Abstract
PURPOSE: Good performance status is widely known as a superior prognostic predictor. However, some patients have large survival differences despite having good performance status that are influenced by certain prognostic factors. The purpose of this study was to explore baseline host- or tumor-related factors and to establish a prognostic model for metastatic or recurrent gastric cancer patients with good performance status who received first-line chemotherapy. METHODS: A total of 310 metastatic or recurrent gastric cancer patients with good performance status who received first-line chemotherapy were enrolled. Prognostic significance was determined using multivariate Cox regression analysis. Incorporating all pretreatment indicators, a prognostic model was established. Overall survival outcomes were compared with different risk groups using the Kaplan-Meier method and log-rank test. RESULTS: In multivariate analysis, no previous gastrectomy [hazard ratio (HR) = 1.42; 95% confidence interval (CI) = 1.08-1.85], number of distant metastatic sites (HR = 1.47; 95% CI = 1.11-1.96), bone metastasis (HR = 2.20; 95% CI = 1.16–4.18), liver metastasis (HR = 1.77; 95% CI = 1.31-2.39), and an elevated neutrophil lymphocyte ratio (HR = 1.37; 95% CI = 1.04-1.79) were independent prognostic factors of overall survival. Patients were categorized into three risk groups according to their risk scores. Median survival times for the low-risk (0 point), intermediate-risk (1-3 points), and high-risk (≥ 4 points) groups were 19.7, 10.7 and 5.1 months, respectively (P < .001). CONCLUSIONS: A prognostic model was developed that could facilitate risk stratification for metastatic or recurrent gastric cancer patients with good performance status who received first-line chemotherapy to help clinicians choose an applicable treatment based on the estimated prognosis.
Introduction
Gastric cancer is the second most common cause of cancer-related death in the world [1]. In China, this disease claimed approximately 297,496 lives in 2011 [2]. Although the only potential curative treatment for gastric cancer is surgery, most gastric patients are usually unable to receive curative surgical resection because of regional advanced or distant metastatic disease at the time of diagnosis. Palliative chemotherapy is still a major treatment in metastatic or recurrent gastric cancer [3], [4].
However, metastatic or recurrent gastric cancer patients who receive palliative chemotherapy have varying survival outcomes. To date, several studies have reported on prognostic indicators associated with survival including host- and tumor-related factors. Some prognostic models incorporating these prognostic factors have been developed. This kind of prognostic tool can be simply used to help oncologists guide treatment plans and improve prognostic accuracy [5]. Lee et al. developed the first prognostic model for metastatic gastric cancer patients receiving first-line chemotherapy [6]. Then, other prognostic models were gradually reported that focused on patients treated with different specific first-line chemotherapeutic regimens, such as cisplatin based, S-1 plus cisplatin, and docetaxel and cisplatin plus fluorouracil [7], [8], [9].
Although several prognostic models have been reported, some issues still need to be resolved. For example, patients analyzed in previous models were too indiscriminate, including those patients with esophageal cancer or squamous cell carcionomas [10]. More importantly, the patient group in these prognostic models included those that had not only good performance status (PS) but also poor PS.
Eastern Cooperative Oncology Group (ECOG) PS is an important parameter which is widely used to assess responses to chemotherapy and survival [11]. A good PS (0-1) has always been considered to have a better beneficial prognostic impact than a poor PS (2-3) [6], [12], [13]. However, some patients have a poor prognosis despite having a good PS. In a study analyzing 148 cancer patients with a good PS, the report showed that patients had a wide range of overall survival (OS) (29-2421 days) even though they had a PS = 0 [14]. One potential explanation is that there are several variables influencing survival, for example, histopathological factors, biological behavior of the malignancy, and others. Even if it is the same host-related factor, it could have different tumor-related effects. Therefore, prognostic factors are probably different between good and poor PS patient populations. Meanwhile, some subgroup analyses of clinical trials showed that patients with a good PS belonged to mixed groups who did not show good survival outcomes. The S-1 plus cisplatin versus S-1 in random control trial in the treatment for stomach cancer and S-1 alone versus S-1 and docetaxel combination in random control trial in the treatment for stomach cancer studies found that the good PS group did not show a statistically significant survival benefit from designated chemotherapy regimens [15], [16]. In other words, even though some patients had the same good PS, appropriate treatments for both good and poor PS patients should be tailored. Nevertheless, few studies have analyzed prognostic factors among cancer patients with a good PS [14], [17]. To optimize treatment for this subset of patients, it will be necessary to identify prognostic factors that can stratify patients within this group.
