Abstract
Cardiac mitochondrial dysfunction is considered to be the main manifestation in the pathology of ischemia reperfusion injury, and by restoring its functional activity, hydrogen sulfide (H2S), a novel endogenous gaseotransmitter renders cardioprotection. Given that interfibrillar (IFM) and subsarcolemmal (SSM) mitochondria are the two main types in the heart, the present study investigates the specific H2S-mediated action on IFM and SSM during ischemic reperfusion in the Langendorff rat heart model. Rats were randomly divided into five groups, namely normal, ischemic control, reperfusion control (I/R), ischemic post-conditioning (POC), and H2S post-conditioning (POC_H2S). In reperfusion control, cardiac contractility decreased, and lactate dehydrogenase, creatine kinase, and infracted size increased compared to both normal and ischemic group. In hearts post-conditioned with H2S and the classical method improved cardiac mechanical function and decreased cardiac markers in the perfusate and infarct size significantly. Both POC and POC_H2S exerts its cardioprotective effect of preserving the IFM, as evident by significant improvement in electron transport chain enzyme activities and mitochondrial respiration. The in vitro action of H2S on IFM and SSM from normal and I/R rat heart supports H2S and mediates cardioprotection via IFM preservation. Our study indicates that IFM play an important role in POC_H2S mediated cardioprotection from reperfusion injury.
Keywords: Hydrogen sulfide, Ischemic post-conditioning, Myocardial ischemia reperfusion, Interfibrillar mitochondria, Subsarcolemmal mitochondria
Introduction
Cardiovascular disease is still the leading cause of morbidity and mortality in the world. The coronary interventions to reestablishing the coronary blood flow, such as thrombolysis, percutaneous coronary angioplasty, and coronary artery bypass graft are essential to salvage viable myocardium, but resulted in damage to ischemic myocardial tissue, termed “reperfusion injury” (Carden and Granger 2000). Hydrogen sulfide post-conditioning was reported to limit reperfusion injury by the activation of intrinsic prosurvival signaling cascades (Luan et al. 2012). Experimental evidences suggest that H2S can readily scavenge the reactive oxygen species during reperfusion and also activate RISK (reperfusion injury salvage kinase) pathway, one of the well-established signaling cascade for cardioprotection (Li et al. 2015). However, according to the recent studies, cardioprotection rendered by post-conditioning may occur independently of the RISK pathway activation, therefore, confirming the existence of multiple protective pathways. One such pathway is the survivor activating factor enhancement (SAFE) pathway, found to effectively protect isolated I/R rat hearts (Lacerda et al. 2009). Intensive analysis of the signal transduction pathways identified similarities between the RISK and SAFE pathways, where the stimulus from signaling molecule converges to the mitochondria, the organelle where many of the prosurvival and death signals exits (Maria-Giulia et al. 2011).
Cardiac mitochondria consists of two main types, namely subsarcolemmal mitochondria (SSM), located directly beneath the sarcolemma, and interfibrillar mitochondria (IFM), between the myofibrils. The two mitochondrial types differed not only in their respective location in the cell, but also in their biochemical properties, viz., higher oxidation potential and higher enzyme activities of complex I, succinate dehydrogenase, etc. in IFM than SSM (Palmer et al. 1977). However, in I/R-subjected rat hearts, both subpopulations behave differently, in which SSM favor FADH2 while IFM prefer NADH as the main reducing equivalent for the electron transport during ischemia and reperfusion (Kurian et al. 2012a, b). Similarly, IFM has a higher coupling efficiency than SSM when energized by GM, but lower than SSM when energized with succinate (Kurian et al. 2012a, b) in I/R rat hearts.
