Abstract
Background
Two thirds of stroke survivors experience motor impairment resulting in long-term disability. The anatomical substrate is often the disruption of cortico-subcortical pathways. It has been proposed that reestablishment of corticosubcortical communication relates to functional recovery.
Objective
Here, we applied a novel training protocol to augment ipsilesional cortico-subcortical connectivity after stroke. Chronic stroke patients with severe motor impairment were provided online feedback of blood-oxygenation level dependent (BOLD) signal connectivity between cortical and subcortical regions critical for motor function using real-time functional magnetic resonance imaging (rtfMRI) neurofeedback.
Results
In this proof of principle study, 3 out of 4 patients learned to voluntarily modulate cortico-subcortical connectivity as intended.
Conclusions
Our results document for the first time the feasibility and safety for patients with chronic stroke and severe motor impairment to self-regulate and augment ipsilesional cortico-subcortical connectivity through neurofeedback using rtfMRI.
Keywords: stroke, real-time functional magnetic resonance imaging (rtfMRI), neurofeedback, connectivity, brain-machine interface (BMI)
Introduction
Stroke is a leading cause of long-term adult disability worldwide (Feigin et al. 2014). Functional recovery after stroke remains highly variable and leaves approximately 2/3 of stroke survivors dependent on assistance in their daily life (Kwakkel et al. 2003). In particular, effective treatment for individuals with severe hemiparesis (i.e., approximately every second stroke survivor) is lacking. Only recently, novel approaches using behavior-oriented physiotherapy with brain-machine interface (BMI) training have provided evidence that motor function in stroke can be improved even in severe, chronic cases (Ramos-Murguialday et al. 2013).
While the exact mechanisms underlying such recovery are still under investigation (Buch et al., 2012; Soekadar et al. 2014), it is thought that simultaneous activation of inputs and outputs to the motor cortical areas trigger Hebbian plasticity, which strengthens cortical-subcortical connectivity. Improvements in this cortical-subcortical connectivity have been linked to better motor recovery after stroke (Chollet, et al., 1991; Jang et al., 2012). However, previous noninvasive neurofeedback techniques have relied on electro- or magnetoencephalography (EEG/MEG), which are very limited in their capacity to record neural activity from deeper, subcortical signal sources. The development of real-time neurofeedback of blood-oxygenation level dependent (BOLD) signals using functional magnetic resonance imaging (rtfMRI) overcomes this limitation by providing full brain coverage, allowing feedback from both cortical and subcortical regions (Weiskopf et al., 2003) which is unachievable with EEG/MEG-based methods. We thus tested the feasibility of directly modulating cortico-subcortical connectivity with real-time fMRI neurofeedback, which may provide an effective new strategy in neurofeedback-based stroke rehabilitation.
Several recent studies have suggested that connectivity-based neurofeedback is more informative and relevant to behavior than feedback of a single region’s activity. In a post-hoc analysis, researchers showed that correlation-based feedback yields more information about task difficulty than single region-of-interest (ROI-based feedback; Zilverstand et al. 2014). Accordingly, Kim et al. (2015) found that a combination of ROI and correlation-based feedback yielded better behavioral effects than single ROI feedback alone. While those studies used a running correlation window, other studies have also shown success with providing intermittent, averaged connectivity feedback over a run (e.g., using a dynamic causal modeling approach to provide effective connectivity feedback every 150 seconds based on 90 seconds of image acquisition (Koush et al., 2013) or a correlation-based connectivity method that provided feedback every 14 seconds (Megumi et al., 2015)).
Here, we use a connectivity technique that provides online, continuous connectivity feedback, updated every 1.5 seconds, based on a sliding window correlation between two distant brain areas (cortical/subcortical). Using this design, we tested for the first time whether chronic stroke patients with severe motor deficits can learn to self-regulate the functional connectivity between perilesional motor cortical and subcortical areas simultaneously.
Methods
Participants
Four individuals (2 female; average age 49±5.94 years) with chronic stroke (stroke onset > 6 months) and moderate to severe right hemiparesis (Fugl-Meyer Upper Extremity (F-MUE) Assessment Score = 19.75±6.99) participated in the study (for more details, see supplementary materials). Participants gave written informed consent before participating in the study. The study was approved by the University of Tübingen Medical Faculty’s local ethics committee and was performed in accordance with the 1964 Declaration of Helsinki.
rtfMRI neurofeedback
Participants participated in two 2-hour neurofeedback sessions (separated by at least 24 hours, but within 1 week) on a 3T Siemens Prisma scanner. After evaluation of the F-MUE. assessment score, participants underwent a high-resolution anatomical scan and a functional localizer run in which they alternated between blocks of imagining movement of their paralyzed limb (45s) and rest (22.5s). Twelve training runs were planned for each day of feedback for a total of 24 runs across both days; in case the neurofeedback training became too effortful for the stroke participants, sessions could be halted upon request. Actual number of feedback runs completed by each participant is indicated in Table 1, with an average of 18±2.94 runs completed across two days.
