Abstract
Background
Primary care providers (PCPs) have few tools for enhancing patient self-efficacy, a key mediator of myriad health-influencing behaviors.
Objective
To examine whether brief standardized patient instructor (SPI)-delivered training increases PCPs’ use of self-efficacy-enhancing interviewing techniques (SEE IT).
Design
Randomized controlled trial.
Participants
Fifty-two family physicians and general internists from 12 primary care offices drawn from two health systems in Northern California.
Interventions
Experimental arm PCPs received training in the use of SEE IT training during three outpatient SPI visits scheduled over a 1-month period. Control arm PCPs received a single SPI visit, during which they viewed a diabetes treatment video. All intervention visits (experimental and control) were timed to last 20 min. SPIs portrayed patients struggling with self-care of depression and diabetes in the first 7 min, then delivered the appropriate intervention content during the remaining 13 min.
Main Measures
The primary outcome was provider use of SEE IT (a count of ten behaviors), coded from three audio-recorded standardized patient visits at 1–3 months, again involving depression and diabetes self-care. Two five-point scales measured physician responses to training: Value (7 items: quality, helpfulness, understandability, relevance, feasibility, planned use, care impact), and Hassle (2 items: personal hassle, flow disruption).
Key Results
Pre-intervention, study PCPs used a mean of 0.7 behaviors/visit, with no significant between-arm difference (P = 0.23). Post-intervention, experimental arm PCPs used more of the behaviors than controls (mean 2.7 vs. 1.0 per visit; adjusted difference 1.7, 95 % CI 1.1–2.2; P < 0.001). Experimental arm PCPs had higher training Value scores than controls (mean difference 1.05, 95 % CI 0.68–1.42; P < 0.001), and similarly low Hassle scores.
Conclusions
Primary care physicians receiving brief SPI-delivered training increased their use of SEE IT and found the training to be of value. Whether patients visiting SEE IT-trained physicians experience improved health behaviors and outcomes warrants study.
ClinicalTrials.gov Identifier
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-016-3644-z) contains supplementary material, which is available to authorized users.
KEY WORDS: continuing professional development, health behavior, patient engagement, physician behavior, primary care
Behaviors such as following a prudent diet, exercising regularly, and adhering to medications can improve chronic illness outcomes, yet patients struggle with these behaviors, in part due to low motivation.1 While research indicates primary care providers can be trained to enhance patients’ behavioral motivation,2 – 5 evidence-based methods such as motivational interviewing are broad in scope, difficult to learn, and lengthy to apply.6 – 14 As a result, the approaches are not widely employed in practice, where primary care providers must address multiple issues in office visits, seldom limited to behavioral change.15 – 17 There is an urgent need for effective, focused, time-efficient interviewing approaches that busy practitioners can routinely employ to motivate healthy behaviors.18
One promising approach is training primary care providers to enhance patient self-efficacy, or confidence in their ability to complete the tasks leading to a behavioral goal.19 Theory and research indicate that self-efficacy influences myriad behaviors and can be strengthened by interventions, improving health outcomes.19 – 29 We had previously developed a brief intervention for training resident primary care physicians (PCPs) to use self-efficacy-enhancing interviewing techniques (SEE IT).30 The intervention was grounded in self-efficacy theory, and informed by prior interventions shown to bolster self-efficacy and improve health behaviors and outcomes. Standardized patient instructors (SPIs) delivered the training in outpatient visits, scheduled during usual office hours, juxtaposed with real patient visits. In a randomized controlled trial (RCT), residents receiving the intervention increased their use of SEE IT and had favorable responses to training.30
Whether these findings extend to practicing PCPs, who might be less amenable to learning new interviewing techniques, remained unclear. We addressed this issue in the current RCT, examining the impact of the SPI-delivered intervention on practicing PCPs’ use of SEE IT and soliciting their responses to the training. SPI delivery maximizes the salience and impact of training by allowing providers to immediately practice and assimilate new skills in their daily work environment. Also, we anticipated busy practicing PCPs would appreciate training using SPIs, because it entails no time commitment outside of usual work hours.15 Beyond the success of our prior RCT of SEE IT targeted to resident PCPs,30 other SPI-delivered interventions have improved surgeons’ informed consent skills31 and PCPs’ skills in assessing human immunodeficiency virus risk32 and addressing patient requests for low-value testing.33
We hypothesized that, compared with practicing PCPs receiving an attention control intervention, those receiving SPI-delivered SEE IT training would: (1) demonstrate greater use of SEE IT during three post-intervention evaluation standardized patient visits; (2) perceive greater value in their training; and (3) indicate similarly low hassle in participating.