Hydrogen sulfide (H2S) produced by cystathionine-gamma-lyase (CSE) from L cysteine is considered to be a novel gaseotransmitter found in almost all tissues, mainly the myocardium, fibroblast, and blood vessels, where it exerts an important effect on physiology and pathophysiology processes (Geng et al. 2004). H2S produces cardioprotective effects via its antioxidant property, brings about a decrease in intracellular Ca2+, may antagonize the negative consequences of sympathetic over activation during ischemia by generating negative feedback to cAMP production, open the mitochondria KATP channel (Dudek et al. 2014), and block calcium channels (Pan et al. 2008). These various H2S-mediated physiological actions are directly and indirectly dependent on the mitochondria (Modis et al. 2014). Previous studies have demonstrated that cardioprotective mechanism mediated by post-conditioning is dependent on the mitochondria in the heart (John et al. 2007). However, the distinct role of cardiac mitochondrial subpopulations (IFM and SSM) in H2S post-conditioning protection of the isolated rat heart against I/R injury is unknown. In the present study, we evaluate the impact of H2S post-conditioning on the mitochondria with respect to cardiac mitochondrial subpopulations, namely IFM and SSM.
Materials and methods
Materials
Decylubiquinone, antimycin, rotenone, and cytochrome c were purchased from Sigma–Aldrich. All other chemicals were obtained from Himedia (Mumbai, India).
Animals
The study protocol was approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA Approval No 279/SASTRA/IAEC/RPP), India. Animals were housed in polycarbonate cages and controlled temperature of 25 ± 3 °C and 60 ± 10 % relative humidity with a 12-h light/dark cycle was maintained. Rats were acclimatized a week before the start of the study, fed on a standard laboratory diet and drinking water ad libitum.
Isolated perfused rat heart preparation
Male Wistar rats (250–300 g) were anesthetized by intraperitoneal injection of sodium pentobarbital (80 mg/kg). Heparin (1000 IU) was administered i.p. 30 min before anesthesia to prevent coagulation during excision of the heart. The heart was excised, mounted on a Langendorff apparatus (AD instruments, Australia), and perfused retrogradely via the aorta with KH (Kreb’s Hensleit) buffer composed of NaCl (118.5 mM), KCl (5.8 mM), NaHCO3 (25 mM), KH2PO4 (1.2 mM), MgSO4 (1.2 mM), glucose (11 mM), and CaCl2 (2.5 mM) with pH = 7.4, maintained at 37 °C; bubbled with 5 % CO2, and 95 % O2, at a constant flow rate of 8 ml/min as described elsewhere (Chevion et al. 1993).
The experimental design for the H2S as a post-conditioning agent is shown in Fig. 1. In the normal perfusion group, hearts were perfused for 120 min. In the ischemic control, hearts were subjected to a 30-min global ischemia and stored for further analysis. In the reperfusion control group, hearts were subjected to global ischemia for 30 min and then reperfused for 60 min. The post-conditioning control group (POC control) involves those hearts subjected to global ischemia, followed by three cycles of a 3-min reperfusion and 2-min ischemia followed by reperfusion (60 min). NaHS (H2S donor) post-conditioning group (POC_H2S) consists of the heart subjected to global ischemia followed by a 15-min NaHS (20 μM) perfusion and 60-min reperfusion with KH buffer. Once the perfusion protocol was over, hearts were stored immediately at −80 °C for further biochemical analysis. Each experimental group consists of ten animals.
Fig. 1.
Experimental design of the perfusion protocol. Experimental groups includes Normal, Ischemic control, Reperfusion control (I/R), POC control, and POC_H2S. Each group consists of ten animals
The following hemodynamic parameters were recorded using Power Lab from AD instruments Ltd, Australia: left ventricular end diastolic pressure (LVEDP) in mmHg, developed pressure (LVDP) in mmHg, heart rate (HR) in beats per minute (bpm), and rate pressure product (RPP = HR × DP).
Estimation of cardiac injury markers
In order to evaluate the presence of necrotic cell death, lactate dehydrogenase (LDH) and creatine kinase (CK) enzymatic activity were measured spectrophotometrically in cardiac tissue using previously described method (Kurian et al. 2005). Protein content was determined spectrophotometrically at 595 nm using Bio-Rad reagent.
Isolation of mitochondrial subpopulation
The differential centrifugation technique was used to isolate rat heart mitochondrial subpopulations, namely interfibrillar mitochondria (IFM) and subsarcolemmal mitochondria (SSM), essentially according to the method described elsewhere (Kurian et al. 2012a, b). IFM and SSM were purified using a 60 % percoll gradient and western blot analysis of HSP 60; the marker protein (data not included) against β-actin was used to determine the purity.
