Abstract
Methylmercury (MeHg) is a global contaminant of concern and human exposures are largely realized via seafood consumption. While it is assumed that 95 to 100% of the ingested MeHg from seafood reaches systemic circulation, recent in vitro studies have yielded results to suggest otherwise. Of the published studies to have characterized the bioaccessibility or bioavailability of MeHg from seafood, only a handful of seafood species have been characterized, there exists tremendous variability in data within and across species, few species of relevance to North America have been studied, and none of the in vitro studies have adapted results to an epidemiology study. The objective of the current study was two-fold: a) to characterize in vitro MeHg bioaccessibility and bioavailability from ten commonly consumed types of seafood in North America; and b) to apply the bioaccessibility and bioavailability data from the in vitro study to an existing human MeHg exposure assessment study. Raw seafood samples (cod, crab, halibut, salmon, scallop, shrimp, tilapia, and three tuna types: canned light, canned white, fresh) were purchased in Montreal and their MeHg concentrations generally overlapped with values reported elsewhere. The bioaccessibility of MeHg from these samples ranged from 50.1±19.2 (canned white tuna) to 100% (shrimp and scallop) of the amount measured in the raw undigested sample. The bioavailability of MeHg from these samples ranged from 29.3±10.4 (crab) to 67.4±9.7% (salmon) of the value measured in the raw undigested sample. There were significant correlations between the initial MeHg concentration in seafood with the percent of that Hg that was bioaccessible (r= -0.476) and bioavailable (r=-0.294). When the in vitro data were applied to an existing MeHg exposure assessment study, the estimated amount of MeHg absorbed into systemic circulation decreased by 25% and 42% when considering bioaccessibility and bioavailability, respectively. When the in vitro data were integrated into a regression model relating dietary MeHg intake from seafood with hair and blood Hg biomarkers, there were no differences in key model parameters when comparing the default model (that assumes 100% bioavailability) with models adjusted for the in vitro bioaccessibility and bioavailability data. In conclusion this work adds to a growing number of studies that together suggest that MeHg bioavailability from seafood may be less than 100%, but also documents the challenges when integrating such in vitro data into human exposure and risk assessments.
Keywords: Mercury, Hg, fish, shellfish, diet, biological availability, bioavailability, Caco-2 cells, risk assessment
1.0 Introduction
Mercury (Hg) is a global contaminant of concern to human and environmental health. Humans are exposed to Hg mainly via seafood consumption given that all seafood is contaminated to some degree with methylmercury (MeHg). Balancing the risks and benefits of seafood consumption is challenging because of MeHg contamination (Mergler et al., 2007). On one hand, seafood is a source of high quality protein and micronutrients. On the other hand, MeHg is a proven neurodevelopmental toxicant and there is increasing evidence that it may also affect the cardiovascular and immune systems (Somers et al., 2015; Goodrich et al., 2013).
To assess the health risks associated with MeHg exposure standard models are used. These models consider the concentration of MeHg in edible portions of seafood tissue as well as information regarding seafood portion size and consumption frequency. Additionally, the risk assessment models utilize a correction factor for the amount of ingested MeHg that is estimated to reach systemic circulation. This correction factor is assumed to be 95% to 100%. The assumption that nearly all ingested MeHg is absorbed into systemic circulation is based primarily on two relatively older studies that used radioisotopes. The first study exposed three middle-aged male volunteers to aqueous methyl mercuric nitrate (MeHgNO3) (Aberg et al., 1969). The second study included 15 volunteers who were fed small portions (10 g) of seafood spiked with MeHgNO3 (Miettinen et al., 1971). Both studies obtained an absorption rate of about 95%. Relevant limitations of these studies were that they had small sample sizes, focused on nitrates of Hg (versus more relevant forms such as sulfur-bound), and assessed acute exposures which do not reflect a realistic exposure pattern.
A growing body of evidence has questioned the validity of assuming that 95-100% of the ingested MeHg reaches systemic circulation. Of note, results from recent in vitro studies have suggested that less than 95 to 100% of the MeHg found in seafood is absorbed (Table 1). Using simulated human gastrointestinal tract models these studies have characterized the bioaccessibility of MeHg (i.e., fraction of ingested MeHg that is solubilized into the gastrointestinal enzyme solution). For example, the bioaccessibility of MeHg from swordfish and tuna ranged from 38 to 83% and 9 to 95% respectively (Cabanero et al., 2004; Cabanero et al., 2007; He and Wang, 2011; Calatayud et al., 2012; Wang et al., 2013). A study on Arctic char found the bioaccessibility of total Hg (THg) ranged from 33 to 95% (Laird et al., 2009). In terms of the MeHg bioavailability across intestinal cells following seafood digestion (i.e., fraction of ingested MeHg absorbed into/across the intestinal epithelium into systemic circulation), to our knowledge, only one recent in vitro study has examined for this aspect (in swordfish), and found MeHg bioavailability was less than 100% (Calatayud et al., 2012). Studying the proper chemical form of MeHg is also important for risk assessment. For example, MeHg from fish undergoing gastric digestion appears to remain as MeHg-cysteine, without forming MeHgCl (George et al. 2008).Tissue accumulation of MeHg via dietary intake is higher when rats are fed uncontaminated fish spiked with MeHgCl compared to fish that are contaminated with MeHg from the environment (Berntssen et al. 2004), raising questions about studies using chemical forms of mercury other than fish that has been naturally contaminated.
Table 1.
