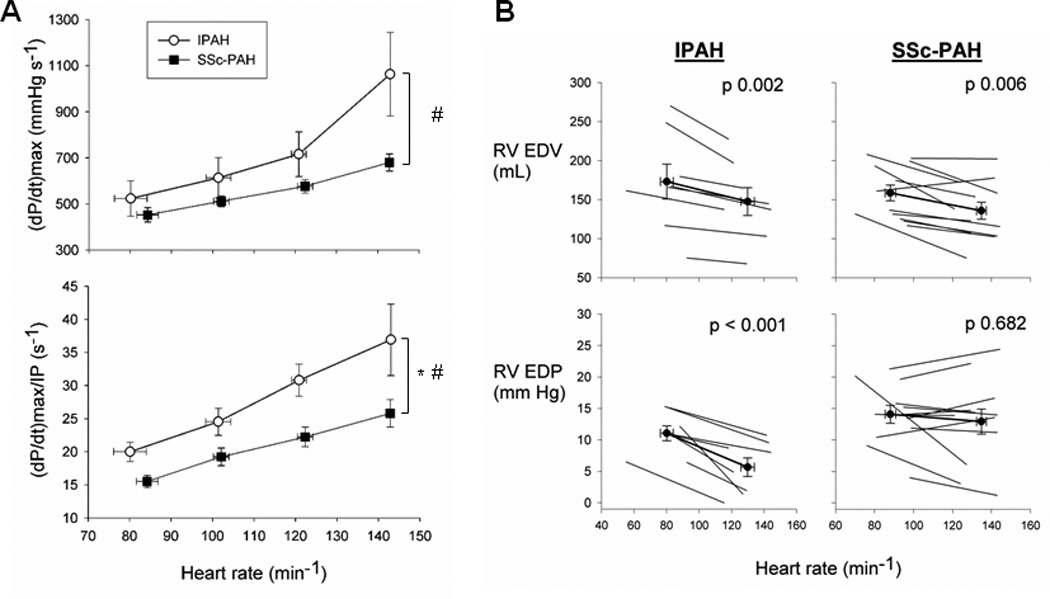
Force-frequency response and diastolic relaxation. (A) Contractility was assessed by dP/dtmax and dP/dtmax normalized to instantaneous pressure developed (dP/dtmax/IP) with escalating pacing rates. * denotes significant difference between disease groups. # denotes significant difference in disease-pacing interaction (pacing effect). SSc-PAH showed diminished contractility and force-frequency response compared to IPAH. The interaction term between groups was significantly different for both dP/dtmax (p=0.002) and dP/dtmax/IP (p=0.03). Additionally, there was a significant disease group difference in dP/dtmax/IP (p=0.006). (B) RV end-diastolic volume (EDV) and pressure (EDP) were charted for each patient in each group. Mean values ± SEM superimposed in bold. IPAH demonstrated decreases in EDP with pacing, while SSc-PAH subjects did not.
A major contributor to the force-frequency relation is recycling of calcium into and out of the sarcoplasmic reticulum.20,21 This can be assessed in vivo by measuring the geometric rate of decay in contractile potentiation following abrupt cessation of atrial pacing (Figure 2A). Plotting dP/dtmax (normalized to steady-state) for beat n+1 versus beat n after stopping pacing yields a linear plot, with the slope being the decay constant, known as recirculation fraction (0.47 or 47% in example, Figure 2B). IPAH patients had a mean recirculation fraction (RF) of 0.50±0.05, similar to that of the normal LV. By contrast, RF for the SSc-PAH group was 0.32±0.05 (p=0.039 vs. IPAH, Figure 2C), consistent with values previously reported for symptomatic LV hypertrophic disease16 and LV systolic heart failure.22
Figure 2.
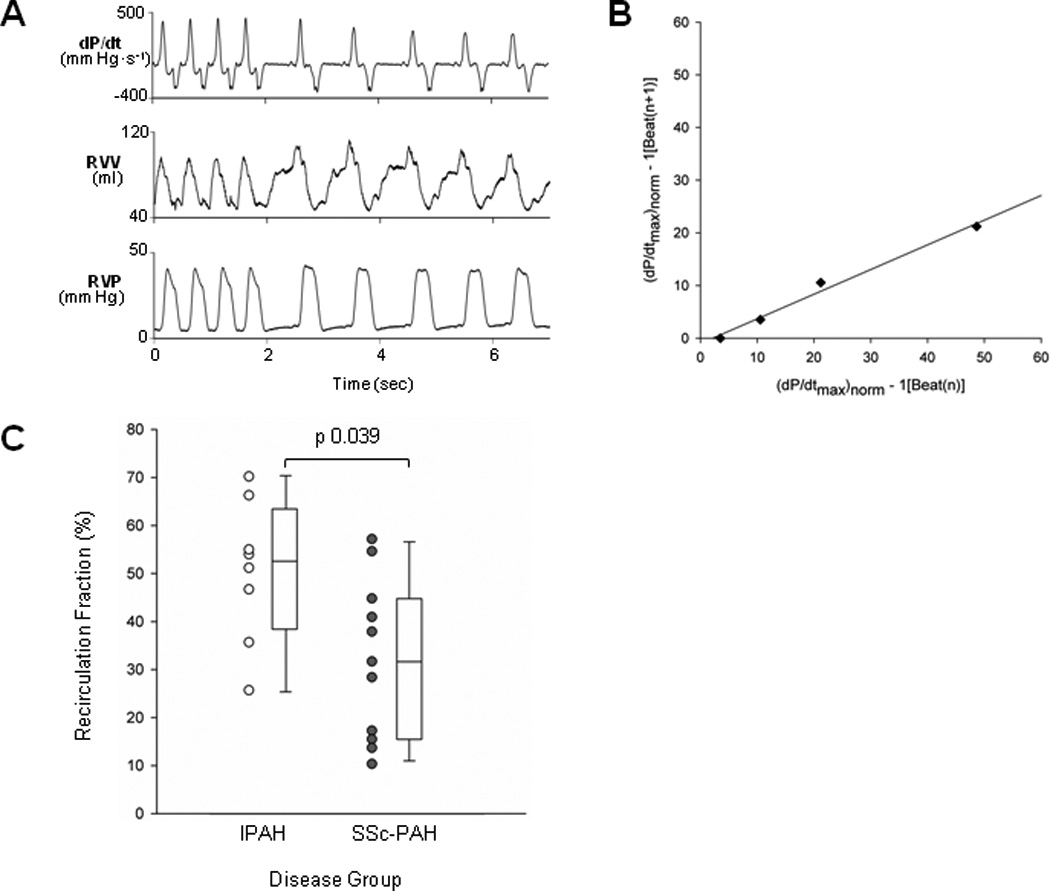
Recirculation fraction (RF). (A) Tracings from an example patient. After the fourth cycle, atrial pacing was terminated and the subsequent five cycles at normal sinus rhythm were recorded. Note RV peak pressure and EDV are nearly identical for post-paced beats. Top panel displays dP/dt. Note that dP/dtmax is greatest on the first post-paced beat and subsequently declines in a geometric fashion. (B) RV dP/dtmax of beat n+1 is plotted versus beat n for post-paced beats. Plotted values are based on pressure derivatives. The slope of this relation provides the RF (0.47 in this example). (C) RF for both disease groups presented as dot and box plots. RF was significantly decreased in SSc-PAH versus IPAH.