To our knowledge, no prognostic model for metastatic gastric cancer patients with a good PS is available. The objective of this study was to explore baseline host- or tumor-related factors that may be associated with survival and establish a prognostic model for metastatic or recurrent gastric cancer patients with a good PS who received first-line chemotherapy.
Patients and methods
Patients
Between April 2007 and December 2013, 371 patients received first-line palliative chemotherapy for metastatic or recurrent gastric cancer at the First Hospital of China Medical University. The criteria for patient inclusion consisted of the following: 1) age ≥ 18 years, 2) histologically confirmed diagnosis of gastric cancer, 3) presence of evaluable disease or measurable lesions, 4) received at least one cycle of chemotherapy, 5) good PS (0-1), and 6) availability of clinicopathological data at the start of chemotherapy. Patients with esophageal cancer, squamous cell carcinomas, or gastroesophageal junction tumors were excluded from the analysis. Of the 371 patients screened, 310 patients conformed with the inclusion criteria. All patients in the study signed informed consents.
Treatment
The 5-fluorouracil (5-FU)–based chemotherapy was as follows: 1) oxaliplatin, capecitabine, or S-1 (n = 74); 2) oxaliplatin, leucovorin, and 5-FU (modified FOLFOX) (n = 56); 3) capecitabine or S-1 (n = 45); 4) capecitabine or S-1 and cisplatin (n = 21); and 5) 5-FU and cisplatin (n = 11).
The taxane-based chemotherapy was as follows: 1) docetaxel or paclitaxel, and capecitabine or S-1 (n = 83); 2) docetaxel or paclitaxel, cisplatin, and 5-fluorouracil (n = 10); 3) docetaxel and cisplatin (n = 1); and 4) docetaxel (n = 1).
Others included 1) an irinotecan-based regimen (n = 4); 2) epirubicin, 5-FU, and cisplatin (n = 3); and 3) epirubicin, oxaliplatin, and capecitabine (n = 1).
Statistical Analysis
OS was the primary end point of this study. OS was counted from the time of metastasis to the time of death or the last follow-up visit. Survival data was analyzed using the Kaplan-Meier method. Comparison of survival curves were performed using log-rank analysis. A multivariate prognostic model was performed using all variables found to be significantly associated with OS at a P value ≤ .05 in the multivariate analysis. P values < .05 were considered statistically significant, and all P values corresponded to two-sided significance tests. All statistical analyses were performed using the SPSS 17.0 software package (SPSS, Chicago, IL).
Variables included in the univariate analysis consisted of the following: sex; age; PS; previous gastrectomy; tumor location; weight loss; the number of distant metastatic sites; the presence of ascites; metastasis to liver, bone, and lung at the start of chemotherapy; white blood cell (WBC) count; absolute neutrophil count (ANC); lymphocyte (LN) count; platelet count (PLT); neutrophil/lymphocyte ratio (NLR); platelet/lymphocyte ratio (PLR); hemoglobin; total protein (TP); serum albumin (ALB); total bilirubin (TBIL); alanine aminotransferase (ALT); and alkaline phosphatase (ALP). Laboratory variables, recorded as continuous variables, were dichotomized based on the median value of each variable.