Comparison of in vitro mercury bioaccessibility and bioavailability data of ten most commonly consumed types of seafood in North America. Data are reported as mean ± SD. Values followed by an asterisk (*) are methylmercury data, and all other data are total mercury values. Grey shaded cells indicate that, to our knowledge, no published data are available.
| Seafood type | Bioaccessibility | Bioavailability | Reference | ||
|---|---|---|---|---|---|
|
| |||||
| Total (ng/g w.w.) | Percentage (%) | Total (ng/g w.w.) | Percentage (%) | ||
| Cod | |||||
| Crab | |||||
| Halibut | |||||
| Salmon | 25.6±2.9 | 89.8±0.1 | Costa et al., 2015 | ||
| 46±22; 49±21 | Laird et al., 2013 | ||||
| 25.1±1.6; 29.9±3.5 | 102; 106 | Calatayud et al., 2012 | |||
| Scallop | |||||
| Shrimp | 7.9±0.9; 17±0.5; 16±0.8 | 92; 86; 75 | Calatayud et al., 2012 | ||
| Tilapia | 33; 13.1* | 42.1; 55.5* | Wang et al., 2013 | ||
| Tuna (canned light) | |||||
| Tuna (canned white) | 60±50; 40±40; 40±40*; 40±50* | 18±4; 20±5; 18±4*; 29±10* | Afonso et al., 2015 | ||
| Tuna (fresh) | 680±780; 570±650* | 78±6; 78±10* | Afonso et al., 2015 | ||
| 474±11; 516±30* | 94.8; 132.2* | Ouedrago & Amyot, 2011 | |||
| <20 | Cabanero et al., 2007 | ||||
| 106±11 | 9 | Cabanero et al., 2004 | |||
Only a handful of seafood species have been characterized for the bioaccessibility or bioavailability of MeHg from seafood in previously published studies, which have shown that there exists tremendous variability in the bioaccessibility data both within and across species. Of particular relevance to North America, of the ten most commonly consumed types of seafood (Groth, 2010; Goodrich et al., 2016), MeHg bioaccessibility data exists for only three of them and no MeHg bioavailability data has been described (Table 1). Importantly, none of the in vitro studies have applied their results towards human Hg exposure assessment. The objectives of the current study were two-fold in order to address the aforementioned gaps. The first objective was to characterize in vitro MeHg bioaccessibility and bioavailability from seafood commonly consumed in North America. The guiding hypotheses were that bioaccessibility and bioavailability of MeHg will be less than 100%, and that these characteristics will vary across the types of seafood. The second objective was to apply the bioaccessibility and bioavailability data from the in vitro study to an existing epidemiological study from which we have previously characterized Hg exposure (Goodrich et al., 2016). The underlying hypothesis for this objective was that inclusion of seafood-specific MeHg bioaccessibility and bioavailability factors will strengthen the relationship between estimated Hg uptake from seafood and biomarkers of Hg exposure.
2.0 Methods
2.1 Seafood Samples
Following a review of the literature, ten types of seafood were selected on the basis of their high consumption in North America (Figure 1; Groth, 2010; Goodrich et al., 2016). For each type of seafood, fresh samples (100 g) from six different individual fish or shellfish were purchased from local markets (Montreal, Canada) between December 2013 and July 2014, and each were studied individually throughout the experiment. Skin and bones were removed, and the edible portions (muscle) of the raw samples were individually homogenized by subjecting them to three 30 s bursts in a kitchen coffee grinder (100 g of seafood muscle and equivalent of Milli-Q water). Samples were stored at -20 °C until dual-phase in vitro gastrointestinal digestion.
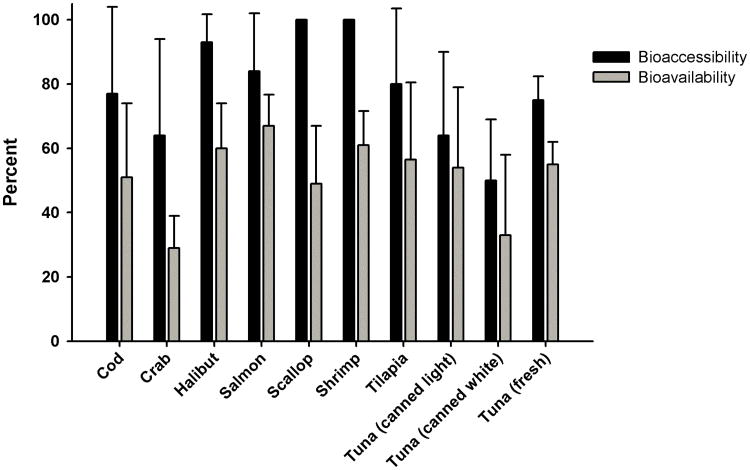
Figure 1.
Bioaccessibility and bioavailability of methylmercury as a percentage of methylmercury concentration measured in the initial (raw, pre-digested) seafood muscle. Percentage data are reported as mean ± SD (n=6/type of seafood).
2.2 In Vitro Bioaccessibility Assay
The bioaccessibility of MeHg from the selected seafood was determined by dual-phase in vitro gastrointestinal digestion based on the physiologically based extraction test (PBET) (Ruby et al., 1996; Ouédrago and Amyot, 2011; Calatayud et al., 2012). All chemicals were purchased from Sigma Aldrich unless indicated. In brief, to each g of seafood muscle tissue, 29.5 mL of 0.1M HCl was added and the mixture was subjected to temperature equalization for 30 min in order to optimize enzymatic activity. Next, 500 μL of freshly prepared gastric enzyme solution (0.0375 g porcine pepsin enzyme/g seafood muscle tissue; enzymatic activity 944 U/mg protein; pH adjusted to 2± 0.2 using 1N HCl) was added and the mixture was incubated in an orbital shaker (120 strokes/min) at 37 °C for one h. The pH of the gastric digests was then raised to 5.3 ± 0.2 by drop wise addition of 1M NaHCO3. Next, 9 mL of freshly prepared intestinal enzyme solution (0.0054 g pancreatin enzyme/g of seafood muscle tissue; enzymatic activity 4 × US Pharmacopoeia specifications/mg pancreatin) and bile extract (0.0216 g bile extract (glycine and taurine conjugates of hyodeoxycholic and other bile salts)/g seafood muscle tissue) were added, and the pH was adjusted to 7.2 ± 0.2 by drop wise addition of 1M NaOH. Following a 2 h incubation at 37 °C, digestive enzymes were heat-inactivated in a 100°C water bath for 4 min to halt digestion, and then digests were cooled in an ice bath prior to centrifugation. To separate the aqueous phase from residual insoluble materials, digests were centrifuged at 5,000 g at 4°C for 30 min. The aqueous phase was isolated, and three aliquots (2 mL) were set aside for future analyses. The pellet (residual materials) was re-suspended in 4 mL of Milli-Q water. All aqueous phases and pellets were stored at -20°C until analysis.