RV Functional Reserve and Volume Compensation during Exercise
Table 1 summarizes baseline functional capacity and metabolic data at peak exercise for both groups. There were no significant differences in baseline New York Heart Association (NYHA) functional class makeup or six-minute walk distance between groups. At peak performance, IPAH subjects exercised nearly twice as long as SSc-PAH subjects (11.3±1.6 vs. 6.1±0.8 min; p=0.009) and achieved greater maximum power (66±8 vs. 36±4 W, p=0.004). There were concordant difference trends in peak VO2max and ventilatory efficiency (VE/VCO2 ratio), though these did not reach statistical significance. Both groups exerted equivalent effort as determined by the respiratory exchange ratio (RER).
Table 1.
Baseline Functional Status and Metabolic Data at Peak Exercise.
| IPAH (n = 9) |
SSc-PAH (n = 15) |
p-value | |
|---|---|---|---|
| Baseline Functional Status | |||
| Baseline NYHA Functional Class | |||
| Class I | 1 (11%) | 1 (7%) | 1.00* |
| Class II | 5 (56%) | 6 (40%) | 0.68* |
| Class III | 3 (33%) | 8 (53%) | 0.42* |
| Baseline 6MWD (m) | 1345 ± 206 | 1132 ± 112 | 0.33 |
| 6MWD (% predicted) | 78 ± 10 | 66 ± 6 | 0.36 |
| Metabolic Data at Peak Exercise | |||
| Total exercise time (min) | 11.3 ± 1.6 | 6.1 ± 0.8 | 0.009 |
| Peak VO2max (ml·kg−1·min−1) | 12.9 ± 1.1 | 10.3 ± 0.8 | 0.07 |
| VE/VCO2 ratio | 46.8 ± 7.6 | 59.1 ± 5.9 | 0.10 |
| RER | 0.88 ± 0.03 | 0.93 ± 0.02 | 0.15 |
| Max Watts achieved (W) | 66 ± 8 | 36 ± 4 | 0.004 |
Fisher’s exact test used to compare proportions. NYHA, New York Heart Association; 6MWD, 6 minute walk distance; Peak VO2max, maximal oxygen consumption; VE/VCO2 ratio, minute ventilation-carbon dioxide production slope; RER, respiratory exchange ratio.
Figure 3 displays representative PV loops from exercise stages 0–3 for both groups, as well as from an example patient with a normal RV and no PH (loops from all patients for stage 0–2 are provided in Figures S2A–S2B). With progressive exercise, IPAH patients displayed an upward and slight leftward shift of the end-systolic PV point (upper left corner of the loop), similar to but with less relative shift than the normal RV. By contrast, with SSc-PAH, PV loops shifted rightward reflecting RV dilatation.
Figure 3.
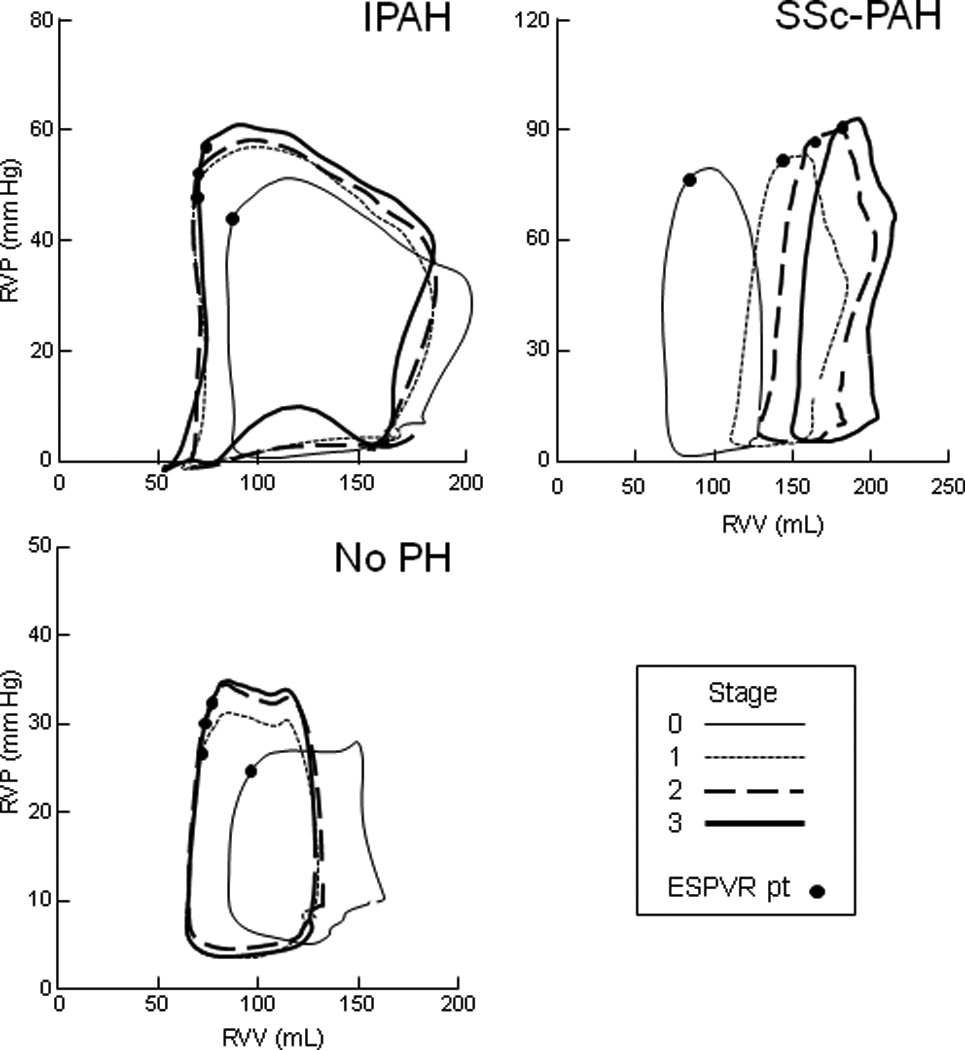
Representative PV loops from rest (stage 0) and exercise stages 1–3 from an IPAH patient and SSc-PAH patient; a patient with no PH was included for comparison. While IPAH subjects augmented contractility and maintained relatively stable volumes, SSc-PAH subjects had difficulty augmenting contractility and instead increased RV volumes, leading to a concurrent right-shift in PV loops. Compared to IPAH, the “no PH” subject showed additional improvement in contractility and RV volume decrease, consistent with published reports of healthy subjects.