Mitochondrial oxidative phosphorylation
Oxygen consumption by mitochondria was measured using a Clarke-type oxygen electrode (Hansatek, UK) at 37 °C in a final volume of 0.5 mL of respiration buffer (80 mM KCl, 50 mM Mops, 1 mM EGTA, 5 mM KH2PO4, and 1 mg/mL BSA, pH 7.4) with glutamate/malate (5 mM/2.5 mM) as substrate (Kurian et al. 2012a, b).
Mitochondrial electron transport chain complex (ETC) activities
ETC enzyme activities in IFM and SSM were measured spectrophotometrically by using specific donor acceptors. Rotenone-sensitive NADH-oxidoreductase and rotenone-sensitive NADH-cytochrome c reductase was used to assess the complexes I and I + III activity, respectively; succinate decylubiquinone DCPIP reductase and succinate cytochrome c reductase assess the complex II and II + III, respectively; Ubiquinol cytochrome c reductase to assess complex III and cytochrome c oxidase (complex IV) were measured as per the protocol described previously (Frazier and Thorburn 2012).
Mitochondrial swelling
The mitochondrial swelling was determined by the rate of change in absorbance at 540 nm under energized (5 mM succinate or 5 mM glutamate plus malate (GM)) as well as non-energized condition (Martens et al. 1986) and expressed as ΔA340/mg protein.
Mitochondrial membrane potential
Mitochondrial membrane potential was estimated as described elsewhere (Scaduto and Grotyohann 1999) using the uptake of the positively charged fluorescent dye Rhodamine 123 and expressed in mV (calculated using Nernst equation).
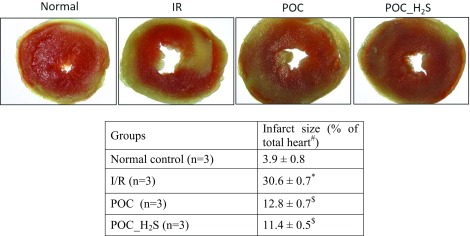
Determination of infarct size
The measurement of infarct size (IS) was done by using TTC staining according to Mensah et al. (2005) with minor changes. The images were acquired using a zoom stereomicroscope (NIKON-SMZ1270) equipped with a high definition CCD camera (NIKON-DS-Fi2), using NIS-elements documentation tool. The percentage of infarcted tissue (triphenyl tetrazolium chloride negative regions) developed was evaluated using the ImageJ analysis tool (NIH-USA).
DNA fragmentation
Cardiac tissue homogenate was prepared in Tris-HCl buffer (pH-8) with EDTA (25 mM) and NaCl (400 mM). DNA was isolated using earlier prescribed protocol (Baise et al. 2002) and the sample (5 μg/well) was run on an agarose gel (1.8 %) for 2 h. Gel pattern was observed for any laddering/smearing pattern and images were taken using Bio-Rad Chemi Doc XRS system.
Measurement of H2S level and its metabolizing enzymes
Estimation of H2S in tissue
H2S assay was performed according to Ang et al. (2012). To the sample, 1 % Zn (O2CCH3) was added and centrifuged. The pellet was washed with milliQ water and again centrifuged. To the water-resuspended pellet, 40 μl of dye (N, N-dimethyl p-phenylene diamine in 30 mM FeCl3) was added and incubated for 10 min. The absorbance was read at 670 nm and expressed as micromole of methylene blue formed/milligram protein. Methylene blue was used to generate a standard curve with known concentration of NaHS.
Cystathionine beta synthase assay
Cystathionine beta synthase (CBS) assay was performed according to Stepien and Pieniazek (1973). The sample was added to the reaction mixture (500 mM phosphate buffer, 50 mM of dithiotheritol (DTT) and 50 mM of l-serine) and incubated on ice after adding freshly prepared NaHS (0.3 M). The mixture was then placed in a water bath at 37 °C for 1 h. Gaitonde’s reagent (1 g of ninhydrin in 16 ml concentrated HCl and 64 ml glacial acetic acid) was added to stop the reaction and transferred into another centrifuge tube. Tubes were then placed in boiling water bath for 5 min then cooled. Two milliliters of ethanol was added and the absorbance was read at 550 nm and expressed as μM of cysteine/mg protein.