METHODS
We conducted the trial activities from January 2013 through December 2014. We obtained ethics approval from the University of California Davis (UCD) and Sutter Health institutional review boards.
Study Setting, Sample Recruitment, and Randomization
We recruited family physicians and general internists from 12 primary care offices in the Sacramento, California area, drawn from the UCD Primary Care Network and Sutter Medical Group. All of the practices were fully focused on providing patient care; none were academic (e.g., residency training) sites. The physicians were solicited for participation via presentations at practice meetings, with follow-up e-mails and phone calls. A research assistant obtained informed consent for participation using a standard consent form. Sutter physicians received $325 for participating. UCD physicians could not receive direct monetary compensation, so we transferred $325 to the primary care network for each UCD physician, which then credited each with an equivalent amount of relative value units (RVUs).
The PCPs were randomly assigned to receive either SEE IT training or an attention control intervention. Recruitment personnel achieved randomization by using a custom-created computer program that implemented randomization in blocks, with random variation in block size (2, 4, 6, or 8).34 Recruitment personnel and SPIs were aware of the PCPs’ random study group allocation. All other personnel (investigators, biostatistician, trainers of the SPIs and evaluation standardized patients, study visit audio-recording coders) were blinded to the PCP study arm.
Procedures
Experimental Intervention
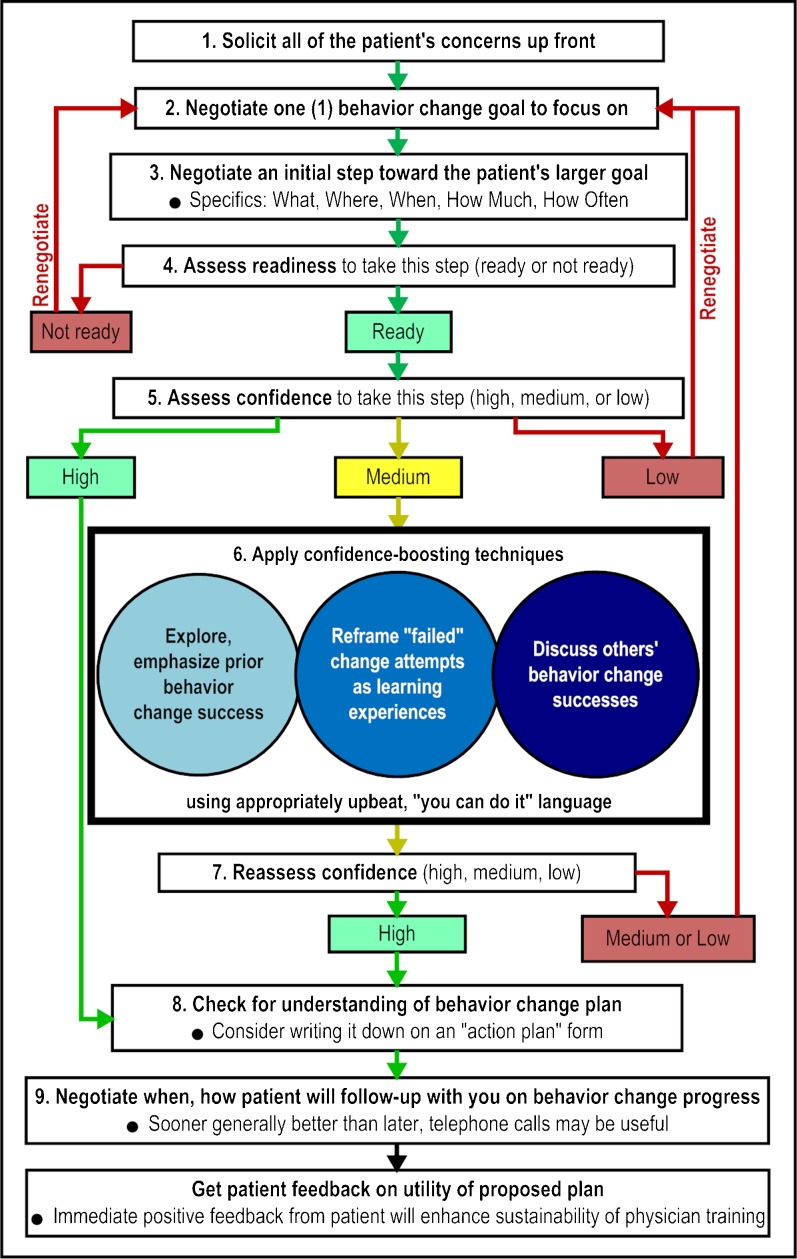
The experimental intervention provided training on the use of nine self-efficacy-enhancing interviewing techniques (SEE IT), presented in a logical sequence (Fig. 1). The techniques were drawn from self-efficacy theory, other relevant behavioral theories, direct observation of primary care, and research-proven approaches to enhancing self-efficacy and promoting healthy behaviors.19 – 29
Figure 1.