Results
Patient Characteristics
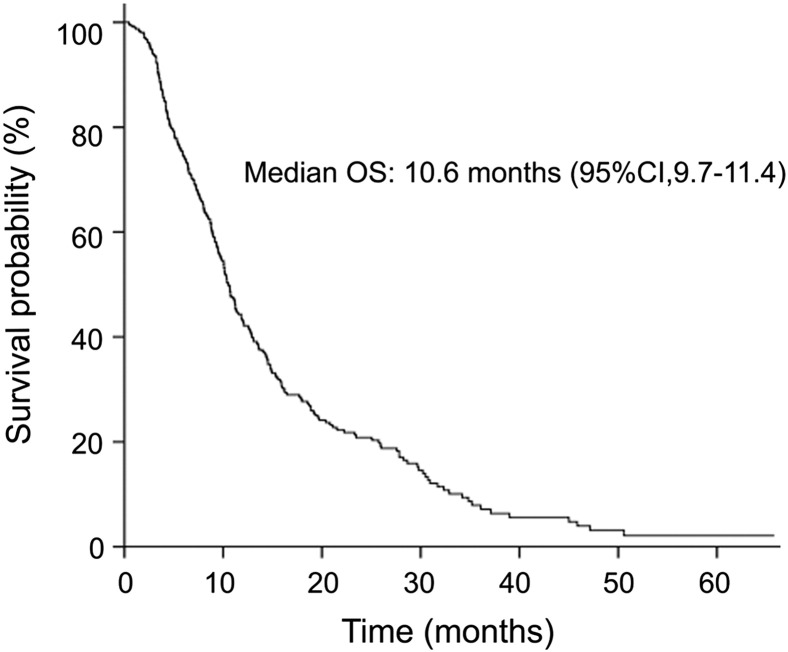
From April 2007 to December 2013, 310 patients were included in this study (Table 1). The median age was 58 years (range, 25-80 years).The percent of patients who had a PS = 0 at the time of receiving first-line chemotherapy was 19.7 (n = 61). Eighty-one percent (251 of 310) of patients had more than one distant metastatic site. Nearly half of the patients had previously received gastrectomies. There were 83 patients (26.8%) who underwent palliative gastrectomies and 66 (21.3%) who underwent radical gastrectomies. By the last follow-up date, 251 patients had died. The median time of OS was 10.6 months [95% confidence interval (CI) = 9.7-11.4] (Figure 1).
Table 1.
Clinical and Laboratory Characteristics of Patients
| No. of Patients (N = 310) | |
|---|---|
| Age (median age, range) | 58.0 (25-80) |
| Sex | |
| Male | 213 (68.7%) |
| Female | 99 (31.3%) |
| PS (ECOG) | |
| 0 | 61 (19.7%) |
| 1 | 249 (80.3%) |
| Previous operation | |
| No | 161 (51.9%) |
| Palliative gastrectomy | 83 (26.8%) |
| Radical gastrectomy | 66 (21.3%) |
| Site of primary | |
| Cardia/Fundus | 42 (13.5%) |
| Body | 76 (24.5%) |
| Pylorus/Antrum | 136 (43.9%) |
| Repeat | 45 (14.5%) |
| Unkown | 11 (3.5%) |
| Grade | |
| Well differentiated | 22 (7.1%) |
| Moderately differentiated | 60 (19.4%) |
| Poorly differentiated | 130 (41.9%) |
| Signet ring | 44 (14.2%) |
| Other/Unknown | 54 (17.4%) |
| Weight loss | |
| Yes | 132 (42.6%) |
| No | 170 (54.8%) |
| Number of distant metastatic site | |
| 0 | 59 (19.0%) |
| 1 | 160 (51.6%) |
| 2 | 69 (22.3%) |
| 3 | 15 (4.8%) |
| 4 | 7 (2.3%) |
| Metastasis | |
| Liver | 76 (24.5%) |
| Ascitic | 38 (12.3%) |
| Lung | 22 (7.1%) |
| Bone | 11 (3.5%) |
| Complete blood count (median ± SD) | |
| WBC, 109/l (N = 307) | 6.4 ± 2.32 |
| ANC, 109/l (N = 297) | 3.8 ± 2.08 |
| LN, 109/l (N = 296) | 1.7 ± 0.64 |
| Hemoglobin, g/l (N = 307) | 114.0 ± 20.70 |
| PLT, 109/l (N = 307) | 230.0 ± 99.86 |
| PLR (N = 298) | 139.6 ± 81.09 |
| NLR (N = 296) | 2.2 ± 1.70 |
| Blood chemistry (median ± SD) | |
| TP, g/l (N = 296) | 63.5 ± 6.75 |
| ALB, g/l (N = 296) | 38.2 ± 5.00 |
| ALP, U/l (N = 301) | 76.0 ± 97.74 |
| ALT, U/l (N = 303) | 15.0 ± 20.82 |
| TBIL, μmol/l (N = 302) | 8.4 ± 4.49 |
Figure 1.