Rhodanese assay
Rhodanese assay was performed as per Lee et al. (1995). The reaction mixture consists of phosphate buffer (200 mM), sodium cyanide (500 mM), sodium thiosulfate (500 mM). The reaction mixture was vortexed and 15 % formalin was added. The above reaction mixture was then incubated for 5 min and 410 mM of ferric nitrate dissolved in 14 % nitric acid was added and the absorbance was recorded at 460 nm and reported as micromole of thiocyanate/milligram protein.
In vitro H2S impact on IFM and SSM
Isolated SSM and IFM from normal I/R- and POC-treated rat hearts were checked for oxygen consumption in the presence of exogenous NaHS (1, 10, and 20 μM) as substrate and the following parameters like RCR, P/O ratio, O2/H2S, and state 3 respiration rate were estimated as described earlier.
Statistical analysis
GraphPad Prism 5.0 was used for all statistical analysis. The comparison between values of the same group, at various time points of the experiment was conducted using ANOVA. Significant differences in variables between the groups for a specific time point were performed using one-way analysis of variance followed by Dunnet’s bonforoni test (post hoc analysis). Differences were considered statistically significant, if p < 0.05. Values were presented as the mean ± SE.
Results
Effect of NaHS post-conditioning on hemodynamics, myocardial infarct size, and cardiac injury markers
Hemodynamic function, measured at the end of reperfusion, was significantly impaired in I/R rat heart, compared with sham control, evidenced by increased LVEDP, low LVDP, and low RPP (Table 1) and decreased rates of contraction (+dP/dt) and relaxation (−dP/dt) (data not shown). The LVEDP was markedly decreased, and other cardiac parameters like LVDP and RPP were significantly elevated in both POC and POC_H2S hearts during reperfusion (Table 1) relative to control hearts. To determine the degree of necrosis in these I/R hearts, we assessed the infarct size by TTC staining and measured the infarct size. The global ischemia in the heart followed by reperfusion exhibit an eightfold increase in the infarct size area, while POC and POC_ H2S hearts display a threefold raise compared with the normal (Table 2). Similarly, the levels of lactate dehydrogenase (LDH) and creatinine kinase (CK) enzymes during the first 10 min of reperfusion after global ischemia was measured (Fig. 2b & d). We observed a significant elevation in total LDH and CK activities in the perfusate and a corresponding decline was noticed in cardiac tissues during reperfusion (Fig. 2a, c), compared with normal control, resulting in increased necrosis. Both POC and H2S post-treated hearts have shown reduced infarct size (area measured by TTC) as well as improved the LDH and CK levels in the myocardium, evidenced by low LDH and CK levels in the coronary perfusate (Fig. 2). Furthermore, the lysate from a subset of experimental hearts were assayed for DNA fragmentation and the results showed the absence of DNA fragmentation in POC and POC_ H2S hearts, whereas reperfusion and ischemia control group exhibit prominent DNA damage, observed as a smearing pattern (Fig. 2e)
Table 1.
Hemodynamic measurement at the end of reperfusion
| Groups | LVEDPa | LVDP1 | RPPb |
|---|---|---|---|
| Normal control (n = 6) | 6 ± 2 | 98 ± 4 | 95 ± 2 |
| I/R (n = 6) | 45 ± 3* | 42 ± 3* | 32 ± 2* |
| POC (n = 6) | 18 ± 4$ | 95 ± 4$ | 87 ± 3$ |
| POC_H2S (n = 6) | 17 ± 3$ | 96 ± 3$ | 88 ± 2$ |
EDP end diastolic pressure, DP developed pressure, RPP rate pressure product
Data are represented as mean ± SE. *p < 0.05 vs control; $ p < 0.05 vs I/R
arepresents values denoted in mmHg
brepresents values denoted in mmHg × bpm × 10^3
Table 2.
Infarct size measured after completion of the experiment using TTC staining
Data are represented as mean ± SE. *p < 0.05 vs control; $ p < 0.05 vs I/R
Fig. 2.