Digests were processed in batches of 15, and each batch included one internal positive control (n=6 pooled fresh tuna) and two negative controls, which underwent the same digestion with either no seafood substrate or with the omission of enzymes. Samples were collected during 0 and 15 min of pepsin digestion and at 120 min of pancreatic digestion. Protein content of the digests was assessed via the Bradford method (Gotham et al., 1988).
2.3 In Vitro Bioavailability Assay
The bioavailability of MeHg was assessed for seafood muscle tissue previously subjected to dual-phase in vitro gastrointestinal digestion. Aqueous phases were subjected to a Caco-2 (colon epithelial cell line) retention and transport assay according to methods detailed by Calatayud et al. (2012). Briefly, cells were seeded at a density of 112 000 cells/mL and grown in 75 cm2 flasks, with 10 mL Dulbecco's Minimum Essential Medium (DMEM) at pH 7.4. DMEM was supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Gibco), 0.6 g/L L-glutamine (BioWhittaker Europe, Verviers, Belgium), 1 mM sodium pyruvate (BioWhittaker), 100 U/mL of penicillin, and 0.1 mg/mL of streptomycin (BioWhittaker). All incubations occurred at 37°C, 5% CO2, and 95% relative humidity. Culture medium was changed every two days. Upon reaching 80-90% confluency, cells were harvested using Trypsin/EDTA (2.5 g/L Trypsin; 0.2 g/L EDTA) and re-seeded.
For bioavailability experiments, Caco-2 cells were seeded onto Transwell inserts (polycarbonate membrane filters, 24 mm diameter, 0.4 um pore size, 1×108 pores/cm) at 5 × 104 cells/cm2. Translucent inserts were placed in 6-well plates, resulting in apical (upper) and basolateral (lower) chambers. Supplemented DMEM (sDMEM) was added to apical chambers (1.5 mL), and basolateral chambers (2.6 mL). Medium was changed every two days until cell differentiation (21-25 days after seeding). Upon differentiation, medium was aspirated from apical and basolateral chambers. Next, aqueous phase (1.5 mL) that contained the bioaccessible MeHg from the in vitro digestions was added to apical chambers and sDMEM (2 mL) was added to basolateral chambers. Cells were incubated for 2 h. Medium was collected from both apical and basolateral chambers for MeHg analysis. Caco-2 cell monolayers were washed with Trypsin/EDTA (three times), detached using Trypsin/EDTA, and recovered in 0.5 mL of sDMEM for MeHg analysis. At this point, viability was assessed using a TC20™ Automated Cell Counter (TC20, Bio-Rad Laboratories Inc.). Integrity of cell monolayers was monitored throughout experiments using transepithelial electrical resistance (TEER; ERS-2 Epithelial Volt-Ohm Meter, Millicell), and only Caco-2 cell monolayers with TEER values >250 ohm/cm were used.
2.4 Methylmercury Determination
Methylmercury levels in seafood muscle tissue as well as from bioaccessibility and bioavailability studies were determined using cold vapour atomic fluorescence (CVAFS, Tekran 2700, Toronto, Canada) according to U.S. Environmental Protection Agency (US EPA) Method 1630. Briefly, 1 mL of sample was digested with 8 mL of 25% KOH in methanol, and next diluted 1:1000 with Milli-Q water and pH adjusted to 4.0-4.5. All samples were ethylated with sodium tetraethyl borate (NaBEt4) prior to analysis. Quality control measures included calibration blanks, replicates and certified reference material (fish protein: DORM-4, National Research Council Canada, Ottawa, Canada) in every batch of 30 samples. The recovery of DORM-4 averaged 108.90 ± 15.26% (n=27) of the certified value, which meets EPA Method 1630 Quality Control Acceptance Criteria for accuracy. The relative standard deviation (precision) for analytical triplicates of fresh tuna internal controls averaged 6.07 % (n=12). The detection limit for this analysis was 0.02 ppt. Methylmercury content of chemical reactants (pepsin, pancreatin, and bile extract) were close to Tekran 2700 detection limit and were therefore not considered a significant source of contamination in our experiment.
2.5 Calculations and Statistical Analyses of In Vitro Results
Methylmercury in the aqueous phase of the bioaccessibility assays was defined as “bioaccessible”. Bioaccessibility (%) was calculated by dividing the amount of MeHg in the aqueous phase by the amount of MeHg in the initial seafood before its in vitro gastrointestinal digestion using the following equation:
MeHg in the pellet was measured and recovery was assessed according to the following equation:
Methylmercury transported into and/or across the Caco-2 cell monolayer and into the basolateral chamber was considered bioavailable. Absorption (%) as percent of MeHg in the aqueous phase was calculated by subtracting the MeHg remaining in the apical chamber from the MeHg in the aqueous phase that was added to the apical chamber and dividing this by the MeHg in the aqueous phase that was added to the apical chamber using the following equation:
Bioavailability, as a percent of MeHg in the initial seafood sample, was calculated by multiplying the previously established percent bioaccessibility and percent absorption.
Results were expressed as means ± standard deviation (SD). Assumptions of normality were confirmed for all samples (except halibut). To determine whether bioaccessibility or bioavailability of MeHg for a given type of seafood was less than 100% a one-tailed Student's t-test was performed with H0=bioaccessibility or bioavailability=100% and HA=bioaccessibility or bioavailability<100% for each type of seafood. To evaluate differences in bioaccessibility and bioavailability among types of seafood a one-way ANOVA was used. Pairwise t tests were performed. Differences among means were considered statistically significant at p<0.05. Statistical analyses were conducted in R Version 3.1.2. (R Foundation for Statistical Computing, Vienna, Austria).
2.6 Epidemiological exposure assessment study
For the second study objective, the resulting bioaccessibility and bioavailability factors obtained from the above experiments were incorporated into an existing epidemiological study as detailed by Goodrich et al. (2016). Briefly, this study included 630 dental professionals recruited during the American Dental Association (ADA) Annual Convention. Self-administered surveys provided demographic information (i.e., age, sex, weight) and MeHg intake information from seafood that included portion size (g/meal), type of seafood, and frequency of consumption (meals/month). Seafood consumption patterns and Hg exposures in this specific population were found to be similar to the U.S. and Canadian general populations.
