Abstract
BACKGROUND: Although pazopanib treatment has become the standard chemotherapy in salvage setting for metastatic sarcoma patients, most patients progress after pazopanib treatment in 4 to 6 months. After failure to pazopanib, patients have limited options for treatment. Therefore, subsequent therapy in patients who failed to pazopanib is urgently needed and the use of patient derived cells or patient derived tumors for accompanying testing with various pharmacological inhibitors could offer additional treatment options for these patients. METHODS: Patient derived tumor cells were collected from ascites at the time of progression to pazopanib and a 13-drug panel was tested for drug sensitivity. We confirmed the results using in vitro cell viability assay and immunoblot assay. We also performed the genomic profiling of PDX model. RESULTS: The growth of patient derived tumor cells was significantly reduced by exposure to 1.0 μM AZD2014 compared with control (control versus AZD2014, mean growth = 100.0% vs 16.04%, difference = 83.96%, 95% CI = 70.01% to 97.92%, P = .0435). Similarly, 1.0 μM BEZ235 profoundly inhibited tumor cell growth in vitro when compared to control (control versus BEZ235, mean growth = 100.0% vs 7.308%, difference = 92.69%, 95% CI = 78.87% to 106.5%, P < .0001). Despite the presence of CDK4 amplification in the patient-derived tumor cells, LEE011 did not considerably inhibit cell proliferation when compared with control (control vs LEE011, mean growth = 100.0% vs 80.23%, difference = 19.77%, 95% CI = 1.828% to 37.72%, P = .0377). The immunoblot analysis showed that BEZ235 treatment decreased pAKT, pmTOR and pERK whereas AZD2014 decreased only pmTOR. CONCLUSION: Taken together, upregulation of mTOR/AKT pathway in sarcoma patient derived cells was considerably inhibited by the treatment of AZD2014 and BEZ235 with downregulation of AKT pathway (greater extent for BEZ235). These molecules may be considered as treatment option in STS patient who have failed to pazopanib in the context of clinical trials.
Introduction
Soft tissue sarcomas (STS) comprise a heterogeneous group of malignant neoplasms which are derived from mesenchymal origin. STS can be located anywhere in the human body. There are more than 50 different sub-types according to the recent revised WHO classification [1], [2]. The incidence of STS is low accounting for approximately 1% of adult cancers worldwide [3]. Approximately 10,000 and 3,300 newly diagnosed cases are reported annually in the USA and the UK, respectively [4], [5]. In patients with localized disease, surgical resection with or without radiotherapy and chemotherapy is the preferred therapeutic approach and the estimated 5-year survival rate is about 70% [6], [7]. STS recur frequently as locally inoperable or metastatic disease and systemic therapy has a prominent role in the multidisciplinary management in this setting [6]. However, patients with metastatic disease have an estimated 3-year survival rate of 20% to 45% [7]. It is of note that there has been no significant improvement in the survival rate of patients with metastatic disease over the last few years despite the continuous development of systemic therapy regimens in the first-line setting [8], [9], [10]. Although toxicity and resistance appear to be the major drawbacks associated with systemic therapy in the first-line setting, it should be noted that there have been no robust data regarding the use of systemic therapy in the second-line setting [11]. Thus, there is unmet clinical need for the treatment of patients with advanced STS in both the first- and the second-line settings. In our previous report on 43 patients treated with pazopanib as salvage treatment, the overall response rate was 17.1% and PFS was 5.0 months (95% CI, 3.6 to 6.4 months) [12]. Therefore, subsequent therapy in patients who failed to pazopanib is urgently needed. Few reports have reported on the potential efficacy of phosphatidylinositol-3 kinase inhibitors (PIK3) in sarcoma preclinical models [13], [14], [15], [16]. The use of patient-derived xenografts (PDX) for advanced sarcoma has also been described [17].
Herein we report the case of a patient with refractory undifferentiated pleomorphic sarcoma and the potential of BEZ235, a PIK3/mTOR inhibitor, after failing to pazopanib in a patient-derived tumor model established from tumor material after failed first-line therapy with pazopanib.