Table 1.
Demographic information and individual results of rtfMRI control learning across participants. All rtfMRI connectivity control values are correlation coefficients derived from a Pearson correlation between the regions of interest during the neurofeedback runs (see methods/supplementary material for details). Scores for early and late training are calculated from the first three and last three rtfMRI neurofeedback runs, respectively. Change in connectivity control and transfer score are shown in absolute values and in () as a percentage relative to early training.
| ID | AGE | GENDER | F- M(UE) |
Total # of feedback runs (sess 1, sess 2) |
rtfMRI connectivity control, early training |
rtfMRI connectivity control, late training |
Change in rtfMRI connectivity control (% early training) |
Transfer score (% early training) |
|---|---|---|---|---|---|---|---|---|
| S01 | 48 | Female | 15 | 22 (10,12) | 0.11±0.04 | 0.25±0.06 | +0.14 (+127%) | 0.19 (+73%) |
| S02 | 54 | Male | 13 | 15 (10,5) | 0.31±0.09 | 0.56±0.05 | +0.25 (+81%) | 0.43 (+39%) |
| S03 | 41 | Female | 23 | 17 (9,8) | 0.33±0.02 | 0.45±0.07 | +0.12 (+36%) | 0.28 (−15%) |
| S04 | 53 | Male | 28 | 18 (9,9) | 0.50±0.12 | 0.45±0.05 | −0.05 (−10%) | 0.48 (−4%) |
Regions of interest (ROIs) were defined individually in subject space for each participant, based on voxels of peak functional activation in perilesional areas of the primary motor cortex (M1) and ipsilesional thalamus during active imagining during the localizer run. During rtfMRI neurofeedback training, a Pearson correlation was calculated across a 12-second sliding window (8 volumes) from the BOLD signal time-course in the two ROIs. It should be noted that there is an important trade-off concerning the length of the correlation window, as a Pearson correlation is more stable with a greater number of samples. However, the user’s ability to adjust their behavior in response to the feedback becomes more difficult with longer time windows, as they would need to interpret the current feedback window as a reflection of their current behavior plus past behavior for a longer time. A longer sampling window also means that the participant can make large changes to their neural activity at a given instant, but only see modest changes in the feedback as the instantaneous change only has a small contribution to the weight of the overall signal. Thus, we used a sample of 8 volumes (12 seconds) to achieve a balance between a stable correlation and instrumental feedback. The correlation value was depicted as a thermometer bar, using Turbo BrainVoyager software (Turbo-BrainVoyager, Brain Innovation B.V., Maastricht, The Netherlands) to retrieve the reconstructed fMRI images and perform an online 3D motion correction and a custom-made Matlab software module to calculate the correlation and present the visual feedback (Fig. 1; Matlab 6.5, The Mathworks, Natick, MA).
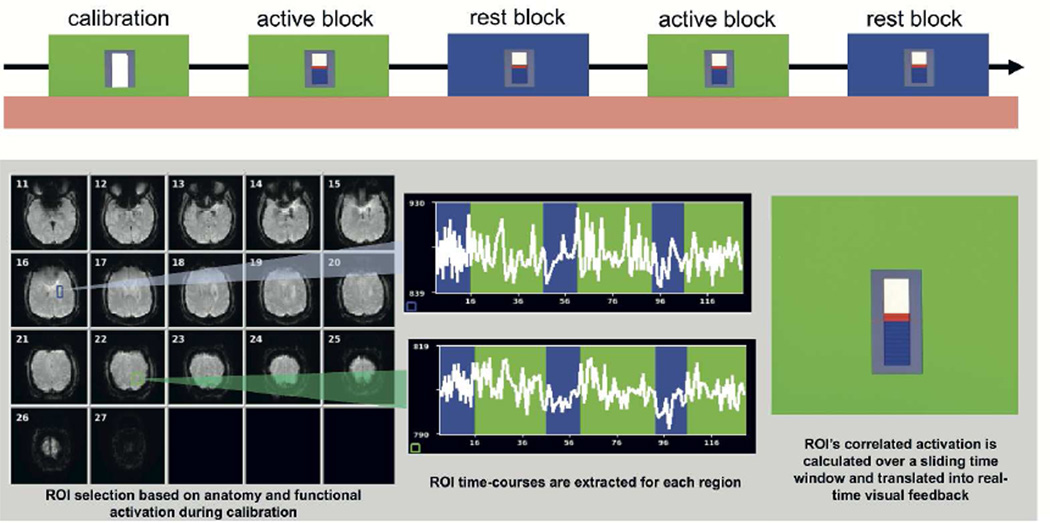
Figure 1.