As described by Goodrich et al. (2016), MeHg intake in this cohort was calculated by combining information on frequency of consumption of different types of seafood, portion sizes, and Hg concentrations in different types of seafood. With this information, monthly MeHg intake (μg/month) was estimated from seafood consumption for each participant based on the formula as follows:
U = Average unit portion size of seafood meals (g/portion)
Pi = Frequency of eating a particular type of seafood (portions/month) where i=1, 2, 3…n
Ci = Seafood type-specific mean Hg concentrations from a database
Herein we modified the aforementioned formula to consider bioaccessibility and bioavailability information from the in vitro studies. This was achieved by multiplying the Ci variable with the corresponding species-specific bioaccessibility and bioavailability percentages.
Linear regression exposure assessment models for hair and blood Hg were developed as outlined by Goodrich et al. (2016) with some modifications. Models were corrected for age but not body weight or red blood cell counts, and the MeHg values for the 10 seafood species were taken from the current study and not the U.S. FDA database.
3.0 Results
3.1 Methylmercury content of seafood
Mean concentrations of MeHg among the seafood studied varied considerably from 0.7±0.4 ng/g wet weight (ww) (scallop) to 527.4±84.1 ng/g ww (canned white tuna) (Table 2). The MeHg concentrations in fresh tuna and canned white tuna were significantly higher than those found in the other eight species studied. The MeHg concentrations were found to be within the range of THg concentrations reported by the U.S. FDA for most species with some exceptions noted (e.g., lower MeHg reported in our study for shrimp, crab, scallop; higher MeHg reported in our study for canned white tuna).
Table 2.
Mean concentrations of methylmercury (ng MeHg/g wet weight) in the initial (raw, pre-digested) seafood muscle tissue, as well as in the aqueous phase (bioaccessible fraction), and transported into and/or across the Caco-2 cell monolayer (bioavailable fraction). Data are reported as mean ± SD (n=6/type of seafood). For a given column, significant differences among types of seafood are denoted by differing superscript letters. For a given row, significant differences (one-tailed Student's t tests) in MeHg concentration between initial raw samples and those following bioaccessibility and bioavailability studies are denoted by asterisks (*). Significant differences between MeHg concentration in the bioaccessible and bioavailable fractions are denoted by a hash symbol (#).
| Seafood type | Initial [MeHg] | Bioaccessible [MeHg] | Bioavailable [MeHg] |
|---|---|---|---|
| Cod | 139.8±90.6b | 91.4±38.6b* | 56.0±18.1a*# |
| Crab | 19.3±8.8a | 11.2±5.6a* | 5.6±3.6a*# |
| Halibut | 81.5±80.2a,b | 70.1±61.1a,b | 40.7±27.9a*# |
| Salmon | 14.3±13.9a | 10.2±9.0a | 8.8±8.1a |
| Scallop | 0.7±0.4a | 0.7±0.4a | 0.3±0.2a*# |
| Shrimp | 3.8±1.5a | 3.8±1.5a | 2.3±0.8a*# |
| Tilapia | 16.4±12.5a | 10.8±6.9a | 7.0±4.3a*# |
| Tuna (canned light) | 83.2±56.7a,b | 44.8±21.7a,b,* | 39.7±24.9a* |
| Tuna (canned white) | 527.4±84.1c | 255.3±80.6c* | 158.6±99.2b*# |
| Tuna (fresh) | 492.7±76.7c | 367.0±50.6d* | 266.6±29.7c*# |
3.2 Bioaccessibility of methylmercury
In order for the MeHg from seafood to be solubilized into digestive fluids, the seafood muscle tissue to which MeHg is bound has to be broken down via gastric pepsin and intestinal pancreatin. To validate this latter aspect, samples from fresh tuna digests (positive control samples) that underwent the simulated gastrointestinal digestion process showed that protein content decreased by 62%, 74% and 100% at 15 min, 30 min, and 3 h, respectively.
For each of the seafood samples bioaccessible MeHg concentrations were calculated following the dual enzyme digestion process (Table 2). The MeHg concentrations in the bioaccessible fractions of fresh tuna and canned white tuna were significantly higher than those found in the other eight types of seafood. Bioaccessible MeHg concentrations were significantly lower than concentrations measured in the initial raw samples for the three types of tuna studied (fresh, canned white, canned light), cod, and crab. For seafood with low MeHg concentrations (shrimp and scallop), MeHg was measured in the bioaccessible fraction was greater than in the initial raw tissue. This latter observation was likely due to a measurement error related to the low amounts of MeHg in these sample (3.8 and 0.7 ng/g for shrimp and scallop, respectively) compared to other seafood studied. Thus, for all analyses, bioaccessible MeHg was assumed to equal to the amount of MeHg in the initial raw seafood muscle tissue and thus set at 100%.
Percent bioaccessibility of MeHg ranged from 50.1±19.2 (canned white tuna) to 100% (shrimp and scallop) (Figure 1). There was a significant difference in percent bioaccessibility of MeHg when comparing canned white tuna with halibut, shrimp, and scallop.
3.3 Bioavailability of methylmercury
Bioavailable MeHg concentrations were measured for all seafood samples (Table 2). The MeHg concentrations in the bioavailable fractions of fresh tuna and canned white tuna were significantly higher than those found in the other eight types of seafood. Bioavailable MeHg concentrations were significantly lower than concentrations measured in the initial raw samples for all the seafood studied except for salmon. In addition, bioavailable MeHg concentrations were significantly lower than bioaccessible MeHg concentrations for all seafood except for salmon and canned light tuna. The relatively lower bioavailable MeHg concentrations was expected as bioavailability is determined by subjecting the bioaccessible fraction to the cellular retention and transport assay.
Percent bioavailability of MeHg (when compared to the concentration in the initial raw seafood) ranged from 29.3±10.4 (crab) to 67.4±9.7% (salmon) (Fig. 1). There was a significant difference in percent bioavailability of MeHg when comparing salmon with crab and canned white tuna.