Methods
Written Informed Consent
The patient was enrolled as part of the SMC Oncology Biomarker study (NCT#01831609, clinicaltrials.gov) [18], which is reported elsewhere. Effusions were obtained for therapeutic purposes after obtaining written informed consent, and all procedures were carried out according to guidelines from the Declaration of Helsinki. The Institutional Review Board at the Samsung Medical Center approved the protocol.
Patient-Derived Cell Culture and Reagents
Malignant ascites were collected after signature of the informed consent form. Collected effusions (1 to 5 L) were divided into 50-mL tubes, centrifuged at 1500 rpm for 10 min, and washed twice with PBS as previously described [18]. Extracted cells were cultured in RPMI media supplemented with 10% fetal bovine serum, 0.5 g/ml of hydrocortisone (Sigma Aldrich), 5 g/ml of insulin (PeproTech, Rocky Hill, NJ, USA), 5 ng of EGF and FGF (PeproTech). BEZ235, AZD2014, lapatinib, LEE011 and pazopanib were purchased from Selleck Chemical (Houston, TX, USA). After pathologic confirmation, cells were seeded at 1 × 106 cells/10 mm dishes or 5000 cells/well/96well plate and treated with 1 μM of each inhibitor after passage #2. Treated cells were incubated for 72 hours at 37°C in a humidified atmosphere of 5 % carbon dioxide. Inhibition of tumor derived cell line proliferation was determined using CellTiter-Glo® (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
For 3-dimensional drug screening system, we utilized the S + Chip Analyzer (Samsung Electro-Mechanics Co., Ltd, South Korea) to analyze the image of the cells grown in 3D droplets on micropillars as previously described [19]. Briefly Cultured primary patient derived cells (passage 1 to 4, 2D) were seeded into 3D culture media; DMEM F/12 containing 1% antibiotic-anti-mycotic solution, 10 mM of HEPES, B27, N2, Glutamax (Gibco BRL), 1 mM of N-acetyl-L-cysteine (Sigma Aldrich), 10 μg/ml of insulin (PeproTech, Rocky Hill, NJ, USA), 20 ng/ml of bFGF, and 50 ng/ml of EGF (PeproTech). After 3 to 5 days, the organoids were dissociated by using accutase (Gibco BRL) to the single cells and prepared to load on the micropillar chip. 40 nl droplet for cell/alginate mixture and 950 nl for media or compounds were dispensed with S + microarrayer (S + Chip Scanner, Samsung Electro-Mechanics Co., Ltd, South Korea). After 1 day incubation, the micropillar chip containing cells moves to new microwell chip filled with various test compounds and combined chips are incubated during 7 days for compound efficacy test as shown in previously published method [20].
PDX Models Established from PDCs
PDCs were transferred to Oncotest as frozen vials. On site, the cells were thawed, the freezing medium was removed, and the cells were resuspended and transferred into T75 flasks. Cells were grown for 3 to 7 days in RPMI/10% FBS until the culture reached around 80% confluence. Cells were collected and counted, and 5 × 106 cells were injected into the hind flanks of NOD scid gamma (NOG) mice (Taconic). Tumors developed within 25 to 85 days after injection; these tumors were explanted, and viable portions of the tumors were cut into pieces and implanted subcutaneously into female NMRI nu/nu mice (Harlan Laboratories). This process was repeated in order to serially passage the respective models. From each passage, formalin-fixed, paraffin-embedded (FFPE) blocks were prepared, and tumor slices were stained with hematoxylin and eosin (H&E). Slides were scanned with a Hamamatsu slide scanner, and images were extracted using the Nanozoomer program from Hamamatsu. All animal handling and experiments with animals were in accordance with the guidelines set by the Samsung Biological Research Institute.
Western Blot Analyses
Total proteins from PDCs were isolated using RIPA buffer (Sigma-Aldrich) containing a protease inhibitor cocktail (Roche, Mannheim, Germany) and phosphatase inhibitor cocktail (Roche), and protein concentrations were determined according to the Bradford procedure using a Quick Start Bradford Protein Assay (Bio-Rad, Hercules, CA, USA). Thirty micrograms of proteins were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on 10% gels and then electrotransferred to nitrocellulose membranes incubated with the following specific antibodies: Cyclin D1 (sc-718) (M-20) from Santa Cruz (USA) and PI3K (#4255) (p110α), phospho-mTOR (#2971) (Ser2448), mTOR (#2983) (7C10), phospho-Akt (#4060) (Ser473), Akt (#9272), phospho-ERK1/2 (#4370) (Thr202/Tyr204), ERK1/2 (#9102) from Cell Signaling Technologies (Beverly, MA, USA). Horseradish peroxidase-conjugated anti-rabbit or mouse IgG (Vector, Burlingame, CA, USA) was used as a secondary antibody, and signals were detected by chemiluminescence using ECL Western Blotting Substrate (Thermo Scientific, Rockford, IL, USA).