Because group differences in peak performance could confound comparisons at peak exertion, statistical comparisons of exercise RV functional reserve between IPAH and SSc-PAH were made at matched, sub-maximal workloads (stage 0–2, 0–25 Watts). Summary data for Ees, Ea and their ratio are shown (Figure 4A; Table 2) along with percent changes of each variable at Stage 2 versus baseline (Figure 4B). Ees rose in IPAH but was virtually unaltered in SSc-PAH (group difference p<0.001, interaction term p=0.042), while Ea increased similarly in both groups. Thus the Ees/Ea ratio declined slightly in SSc-PAH but remained steady in IPAH (group difference p<0.001, interaction term p=0.078).
Figure 4.
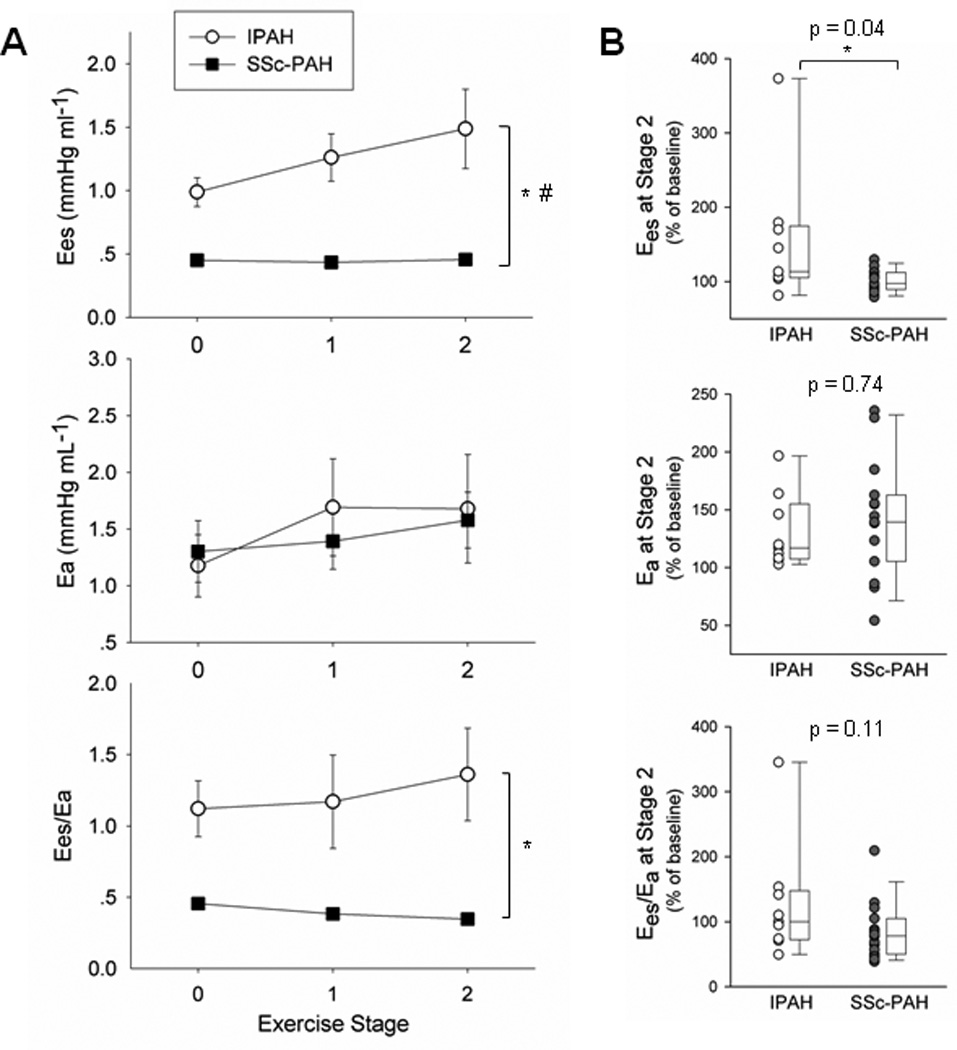
Exercise changes in contractility, afterload, and RV-PA coupling. (A) Ees, Ea, and Ees/Ea were plotted for IPAH and SSc-PAH from exercise stage 0–2, while percent change at stage 2 normalized to baseline for each subject was graphed alongside using dot and box plots (B). Despite similar increases in Ea in both groups, IPAH subjects augmented Ees while SSc-PAH subjects did not. RV-PA coupling (Ees/Ea) was maintained in the former but remained poor in the latter. * denotes significant difference between disease groups. # denotes significant difference in disease-exercise interaction (exercise effect). See Table 2 for p-values.
Table 2.