3.4 Associations between methylmercury concentrations and bioavailability
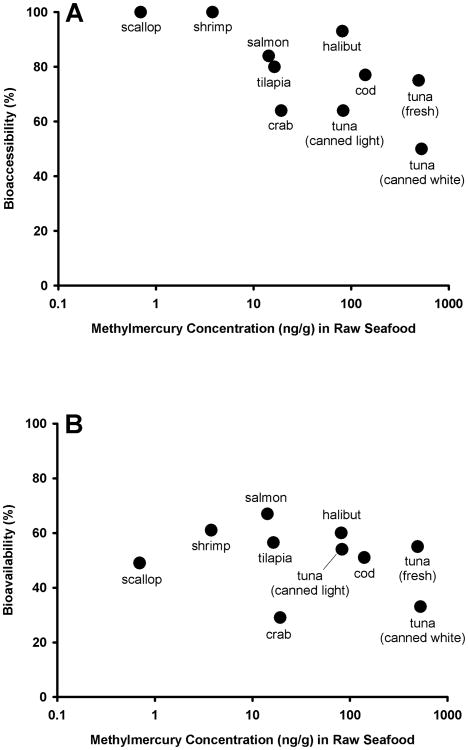
Bivariate correlations were performed to understand the association between MeHg content in seafood and its bioavailability (Figure 2). There were significant correlations between the initial MeHg concentration in seafood with the percent of that MeHg that was bioaccessible (r= -0.476, p<0.01) and bioavailable (r=-0.294, p<0.05) when all individual samples of each type of seafood were compared. This relationship remained when comparing seafood-specific mean values as depicted in Figure 2.
Figure 2.
Methylmercury concentration (ng/g wet weight) in the initial (raw, pre-digested) seafood muscle compared with the percent of that mercury that was bioaccessible (A) and bioavailable (B). For both axes mean data for each seafood are reported as points (n=6/type of seafood).
3.5 Exposure assessment study
Mean uptake of MeHg was calculated from dietary survey information as described by Goodrich et al. (2016). The estimated uptake of MeHg from seafood was 163.2 (185.3) μg/month (median followed by median absolute deviation in brackets). When the in vitro bioaccessibility and bioavailability data from the top 10 consumed species are applied to the calculations, the estimated uptake of MeHg from seafood dropped to 122.0 (142.4) μg/month when corrected for bioaccessibility and 95.0 (114.5) μg/month when corrected for bioavailability. These corrections for bioaccessibility and bioavailability represent reductions in estimated MeHg uptake by 25% and 42%, respectively.
Regression models, as detailed by Goodrich et al. (2016), were run with THg levels in blood and in hair as dependent variables (natural log-transformed). Unlike the work outlined in Goodrich et al., here the Hg uptake variable was “μg Hg/month” versus “μg Hg per kg body weight per day”. When these models were run assuming 100% bioavailability (as normally done; models #1 in Table 3), as well as by correcting each of the ten most consumed types of seafood by the corresponding in vitro results for bioaccessibility (model #2) and bioavailability (model #3), there were no significant changes.
Table 3.
Total methylmercury (MeHg) uptake models predicting blood and hair mercury (Hg). Total MeHg uptake (μg/month) from all seafood for subjects with measures of blood and/or hair Hg was calculated using three different corrections: assuming 100% bioavailability (model #1); accounting for seafood-specific bioaccessibility (model #2); accounting for seafood-specific bioavailability (model #3). All βs (SD in brackets) and r2 s were significant (p<0.001).
| Hg Biomarker | Model | Β (SD) | r2 | |
|---|---|---|---|---|
| Blood | #1 | Assuming 100% bioavailability | 0.21(0.02) | 0.20 |
| #2 | Accounting for bioaccessibility | 0.24(0.02) | 0.21 | |
| #3 | Accounting for bioavailability | 0.24(0.02) | 0.21 | |
| Hair | #1 | Assuming 100% bioavailability | 0.36(0.03) | 0.36 |
| #2 | Accounting for bioaccessibility | 0.37(0.03) | 0.37 | |
| #3 | Accounting bioavailability | 0.37(0.03) | 0.37 |
Discussion
The major objective of this study was to determine MeHg bioaccessibility and bioavailability for the most commonly consumed types of seafood in North America. To our knowledge, five types of seafood of relevance to the North American diet have been studied in terms of MeHg bioaccessibility and no types of seafood have been studied in terms of MeHg bioavailability (Table 1).
The seafood samples studied in the current investigation were obtained from local markets. The MeHg content of these samples are consistent with concentrations found in seafood consumed by the general North American population when comparing results with the U.S. FDA database. Bioaccessible concentrations of MeHg measured in the present study generally fall into the reported range for a given type of seafood for two of three species. For instance, the bioaccessible concentration of MeHg from fresh tuna measured in our work, 493 ng/g ww, fell within the reported range of 106 ng/g ww to 680 ng/g ww seen in the literature (Cabanero et al., 2007; Ouedrago et Amyot, 2011; Afonso et al., 2015). The bioaccessible concentration of MeHg from tilapia measured in our work, 11 ng/g ww, was similar to the 13 ng/g ww seen in the literature (Wang et al., 2013). However, the bioaccessible concentration of MeHg from canned white tuna measured in our work, 255 ng/g ww, was much higher than what has been previously reported (60 or 40 ng/g ww for THg; 40 ng/g ww for MeHg) (Afonso et al., 2015). The differences between our results and the work by Afonso et al. (2015) may be due, in part, to the use of alternate in vitro gastrointestinal digestion protocols as this has been shown to affect the bioaccessibility of MeHg. In addition, the source and processing of canned tuna may have some influence on the initial MeHg concentration and bioaccessibility. The wide variability in bioaccessibility findings across studies serves to emphasize major challenges in this emerging area of study, namely a lack of standardized methods and a need for evaluating in vivo/in vitro correlations, for example as more commonly conducted for studies characterizing the bioavailability of pharmaceuticals (Skolnik et al. 2010) or bioaccessibility of lead from soil (Van de Wiele et al. 2007).