Targeted Sequencing
Genomic DNA from single cell suspensions of PDX tumors were extracted, and a SureSelect customized kit (Agilent Technologies, Santa Clara, CA, USA) was used for capturing 381 cancer-related genes. Illumina HiSeq 2500 was used for sequencing with 100 bp paired-end reads. The sequencing reads were aligned to the human genome reference sequence (hg19) using BWA-mem (v0.7.5), SAMTOOLS (v0.1.18), Picard (v1.93), and GATK (v3.1.1) for sorting SAM/BAM files, duplicate marking, and local realignment. Local realignment and base recalibration were carried out based on dbSNP137, Mills indels, HapMap, and Omni. SNVs and InDels were identified using Mutect (v1.1.4) and Pindel (v0.2.4), respectively. ANNOVAR was used to annotate the detected variants. Copy number variations (CNVs) were called by an in-house algorithm. The module ‘Depth of Coverage’ in GATK v3.1-1 was used to calculate sequencing coverage in each exon. The median values of mean coverage for total exons were calculated and divided by the respective gained average. The median value of each exon in normal reference datasets was used as a reference value. Then the normalized median value of exons of each patient was divided by the reference value and transformed to the binary logarithm.
Statistical Method
For PDC viability curves, results are expressed as the means. Paired one way ANOVA tests were used to calculate the P values. Statistical significance was assessed using one way ANOVA tests and described in the figure.
Results
Patient History
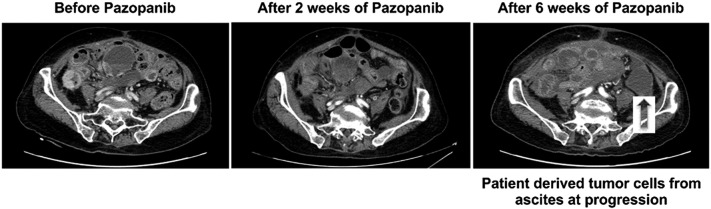
A 56-year-old woman presented with abdominal pain in May 2012, with a computed tomography (CT) scan showing a huge retroperitoneal mass. She was diagnosed with a retroperitoneal French Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grade 3 undifferentiated pleomorphic sarcoma after palliative surgical excision of tumor. She achieved partial remission initially with VAC/IE (vincristine, adriamycin, cyclophosphamide, ifosfamide, etoposide), but a new cystic retroperitoneal mass and right-sided hydronephrosis appeared on the follow-up CT scan in January 2013 after the 7th treatment-cycle. She underwent second surgery to extirpate the recurrent mass. After the second operation, she had rapidly progressive disease on 2 cycles of gemcitabine/docetaxel and 2 cycles of VIP (vincristine, ifosfamide, cisplatin). After 2 weeks of treatment with pazopanib, a CT scan demonstrated hemorrhagic necrosis of tumor and her abdominal pain was substantially decreased. After 6 weeks on pazopanib, the patient had progressive disease with newly developed ascites (Figure 1, right panel). At this time, the patient derived tumor cells from ascites were collected and cultured. After stopping pazopanib, the patient was on supportive care until her death due to disease progression.
Figure 1.
The CT evaluation of the patient during the course of pazopanib treatment in metastatic sarcoma patient.