Overall survival of all patients. The median time of OS was 10.6 months (95% CI = 9.7-11.4).
Univariate Analyses
We obtained complete information on all parameters on 296 of the 310 patients, and therefore, they were used in the prognostic analyses. In univariate analysis, statistically significant factors that adversely affected OS included no previous gastrectomy, bone and liver metastasis, number of distant organ metastasis (≥ 2), the presence of ascites, WBC count > 6.4*109/l, ANC > 3.8*109/l, PLT > 230*109/l, NLR > 50th percentile, and PLR > 50th percentile (Table 2).
Table 2.
Univariate Analyses with Regard to OS in 296 Patients
| Variable | MST (Months) | 1-Year Survival | P Value | HR (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 10.8 | 49.5% | .145 | 1.221 |
| Female | 10.1 | 38.0% | (0.934-1.598) | |
| Age | ||||
| ≤ 58 | 11.2 | 48.1% | .832 | 1.028 |
| > 58 | 10.6 | 43.4% | (0.797-1.327) | |
| ECOG | ||||
| 0 | 12.9 | 50.9% | .177 | 1.238 |
| 1 | 10.4 | 44.8% | (0.908-1.686) | |
| Previous gastrectomy | ||||
| Yes | 12.1 | 53.8% | < .001 | 1.627 |
| No | 9.4 | 38.4% | (1.258-2.104) | |
| Weight loss | ||||
| Yes | 10.4 | 44.0% | .134 | 0.818 |
| No | 11.2 | 47.4% | (0.628-1.064) | |
| Number of distant organ metastasis | ||||
| 0-1 | 11.9 | 51.7% | < .001 | 1.728 |
| ≥ 2 | 8.0 | 31.8% | (1.310-2.279) | |
| Ascitic | ||||
| No | 11.2 | 47.7% | .042 | 1.459 |
| Yes | 8.7 | 33.3% | (1.014-2.099) | |
| Liver metastasis | ||||
| No | 11.5 | 49.6% | < .001 | 1.920 |
| Yes | 8.8 | 34.7% | (1.430-2.576) | |
| Lung metastasis | ||||
| No | 10.7 | 47.1% | .631 | 1.117 |
| Yes | 8.7 | 31.8% | (0.712-1.753) | |
| Bone metastasis | ||||
| No | 10.7 | 46.5% | .027 | 2.047 |
| Yes | 4.4 | 30.0% | (1.084-3.864) | |
| WBC | ||||
| ≤ 6.4*109/l | 11.9 | 51.7% | .033 | 1.318 |
| > 6.4*109/l | 9.7 | 40.0% | (1.022-1.700) | |
| ANC | ||||
| ≤ 3.8*109/l | 12.9 | 54.4% | .001 | 1.555 |
| > 3.8*109/l | 9.2 | 37.4% | (1.205-2.007) | |
| LN | ||||
| ≤ 1.7*109/l | 9.1 | 40.3% | .234 | 0.857 |
| > 1.7*109/l | 11.8 | 51.7% | (0.665-1.105) | |
| Hemoglobin | ||||
| ≤ 114 g/l | 10.7 | 45.7% | .917 | 1.014 |
| > 114 g/l | 10.8 | 46.2% | (0.785-1.308) | |
| PLT | ||||
| ≤ 230*109/l | 11.9 | 51.3% | .049 | 1.291 |
| > 230*109/l | 9.8 | 40.3% | (1.001-1.665) | |
| PLR | ||||
| ≤ 50th percentile | 12.6 | 53.7% | .009 | 1.1.406 |
| > 50th percentile | 9.5 | 38.1% | (1.090-1.814) | |
| NLR | ||||
| ≤ 50th percentile | 13.6 | 57.4% | < .001 | 1.623 |
| > 50th percentile | 8.8 | 34.5% | (1.256-2.096) | |
| TP | ||||
| ≤ 63.5 g/l | 10.4 | 43.2% | .713 | 0.953 |
| > 63.5 g/l | 11.1 | 48.6% | (0.736-1.233) | |
| ALB | ||||
| ≤ 38.2 g/l | 10.1 | 41.8% | .064 | 0.785 |
| > 38.2 g/l | 11.3 | 50.0% | (0.607-1.014) | |
| ALP | ||||
| ≤ 76 U/l | 11.1 | 48.6% | .383 | 1.121 |
| > 76 U/l | 10.4 | 42.4% | (0.867-1.449) | |
| ALT | ||||
| ≤ 15 U/l | 10.7 | 44.3% | .210 | 0.848 |
| > 15 U/l | 10.8 | 47.8% | (0.654-1.098) | |
| TBIL | ||||
| ≤ 8.4 μmol/l | 11.1 | 46.7% | .329 | 0.880 |
| > 8.4 μmol/l | 10.3 | 44.8% | (0.680-1.138) |
MST, median survival time.