In our work, bioaccessible MeHg concentrations were significantly lower than pre-digestion concentrations of MeHg in initial raw seafood muscle tissue for several types of seafood including all varieties of tuna, cod, and crab. In our study percent bioaccessibility of MeHg for salmon and for shrimp ranged from 85 to 100% compared to the literature where percent bioaccessibility of THg for these same types of seafood ranged from 75 to 105% (Calatayud et al., 2012; Costa et al., 2015). For fresh tuna, a variety of values have been reported (9 to 132%; Table 1) compared to our value of 75%. For two types of seafood analyzed in our study, compared to the literature we found higher percent bioaccessibilities of MeHg. One type was canned white tuna for which we calculated 50% bioaccessibility of MeHg compared to the literature, where 18% and 29% were reported for percent bioaccessibility THg and percent bioaccessibility of MeHg respectively. Another type was tilapia for which we calculated 80% bioaccessibility of MeHg compared to the literature, where 56% and 42% were reported for percent bioaccessibility THg and percent bioaccessibility of MeHg respectively. Again, here the differences between our results and the literature may be due, in part, to the use of alternate in vitro gastrointestinal digestion protocols which have been shown to affect the bioaccessibility of MeHg, as well as potential differences in the measurement of Hg (THg and MeHg), the source of sample, or inter-individual variability.
Bioaccessibility of MeHg from seafood muscle tissue can vary among and within types of seafood. In our study, among types of seafood, percent bioaccessibility of MeHg ranged from ∼50% (canned white tuna) to 100% (shrimp and scallop). This variation is well reflected in the literature where within a given study using one standard method, percent bioaccessibility of MeHg for a similar number of different types of seafood has been seen to range from 21.4% (yellow seafin) to 48.7% (catfish) (Wang et al., 2013), from 51% (squid) to 103% (salmon) (Calatayud et al., 2012), and from 18% (mackerel) to 48% (golden thread) (He and Wang, 2011). In our study, within types of seafood, the standard deviations for percent bioaccessibility were as high as 30% (crab). This variation within types of seafood was also seen in the literature. For instance, for canned white tuna, the SD calculated in our work was 80.6 ng/g ww, and the SD calculated in the literature was 50 ng/g ww (Afonso et al., 2015). High variability may be due to differences in protein, peptide, and amino acid profiles of the individual pieces of seafood muscle tissue, that were each purchased on separate days from a variety of Montreal markets. The proteins to which MeHg is bound within the muscle tissue, as well as the sub-cellular fraction in which MeHg ends up within the seafood protein matrix might explain these differences (Pavlisko and Coppes, 1999; He and Wang 2011). Methylmercury bound to soluble proteins may be more easily solubilized into digestive fluids since these proteins are more accessible to digestive proteases compared to myofibrillar proteins (Pavlisko and Coppes, 1999), thus seafood with a higher soluble protein content may release MeHg more readily into digestive fluids and result in higher bioaccessibility of MeHg. Here we documented enzymatic digestion of fresh tuna protein, and found that by the end of the three-hour in vitro gastrointestinal digestion, 100% of the initial protein from raw seafood muscle tissue was broken down. While this illustrates the effectiveness of our digestion method, we did not extend this work to the other species which represents a limitation of this study. Another explanation for differences in the solubilization of MeHg has been proposed by He and Wang (2011) who suggested that these differences were not based on type of protein in the muscle tissue or extent of protein breakdown, but on MeHg fractionation within the muscle cell. He and Wang (2011) found that within eight out of the nine fish species they studied, bioaccessibility was negatively correlated with the extent to which MeHg was partitioned into the metal-rich granule fraction and the trophically available fraction, and bioaccessibility was positively correlated with partitioning into the cellular debris fraction. None of these species were analyzed in our study, and we did not evaluate subcellular fractionation of MeHg however it would be an interesting study to pursue. Overall, much remains to be elucidated in terms of resolving this variability among and within types of seafood.
In particular, our results show that all varieties of tuna studied had MeHg bioaccessibilities between 50% and 75%. Many recognize this types of seafood as the major contributor to population-level MeHg exposures, and in light of our findings more work should be done to determine its true contribution to overall exposure and how the human body processes MeHg from this particular source. Moreover the differences in bioaccessibility of MeHg among tuna varieties may warrant further study with respect to the effects of the industrial process of canning on the bioaccessibility of MeHg. Costa et al. (2015) found that after digestion, the percent bioaccessibility of THg from canned white tuna (20%) was lower than that from fresh tuna (78%). This matches the pattern in our work. Compared to fresh tuna, in canned seafood the lower bioaccessible MeHg might be related to protein denaturation. Denatured proteins may become less accessible to protease action and consequently less THg is released into the aqueous phase (Afonso et al. 2015). Additional research looking at industrial processing techniques for seafood, such as canning, is thus needed. In addition to processed seafood, with the exception of the two types of canned tuna studied here, we only examined raw seafood, and more work is needed to understand the role of cooking of MeHg bioaccessibility as explored by Ouedrago and Amyot (2011).
Moving beyond the bioaccessibility work, here we used a Caco-2 cell model to evaluate intestinal cell retention and transport of MeHg to offer a more reliable approximation of MeHg bioavailability in vivo. To our knowledge only one prior study has used a similar in vitro approach to assess MeHg bioavailability from seafood, specifically with swordfish (Calatayud et al., 2012).
Our results show that bioavailable MeHg concentrations were significantly less than MeHg concentrations in the raw undigested sample for all types of seafood except for salmon. Across all seafood samples a decrease in concentration from one digestion process to the next was observed. Comparisons with the literature are challenged as to our knowledge the only previous research on bioavailable concentrations of MeHg from seafood muscle tissue was conducted for swordfish, and found that bioavailable MeHg concentrations were lower than both pre-digestion concentrations and bioaccessible MeHg concentrations (Calatayud et al., 2012). The significant difference observed between the bioaccessible concentration of MeHg and the bioavailable concentration of MeHg within a seafood type suggest that it is equally important to account for each of these physiological processes when trying to understand MeHg exposure from seafood. Overall, lower concentrations of bioavailable MeHg imply a lower internal dose of MeHg than is currently assumed, which in turn could alter risk assessment models.