Disease and Disease-Stage Interaction Differences with Exercise.
| Dependent Variable | Exercise Stage |
IPAH | SSc-PAH | Disease Group Difference |
Disease-Stage Interaction |
|---|---|---|---|---|---|
| Exercise Variable | Mean (SD) | Mean (SD) | (p-value) | (p-value) | |
| Ees (mm Hg·ml−1) | 0 | 0.99 (0.34) | 0.45 (0.14) | < 0.001 | 0.042 |
| 1 | 1.26 (0.56) | 0.43 (0.17) | |||
| 2 | 1.49 (0.94) | 0.46 (0.16) | |||
| Ea (mm Hg·ml−1) | 0 | 1.18 (0.82) | 1.30 (1.05) | 0.836 | 0.169 |
| 1 | 1.69 (1.28) | 1.39 (0.96) | |||
| 2 | 1.68 (1.44) | 1.58 (0.96) | |||
| Ees/Ea ratio | 0 | 1.12 (0.59) | 0.46 (0.17) | < 0.001 | 0.078 |
| 1 | 1.17 (0.98) | 0.38 (0.16) | |||
| 2 | 1.36 (0.97) | 0.35 (0.15) | |||
| ΔESV (ml) | 0 | 0 (0) | 0 (0) | 0.004 | 0.002 |
| 1 | −1 (20) | 27 (26) | |||
| 2 | −6 (22) | 36 (32) | |||
| ΔEDV (ml) | 0 | 0 (0) | 0 (0) | < 0.001 | < 0.001 |
| 1 | −11 (20) | 26 (22) | |||
| 2 | −8 (25) | 31 (25) |
Presented are the mean and standard deviation of each dependent variable (at exercise stages 0–2 for both groups) and p-values for the comparisons of disease group and disease-exercise stage effect (i.e. interaction effect). Corresponding dependent variables are depicted in Figures 4 and 5. Ees, End-systolic elastance; Ea, Effective arterial elastance; Ees/Ea, right ventricular-pulmonary arterial coupling; ESV, End-systolic volume, EDV, End-diastolic volume.
The failure of contractile augmentation during exercise in SSc-PAH patients was offset in part by RV dilation. At the end of stage 2 of exercise, HR, SV, and CO were similar between the two groups (Figure S3A). Yet SSc-PAH patients maintained SV by increasing RV preload, whereas IPAH patients did not dilate (Figure 5A; Table 2). Consequently, RV ejection fraction (EF) was preserved in IPAH, but trended lower in SSc-PAH subjects (p=0.056 at Stage 2, Figure 5B).
Figure 5.
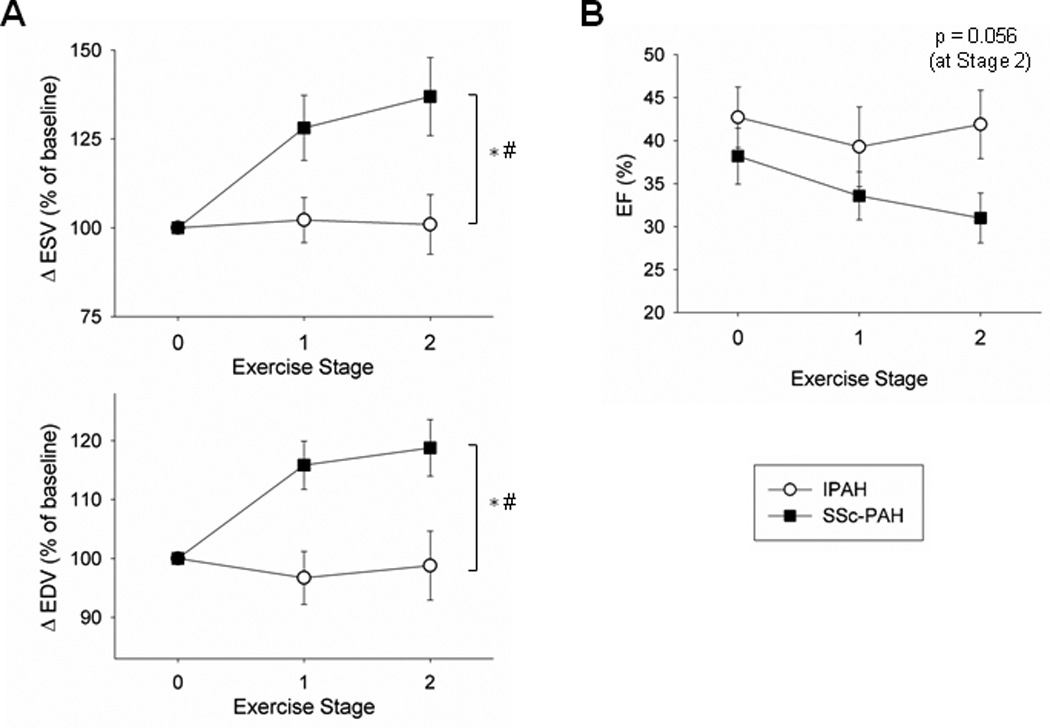
Exercise changes in RV ESV and EDV. (A) Percent change in ESV and EDV were plotted for each group from exercise stage 0–2. SSc-PAH subjects acutely increased ESV and EDV while IPAH subjects maintained stable RV volumes with increasing workload. (B) RV EF also declined in SSc-PAH versus IPAH with increasing exercise, with a trend towards a decrease by stage 2 (p=0.056). * denotes significant difference between two disease groups. # denotes significant difference in disease-exercise interaction (exercise effect). See Table 2 for p-values.
To ensure there was no systematic bias in SV and CO calculations based on PV catheter data during exercise, we compared CO calculated by the calibrated catheter signal to that by direct Fick method. Both were correlated throughout exercise, with no significant difference in the relationship between patient groups (Figure S3B); however, there was a modest systematic underestimation of CO by catheter as CO increased (Figure S3C). Analyses were repeated comparing patients with NYHA functional class III to class I–II patients to test whether any disparities in functional class composition among disease groups may have contributed to between group differences. However, NYHA functional class did not prove to be a significant contributor to any key findings (Figures S4–S6 and Table S4).
Resting RV-PA Coupling ratio predicts RV Dilatation during Exercise
We found a significant correlation between resting RV-PA coupling (Ees/Ea, log transformed to yield a Gaussian distribution) and ventilator efficiency (VE/VCO2), a known correlate of RV functional reserve (r = −0.35; p-value 0.049, adjusted for age and sex, Figure 6A).23 To improve the generalizability of this analysis, we expanded the cohort to include subjects without PAH (total n=33). Ventilatory efficiency also correlated with other indices of RV load and function (Figure S7A) but not LV performance (Figure S7B), similar to other previously reported data.23 We also tested for a correlation between resting coupling and exercise RVSP augmentation (which has been shown to predict mortality in PH7), but did not observe such a correlation among our PH subjects (Figure S7C).
Figure 6.