Determination of cardiac injury. a Lactate dehydrogenase (LDH) in cardiac tissue. b LDH in the perfusate. c Creatinine Kinase (CK) in the cardiac tissue. d CK in the perfusate. e DNA fragmentation [lane-1, 100 bp DNA ladder; lane-2, normal perfusion; lane-3, Ischemic control; lane-4, reperfusion control; lane-5, POC control; lane-6, POC_H2S]. Values were expressed as mean ± SE of n = 4–6 independent assays. *p < 0.05, statistically different from the normal controls; # p < 0.05, statistically different between subpopulations (i.e., IFM vs SSM). DNA fragmentation was visualized on a 1.5 % agarose gel stain
Conditioning the post-ischemic heart with NaHS alters OXPHOS complex enzymes
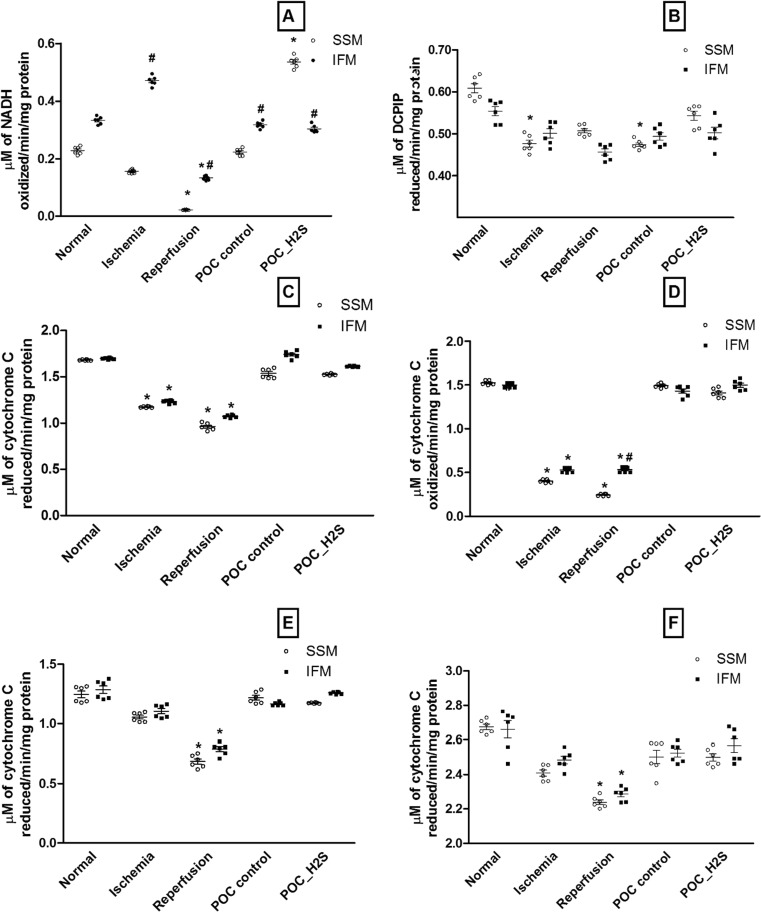
To identify the possible cardioprotective mechanism of hydrogen sulfide in relation to mitochondrial subpopulation, we evaluated the activity of its electron transport chain enzymes. The complex I activity as shown in Fig. 3a, indicates the prominent difference between IFM and SSM across all experimental groups. Furthermore, the complex I activity was progressively declined in SSM during both ischemia and I/R, while in IFM, the activity was reduced significantly (p < 0.05) during I/R alone. However, complex II (Fig. 3b), III (Fig. 3c), and IV (Fig. 3d) activities were similar in both IFM and SSM, although their activities were low during reperfusion. Indeed, the complex IV activity of the ischemic heart was significantly reduced (p < 0.05). The complex I + III (Fig. 3e) and complex II + III (Fig. 3f) measurement, an index of the respirosome activity, showed significant low activity during I/R with significant difference within the subpopulation as well. Classical post-conditioning improves the IFM significantly throughout the electron transport chain while SSM was not recovered efficiently, especially with SQR and SSCR activity. On the other hand, H2S post-conditioning improves all electron transport chain enzyme activities in both IFM and SSM (Fig. 3).