In the present work, cellular retention and cellular transport of MeHg from seafood matrices were calculated together as one measure of percent bioavailability and ranged from 29% (crab) to 67% (salmon). The range of MeHg bioavailabilities calculated in our work overlapped with the range reported by Calatayud et al. (2012) who found that the percent cellular retention of MeHg was 49 to 69% from the swordfish food matrix and the percent cellular transport of MeHg was less than 14%. Given these findings regarding high cellular retention and relatively low cellular transport, the majority of the MeHg considered bioavailable in our work was most likely retained in cells rather than transported across the Caco-2 cell monolayer. Higher cellular retention might indicate greater bioavailability if the intracellular content is finally transported across the cell barrier. High cellular retention of MeHg might also indicate that the intestinal epithelium acts as a barrier for absorption, though eventual transport across the cell barrier seems to be the more likely outcome (Vazquez et al. 2014). In addition, the retention of MeHg by the intestinal epithelium might have specific toxic effects at a local level (Vazquez et al., 2014; Vazquez et al., 2015).
The in vitro models used here are among the simplest models available for studies on bioaccessibility and bioavailability. Their simplicity has meant that these models are more widely used than other approaches and this allows for better comparisons to the existing literature. Similarly, these simpler models allow for greater sample size at lower cost than other, more complex models such as the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) or the TNO gastro-Intestinal Model (TIM) (see the review by Van de Wiele et al. 2007). However, simpler bioaccessibility models are also limited. Key limitations include less realistic modeling of peristaltic movements, lack of absorption concurrent with digestion, absence of other food components that would be more representative of a full meal, and less opportunity for modeling human genetic variability.
Given the financial and labor costs associated with collecting, processing, and analyzing samples for this study, the sample size for each type of seafood is somewhat limited. As a result, statistical power was limited, and given the variability observed within seafood types this presents a challenge for the interpretation of the present work. Regardless, the current study includes a larger number of species than most other reports of MeHg bioaccessibility and bioavailability from seafood, and to our knowledge the work represents that largest focused on the North American context.
The final aspect of our work took the resulting MeHg bioaccessibility and bioavailability factors from our in vitro work and incorporated them into to an existing epidemiological study (Goodrich et al., 2016). While this study population consisted of dental professionals and thus may not best reflect the situation in other vulnerable groups (e.g., pregnant women, children), the study group was designed generally to better understand the relationship between estimated MeHg uptake (i.e., seafood consumption) and MeHg biomarkers (i.e., blood THg and hair THg). In all multiple linear regression models predicting Hg biomarker levels from seafood consumption (accounting for age) estimated Hg uptake from seafood was a statistically significant predictor, although inclusion of the in vitro bioavailability data did not improve the strength of the models. Thus, we were unable to support our second hypothesis. Perhaps this is not surprising as the precision and accuracy associated with Hg exposure assessment models are extremely challenged, and the wide variances encountered likely dwarf the possible influence of bioavailability. Foremost is that these exposure models depend upon a participant's ability to accurately recall foods (i.e., types, portion size) that they have previously consumed. Another challenge is that the models utilize an average MeHg value for each type of seafood and do not account for the great variability in MeHg concentrations within a particular type of seafood. For example, we previously measured THg levels in 9 different varieties of canned tuna and found the value to range nearly 50-fold, from 0.015 to 0.7 ug/g (Basu et al., 2014). This type of variability extends to the bioaccessibility and bioavailability data as indicated in the current study. In addition, here we focused what we earlier justified to be the top10 most consumed seafood, and as such bioavailability and bioaccessibility data were not available for the 18 additional types of seafood participants were surveyed about including high MeHg species such as swordfish and shark.
Highlights.
We assume that nearly all the methylmercury in consumed seafood is absorbed
First study of mercury bioavailability in a North American context
In vitro evidence that less than 100% of mercury in seafood is bioavailable
Incorporation of in vitro results into a human exposure assessment is challenged
Acknowledgments
We acknowledge the support of Jenny Eng, Gordana Stankovic Martincevic, Marie Perkins, Adeline Arini, Behnam Azadi, Krittika Mittal, Kebba Sabally, Stephane Bayen, Lara Khadr, and Autumn Poisson. We acknowledge the American Dental Association for their support especially Stephen Gruninger. This work was supported by a Graduate Excellence Fellowship from the McGill School of Dietetics and Human Nutrition (to MS). NB received support from the NSERC Discovery Grants program, Canada Research Chair, and Canada Foundation for Innovation (CFI). In addition, the epidemiological work was supported by grants from the U.S. National Institutes of Health (2UL1TR000433; P30 ES017885), the University of Michigan Office of the Vice President for Research, and the University of Michigan School of Public Health.
Footnotes
No conflict of interest is declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg B, Ekman L, Falk R, Greitz U, Persson G, Snihs JO. Metabolism of methyl mercury compounds in man: excretion and distribution. Archives of Environmental Health - An International Journal. 1969;19:478–484. doi: 10.1080/00039896.1969.10666872. [DOI] [PubMed] [Google Scholar]