Study self-efficacy-enhancing interviewing techniques and their presentation sequence.
Standardized patient instructors (SPIs) delivered the training over three outpatient visits, scheduled during the PCPs’ regular office hours. We notified study PCPs in advance of SPI visits. We employed pre-notification because PCPs tend to have less favorable views of unannounced SPI visits.32 The SPIs audio-recorded all experimental intervention visits using pocket digital recorders.
The experimental training visits were timed to last 20 min. In the first 7 min, the SPI portrayed a patient with co-existing depression and diabetes who was struggling with medication adherence and other behaviors known to help control these conditions. Each of the three SPI visits featured a completely different fictional patient and scenario. Each actor was trained to portray only one patient/scenario. Across the three SPI visits, case presentation sequence varied non-randomly among PCPs. Random case sequencing was not feasible due to scheduling constraints. SEE IT steps 1–3 were presented in the first SPI visit, steps 4–6 in the second visit, and steps 7–9 in the third visit.
A pocket digital timer alarm prompted the SPI to come out of patient role at 7 min, introduce themselves, briefly review the purpose of the remaining 13 min of the visit, address questions, and then deliver the training. The SPIs employed standard scripts implemented on flip cards, and provided each physician with a copy of the SEE IT diagram (Fig. 1), referring to it periodically for orientation and clarification. For each targeted interviewing technique, the SPIs employed the following approach: first, state the technique; if PCP used the technique during the patient portion of the visit, note and reinforce its use; if PCP did not use the technique, gently point this out, identify where it might have been used in the foregoing encounter, and demonstrate how to use it; briefly resume patient role and allow the PCP to practice the technique; solicit and answer questions before moving to the next technique. Videos simulating the three experimental SPI training visits (drawing on audio recordings from the trial) are available at: http://bit.ly/1HuSNgN (visit 1); http://bit.ly/1K7EX7K (visit 2); and http://bit.ly/1M613ai (visit 3).
Attention Control
SPIs delivered the attention control intervention in a single visit. Similar to the experimental SPI visits, the SPI initially portrayed a patient with depression and diabetes struggling with self-care, then came out of role at 7 min to deliver the intervention, audio-recording the visit with a pocket digital device. However, the control intervention was an 8-min video summarizing new medications for type 2 diabetes, viewed on a laptop.35 The video contained no material on self-efficacy, health behavior, or patient interviewing, and the SPI did not discuss these issues. The SPI also provided the PCP with a printed copy of the journal article from which the video content was drawn.36
Post-intervention Standardized Patient Visits
All study PCPs received three pre-announced evaluation standardized patient visits 1–3 months post-intervention. Each visit again featured a different “patient” struggling with medication adherence and other depression- and diabetes-related health behaviors, with non-random variation (due to office scheduling constraints) in case sequence among physicians. Unlike the SPIs, evaluation standardized patients stayed in patient role throughout. PCPs in both study groups were notified in advance of the visits, since providers tend to prefer pre-announced standardized patient visits,32 and because we sought simply to determine whether the PCP had learned how to use the targeted interviewing skills. Standardized patients audio-recorded evaluation visits using pocket digital recorders.
SPI and Standardized Patient Training
Seven individuals were trained as SPIs, while nine additional individuals were trained to make evaluation standardized patient visits. Both genders and a range of ages, races, and ethnicities were represented in the SPI and standardized patient pools. All had prior amateur and/or professional acting experience, and many had worked as standardized patients (but not as SPIs). The training processes were developed and implemented by a physician assistant with over 30 years of SPI and standardized patient training experience, and were maintained by two subsequent trainers.
The trainers conducted monthly fidelity audits of about 10 % of the SPI and evaluation standardized patient visit recordings, using a standardized checklist (options for all items: excellent; acceptable but with room for improvement; or not acceptable). With regard to SPI and SP patient role portrayal (31 visits audited), fidelity was as follows: stayed in character, 31 (100 %) excellent; realistically disclosed self-care issues, 25 (81 %) excellent, 5 (16 %) acceptable, 1 (3 %) not acceptable; and conveyed appropriate depression level, 25 (81 %) excellent, 6 (19 %) acceptable. For SPI presentation of interviewing techniques (N = 27 technique presentations [9 visits audited × 3 techniques presented in each], fidelity ratings were 22 (81.5 %) excellent and 5 (18.5 %) acceptable. Trainers provided corrective feedback to actors based on the audits when indicated.