Fig. 3.
OXPHOS complex enzyme activities. a Complex I. b Complex II. c Complex III. d Complex II + III. e Complex IV. f Complex I + III. Complex I was measured as rotenone-sensitive NADH-decylubiquinone oxidoreductase activity, complex II was measured as succinate decylubiquinone DCPIP reductase activity, complex III was measured as ubiquinone- cytochrome c reductase activity, combined complex II and III was measured as succinate cytochrome c reductase activity, complex IV was measured as cytochrome c oxidase activity, and complex (I + III) was measured as rotenone sensitive NADH-cytochrome c oxidoreductase activity. Activity was expressed as micromole of NADH oxidized/min/milligram protein for complex I; micromole of DCPIP reduced/min/milligram protein for complex II; micromole of cytochrome c reduced/min/milligram protein for complex (I + III), complex (II + III) and complex III; micromole of cytochrome c oxidized/min/milligram protein for complex IV. Values were expressed as mean ± SE of n = 4–6 independent assays. *p < 0.05, statistically different from the normal controls; # p < 0.05, statistically different between subpopulations (i.e., IFM vs SSM)
Effect of POC_H2S on the mitochondrial respiration, swelling, and membrane permeability transition pore
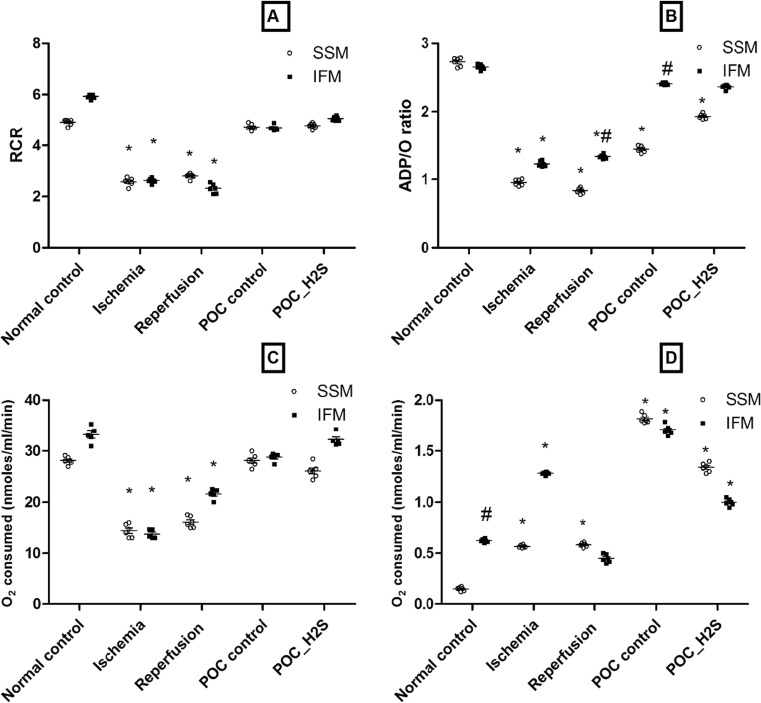
To evaluate the physiology and integrity of isolated IFM and SSM, parameters like RCR, P/O, state 3 and state 4 (Fig. 4), mitochondrial permeability transition and swelling (Fig. 5) were measured. The ratio of state 3 to state 4 respiration (denoted as RCR), generally considered to be the key parameter to assess the isolated mitochondrial integrity, was found to be deteriorated during ischemia and I/R (Fig. 4a). The concomitant assay to determine the oxidative phosphorylation is P/O ratio, which was observed to be diminished with ischemia and I/R treatment (Fig. 4b). As shown in Fig. 5c, d, both IFM and SSM exhibit a low oxygen consumption rate during ischemia and reperfusion. The physiological parameters were significantly improved with IFM only, by means of POC and H2S treatment, as SSM showed impaired P/O ratio (Fig. 4).
Fig. 4.