Real-time functional magnetic resonance imaging (rtfMRI) neurofeedback was provided by extracting the average time course of the voxels in each of two ROIs, and performing a Pearson correlation over a 16-second sliding window. Top: Visual feedback was presented in the form of a thermometer bar that increased in height depending on the correlated activation of both ROIs. An example of a rtfMRI neurofeedback scanning run is depicted at the top with a baseline calibration period (30 seconds) was followed by alternating blocks of active neurofeedback (‘active blocks’, 60 seconds, signified by a green background) and rest (‘rest blocks’, 30 seconds, signified by a blue background). Bottom: The BOLD timecourse of activation was calculated from each region of interest and the correlation between these regions was feedback in the form of a thermometer.
Before the sessions, participants were given suggestions on how to modulate the signal (e.g., by imagining hand motions), but were instructed to use whatever strategy worked best for them without moving. Strategies were recorded after each run. Neurofeedback runs started with a 15-volume (22.5s) calibration period during which participants were asked to rest. Then active and rest blocks alternated three times each throughout the run (Figure 1). During active blocks (signaled by a green background for 30 volumes (45 seconds)) participants were asked to increase the height of the bar when the screen’s background color changed to green, using strategies they could develop over the course of training. During rest blocks (signaled by a blue background for 15 volumes (22.5 seconds)), participants were to rest and try not to think about movement. To evaluate neurofeedback training-related differences in rtfMRI connectivity, we looked specifically at “early training” (first three neurofeedback runs on the first day) and “late training” (last three neurofeedback runs on the second day), and compared early versus late training using a paired t-test. At the end of the second session, a ‘transfer run’ without feedback was conducted to test the participants’ ability to control BOLD connectivity in the absence of feedback (‘transfer effect’), which was compared to early training. Finally, a resting state scan was performed before and after training sessions to assess changes in intrinsic connectivity (for additional methods details, see supplementary material).
Results
Overall, participants demonstrated a significant increase in rtfMRI connectivity control (see Fig. 2, Table 1; group analysis: t=−2.70, p=0.02, early training correlation Pearson’s r = 0.31±0.16, late training r = 0.43±0.13, average increase in rtfMRI control r = +0.12±0.15). As seen in the individual full time courses of training (Supplementary Figure 3), three out of the four participants demonstrated an increase in rtfMRI connectivity control. The fourth individual, who did not improve, started with a high baseline level of control and did not change significantly during the practice (baseline r = 0.50±0.12, post r = 0.45±0.05). We additionally tested for significance of these training effects over time (e.g., slope across runs) using a permutation test on the ordering of the runs. We generated 10,000 permutations of the orders of the runs and linear regression was performed. A pairwise t-test was then performed on the slopes of the generated permutations of runs and the original sequence of runs. These were significant for all participants (subj01: t=280.73, p<0.01; subj02: t=280.62, p<0.01; subj03: t=240.17; p<0.01; subj04: t=−44.88, p<0.01).
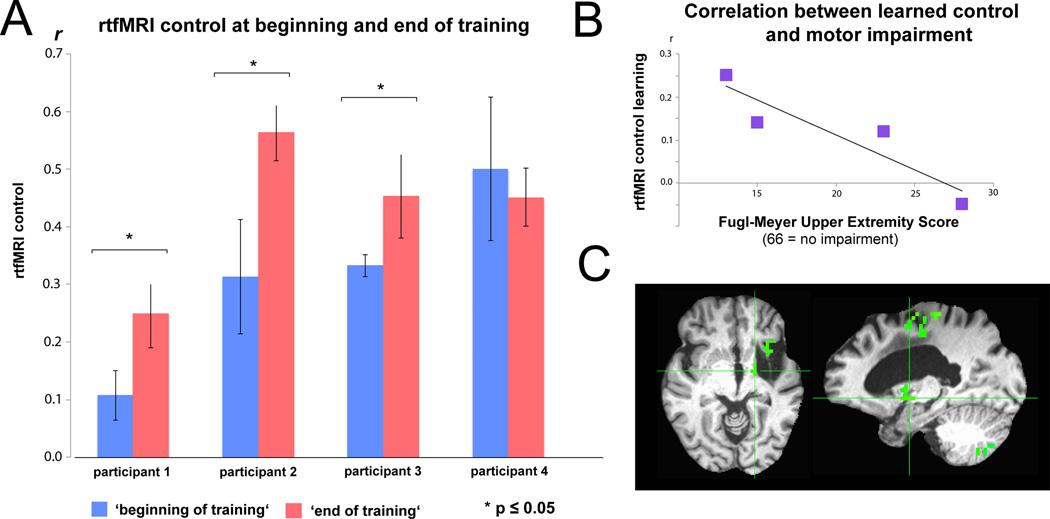
Figure 2.