- Afonso C, Costa S, Cardoso C, Oliveira R, Lourenço H, Viula A, et al. Nunes M. Benefits and risks associated with consumption of raw, cooked, and canned tuna (Thunnus spp.) based on the bioaccessibility of seleniuand methylmercury. Environ Res. 2015 doi: 10.1016/j.envres.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Basu N, Tutino R, Zhang Z, Cantonwine D, Goodrich J, Somers E, Rodriguez L, Schnaas L, Solano M, Mercado A, Peterson K, Sanchez B, Hernández-Avila M, Hu H, Tellez-Rojo M. Mercury Levels in Pregnant Women, Children, and Seafood from Mexico City. Environmental Research. 2014;135:63–69. doi: 10.1016/j.envres.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntssen MH, Hylland K, Lundebye AK, Julshamn K. Higher faecal excretion and lower tissue accumulation of mercury in Wistar rats from contaminated fish than from methylmercury chloride added to fish. Food and Chemical Toxicology. 2004;42:1359–1366. doi: 10.1016/j.fct.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Cabanero AI, Madrid Y, Camara C. Selenium and mercury bioaccessibility in fish samples: an in vitro digestion method. Analytica Chimica Acta. 2004;526:51–61. [Google Scholar]
- Cabanero AI, Madrid Y, Camara C. Mercury-selenium species ratio in representative fish samples and their bioaccessibility by an in vitro digestion method. Biological Trace Element Research. 2007;119:195–211. doi: 10.1007/s12011-007-8007-5. [DOI] [PubMed] [Google Scholar]
- Calatayud M, Devesa V, Virseda JR, Barbera R, Montoro R, Velez D. Mercury and selenium in fish and shellfish: occurrence, bioaccessibility and uptake by Caco-2 cells. Food Chem Toxicol. 2012;50:2696–2702. doi: 10.1016/j.fct.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Costa S, Afonso C, Cardoso C, Batista I, Chaveiro N, Nunes ML, Bandarra NM. Fatty acids, mercury, and methylmercury bioaccessibility in salmon (Salmo salar) using an in vitro model: Effect of culinary treatment. Food Chemistry. 2015;185:268–276. doi: 10.1016/j.foodchem.2015.03.141. [DOI] [PubMed] [Google Scholar]
- Goodrich JM, Chou HN, Gruninger S, Franzblau A, Basu N. Exposures of Dental Professionals to Elemental Mercury and Methylmercury. Journal of Exposure Science and Environmental Epidemiology. 2016;26(1):78–85. doi: 10.1038/jes.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J, Wang Y, Gillespie B, Werner R, Franzblau A, Basu N. Methylmercury and elemental mercury differentially associate with blood pressure among dental professionals. International Journal of Hygiene and Environmental Health. 2013;216:195–201. doi: 10.1016/j.ijheh.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham SM, Fryer PJ, Paterson WR. The measurement of insoluble proteins using a modified Bradford assay. Anal Biochem. 1988;173:353–358. doi: 10.1016/0003-2697(88)90199-6. [DOI] [PubMed] [Google Scholar]
- Groth E., III Ranking the contributions of commercial fish and shellfish varieties to mercury exposure in the United States: implications for risk communication. Environ Res. 2010;110:226–236. doi: 10.1016/j.envres.2009.12.006. [DOI] [PubMed] [Google Scholar]
- He M, Wang WX. Factors affecting the bioaccessibility of methylmercury in several marine fish species. Journal of Agricultural and Food Chemistry. 2011;59:7155–7162. doi: 10.1021/jf201424g. [DOI] [PubMed] [Google Scholar]
- Laird BD, Shade C, Gantner N, Chan HM, Siciliano SD. Bioaccessibility of mercury from traditional northern country foods measured using an in vitro gastrointestinal model is independent of mercury concentration. Science of the Total Environment. 2009;407:6003–6008. doi: 10.1016/j.scitotenv.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Laird BD, Weiseth B, Packull-McCormick SR, Peak D, Dodd M, Siciliano SD. Solid–liquid separation method governs the in vitro bioaccessibility of metals in contaminated soil-like test materials. Chemosphere. 2015;134:544–549. doi: 10.1016/j.chemosphere.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Mergler D, Anderson H, Chan L, Mahaffey K, Murray M, Sakamoto M, Stern A. Methylmercury exposure and health effects in humans: a worldwide concern. AMBIO: A Journal of the Human Environment. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Miettinen J, Rahola T, Hattula T, Rissanen K, Tillander M. Elimination of 203Hg-methylmercury in man. Annals of clinical research. 1971;3(2):116–122. [PubMed] [Google Scholar]
- Ouedrago O, Amyot M. Effects of various cooking methods and food components on bioaccessibility of mercury from fish. Environ Res. 2011;111:1064–1069. doi: 10.1016/j.envres.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Pavlisko A, Coppes Z. Digestion of myofibrillar and sarcoplasmic fractions from menhaden muscle by the action of menhaden (Brevoortia spp.) and white croaker (Micropogonias furnieri) trypsins. Journal of Food Biochemistry. 1999;23(5):547–558. [Google Scholar]
- Ruby MV, Davis A, Schoof R, Eberle S, Sellstone CM. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environmental Science & Technology. 1996;30:422–430. [Google Scholar]
- Skolnik S, Lin X, Wang J, Chen XH, He T, Zhang B. Towards prediction of in vivo intestinal absorption using a 96-well Caco-2 assay. Journal of Pharmaceutical Sciences. 2010;99(7):3246–3265. doi: 10.1002/jps.22080. [DOI] [PubMed] [Google Scholar]
- Somers EC, Ganser MA, Warren JS, Basu N, Wang L, Zick SM, Park SK. Mercury Exposure and Antinuclear Antibodies among Females of Reproductive Age in the General United States Population. Environmental Health Perspectives. 2015 doi: 10.1289/ehp.1408751. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Escribano S, Denis S, Blanquet-Diot S, Calatayud M, Barrios L, Vélez D, Alric M, Montoro R. Comparison of a static and a dynamic in vitro model to estimate the bioaccessibility of As, Cd, Pb and Hg from food reference materials Fucus sp. (IAEA-140/TM) and Lobster hepatopancreas (TORT-2) Science of the Total Environment. 2011;409(3):604–611. doi: 10.1016/j.scitotenv.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Van de Wiele TR, Oomen AG, Wragg J, Cave M, Minekus M, Hack A, Cornelis C, Rompelberg CJ, De Zwart LL, Klinck B, Van Wijnen J, Verstraete W, Sips AJ. Comparison of five in vitro digestion models to in vivo experimental results: lead bioaccessibility in the human gastrointestinal tract. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2007;42(9):1203–1211. doi: 10.1080/10934520701434919. [DOI] [PubMed] [Google Scholar]
- Vázquez M, Vélez D, Devesa V. Participation of b 0,+ and B 0,+ systems in the transport of mercury bound to cysteine in intestinal cells. Toxicology Research. 2015;4:895–900. [Google Scholar]
- Vázquez M, Vélez D, Devesa V. In Vitro Characterization of the Intestinal Absorption of Methylmercury using a Caco-2 cell model. Chemical Research in Toxicology. 2014;27(6):254–264. doi: 10.1021/tx4003758. [DOI] [PubMed] [Google Scholar]
- Wang HS, Xu WF, Chen ZJ, Cheng Z, Ge LC, Man YB, et al. Wong MH. In vitro estimation of exposure of Hong Kong residents to mercury and methylmercury via consumption of market fishes. Journal of Hazardous Materials. 2013;248:387–393. doi: 10.1016/j.jhazmat.2012.12.060. [DOI] [PubMed] [Google Scholar]