Measures
PCP Use of SEE IT
The patient role portion of the first experimental group SPI visit and of the sole SPI visit in the control group represented the baseline (pre-intervention) interaction (henceforth “visit 1”). The three evaluation standardized patient visits were the post-intervention interactions (henceforth “visits 2, 3, and 4”). Three trained coders, blinded to physician study group, listened individually to each recording and applied the Doctors’ Observable Use of Self-Efficacy-Enhancing Interviewing Techniques (DO U SEE IT) measure, a count of ten behaviors, employed in our prior resident-focused RCT.30 Six of the ten DO U SEE IT items corresponded to SEE IT steps 1–4, 8, and 9 in Figure 1; three items corresponded to the three “sub-behaviors” of SEE IT step 6 (blue circles in Fig. 1); and a final item assessed both steps 5 and 7, given their overlap. All ten items employed a yes/no response scale (PCP demonstrated/did not demonstrate behavior), with one point assigned for each “yes” response (score range 0–10). We maintained coder blinding by removing PCP–patient introductions from recordings and, for visits 2–4, by truncating the recordings just before the SPIs left the patient role. The mean chance-corrected agreement (kappa) among the three coders for each item was 0.60 (range 0.11–0.83, all P < 0.01). Subsequently, one of the coders, pre-designated as master coder, reconciled any between-coder discrepancies via group re-review and discussion of recordings until consensus was reached. The consensus DO U SEE IT scores were employed in the current analyses. Cronbach’s alpha (α) for the measure was 0.94.
PCP Responses to Intervention
Following their participation, PCPs completed an 11-item measure of their responses to study intervention and trial participation (Online Appendix). All items employed five-point Likert response scales. We conducted an exploratory factor analysis of this newly developed measure, which indicated two factors. Seven items formed a Value scale (mean score 4.03, standard deviation [SD] 0.80, higher scores = higher Value; α = 0.96). Items in this scale concerned training quality, helpfulness, understandability, practice relevance, feasibility of application, plans for future use, and anticipated impact on patient care. Two items—personal hassle and disruption of patient flow—formed a Hassle scale (mean 2.17, SD 0.87, higher scores = higher Hassle; α = 0.73). The two remaining items, SPI case realism and PCP willingness to participate in future similar studies, did not load with other items, so we examined them individually.
We also collected information regarding PCP age, gender, race/ethnicity, and specialty.
Analyses
Data analyses were conducted using Stata (version 14.0; StataCorp LP, College Station, TX, USA). To examine the effects of the experimental intervention on PCP use of SEE IT, we used mixed Poisson regression models, accommodating the repeated measures on each physician. The dependent variable in the primary analysis was the total DO U SEE IT score in each visit from the three post-intervention evaluation standardized patient visits (visits 2–4). The key independent variable was the randomly assigned study group (experimental SEE IT training versus attention control). Secondary analyses adjusted for the baseline (visit 1) SEE IT score. Further models examined visit-by-intervention group interaction terms (as an alternative assessment of the interaction effect and growth of the effect over time). We also explored whether PCP characteristics (age, gender, race/ethnicity, specialty) or patient case scenario were associated with the experimental intervention effect on DO U SEE IT score and, in analyses limited to the experimental arm, whether there was an effect of SPI (actor) on DO U SEE IT score. To explore for possible differential effects of SEE IT training on component interviewing behaviors, we conducted analyses considering three sub-groupings of the behaviors: SEE IT steps 1–3, 4–6, and 7–9, corresponding to their presentation in SPI visits 1, 2, and 3, respectively. We used t tests to examine PCP responses to their randomly assigned study intervention.
RESULTS
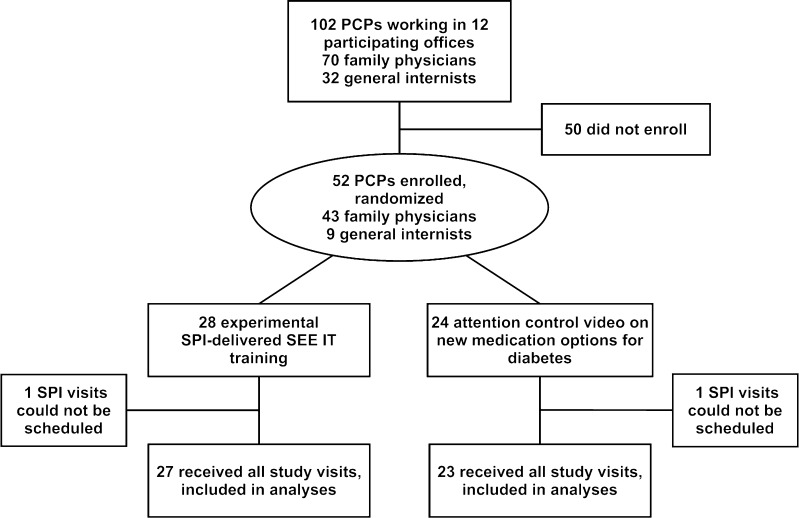
Of the 102 PCPs in the participating offices, 52 (51 %) enrolled in the RCT. Figure 2 depicts the flow of PCPs through the trial, while Table 1 summarizes PCP characteristics by trial arm. None of the differences in characteristics between groups were statistically significant (P ≥ 0.17 for all comparisons).