A: Real-time functional magnetic resonance imaging (rtfMRI) connectivity control at the beginning (first three runs of session 1) and end of training (last three runs on session 2). r = Pearson correlation value of regions of interest’s (ROIs) blood-oxygenation-level-dependent (BOLD) signals. B: Individuals with lower Fugl-Meyer Upper Extremity (F-MUE) scores, reflecting less remaining motor function, showed a trend towards better learning than individuals with higher scores (r = −0.914, p = 0.08). C: Exemplary whole brain analysis showing increased correlated activation between perilesional M1 and ipsilesional thalamic areas at the end of training (cluster-corrected for multiple comparisons, p<0.05).
Two of the individuals who improved during training also showed an increased correlation between the ROIs’ BOLD activity during in the absence of feedback (‘transfer run’) compared to early training (Table 1; subj01: transfer=0.19, early training=0.11, increase=73%; subj02: transfer=.43, early training=.31, increase=39%).
Importantly, individuals with greater motor impairment at inclusion showed a greater trend towards learning across sessions (r = −0.914, p = 0.08, Fig.2B) and an increased correlation during the no feedback (‘transfer’) run. Furthermore, a whole brain analysis comparing late to early training during active versus rest blocks confirmed that there was greater correlated activation in perilesional M1 and ipsilesional thalamus after training in those with greater motor impairments (Fig.2C). Finally, all 4 subjects showed an increase in cortical-subcortical resting state connectivity using ROIs from M1 and thalamus after feedback training (p = 0.005, group baseline r = 0.35±0.21, post r = 0.53±0.24, increase r = 0.18±0.05).
Regarding strategies employed to control the feedback, while most participants tried several before settling on one, they all used motor imagery of the affected side, such as apple picking, writing one’s name, opening and closing one’s hand, pushing a red ball, skiing, dancing, and playing with one’s grandson.
Discussion
Here we document the feasibility and safety of improving ipsilesional cortico-subcortical communication after stroke using real-time fMRI neurofeedback of functional connectivity. Individuals with severe motor deficits can learn to modulate connectivity between two distant cortical and subcortical ROIs successfully. Learning such skill was associated with increased motor cortico-thalamic connectivity during training and at rest, with sustained higher connectivity in the absence of feedback. Importantly, a trend suggested that individuals with greater motor impairment showed the largest increases in learning and cortico-thalamic connectivity, suggesting that this group may benefit most from this paradigm. However, as this trend was only marginally significant with a small sample size, future studies should examine this more carefully. Overall, these preliminary results suggest that real-time fMRI neurofeedback of cortical-subcortical connectivity is a feasible and promising strategy in the context of stroke neurorehabilitation.
While this proof-of-principle study suggests that rtfMRI feedback of multiple brain regions can be used to voluntarily increase neural connectivity between distant brain areas compromised after stroke or other brain injuries, we do note that the sample size and number of sessions was limited due to the pilot nature of this study. Further research should investigate these effects in larger diverse samples and examine whether long-term application of this learning paradigm can facilitate lasting motor recovery or behavioral changes. It is important to note that limitations of this method include some disadvantages of using rtfMRI, such as the expense of fMRI scans and relative inaccessibility, due to the high cost of system maintenance. In addition, individuals who meet any exclusion criteria for MRI scans, such as having metal implants or claustrophobia, would be prohibited from participating due to safety. On the other hand, advances in technology are making techniques such as mobile EEG both reliable and affordable. While EEG cannot measure activity in subcortical structures, preliminary work has shown that it is feasible to use simultaneous EEG with fMRI to identify an “EEG signature” of subcortical fMRI structures. Once identified, EEG could be used for neurofeedback training, lowering costs and improving accessibility. Also, future work may be able to assess personalized ‘blueprints’ of functional connectivity in each individual and compare this with standardized connectivity maps based on data of stroke patients with good motor recovery or healthy controls. Such approaches could further pave the way for individualized neural connectivity training to foster lasting motor restoration.