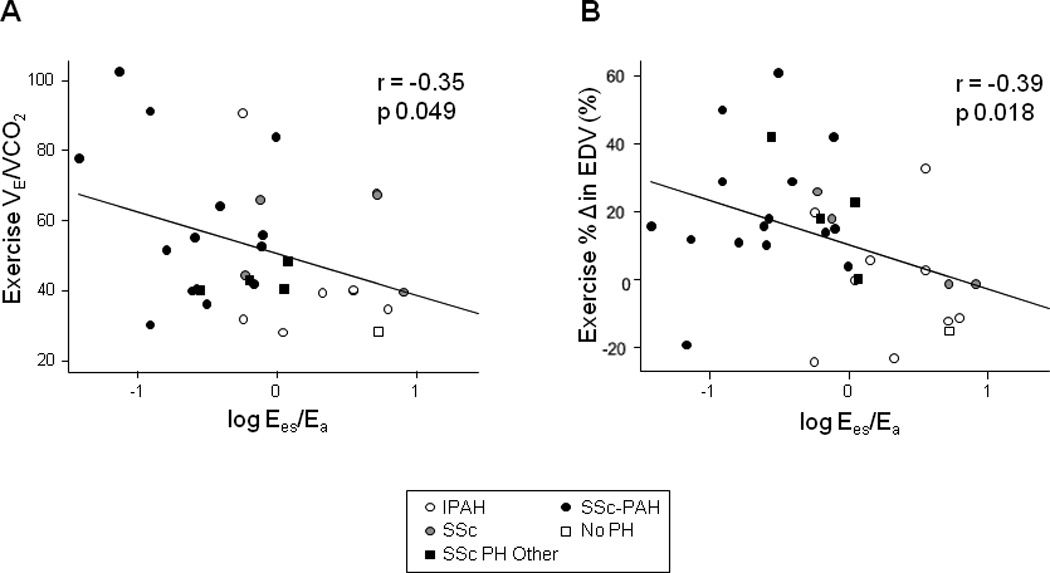
VE/VCO2 and exercise change in EDV correlated with resting RV-PA coupling. Comparison using regression and Pearson correlation coefficients revealed moderate, negative correlations between (A) the log transformation of resting Ees/Ea and VE/VCO2 ratio as well as (B) percent change in EDV at 25W workload (p=0.049 and p=0.018, respectively, adjusted for age and sex).
Given the association between reduced RV-PA coupling and RV volume increase seen in SSc-PAH subjects during exercise (Figures 4–5), we next tested whether coupling at rest predicted exercise-induced EDV change in the larger cohort. Overall, there was indeed a moderate negative correlation between resting Ees/Ea (log transformed) and percent change in EDV with exercise (r = −0.39; p-value 0.018, adjusted for age and sex, Figure 6B).
DISCUSSION
Comparing SSc-PAH to IPAH offers a unique opportunity to investigate deficient RV compensation in PAH, given the disproportionate rates of RV failure and death in SSc-PAH.3,4 Commonly used hemodynamic and imaging measures of resting RV function (e.g. ejection fraction) have not adequately explained why patients with SSc-PAH are more vulnerable than those with IPAH. More recently, we showed that while SSc-PAH subjects have similar pulmonary vascular load, their RV resting contractility is depressed.6 However, the RV reserve response, perhaps more important given its ability to aid in assessment and prognosis beyond resting function alone, was unknown in SSc-PAH as compared to IPAH, and no prior study had determined this. Here, we used continuous PV analysis, and show for the first time that despite similar resting RV function, LV function, and overall chamber morphology, SSc-PAH subjects have markedly depressed RV functional reserve reflected by depressed force-frequency responsiveness, diminished contractile and diastolic reserve, and abnormal RV-PA coupling during exercise. This highlights the central role that intrinsic RV dysfunction plays in SSc-PAH. Furthermore, abnormal RV-PA coupling predicted abnormal RV dilation responses during exercise not only in the primary PAH comparison but also in a larger cohort that included subjects without PAH.
RV Contractility and Diastolic Dysfunction and its Relationship to Calcium Cycling
The force-frequency response (FFR) of the normal and diseased LV has been well studied, but very few analyses have been reported for the RV, and none specific to patients with PAH.24–27 The normal LV augments contractility by approximately 100% (across a similar HR rise as was tested here), and this response is markedly depressed in patients with LV hypertrophic heart disease.16 Data on the normal human RV FFR remains lacking, but in the current study, the percent increase in RV FFR in IPAH was similar to that seen in the LV,16 while in SSc-PAH, RV FFR was significantly blunted. In addition to FFR abnormalities, SSc-PAH patients also maintained higher end-diastolic pressures at faster rates despite the similar declines in RV preload, indicating inadequate diastolic relaxation during tachycardia and impaired RV diastolic reserve. This is intriguing because neither our prior study6 nor the current one (Table S2) detected significant differences in resting RV diastolic function between IPAH and SSc-PAH.
A major regulator of the FFR is calcium cycling into and out of the sarcoplasmic reticulum (SR), and SR dysfunction is a prominent contributor to a reduced FFR.20,21,25 The recirculation fraction is an admittedly indirect way to assess SR calcium cycling, but one that has proven useful when direct measurements are impossible—as in this in vivo human study. It has been validated by studies showing its modulation by activators and inhibitors of SR calcium uptake.28,29 In healthy humans the RF is ~50%, but declines to ~35% in both human LV hypertrophy and systolic heart failure.16,22 Assessment of RF in the normal RV is limited to a few animal studies,30,31 with one also reporting LV values that were similar to those of the RV.31 It is interesting that the RF in hypertrophied RVs of IPAH patients was also ~50%, as this suggests these RVs were still fairly compensated, consistent with their stronger FFR and exercise-induced contractile reserve. RF in SSc-PAH patients was more like human LV failure, revealing greater underlying RV disease in this syndrome. These data suggest that drugs augmenting calcium cycling might prove beneficial to SSc-PAH patients.
RV Reserve and Compensatory Mechanisms during Exercise
Exercise stimulates the cardiovascular system to increase HR, augment RV and LV contractility, vasodilate peripheral skeletal vascular beds, and reduce venous capacitance. The latter offsets tachycardia-induced declines in diastolic filling as observed with pure atrial pacing. In the pulmonary vasculature, pulmonary vascular resistance typically declines despite a marked rise in CO; this in turn blunts the mPAP increase seen by the RV. However, in PAH patients, PVR does not fall appropriately, and may even rise during exercise, increasing the load imposed on the RV.32 This puts even more premium on the capacity of the RV to enhance contractility.