Prognostic Model
Multivariate regression analysis included the variables that were found to have prognostic significance in univariate analysis, as listed above and in Table 2. The forward conditional Cox regression model was used to delineate significant prognostic factors for survival. Five factors were found to play an independent prognostic role: no previous gastrectomy, number of distant metastatic sites, bone metastasis, liver metastasis, and an elevated NLR (Table 3).
Table 3.
Multivariate Analysis with Regard to OS in 296 Patients
| Factor | HR | 95% CI | P Value |
|---|---|---|---|
| Liver metastasis | 1.766 | 1.308-2.385 | < .001 |
| No previous gastrectomy | 1.416 | 1.082-1.853 | .011 |
| Bone metastasis | 2.198 | 1.156-4.181 | .016 |
| NLR > 50th percentile | 1.367 | 1.042-1.794 | .024 |
| Number of distant metastatic site ≥ 2 | 1.474 | 1.110-1.957 | .007 |
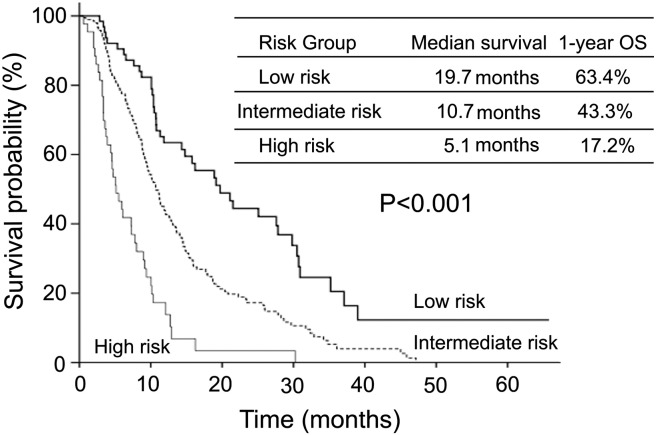
Then, a multivariate analysis prognostic model was constructed by incorporating all five prognostic indicators. The risk scoring system was distributed based on hazard ratios (HRs) from the final multivariate analysis model, with 1 point for an HR < 1.5 and 2 points for an HR > 1.5. According to the risk scores, patients were assigned to three risk categories: low risk (0 point), intermediate risk (1-3 points), and high risk (≥ 4 points). Median survival times for low-, intermediate-, and high-risk groups were 19.7, 10.7, and 5.1 months, respectively. Survival curves according to the prognostic model are shown in Figure 2. One-year survival rates for low-, intermediate-, and high-risk groups were 63.4%,43.3%, and 17.2%.When compared with the low-risk group, the intermediate-risk group had a 2.1-fold (HR = 2.07; 95% CI = 1.46-2.94) increased risk of death, wheresa the high-risk group demonstrated a 3.7-fold (HR = 3.66; 95% CI = 2.53-5.28) increased risk of death. There were significant survival differences among the three risk groups (P < .001).
Figure 2.
Kaplan-Meier survival curves for survival probability according to risk groups. The median survival differences between low-, intermediate-, and high-risk groups (19.7, 10.7, and 5.1 months, respectively; P < .001) were statistically significant.
Discussion
Many variables potentially influence survival such as tumor grade, tumor burden, nutritional status, and PS. PS is the most important parameter for predicting survival in patients with metastatic gastric cancer [10]. In a study analyzing 1455 metastatic gastric cancer patients who received first-line chemotherapy, patients with a PS of 2 to 4 were found to be associated with a 1-year survival rate of 17.1% compared with 39.2% for patients with a PS of 0 to 1 [6]. Evidently, patients with a good PS were better able to tolerate the prescribed dose and course of chemotherapy and usually had relatively good survival outcomes. In our study, the median time of OS for all patients with a good PS was 10.6 months, longer than predicted by other prognostic models for metastatic gastric cancer patients (7.3-8.6 months) [6], [7], [10]. This highlights that having a good PS is a superior prognostic predictor.