Patient Derived Sarcoma Cells
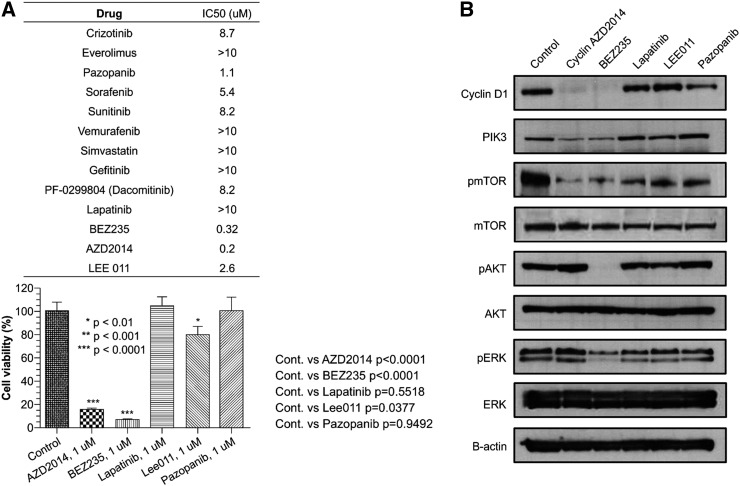
Malignant tumor cells were harvested from malignant ascites developed at resistance to pazopanib in this patient as described in the Methods section. First, we tested if the patient derived tumor cells were resistant to pazopanib as these cells were cultured at the time of pazopanib resistance. Using high-throughput 3-dimensional culture system, we surveyed anti-tumor activity of 13-drug panel (Figure 2A, left upper panel). We validated the results from 3-dimensional drug screening results and confirmed with in vitro cell viability assay (Figure 2A, left lower panel). Based on the initial results from 3-dimentionsal multi-drug testing, we tested the anti-tumor effect of AZD2014 (TORC1/TORC2 inhibitor), BEZ235 (mTOR/PIK3 inhibitor), lapatinib (HER1, HER2 inhibitor), LEE011 (CDK4 inhibitor), pazopanib which showed IC 50 less than 3.0 μM, in vitro cell viability assay. In accordance to the results from the 3-dimensional culture assay, we confirmed that the cells were very sensitive to AZD2014 and BEZ235 while the cells were relatively resistant to lapatinib, LEE011 and pazopanib. The growth of tumor cells was significantly reduced by exposure to 1.0 μM AZD2014 compared with control (control versus AZD2014, mean growth = 100.0% vs 16.04%, difference = 83.96%, 95% CI = 70.01% to 97.92%, P = .0435). Similarly, 1.0 μM BEZ235 profoundly inhibited tumor cell growth in vitro when compared to control (control versus BEZ235, mean growth = 100.0% vs 7.308%, difference = 92.69%, 95% CI = 78.87% to 106.5%, P < .0001). In contrast, despite of the presence of CDK4 amplification (Table 1) in the patient-derived tumor cells, LEE011 did not considerably inhibit cell proliferation when compared with control (control versus LEE011, mean growth = 100.0% vs 80.23%, difference = 19.77%, 95% CI = 1.828% to 37.72%, P = .0377).
Figure 2.
(A) Using high-throughput 3-dimensional culture system, the anti-tumor activity of 13-drug panel (left upper panel) was tested using PDC line. The results from 3-dimensional drug screening results were further confirmed with in vitro cell viability assay (left lower panel). The growth of tumor cells was significantly reduced by exposure to 1.0 μM AZD2014 compared with control (control versus AZD2014, mean growth = 100.0% vs 16.04%, difference = 83.96%, 95% CI = 70.01% to 97.92%, P = .0435). 1.0 μM BEZ235 profoundly inhibited tumor cell growth in vitro when compared to control (control versus BEZ235, mean growth = 100.0% vs 7.308%, difference = 92.69%, 95% CI = 78.87% to 106.5%, P < .0001). (B) The effects of AZD2014, BEZ235, lapatinib, LEE011, pazopanib on PI3K/AKT signaling in sarcoma PDC line were determined by immunoblotting analysis. Cells were treated with 1 μM of the indicated drugs for 72 h.
Table 1.
Genomic profiling of the PDX established from PDC
| Genomic alterations by targeted sequencing | |||||
|---|---|---|---|---|---|
| SNV/INDEL | FGFR1 N457K | FBXW7 P255K | FBXW7 S148T | PIK3R1 M56I | BRCA1 Q309R |
| CNV | CDK4 AMP | KIT AMP | MDM2 AMP | PTEN del | |
Next, we tested if downstream targets were downregulated upon exposure to each drug. As shown in Figure 2B, we observed that BEZ235 treatment completely downregulated the AKT phosphorylation of tumor cells as well as pmTOR and pERK. AZD2014, a pharmacologically much “cleaner” TORC1/TORC2 inhibitor, also down-regulated pmTOR and PI3K but not pAKT. Upon exposure to AZD2014 and BEZ235, cyclin D1 was virtually undetectable whereas lapatinib, LEE011 and pazopanib did not have any effect on its expression. Taken together, upregulation of mTOR/AKT pathway in pazopanib-resistant tumor derived cells was considerably inhibited by the treatment of AZD2014 and BEZ235 with downregulation of AKT pathway (greater extent for BEZ235). In contrast, LEE011 did not have significant inhibitory effect on CDK4-amplified sarcoma cells in this particular case.