A few prior studies have compared RV functional reserve in PH patients to healthy controls.11,12 Spruijt and colleagues11 used single-beat Ees estimates and concluded that while contractility rose during exercise in normal subjects, it failed to do so in a cohort of primarily PAH patients, thereby reducing RV-PA coupling. Claessen et al12 exercised healthy controls and patients with chronic thromboembolic PH (CTEPH) while imaging them using CMR. RV EF increased and RV end-systolic volume (ESV) decreased in controls while the opposite occurred in CTEPH patients. In the present study, although we could not study truly normal subjects given the nature of the protocol, it is likely that our IPAH patients had reduced reserve compared to a healthy group. It is noted that our IPAH results differ slightly from the Spruijt study, since in our cohort, Ees rose and RV-PA coupling was preserved with exercise. That is not to suggest that our IPAH subjects are normal, as they demonstrate several abnormalities including a rise in PVR with exercise, diminished exercise endurance, peak oxygen consumption, and ventilatory efficiency, and less RV volume decline as observed in normal subjects.11,12 The discrepancy between studies may relate to differences in the specific cohorts studied or the model-based calculation of RV Ees used in the Spruijt study. The latter may underestimate Ees in disease states such as PH or with dynamic changes in RV function.33–35
SSc-PAH subjects demonstrated deficiencies in Ees augmentation and RV-PA coupling during exercise when compared to IPAH. This correlated with a disparity in RV end-systolic and end-diastolic volume change with exercise. The discrepancy in volume change is consistent with a preload mechanism (i.e. Frank-Starling effect, or heterometric adaption) being preferentially invoked in SSc-PAH when contractility (homeometric adaption) and afterload reduction mechanisms are blunted. A similar difference is observed in the LV with aging, where a decline in contractile and chronotropic reserve and increase in vascular stiffening (pulsatile load) is countered by greater LV dilation during exercise in the elderly, enabling them to achieve a similar CO rise as in younger individuals.36 The RV is also known to dilate with endurance training, although this is a chronic change to sustained exercise.37 Transient ventricular dilation would not induce heart failure itself, though in the RV in the setting of abnormal afterload (e.g. PAH) and underlying myocardial defects, it is possible that this would only worsen maladaptive remodeling and outcomes in SSc-PAH.
The cause of the RV defect in SSc-PAH remains speculative. Interstitial fibrosis is observed in scleroderma myocardium,38 although how this would relate to depressed contractile reserve per se is uncertain. For the cohort studied here, CMR analysis revealed no differences in delayed gadolinium uptake (to suggest a difference in RV fibrosis) or RV remodeling, aside from a difference in mid-RV free wall thickness. Perhaps related, in a larger cohort, RV mass has been shown to be lower in SSc-PAH compared to IPAH when adjusted for pulmonary vascular load.39 Other possibilities include weakened myocyte contractility and/or abnormal angiogenesis in SSc; these are being studied in an ongoing investigation.
Right ventricular-pulmonary vascular (RV-PA) coupling is a powerful, load-independent indicator of RV function in PH that detects RV dysfunction well before overt decreases in RV EF.6,35 Accurate clinical measurement of this ratio, however, has required invasive PV measurements.13 Various non-invasive measurements of RV functional reserve have been proposed. Here, resting RV-PA coupling did not correlate with one such measurement—exercise change in RVSP—although our PH cohort was small and unlike the relevant derivation cohorts.7,40 On the other hand, RV-PA coupling did correlate with ventilatory efficiency, which has been shown to correlate with RV functional reserve in conditions such as left-sided heart failure and pulmonary hypertension.23,41 The correlation reported here between depressed RV-PA coupling and exercise-induced RV dilation suggests a potentially new method to estimate coupling. With recent advances in three-dimensional echocardiography or CMR during exercise,12,42,43 one could assess RV dilatation with stress and use this as a surrogate for RV-PA coupling. Further work is needed to validate these findings in a range of healthy and diseased subjects and correlate them with clinical outcomes.
Study Limitations
The invasive nature of our protocol precluded analysis of healthy controls. We also had an unequal sample size between IPAH and SSc-PAH groups, which reflect some referral bias. However, we used non-parametric methods to deal with unequal variance as necessary. Our CMR protocol did not include T1-mapping to assess RV extracellular volume, which could have shed light on differences in fibrosis. The volume catheter provides an uncalibrated signal, and some uncertainty in calibration exists, particularly during maneuvers such as exercise. However, we found no systematic disparities between patient groups and comparisons of direct Fick measured and catheter-based CO, so calibration noise did not likely contribute to group disparities. Furthermore, volume calibration had no bearing on relative changes in volume or RV-PA coupling for any given subject.
CONCLUSION
While patients with IPAH can mount significant improvement in contractility with both heart rate and exertional stress, patients with SSc-PAH cannot. This in part is likely related to depressed calcium cycling in the latter group. Likely as a consequence, RV end-diastolic and end-systolic volumes substantially increase in SSc-PAH patients during exercise, whereas these are minimally altered in IPAH patients. The marked differences in RV functional reserve between groups despite similarities in pulmonary vascular load direct our focus towards innate RV pathology in SSc-PAH. Finally, a significant correlation existed between resting RV-PA uncoupling and increased chamber volumes during exercise, which held true even in a larger cohort. Assessing RV volume change with exercise may present a novel avenue for non-invasive assessment of RV-PA coupling.
Supplementary Material
Clinical Perspectives.
This prospective study shows that the right ventricle (RV) in systemic sclerosis-associated pulmonary arterial hypertension (SSc-PAH) exhibits marked deficiencies in RV functional reserve when compared to the RV in patients with idiopathic PAH (IPAH). This was found despite both groups having similar standard measures of RV function, morphology, and pulmonary vascular load, although SSc-PAH subjects did have reduced RV contractility and abnormal RV-pulmonary arterial (PA) coupling as reflected by pressure-volume analysis. SSc-PAH subjects demonstrated a blunted RV systolic and diastolic response to increasing heart rate (force-frequency response) and the recirculation fraction, an in vivo reflector of calcium cycling, was depressed. During supine bicycle exercise, SSc-PAH subjects failed to augment RV contractility in response to increases in RV afterload, leading to progressive RV-PA uncoupling. This was countered by increases in RV systolic and diastolic volume compatible with use of Frank-Starling compensation. These results highlight the profound role that intrinsic RV pathology plays in SSc-PAH patients, who have disproportionately poor outcomes as compared to IPAH. Acute RV dilation in exercising SSc-PAH subjects correlated with poor resting RV-PA coupling. While the latter is recognized to have prognostic value, it remains difficult to measure non-invasively. Our results show that image-analysis of RV dilation during submaximal exercise may provide such an assessment.