There was no previous prognostic model identified that could be used to guide treatment for patients with a good PS. Our study was a single-institution, retrospective study that analyzed data pooled from patients with a good PS receiving first-line palliative chemotherapy for metastatic gastric cancer. Five statistically significant independent prognostic factors of poor survival (no previous gastrectomy, distant organ metastasis ≥ 2, NLR > 50th percentile, bone metastasis, and liver metastasis) were identified. Using these factors, a prognostic model to predict survival was developed that involved grouping patients into three risk categories: low, intermediate, and high, with significant survival differences.
It is recognized increasingly that tumors that occur or spread are associated not only with tumor-related factors but also host inflammatory factors [18]. These various factors have an important impact in building a prognostic model. NLR was a useful host inflammatory biomarker for predicting inferior OS. Previous studies revealed that an elevated NLR was an independent factor of OS in multivariate analysis in patients with advanced gastric cancer [19], [20], [21]. A current meta-analysis showed that OS was significantly worse in patients with an elevated NLR value regardless of whether they had resectable or metastatic gastric cancer [22].
In the present analysis, patients with an elevated NLR (NLR > 50th percentile) had a significantly poorer OS than those with a low NLR. This is the first time that NLR has been incorporated in the development of a prognostic model for metastatic gastric cancer. In recent years, clinicians invested in resources and effort to develop biomarkers for cancer patients. The NLR has distinct advantages because it is easily obtained, convenient to monitor, and cost-effective. This parameter should be extensively used to analyze clinical study results and in the design of future clinical trials. Other host-response factors, such as PLR and hemoglobin and albumin level, were also prognostically relevant in this study. Elevated PLR was statistically significant in univariate analysis but not in multivariate analysis. One possible reason why many studies have previously found low hemoglobin and low albumin levels to be independent poor prognostic factors for advanced gastric cancer was that, in these studies, patients usually had a poor PS [6], [9], [23], [24]. Although all patients involved in our study had a good PS, these two factors were not identified as prognostic in the present analysis.
Liver metastasis is usually found in gastric cancer because the presence of liver metastasis is readily evident clinically or radiologically. For example, liver nodules are easily seen on ultrasound or computed tomography scans. Although bone is not a common site of metastasis in gastric cancer, previous studies have found that liver and bone metastases were extremely poor prognostic factors in gastric cancer patients [6], [7], [8]. In our study, we found that liver and bone metastases were independent inferior prognostic factors in patients with a good PS. In a study evaluating 1080 patients with esophagogastric cancer, a similar conclusion was reached for patients with good and poor PS [6]. Liver and bone metastases were two common poor prognostic factors for patients with good and poor PS.
The role of radical gastrectomy in operable gastric cancer patients is relatively doubtless; however, the role of gastric resection in patients with metastatic gastric cancer remains dismal. Some studies have demonstrated that not having a prior gastrectomy was a statistically significant prognostic factor of poor survival [8], [25]. Reasons considered to explain the benefit of palliative gastrectomy were as follows: First, palliative gastrectomy could relieve potentially life-threatening complications such as perforation, obstruction, or bleeding. Second, surgery could improve outcomes by reducing tumor burden, increasing the quality of life and responsiveness to adjuvant chemoradiotherapy. Third, surgical resection for primary sites may reduce immunosuppressive cytokines produced by the tumor [26], [27], [28]. A recent meta-analysis of 10 studies showed that gastric resections were associated with a five-fold higher OS rate versus nonsurgical treatment [29]. In our analysis, patients with previous gastrectomy, either palliative or radical, had obvious survival superiority when compared with those without previous gastrectomy, and its prognostic significance was retained in multivariate analysis.