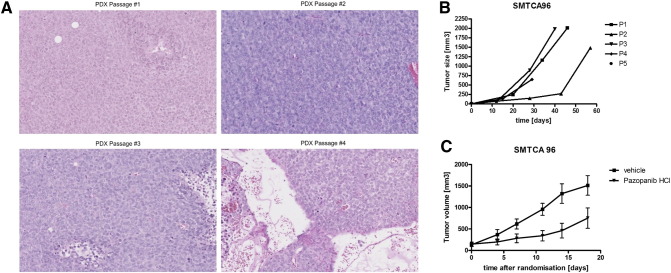
After successful PDC establishment from malignant ascites, we implanted these PDCs in NOG mice to evaluate whether PDCs could be converted into a PDX model. As shown in Figure 3A, the PDX model could serially passaged for at least 5 times. The histologic examination demonstrated high grade sarcoma cells. At passage 5, single cell suspensions from the PDX tumor were generated for further experiments. The genomic profiling of these cells using targeted deep sequencing showed FGFR1 (N457K), PIK3R1 (M56I), BRCA1 (Q309R) mutations and CDK4/MDM2/KIT amplifications and a PTEN deletion (Table 1).
Figure 3.
(A) H&E stains of subsequent PDX passages of SMTCA96. The histological architecture is stable over the first four passages: homogenous growing cells surrounding some small sinusoids could be observed. The picture in passage 4 depicts the edges of the subcutaneously growing tumor including relevant quantities of surrounding mouse tissue. (B) Growth curves of the PDX model SMTCA 96. As it is frequently observed, the initial establishment and growth of human xenografts in immunocompromised mice takes a longer time frame than later passages. (C) NMRI nu/nu mice were implanted with fragments of SMTCA 96. Once these pieces engrafted, cohorts were randomized and treatment with several drugs was started. Pazopanib was administered twice daily at 50 mg/kg/d for two weeks. Despite this continuous treatment, tumor growth was only slowed down. Once the therapy cycle finished the tumor growth rate was similar to the control group. This result is in line with both the patient data and the in vitro data from the PDCs were the cells showed still some effect towards treatment with the inhibitor. In contrast, doxorubicin, another drug registered for sarcoma did not affect tumor growth in vivo at all (data not shown).
Discussion
In this study, we successfully established patient derived tumor cells from a metastatic, high grade sarcoma patient who initially responded to pazopanib but has developed resistance after 6 weeks of treatment. Using a 3-dimensional high throughput drug screening system and in vitro cell viability assay, we demonstrated that both an AKT/mTOR and TORC1/2 inhibitor were effective in inhibiting cell proliferation of the obtained sarcoma cell line. In contrast, the cells were relatively resistant to pazopanib, LEE011 or lapatinib. The AKT pathway was significantly downregulated upon drug treatment with AZD2014 or BEZ235. Our results suggest that AKT/mTOR inhibition may offer therapeutic intervention in sarcomas that failed to pazopanib. Except one case report about reversal of acquired resistance to pazopanib with mTOR inhibitor [21], no other study has yet been reported indicating use of BEZ235 or AZD2014 in pazopanib resistant STS. However, this needs further validation on a larger number of samples to be considered as a potential therapy.