Figure 2.
Flow of participants through the trial. PCP primary care physician, SEE IT self-efficacy enhancing interviewing techniques, SPI standardized patient instructor.
Table 1.
Characteristics of Participating Primary Care Physicians by Study Group
| Characteristic, no. (%) | Experimental SEE IT training group (N = 28)a | Attention control video group (N = 24)a |
|---|---|---|
| Age, mean (SD) | 44.4 (8.3) | 47.3 (8.9) |
| Female, no. (%) | 16 (64) | 12 (52) |
| Race/Ethnicity category, no. (%) | ||
| Non-Hispanic | ||
| White | 13 (52) | 11 (48) |
| Asian | 6 (24) | 8 (35) |
| Other race | 4 (16) | 0 (0) |
| Hispanic (any race) | 2 (8) | 4 (17) |
| Health system, no. (%) | ||
| University of California Davis | 15 (54) | 11 (46) |
| Sutter | 13 (46) | 13 (54) |
| Specialty, no. (%) | ||
| Family medicine | 23 (82) | 20 (83) |
| Internal medicine | 5 (18) | 4 (17) |
SD standard deviation, SEE IT self-efficacy enhancing interviewing techniques
aSPI visits could not be scheduled for one physician in each group, leaving 50 (27 experimental, 23 control) who received their randomly assigned intervention
Effects on PCP Use of SEE IT
Fifty PCPs completed their baseline and three post-intervention evaluation visits (Fig. 2). Table 2 shows the unadjusted DO U SEE IT scores by study group and visit. At baseline, study physicians used a mean 0.7 targeted behaviors per visit (median 1, range 0–2), with no significant difference in use between trial arms. Post-intervention, experimental arm PCPs had higher mean use of SEE IT and a wider range of interviewing behaviors used per visit than controls (Table 2).
Table 2.
Unadjusted Physician Use of Self-Efficacy-Enhancing Interviewing Techniques (SEE IT) by Study Group and Visit
| Study visit | Unadjusted mean (SD, median, interquartile range, range) DO U SEE IT score | P value | |
|---|---|---|---|
| Experimental SEE IT training group (N = 27) | Attention control video group (N = 23) | ||
| Visit 1 (baseline) | 0.58 (0.64, 0.5, 0–1, 0–2) | 0.87 (0.55, 1.0, 1–1, 0–2) | 0.07 |
| Visit 2 | 2.81 (1.62, 2.0, 2–4, 1–6) | 1.22 (0.67, 1.0, 1–2, 0–3) | <0.001 |
| Visit 3 | 2.44 (1.72, 2.0, 1–4, 0–6) | 0.91 (0.73, 1.0, 0–1, 0–3) | <0.001 |
| Visit 4 | 2.89 (1.89, 2.0, 1–5, 0–7) | 1.00 (0.60, 1.0, 1–1, 0–3) | <0.001 |
DO U SEE IT Doctors’ Observable Use of Self-Efficacy-Enhancing Interviewing Techniques, SD standard deviation, SEE IT self-efficacy-enhancing interviewing techniques
Across the three post-intervention visits, experimental group PCPs used significantly more of the targeted behaviors than controls (mean 2.7 versus 1.0 per visit; adjusted difference 1.7, 95 % confidence interval [CI] 1.1, 2.2; P < 0.001). The model formulation examining the effect of the intervention through an interaction between experimental group and visit (including baseline visit) yielded consistent results (interaction effect chi-square 14.1, degrees of freedom 3, P = 0.003).
In analyses exploring moderation of the SEE IT training effect by PCP or contextual factors, there were no significant interactions with physician gender, age group, or race/ethnicity, or with evaluation visit number or case scenario. Additionally, in an analysis limited to experimental arm PCPs, there was no significant SPI effect on the primary outcome (F = 1.43, P = 0.25). There were also significant effects of the experimental training on each of the three sub-groupings of interviewing behaviors (SEE IT steps 1–3, 4–6, and 7–9). Further details of these exploratory analyses are available from the authors.