Previous prognostic models revealed that patients categorized in the high-risk group had very poor survival outcomes (2.7-4.1 months) [6], [7], [10]. This subset of patients, who usually had a poor PS, would be provided with the best supportive care. Nevertheless, patients with a good PS in our models had a better predictive survival rate. Notably, patients with high-risk factors had very poor survival, approximately 5.1 months. This group of patients that had a good PS was able to endure high-intensity treatment. Therefore, this subset of patients may be candidates for more cytotoxic drug combination chemotherapy regimens or for adding targeted agents rather than just being provided with best supportive care. This strategy may be crucial in developing new approaches or clinical trials for these patients. That is the difference between our model and previous prognostic models.
To the best of our knowledge, it is the first prognostic model for this specific subset of patients.
Conclusions
Five prognostic factors were identified in patients receiving first-line palliative chemotherapy for metastatic or recurrent gastric cancer. A prognostic model was developed with distinct survival outcomes among the different risk groups. Therefore, the constructed prognostic model could be used to help oncologists identify patients that could possibly benefit from current chemotherapeutic agents and aid them in selecting an appropriate treatment based on the approximated prognosis.
Compliance with Ethical Standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the First Hospital of China Medical University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from each participant before enrollment.
Acknowledgements
This work was supported by the National Science and Technology Major Project (no. 2013ZX09303002) and Science and Technology Plan Project of Liaoning Province (nos. 2011404013-1 and 2014225013).
Footnotes
This work was supported by the National Science and Technology Major Project (no. 2013ZX09303002) and Science and Technology Plan Project of Liaoning Province (nos. 2011404013-1 and 2014225013).
Contributor Information
Yunpeng Liu, Email: cmuliuyunpeng@hotmail.com.
Xiujuan Qu, Email: qu_xiujuan@hotmail.com.
References
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27(1):2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24(18):2903–2909. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 4.Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, Andreozzi F, Ventriglia J, Savastano B, Mabilia A. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635–1649. doi: 10.3748/wjg.v20.i7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CK, Hudson M, Stockler M, Coates AS, Ackland S, Gebski V, Lord S, Friedlander M, Boyle F, Simes RJ. A nomogram to predict survival time in women starting first-line chemotherapy for advanced breast cancer. Breast Cancer Res Treat. 2011;129(2):467–476. doi: 10.1007/s10549-011-1471-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Lim T, Uhm JE, Park KW, Park SH, Lee SC, Park JO, Park YS, Lim HY, Sohn TS. Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann Oncol. 2007;18(5):886–891. doi: 10.1093/annonc/mdl501. [DOI] [PubMed] [Google Scholar]
- 7.Kim JG, Ryoo BY, Park YH, Kim BS, Kim TY, Im YH, Kang YK. Prognostic factors for survival of patients with advanced gastric cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2008;61(2):301–307. doi: 10.1007/s00280-007-0476-x. [DOI] [PubMed] [Google Scholar]
- 8.Koo DH, Ryu MH, Ryoo BY, Lee SS, Moon JH, Chang HM, Lee JL, Kim TW, Kang YK. Three-week combination chemotherapy with S-1 and cisplatin as first-line treatment in patients with advanced gastric cancer: a retrospective study with 159 patients. Gastric Cancer. 2012;15(3):305–312. doi: 10.1007/s10120-011-0117-2. [DOI] [PubMed] [Google Scholar]
- 9.Inal A, Kaplan MA, Kuukoner M, Urakci Z, Guven M, Nas N, Yunce M, Iikdogan A. Prognostic factors in first-line chemotherapy treated metastatic gastric cancer patients: a retrospective study. Asian Pac J Cancer Prev. 2012;13(8):3869–3872. doi: 10.7314/apjcp.2012.13.8.3869. [DOI] [PubMed] [Google Scholar]