BEZ235 is a synthetic small molecule belonging to the class of imidazoquinolines that potently and reversibly inhibits PI3K catalytic activity by competing at its ATP-binding site. Moreover, BEZ235 also inhibits mTOR catalytic activity [22]. Although BEZ235 has shown promising therapeutic activity in several tumors including breast cancer, ovarian cancer [23], [24], [25], [26] and lymphomas, only a few reports are available for sarcomas [27], [28]. Phase I/II trials with BEZ235 are still ongoing and results have not been reported yet. In contrast, AZD2014 is a highly specific mTORC1 and mTORC2 inhibitor [29]. It has displayed activity in models of everolimus resistance in a long term estrogen deprived background in vitro and in vivo, suggesting that inhibition of mTORC1 and 2 could be effective in patients which have become resistant to rapalogues [30]. These targeted agents can be considered as a potential therapeutic option in metastatic sarcoma patients who have failed to pazopanib.
We have sequenced the PDX models generated from PDCs with targeted sequencing and found FGFR1 (N457K), PIK3R1 (M56I), BRCA1 (Q309R) mutations, which are currently not reported to be associated with AKT pathway activation. A PTEN deletion was detected in deep sequencing which may explain sensitivity to PIK3/mTOR inhibitors. In addition, CDK4 amplification was detected in this patient. Recently, a phase II trial of the CDK4 inhibitor PD0332991 in patients with CDK4-amplified liposarcoma have shown a progression free survival at 12 weeks to be 66% and one partial response [31]. In our cell line model with CDK4 amplification, the activity of CDK4 inhibitor was modest.
Our study demonstrated that a preclinical model we have developed can be a useful preclinical screening tool to interrogate chemosensitivity to targeted agents based on genomic aberrations [18], [32]. The cell-based preclinical model was successfully converted to PDX animal model as described previously [18]. Although our results are limited from generalization since it is an observation based on a single case, our study also demonstrates the potential insight which can be drawn from a single patient case when combined with preclinical model and genomic sequencing. Nevertheless, although a preclinical activity of palifosfamide lysine was very active in conventional 2-dimensional model of several immortalized cell lines [33], phase III PICASSO 3 trial which compared palifosfamide/doxorubicin and doxorubicin/placebo in sarcoma patients have failed to demonstrate survival benefit [34].
Taken together, the mTOR/AKT pathway in pazopanib-resistant tumor derived cells was considerably inhibited by the treatment with AZD2014 or BEZ235 with downregulation of AKT pathway (to a greater extent for BEZ235). These molecules may be considered as treatment option for pazopanib-resistant STS patient in the context of clinical trials.
Acknowledgements
This work was supported by a grant from the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C0072, HI14C3418, HI14C2750). Support was also provided by a grant from the 20 by 20 project of Samsung Medical Center (GF01160111) and the Samsung Medical Center (SMX1161251).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.tranon.2016.03.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Supplementary Figure 1.
Growth curves of PDC using 3-dimensional culture system and 2-dimensional in vitro cell viability assay. (A) Using high-throughput 3-dimensional culture system, the anti-tumor activity of 13-drug panel was tested using PDC line. Calcein stained cells with AZD2014 treatment on the micropillar chip and growth curve (IC50) from software are shown. (B) 2-dimensional culture of cells in 96well was treated with AZD2014, BEZ-235, lapatinib, LEE011, and pazopanib for 3 days, and proliferation was measured by cell viability assays.
Supplementary Figure 2.
Calcein stained PDC line images of the micropillar chips in 3-dimensional culture system. * Red character indicates the compounds used in this research.