PCP Responses to Study Training and Participation
Compared with controls, SEE IT-trained PCPs had more favorable Value scores (mean 1.05 points higher, 95 % CI 0.68, 1.42; P < 0.001). Both the experimental and control group providers had low Hassle scores, high ratings of SPI case realism, and high willingness to participate in future studies, with no significant between-arm differences in these responses (details available from authors).
DISCUSSION
In an RCT, we found that a brief intervention, delivered by SPIs over three 20-min outpatient visits conducted during usual office hours, increased the use of SEE IT among practicing primary care physicians (PCPs). The SEE IT training effect did not wane over the evaluation period (1–3 months post-intervention), and was present in both younger and older providers and regardless of PCP gender, race/ethnicity, or specialty. SEE IT-trained PCPs also perceived high value in their training (e.g., quality, relevance, anticipated clinical impact) and little hassle in participating. These findings expand upon the similarly encouraging findings of our prior RCT of SPI-delivered SEE IT training, which focused exclusively on resident PCPs.30
Primary care physicians provide the bulk of care for individuals with depression, diabetes, and other chronic illnesses,37 and research indicates that enhancing self-efficacy can improve health behaviors and outcomes among chronically ill patients.19 – 29 Prior studies also indicate that physicians’ behaviors during standardized patient encounters correlate with their behaviors during real patient visits.38 , 39 In this context, the combined findings of our practicing PCP-focused and resident PCP-focused trials suggest that routinely offering SEE IT training to PCPs, regardless of their experience level, could have considerable clinical impact, potentially leading to improved patient health behaviors and outcomes. Additional studies are warranted to examine whether the effects we observed will generalize to real patients and will lead to improved health behaviors and outcomes.
If SPI-delivered SEE IT training is shown through further study to have clinical impact, it will have the potential for broad dissemination and implementation in practice, given its brevity and delivery during usual patient care hours, and that physicians appear to value the training and perceive little hassle in receiving it. Also of relevance to future dissemination and implementation potential, 51 % of the PCPs working in the participating offices volunteered for our trial, suggesting that the notion of SEE IT training resonates with many PCPs. Eventually, SPI and standardized patient training centers might be established in strategic locations throughout the U.S., providing SPIs and standardized patients (and/or SPI and standardized patient trainers) to practices and health systems in various geographic regions. Such an approach might be implemented under the aegis of medical specialty licensing boards, as part of the growing push to achieve more evidence-based, clinically relevant and practical maintenance of certification programs.40
Our trial had some limitations. The PCPs were enrolled from 12 primary care offices drawn from two health systems in a single, relatively small geographic region. Whether the findings generalize to offices and PCPs elsewhere is unclear. Because the evaluation standardized patient visits in our trial were pre-announced, we have shown that practicing PCPs can demonstrate the targeted interviewing behaviors under prompted circumstances. It is uncertain whether PCPs would use the targeted interviewing behaviors without prompting. Nonetheless, prior SPI trials suggest that visit behavioral outcomes are largely not affected by whether post-intervention evaluation standardized patients are detected.41
We conclude that primary care physicians who received brief SPI-delivered training on the use of SEE IT during their usual outpatient office hours subsequently increased their use of SEE IT, and perceived the quality, utility, and anticipated clinical impact of the training very favorably. These findings warrant replication with real patients in other settings, and determination of whether patients exposed to SEE IT-trained primary care physicians will experience improved health behaviors and outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 20 kb)
Acknowledgments
Contributors
We are grateful to the following individuals for their outstanding contributions: Julie Anchor, Todd Gearou, Mike Kerrigan, Nikki Lausmann, Patris Miller, Betsy Reifsnider, and Stephen Savage, standardized patient instructors; Michael Coleman, Bridggett Davis, DeMarco Davis, Mark (Moe) Davis, Martin Idle, Corey Jackson, Desi Motley, Peter Playdon, and Wanel Thomas, standardized patients; Emily Hanes, Sanjeet Grewal, and Rimaben Cabrera, study visit audio recording coders; Simon Dvorak and Charles Turner, PhD, who programmed the randomization program and electronic questionnaires; and Lizette Macias, who participated in electronic questionnaire programming, trial recruitment and enrollment, and data collection. We are also indebted to all of the participating primary care offices and providers. We dedicate this paper to the memory of our friend and colleague Janice Bishop, who made key contributions to training the study SPIs and standardized patients.