Supplementary Material
Acknowledgments
The authors would like to thank the participants for their time, as well as Birgit Teufel for her assistance. This work was supported by the Intramural Research Program (IRP) of the National Institute of Neurological Disorders and Stroke (NINDS), USA; the DAAD Research Grant [to SLL]; NIH K12 HD055929 [to SLL]; the German Federal Ministry of Education and Research [BMBF, grant number 01GQ0831, 16SV5838K to SRS and NB]; the Deutsche Forschungsgemeinschaft [DFG, grant number SO932-2 to SRS and Koselleck support to NB]; the European Commission under the project WAY [grant number 288551 to SRS and NB]; the Volkswagenstiftung (VW); the Baden-Württemberg Stiftung, Germany; and the New INDIGO Research Grant [to RS].
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Buch ER, Shanechi AM, Fourkas AD, Weber C, Birbaumer N, Cohen LG. Parietofrontal integrity determines neural modulation associated with grasping imagery after stroke. Brain. 2012;135(2):596–614. doi: 10.1093/brain/awr331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chollet F, DiPiero V, Wise RJS, Brooks DJ, Dolan RJ, Frackowiak RSJ. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Annals of neurology. 1991;29(1):63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang SH, Kwon YH, Lee MY, Lee DY, Hong JH. Difference of neural connectivity for motor function in chronic hemiparetic stroke patients with intracerebral hemorrhage. Neuroscience letters. 2012;531(2):80–85. doi: 10.1016/j.neulet.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Kim DY, Yoo SS, Tegethoff M, Meinlschmidt G, Lee JH. The Inclusion of Functional Connectivity Information into fMRI-based Neurofeedback Improves Its Efficacy in the Reduction of Cigarette Cravings. Journal of cognitive neuroscience. 2015 doi: 10.1162/jocn_a_00802. [DOI] [PubMed] [Google Scholar]
- 6.Koush Y, Rosa MJ, Robineau F, Heinen K, Rieger SW, Weiskopf N, Scharnowski F. Connectivity-based neurofeedback: dynamic causal modeling for real-time fMRI. Neuroimage. 2013;81:422–430. doi: 10.1016/j.neuroimage.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJH. Probability of regaining dexterity in the flaccid upper limb: Impact of severity of paresis and time since onset in acute stroke. Stroke. 2003;34:2181–2186. doi: 10.1161/01.STR.0000087172.16305.CD. [DOI] [PubMed] [Google Scholar]
- 8.Megumi F, Yamashita A, Kawato M, Imamizu H. Functional MRI neurofeedback training on connectivity between two regions induces long-lasting changes in intrinsic functional network. Frontiers in human neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramos-Murguialday A, Broetz D, Rea M, Läer L, Yilmaz O, Brasil FL, Liberati G, Curado MR, Garcia-Cossio E, Vyziotis A, Cho W, Agostini M, Soares E, Soekadar SR, Caria A, Cohen LG, Birbaumer N. Brain-machine interface in chronic stroke rehabilitation: A controlled study. Annals Neurology. 2013;74:100–108. doi: 10.1002/ana.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sitaram R, Veit R, Stevens B, Caria A, Gerloff C, Birbaumer N, Hummel F. Acquired control of ventral premotor cortex activity by feedback training an exploratory real-time fMRI and TMS study. Neurorehabilitation and neural repair. 2012;26(3):256–265. doi: 10.1177/1545968311418345. [DOI] [PubMed] [Google Scholar]
- 11.Soekadar SR, Birbaumer N, Slutzky MW, Cohen LG. Brain-Machine Interfaces In Neurorehabilitation of Stroke. Neurobiol Dis. 2014 doi: 10.1016/j.nbd.2014.11.025. [in press]. [DOI] [PubMed] [Google Scholar]
- 12.Weiskopf N, Veit R, Erb M, Mathiak K, Grodd W, Goebel R, Birbaumer N. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): Methodology and exemplary data. NeuroImage. 2003;19:577–586. doi: 10.1016/s1053-8119(03)00145-9. [DOI] [PubMed] [Google Scholar]
- 13.Zilverstand A, Sorger B, Zimmermann J, Kaas A, Goebel R. Windowed correlation: A suitable tool for providing dynamic fMRI-based functional connectivity neurofeedback on task difficulty. Plos ONE. 2014:e85929. doi: 10.1371/journal.pone.0085929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.