References
- 1.Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer. 2014;120:1763–1774. doi: 10.1002/cncr.28657. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CD, Hogendoorn P, Mertens F, Bridge J. 4th ed. IARC Press; Lyon, France: 2013. WHO Classification of Tumours of Soft Tissue and Bone. [Google Scholar]
- 3.Amankwah EK, Conley AP, Reed DR. Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol. 2013;5:147–162. doi: 10.2147/CLEP.S28390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 5.Matthew F, Nicola D, Jackie C, Gill L, Rob G. National Cancer Intelligence Network (NCIN); 2013. Bone and soft tissue sarcomas UK incidence and survival: 1996 to 2010. [Google Scholar]
- 6.Rossi CR, Vecchiato A, Mastrangelo G, Montesco MC, Russano F, Mocellin S, Pasquali S, Scarzello G, Basso U, Frasson A. Adherence to treatment guidelines for primary sarcomas affects patient survival: a side study of the European CONnective TIssue CAncer NETwork (CONTICANET) Ann Oncol. 2013;24:1685–1691. doi: 10.1093/annonc/mdt031. [DOI] [PubMed] [Google Scholar]
- 7.Corey RM, Swett K, Ward WG. Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer Med. 2014;3:1404–1415. doi: 10.1002/cam4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young RJ, Natukunda A, Litiere S, Woll PJ, Wardelmann E, van der Graaf WT. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50:3178–3186. doi: 10.1016/j.ejca.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Kasper B, Ouali M, van Glabbeke M, Blay JY, Bramwell VH, Woll PJ, Hohenberger P, Schoffski P. Prognostic factors in adolescents and young adults (AYA) with high risk soft tissue sarcoma (STS) treated by adjuvant chemotherapy: a study based on pooled European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62771 and 62931. Eur J Cancer. 2013;49:449–456. doi: 10.1016/j.ejca.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Takyar S, Manson SC, Powell S, Penel N. Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: a systematic review. BMC Cancer. 2013;13:385–405. doi: 10.1186/1471-2407-13-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo KH, Kim HS, Lee SJ, Park SH, Kim SJ, Kim SH, La Choi Y, Shin KH, Cho YJ, Lee J. Efficacy of pazopanib monotherapy in patients who had been heavily pretreated for metastatic soft tissue sarcoma: a retrospective case series. BMC Cancer. 2015;15:154–160. doi: 10.1186/s12885-015-1160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Dodd RD, Mito JK, Ma Y, Kim Y, Riedel RF, Kirsch DG. Efficacy of phosphatidylinositol-3 kinase inhibitors in a primary mouse model of undifferentiated pleomorphic sarcoma. Sarcoma. 2012;2012:1–8. doi: 10.1155/2012/680708. 680708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez A, Snyder EL, Marino-Enriquez A, Zhang YX, Sioletic S, Kozakewich E, Grebliunaite R, Ou WB, Sicinska E, Raut CP. Aberrant AKT activation drives well-differentiated liposarcoma. Proc Natl Acad Sci U S A. 2011;108:16386–16391. doi: 10.1073/pnas.1106127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YX, van Oosterwijk JG, Sicinska E, Moss S, Remillard SP, van Wezel T, Buhnemann C, Hassan AB, Demetri GD, Bovee JV. Functional profiling of receptor tyrosine kinases and downstream signaling in human chondrosarcomas identifies pathways for rational targeted therapy. Clin Cancer Res. 2013;19:3796–3807. doi: 10.1158/1078-0432.CCR-12-3647. [DOI] [PubMed] [Google Scholar]
- 16.Yasui H, Naka N, Imura Y, Outani H, Kaneko K, Hamada K, Sasagawa S, Araki N, Ueda T, Itoh K. Tailored therapeutic strategies for synovial sarcoma: receptor tyrosine kinase pathway analyses predict sensitivity to the mTOR inhibitor RAD001. Cancer Lett. 2014;347:114–122. doi: 10.1016/j.canlet.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Stebbing J, Paz K, Schwartz GK, Wexler LH, Maki R, Pollock RE, Morris R, Cohen R, Shankar A, Blackman G. Patient-derived xenografts for individualized care in advanced sarcoma. Cancer. 2014;120:2006–2015. doi: 10.1002/cncr.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JY, Kim SY, Park C, Kim NK, Jang J, Park K, Yi JH, Hong M, Ahn T, Rath O. Patient-derived cell models as preclinical tools for genome-directed targeted therapy. Oncotarget. 2015;6:25619–25630. doi: 10.18632/oncotarget.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DW, Choi YS, Seo YJ, Lee MY, Jeon SY, Ku B, Kim S, Yi SH, Nam DH. High-throughput screening (HTS) of anticancer drug efficacy on a micropillar/microwell chip platform. Anal Chem. 2014;86:535–542. doi: 10.1021/ac402546b. [DOI] [PubMed] [Google Scholar]