Acknowledgments
The authors acknowledge the Johns Hopkins Cardiovascular Interventional Laboratory, and in particular Stacie Richardson, for research protocol assistance.
Funding Sources: The study was supported by the NIH-NHLBI (5-R01-HL114910 and L30 HL110304, T32-HL007227-40) and the Scleroderma Research Foundation.
Footnotes
Disclosures: None.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Vonk Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, Girgis RE, Hassoun PM. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:252–260. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Cano MJ, Escribano P, Alonso R, Delgado J, Carreira P, Velazquez T, Sanchez MAG, Sáenz de la Calzada C. Comparison of baseline characteristics and survival between patients with idiopathic and connective tissue disease-related pulmonary arterial hypertension. J Heart Lung Transplant. 2009;28:621–627. doi: 10.1016/j.healun.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Hassoun PM. The right ventricle in scleroderma (2013 Grover Conference Series) Pulm Circ. 2015;5:3–14. doi: 10.1086/679607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, Boyce D, Kelemen BW, Bacher AC, Shah AA, Hummers LK, Wigley FM, Russell SD, Saggar R, Saggar R, Maughan WL, Hassoun PM, Kass DA. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6:953–963. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grünig E, Tiede H, Enyimayew EO, Ehlken N, Seyfarth H-J, Bossone E, D'Andrea A, Naeije R, Olschewski H, Ulrich S, Nagel C, Halank M, Fischer C. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation. 2013;128:2005–2015. doi: 10.1161/CIRCULATIONAHA.113.001573. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg FC, Arzt M, Lange T, Schroll S, Pfeifer M, Wensel R. Impact of right ventricular reserve on exercise capacity and survival in patients with pulmonary hypertension. Eur J Heart Fail. 2013;15:771–775. doi: 10.1093/eurjhf/hft044. [DOI] [PubMed] [Google Scholar]
- 9.Guazzi M, Naeije R, Arena R, Corrà U, Ghio S, Forfia P, Rossi A, Cahalin LP, Bandera F, Temporelli P. Echocardiography of Right Ventriculoarterial Coupling Combined With Cardiopulmonary Exercise Testing to Predict Outcome in Heart Failure. Chest. 2015;148:226–234. doi: 10.1378/chest.14-2065. [DOI] [PubMed] [Google Scholar]
- 10.Lewis GD, Bossone E, Naeije R, Grünig E, Saggar R, Lancellotti P, Ghio S, Varga J, Rajagopalan S, Oudiz R, Rubenfire M. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–1479. doi: 10.1161/CIRCULATIONAHA.112.000667. [DOI] [PubMed] [Google Scholar]
- 11.Spruijt OA, de Man FS, Groepenhoff H, Oosterveer F, Westerhof N, Vonk Noordegraaf A, Bogaard H-J. The effects of exercise on right ventricular contractility and right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. 2015;191:1050–1057. doi: 10.1164/rccm.201412-2271OC. [DOI] [PubMed] [Google Scholar]
- 12.Claessen G, La Gerche A, Dymarkowski S, Claus P, Delcroix M, Heidbuchel H. Pulmonary vascular and right ventricular reserve in patients with normalized resting hemodynamics after pulmonary endarterectomy. J Am Heart Assoc. 2015;4:e001602. doi: 10.1161/JAHA.114.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houston BA, Tedford RJ. Stressing the stepchild: assessing right ventricular contractile reserve in pulmonary arterial hypertension. Eur Respir J. 2015;45:604–607. doi: 10.1183/09031936.00233614. [DOI] [PubMed] [Google Scholar]
- 14.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, Matucci-Cerinic M, Naden RP, Medsger TA, Carreira PE, Riemekasten G, Clements PJ, Denton CP, Distler O, Allanore Y, Furst DE, Gabrielli A, Mayes MD, van Laar JM, Seibold JR, Czirjak L, Steen VD, Inanc M, Kowal-Bielecka O, Müller-Ladner U, Valentini G, Veale DJ, Vonk MC, Walker UA, Chung L, Collier DH, Ellen Csuka M, Fessler BJ, Guiducci S, Herrick A, Hsu VM, Jimenez S, Kahaleh B, Merkel PA, Sierakowski S, Silver RM, Simms RW, Varga J, Pope JE. 2013 classification criteria for systemic sclerosis: an ACR/EULAR collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M Authors/Task Force Members. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Eur Heart J. 2015;10:1–58. [Google Scholar]
- 16.Liu CP, Ting CT, Lawrence W, Maughan WL, Chang MS, Kass DA. Diminished contractile response to increased heart rate in intact human left ventricular hypertrophy. Systolic versus diastolic determinants. Circulation. 1993;88:1893–1906. doi: 10.1161/01.cir.88.4.1893. [DOI] [PubMed] [Google Scholar]
- 17.Nozawa T, Cheng CP, Noda T, Little WC. Effect of exercise on left ventricular mechanical efficiency in conscious dogs. Circulation. 1994;90:3047–3054. doi: 10.1161/01.cir.90.6.3047. [DOI] [PubMed] [Google Scholar]
- 18.Maughan WL, Sunagawa K, Burkhoff D, Graves WL, Hunter WC, Sagawa K. Effect of heart rate on the canine end-systolic pressure-volume relationship. Circulation. 1985;72:654–659. doi: 10.1161/01.cir.72.3.654. [DOI] [PubMed] [Google Scholar]
- 19.Mathai SC, Bueso M, Hummers LK, Boyce D, Lechtzin N, Le Pavec J, Campo A, Champion HC, Housten T, Forfia PR, Zaiman AL, Wigley FM, Girgis RE, Hassoun PM. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. Eur Respir J. 2010;35:95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 20.Alpert NR, Leavitt BJ, Ittleman FP, Hasenfuss G, Pieske B, Mulieri LA. A mechanistic analysis of the force-frequency relation in non-failing and progressively failing human myocardium. Basic Res Cardiol. 1998;93:23–32. doi: 10.1007/s003950050200. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt U, Hajjar RJ, Gwathmey JK. The force-interval relationship in human myocardium. J Card Fail. 1995;1:311–321. doi: 10.1016/1071-9164(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 22.Seed WA, Noble MI, Walker JM, Miller GA, Pidgeon J, Redwood D, Wanless R, Franz MR, Schoettler M, Schaefer J. Relationships between beat-to-beat interval and the strength of contraction in the healthy and diseased human heart. Circulation. 1984;70:799–805. doi: 10.1161/01.cir.70.5.799. [DOI] [PubMed] [Google Scholar]