Funders
This work was supported by grant R34MH095893 (Jerant) from the National Institute of Mental Health, with supplemental funding provided by the University of California Davis Department of Family and Community Medicine. The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of data; or preparation, review, or approval of the manuscript, or decision to submit the manuscript for publication.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflicts of interest.
REFERENCES
- 1.World Health Organization . Adherence to long-term therapies. Evidence to action. Geneva: World Health Organization; 2003. [Google Scholar]
- 2.Rubak S, Sandbaek A, Lauritzen T, Borch-Johnson K, Christensen B. General practitioners trained in motivational interviewing can positively affect the attitude to behaviour change in people with type 2 diabetes. Scand J Prim Health Care. 2009;27:172–9. doi: 10.1080/02813430903072876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes RD, Ivezaj V. A systematic review of motivational interviewing for weight loss among adults in primary care. Obes Rev. 2015;16:304–18. doi: 10.1111/obr.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015;3 doi: 10.1002/14651858.CD006936.pub3. [DOI] [PubMed] [Google Scholar]
- 5.VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768–80. doi: 10.1007/s10865-013-9527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter KM, Cheng WY, Smith JL, et al. “Old dogs” and new skills: how clinician characteristics relate to motivational interviewing skills before, during, and after training. J Consult Clin Psychol. 2012;80:560–73. doi: 10.1037/a0028362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiIorio C, McCarty F, Resnicow K, et al. Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care. 2008;20:273–83. doi: 10.1080/09540120701593489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. CD006936. [DOI] [PubMed]
- 9.Lundahl B, Burke BL. The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol. 2009;65:1232–45. doi: 10.1002/jclp.20638. [DOI] [PubMed] [Google Scholar]
- 10.Noordman J, van Lee I, Nielen M, Vlek H, van Weijden T, van Dulmen S. Do trained practice nurses apply motivational interviewing techniques in primary care consultations? J Clin Med Res. 2012;4:393–401. doi: 10.4021/jocmr1120w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soderlund LL, Madson MB, Rubak S, Nilsen P. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns. 2011;84:16–26. doi: 10.1016/j.pec.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Miller WR, Yahne CE, Moyers TB, Martinez J, Pirritano M. A randomized trial of methods to help clinicians learn motivational interviewing. J Consult Clin Psychol. 2004;72:1050–62. doi: 10.1037/0022-006X.72.6.1050. [DOI] [PubMed] [Google Scholar]
- 13.Moyers TB, Manuel JK, Wilson PG, Hendrickson SML, Talcott W, Durand P. A randomized trial investigating training in motivational interviewing for behavioral health providers. Behav Cog Psychother. 2008;36:149–62. doi: 10.1017/S1352465807004055. [DOI] [Google Scholar]
- 14.Fu SS, Roth C, Battaglia CT, et al. Training primary care clinicians in motivational interviewing: a comparison of two models. Patient Educ Couns. 2015;98:61–68. doi: 10.1016/j.pec.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Midboe AM, Cucciare MA, Trafton JA, Ketroser N, Chardos JF. Implementing motivational interviewing in primary care: the role of provider characteristics. Trans Behav Med. 2011;1:588–94. doi: 10.1007/s13142-011-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cucciare MA, Ketroser N, Wilbourne P, et al. Teaching motivational interviewing to primary care staff in the Veterans Health Administration. J Gen Intern Med. 2012;27:953–61. doi: 10.1007/s11606-012-2016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guydish J, Jessup M, Tajima B, Manser ST. Adoption of motivational interviewing and motivational enhancement therapy following clinical trials. J Psychoactive Drugs. 2010;Suppl 6:215–26. doi: 10.1080/02791072.2010.10400545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns. 2004;53:147–55. doi: 10.1016/S0738-3991(03)00141-1. [DOI] [PubMed] [Google Scholar]
- 19.Bandura A. Self-efficacy: the exercise of control. New York: Freeman; 1997. [Google Scholar]
- 20.Barnason S, Zimmerman L, Nieveen J, Schmaderer M, Carranza B, Reilly S. Impact of a home communication intervention for coronary artery bypass graft patients with ischemic heart failure on self-efficacy, coronary disease risk factor modification, and functioning. Heart Lung. 2003;32:147–58. doi: 10.1016/S0147-9563(03)00036-0. [DOI] [PubMed] [Google Scholar]