- 20.Lee DW, Choi YS, Seo YJ, Lee MY, Jeon SY, Ku B, Nam DH. High-throughput, miniaturized clonogenic analysis of a limiting dilution assay on a micropillar/microwell chip with brain tumor cells. Small. 2014;10:5098–5105. doi: 10.1002/smll.201401074. [DOI] [PubMed] [Google Scholar]
- 21.Katz D, Zick A, Cohen J, Sonnenblick A, Fridman E, Shaham D, Peretz T. Reversal of acquired resistance to pazopanib in soft tissue sarcoma with addition of an mTOR inhibitor: A case report. J Clin Oncol. 2013;31:e21508. [Suppl., abstr] [Google Scholar]
- 22.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 23.Santiskulvong C, Konecny GE, Fekete M, Chen KY, Karam A, Mulholland D, Eng C, Wu H, Song M, Dorigo O. Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma. Clin Cancer Res. 2011;17:2373–2384. doi: 10.1158/1078-0432.CCR-10-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cebulla J, Huuse EM, Pettersen K, van der Veen A, Kim E, Andersen S, Prestvik WS, Bofin AM, Pathak AP, Bjorkoy G. MRI reveals the in vivo cellular and vascular response to BEZ235 in ovarian cancer xenografts with different PI3-kinase pathway activity. Br J Cancer. 2015;112:504–513. doi: 10.1038/bjc.2014.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gil del Alcazar CR, Hardebeck MC, Mukherjee B, Tomimatsu N, Gao X, Yan J, Xie XJ, Bachoo R, Li L, Habib AA. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin Cancer Res. 2014;20:1235–1248. doi: 10.1158/1078-0432.CCR-13-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuger S, Corek E, Polat B, Kammerer U, Flentje M, Djuzenova CS. Novel PI3K and mTOR Inhibitor NVP-BEZ235 Radiosensitizes Breast Cancer Cell Lines under Normoxic and Hypoxic Conditions. Breast Cancer (Auckl) 2014;8:39–49. doi: 10.4137/BCBCR.S13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobin B, Battaglia S, Lanel R, Chesneau J, Amiaud J, Redini F, Ory B, Heymann D. NVP-BEZ235, a dual PI3K/mTOR inhibitor, inhibits osteosarcoma cell proliferation and tumor development in vivo with an improved survival rate. Cancer Lett. 2014;344:291–298. doi: 10.1016/j.canlet.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Manara MC, Nicoletti G, Zambelli D, Ventura S, Guerzoni C, Landuzzi L, Lollini PL, Maira SM, Garcia-Echeverria C, Mercuri M. NVP-BEZ235 as a new therapeutic option for sarcomas. Clin Cancer Res. 2010;16:530–540. doi: 10.1158/1078-0432.CCR-09-0816. [DOI] [PubMed] [Google Scholar]
- 29.Basu B, Dean E, Puglisi M, Greystoke A, Ong M, Burke W, Cavallin M, Bigley G, Womack C, Harrington EA. First-in-Human Pharmacokinetic and Pharmacodynamic Study of the Dual m-TORC 1/2 Inhibitor AZD2014. Clin Cancer Res. 2015;21:3412–3419. doi: 10.1158/1078-0432.CCR-14-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guichard SM, Curwen J, Bihani T, D'Cruz CM, Yates JW, Grondine M, Howard Z, Davies BR, Bigley G, Klinowska T. AZD2014, an Inhibitor of mTORC1 and mTORC2, Is Highly Effective in ER + Breast Cancer When Administered Using Intermittent or Continuous Schedules. Mol Cancer Ther. 2015;14:2508–2518. doi: 10.1158/1535-7163.MCT-15-0365. [DOI] [PubMed] [Google Scholar]
- 31.Dickson MA, Tap WD, Keohan ML, D'Angelo SP, Gounder MM, Antonescu CR, Landa J, Qin LX, Rathbone DD, Condy MM. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013;31:2024–2028. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Ou SH, Lee JM, Kim HC, Hong M, Kim SY, Jang J, Ahn S, Kang SY, Lee S. Gastrointestinal malignancies harbor actionable MET exon 14 deletions. Oncotarget. 2015;6:28211–28222. doi: 10.18632/oncotarget.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hingorani P, Zhang W, Piperdi S, Pressman L, Lin J, Gorlick R, Kolb EA. Preclinical activity of palifosfamide lysine (ZIO-201) in pediatric sarcomas including oxazaphosphorine-resistant osteosarcoma. Cancer Chemother Pharmacol. 2009;64:733–740. doi: 10.1007/s00280-008-0922-4. [DOI] [PubMed] [Google Scholar]
- 34.Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, Sellers WR, Lengauer C, Stegmeier F. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–4293. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]