- 23.Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail. 2008;1:227–233. doi: 10.1161/CIRCHEARTFAILURE.108.785501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burkhoff D, Yue DT, Franz MR, Hunter WC, Sunagawa K, Maughan WL, Sagawa K. Quantitative comparison of the force-interval relationships of the canine right and left ventricles. Circ Res. 1984;54:468–473. doi: 10.1161/01.res.54.4.468. [DOI] [PubMed] [Google Scholar]
- 25.Banijamali HS, Gao WD, MacIntosh BR, Keurs ter HE. Force-interval relations of twitches and cold contractures in rat cardiac trabeculae. Effect of ryanodine. Circ Res. 1991;69:937–948. doi: 10.1161/01.res.69.4.937. [DOI] [PubMed] [Google Scholar]
- 26.Rossman EI, Petre RE, Chaudhary KW, Piacentino V, Janssen PML, Gaughan JP, Houser SR, Margulies KB. Abnormal frequency-dependent responses represent the pathophysiologic signature of contractile failure in human myocardium. J Mol Cell Cardiol. 2004;36:33–42. doi: 10.1016/j.yjmcc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Ståhlberg M, Kessels R, Linde C, Braunschweig F. Acute haemodynamic effects of increase in paced heart rate in heart failure patients recorded with an implantable haemodynamic monitor. Europace. 2011;13:237–243. doi: 10.1093/europace/euq354. [DOI] [PubMed] [Google Scholar]
- 28.Mörner SE, Arlock P. Mechanical and electrophysiological effects of milrinone on the force-frequency relationship in mammalian myocardium. Acta Physiol Scand. 1994;150:125–132. doi: 10.1111/j.1748-1716.1994.tb09669.x. [DOI] [PubMed] [Google Scholar]
- 29.Juggi JS. Recirculation fraction of the activator Ca2+: index of the extent of Ca2+ loading of rat myocardium during ischemia-reperfusion. Can J Physiol Pharmacol. 1996;74:116–123. [PubMed] [Google Scholar]
- 30.Versluis JP, Heslinga JW, Sipkema P, Westerhof N. Contractile reserve but not tension is reduced in monocrotaline-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2004;286:H979–H984. doi: 10.1152/ajpheart.00536.2002. [DOI] [PubMed] [Google Scholar]
- 31.Black A, Ewert D, Mulligan LJ. Decay of postextrasystolic potentiation in the left and right ventricles of intact canine hearts. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2904–2906. doi: 10.1109/IEMBS.2009.5334445. [DOI] [PubMed] [Google Scholar]
- 32.Naeije R, Vanderpool R, Dhakal BP, Saggar R, Saggar R, Vachiery J-L, Lewis GD. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187:576–583. doi: 10.1164/rccm.201211-2090CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambermont B, Segers P, Ghuysen A, Tchana-Sato V, Morimont P, Dogne J-M, Kolh P, Gerard P, D'Orio V. Comparison between single-beat and multiple-beat methods for estimation of right ventricular contractility. Crit Care Med. 2004;32:1886–1890. doi: 10.1097/01.ccm.0000139607.38497.8a. [DOI] [PubMed] [Google Scholar]
- 34.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol. 2003;284:H1625–H1630. doi: 10.1152/ajpheart.01023.2002. [DOI] [PubMed] [Google Scholar]
- 35.Vanderpool RR, Pinsky MR, Naeije R, Deible C, Kosaraju V, Bunner C, Mathier MA, Lacomis J, Champion HC, Simon MA. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart. 2015;101:37–43. doi: 10.1136/heartjnl-2014-306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodeheffer RJ, Gerstenblith G, Becker LC, Fleg JL, Weisfeldt ML, Lakatta EG. Exercise cardiac output is maintained with advancing age in healthy human subjects: cardiac dilatation and increased stroke volume compensate for a diminished heart rate. Circulation. 1984;69:203–213. doi: 10.1161/01.cir.69.2.203. [DOI] [PubMed] [Google Scholar]
- 37.Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol. 2008;104:1121–1128. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- 38.Fernandes F, Ramires FJA, Arteaga E, Ianni BM, Bonfá ESDO, Mady C. Cardiac remodeling in patients with systemic sclerosis with no signs or symptoms of heart failure: an endomyocardial biopsy study. J Card Fail. 2003;9:311–317. doi: 10.1054/jcaf.2003.51. [DOI] [PubMed] [Google Scholar]
- 39.Kelemen BW, Mathai SC, Tedford RJ, Damico RL, Corona-Villalobos C, Kolb TM, Chaisson NF, Harris TH, Zimmerman SL, Kamel IR, Kass DA, Hassoun PM. Right ventricular remodeling in idiopathic and scleroderma-associated pulmonary arterial hypertension: two distinct phenotypes. Pulm Circ. 2015;5:327–334. doi: 10.1086/680356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaouat A, Sitbon O, Mercy M, Ponçot-Mongars R, Provencher S, Guillaumot A, Gomez E, Selton-Suty C, Malvestio P, Regent D, Paris C, Hervé P, Chabot F. Prognostic value of exercise pulmonary haemodynamics in pulmonary arterial hypertension. Eur Respir J. 2014;44:704–713. doi: 10.1183/09031936.00153613. [DOI] [PubMed] [Google Scholar]
- 41.Schwaiblmair M, Faul C, Scheidt von W, Berghaus TM. Ventilatory efficiency testing as prognostic value in patients with pulmonary hypertension. BMC Pulm Med. 2012;12:23. doi: 10.1186/1471-2466-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claessen G, La Gerche A, Voigt J-U, Dymarkowski S, Schnell F, Petit T, Willems R, Claus P, Delcroix M, Heidbuchel H. Accuracy of Echocardiography to Evaluate Pulmonary Vascular and RV Function During Exercise. JACC Cardiovasc Imaging. 2015 Oct 16; doi: 10.1016/j.jcmg.2015.06.018. pii: S1936-878X(15)00706-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Wang Y, Zhai Z, Guo X, Yang Y, Lu X. Real-Time Three-Dimensional Echocardiography to Assess Right Ventricle Function in Patients with Pulmonary Hypertension. PLoS ONE. 2015;10:e0129557. doi: 10.1371/journal.pone.0129557. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