- 10.Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. J Clin Oncol. 2004;22(12):2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 12.Shitara K, Muro K, Matsuo K, Ura T, Takahari D, Yokota T, Sawaki A, Kawai H, Ito S, Munakata M. Chemotherapy for patients with advanced gastric cancer with performance status 2. Gastrointest Cancer Res. 2009;3(6):220–224. [PMC free article] [PubMed] [Google Scholar]
- 13.Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10(11):1063–1069. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 14.Penel N, Vanseymortier M, Bonneterre ME, Clisant S, Dansin E, Vendel Y, Beuscart R, Bonneterre J. Prognostic factors among cancer patients with good performance status screened for phase I trials. Investig New Drugs. 2008;26(1):53–58. doi: 10.1007/s10637-007-9088-x. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9(3):215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START) J Cancer Res Clin Oncol. 2014;140(2):319–328. doi: 10.1007/s00432-013-1563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriwaki T, Ishige K, Araki M, Yoshida S, Nishi M, Sato M, Yamada T, Yamamoto Y, Ozeki M, Ishida H. Glasgow Prognostic Score predicts poor prognosis among advanced biliary tract cancer patients with good performance status. Med Oncol. 2014;31(11):287. doi: 10.1007/s12032-014-0287-y. [DOI] [PubMed] [Google Scholar]
- 18.Mohri Y, Tanaka K, Ohi M, Yokoe T, Miki C, Kusunoki M. Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg. 2010;34(2):285–290. doi: 10.1007/s00268-009-0302-1. [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73(3-4):215–220. doi: 10.1159/000127412. [DOI] [PubMed] [Google Scholar]
- 20.Qiu MZ, Wei XL, Zhang DS, Jin Y, Zhou YX, Wang DS, Ren C, Bai L, Luo HY, Wang ZQ. Efficacy and safety of capecitabine as maintenance treatment after first-line chemotherapy using oxaliplatin and capecitabine in advanced gastric adenocarcinoma patients: a prospective observation. Tumour Biol. 2014;35(5):4369–4375. doi: 10.1007/s13277-013-1574-5. [DOI] [PubMed] [Google Scholar]
- 21.Mohri Y, Tanaka K, Ohi M, Saigusa S, Yasuda H, Toiyama Y, Araki T, Inoue Y, Kusunoki M. Identification of prognostic factors and surgical indications for metastatic gastric cancer. BMC Cancer. 2014;14:409. doi: 10.1186/1471-2407-14-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang W, Feng LJ. Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo DH, Ryoo BY, Kim HJ, Ryu MH, Lee SS, Moon JH, Chang HM, Lee JL, Kim TW, Kang YK. A prognostic model in patients who receive chemotherapy for metastatic or recurrent gastric cancer: validation and comparison with previous models. Cancer Chemother Pharmacol. 2011;68(4):913–921. doi: 10.1007/s00280-011-1561-8. [DOI] [PubMed] [Google Scholar]
- 24.Kanagavel D, Pokataev IA, Fedyanin MY, Tryakin AA, Bazin IS, Narimanov MN, Yakovleva ES, Garin AM, Tjulandin SA. A prognostic model in patients treated for metastatic gastric cancer with second-line chemotherapy. Ann Oncol. 2010;21(9):1779–1785. doi: 10.1093/annonc/mdq032. [DOI] [PubMed] [Google Scholar]
- 25.Lee SS, Lee JL, Ryu MH, Chang HM, Kim TW, Kang HJ, Kim WK, Lee JS, Kang YK. Combination chemotherapy with capecitabine (X) and Cisplatin (P) as first line treatment in advanced gastric cancer: experience of 223 patients with prognostic factor analysis. Jpn J Clin Oncol. 2007;37(1):30–37. doi: 10.1093/jjco/hyl134. [DOI] [PubMed] [Google Scholar]
- 26.McCarter MD, Fong Y. Role for surgical cytoreduction in multimodality treatments for cancer. Ann Surg Oncol. 2001;8(1):38–43. doi: 10.1007/s10434-001-0038-0. [DOI] [PubMed] [Google Scholar]
- 27.Saidi RF, ReMine SG, Dudrick PS, Hanna NN. Is there a role for palliative gastrectomy in patients with stage IV gastric cancer? World J Surg. 2006;30(1):21–27. doi: 10.1007/s00268-005-0129-3. [DOI] [PubMed] [Google Scholar]
- 28.Pollock RE, Roth JA. Cancer-induced immunosuppression: implications for therapy? Semin Surg Oncol. 1989;5(6):414–419. doi: 10.1002/ssu.2980050607. [DOI] [PubMed] [Google Scholar]
- 29.Lasithiotakis K, Antoniou SA, Antoniou GA, Kaklamanos I, Zoras O. Gastrectomy for stage IV gastric cancer. a systematic review and meta-analysis. Anticancer Res. 2014;34(5):2079–2085. [PubMed] [Google Scholar]