- 21.Lin EH, Von Korff M, Ludman EJ, et al. Enhancing adherence to prevent depression relapse in primary care. Gen Hosp Psychiatry. 2003;25:303–10. doi: 10.1016/S0163-8343(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 22.Rost K, Nutting P, Smith JL, Elliott CE, Dickinson M. Managing depression as a chronic disease: a randomised trial of ongoing treatment in primary care. BMJ. 2002;325:934. doi: 10.1136/bmj.325.7370.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brody BL, Roch-Levecq AC, Gamst AC, Maclean K, Kaplan RM, Brown SI. Self-management of age-related macular degeneration and quality of life: a randomized controlled trial. Arch Ophthalmol. 2002;120:1477–83. doi: 10.1001/archopht.120.11.1477. [DOI] [PubMed] [Google Scholar]
- 24.Dallow CB, Anderson J. Using self-efficacy and a transtheoretical model to develop a physical activity intervention for obese women. Am J Health Promot. 2003;17:373–81. doi: 10.4278/0890-1171-17.6.373. [DOI] [PubMed] [Google Scholar]
- 25.Kukafka R, Lussier YA, Eng P, Patel VL, Cimino JJ. Web-based tailoring and its effect on self-efficacy: results from the MI-HEART randomized controlled trial. Proc AMIA Symp. 2002:410–4. [PMC free article] [PubMed]
- 26.Jerant AF, Kravitz RL, Moore-Hill MM, Franks P. Depressive symptoms moderated the effect of chronic illness self-management training on self-efficacy. Med Care. 2008;46:523–31. doi: 10.1097/MLR.0b013e31815f53a4. [DOI] [PubMed] [Google Scholar]
- 27.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Salbach NM, Mayo NE, Robichaud-Ekstrand S, Hanley JA, Richards CL, Wood-Dauphinee S. The effect of a task-oriented walking intervention on improving balance self-efficacy poststroke: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:576–82. doi: 10.1111/j.1532-5415.2005.53203.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsay SL. Self-efficacy training for patients with end-stage renal disease. J Adv Nurs. 2003;43:370–75. doi: 10.1046/j.1365-2648.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- 30.Jerant AF, Kravitz RL, Azari R, et al. Training residents to employ self-efficacy enhancing interviewing techniques: Randomized controlled trial of a standardized patient intervention. J Gen Intern Med. 2009;24:606–13. doi: 10.1007/s11606-009-0946-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leeper-Majors K, Veale JR, Westbrook TS, Reed K. The effect of standardized patient feedback in teaching surgical residents informed consent: results of a pilot study. Curr Surg. 2003;60:615–22. doi: 10.1016/S0149-7944(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 32.Epstein RM, Levenkron JC, Frarey L, Thompson J, Anderson K, Franks P. Improving physicians’ HIV risk-assessment skills using announced and unannounced standardized patients. J Gen Intern Med. 2001;16:176–80. doi: 10.1111/j.1525-1497.2001.02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenton JJ, Kravitz RL, Jerant A, et al. Promoting patient-centered counseling to reduce use of low-value diagnostic tests: a randomized clinical trial. JAMA Intern Med. 1–7. [DOI] [PubMed]
- 34.Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. 2002;359:515–9. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- 35.Management of Type 2 diabetes: new and future developments in treatment. 2012; http://www.thedoctorschannel.com/view/new-future-developments-treatment-diabetes-cme/collection/diabetes-cme/. Accessed Feb 8 2016.
- 36.Tahrani AA, Bailey CJ, Del Prato S, Barnett AH. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378:182–97. doi: 10.1016/S0140-6736(11)60207-9. [DOI] [PubMed] [Google Scholar]
- 37.Parchman ML, Noel PH, Lee S. Primary care attributes, health care system hassles, and chronic illness. Med Care. 2005;43:1123–9. doi: 10.1097/01.mlr.0000182530.52979.29. [DOI] [PubMed] [Google Scholar]
- 38.Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA. 2000;283:1715–22. doi: 10.1001/jama.283.13.1715. [DOI] [PubMed] [Google Scholar]
- 39.Fiscella K, Franks P, Srinivasan M, Kravitz RL, Epstein R. Ratings of physician communication by real and standardized patients. Ann Fam Med. 2007;5:151–8. doi: 10.1370/afm.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook DA, Holmboe ES, Sorensen KJ, Berger RA, Wilkinson JM. Getting maintenance of certification to work: a grounded theory study of physicians’ perceptions. JAMA Intern Med. 2015;175:35–42. doi: 10.1001/jamainternmed.2014.5437. [DOI] [PubMed] [Google Scholar]
- 41.Franz CE, Epstein R, Miller KN, et al. Caught in the act? Prevalence, predictors, and consequences of physician detection of unannounced standardized patients. Health Serv Res. 2006;41:2290–302. doi: 10.1111/j.1475-6773.2006.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 20 kb)