Mitochondrial polarographic measurement. a Respiratory control ratio. b ADP/O ratio. c State 3 respiration. d State 4 respiration. All measurements were made with glutamate/malate as a substrate. Values were expressed as mean ± SE of n = 4–6 independent assays. *p < 0.05, statistically different from the normal controls; # p < 0.05, statistically different between subpopulations (i.e., IFM vs SSM)
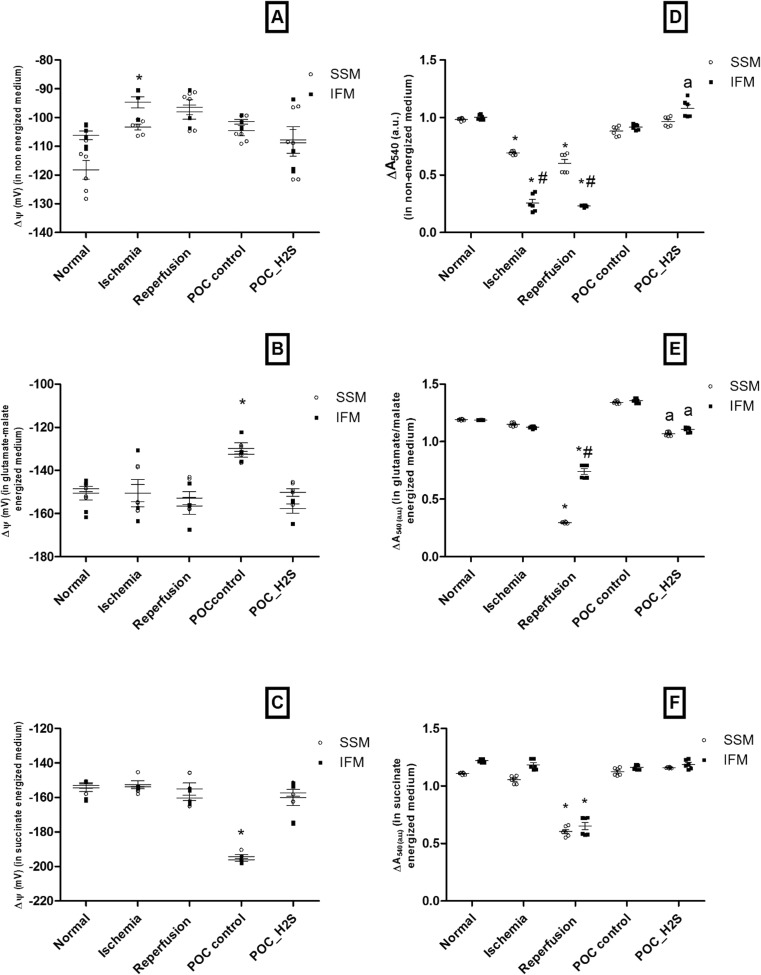
Fig. 5.
Mitochondrial physiology measurement. a–c Membrane potential under non-energized and energized conditions. d–f Mitochondria swelling under non-energized and energized conditions. a, d measurements were made under non-energized condition. b, e Measurements were made after energized with glutamate/malate. c. f measurements were made after energized with succinate. Values were expressed as mean ± SE of n = 4–6 independent assays. *p < 0.05, statistically different from the normal controls; # p < 0.05, statistically different between subpopulations (i.e., IFM vs SSM); a p < 0.05, statistically different from POC control
Figure 5a, membrane potential measured in non-energized condition, showed hypopolarization during ischemia and reperfusion. But, in energized condition, the presence of glutamate (Fig. 5b) or succinate (Fig. 5c) impart hyperpolarization of mitochondrial membrane during ischemia and reperfusion. Mitochondria from post-conditioned heart showed hypopolarization during GM-mediated respiration while it showed hyperpolarization with succinate-mediated respiration. Importantly, H2S conditioning brought the membrane potential to the near normal level in energized condition.
There was an increased swelling in IFM and SSM of I/R rat heart in both energized and non-energized mediums. Non-energized condition imparts severe swelling in IFM than SSM during I/R while energized with GM showed significant swelling in SSM. Both POC and POC_H2S improved the swelling behavior in IFM and SSM (Fig. 5d, e, f).
Direct impacts of hydrogen sulfide on isolated SSM and IFM
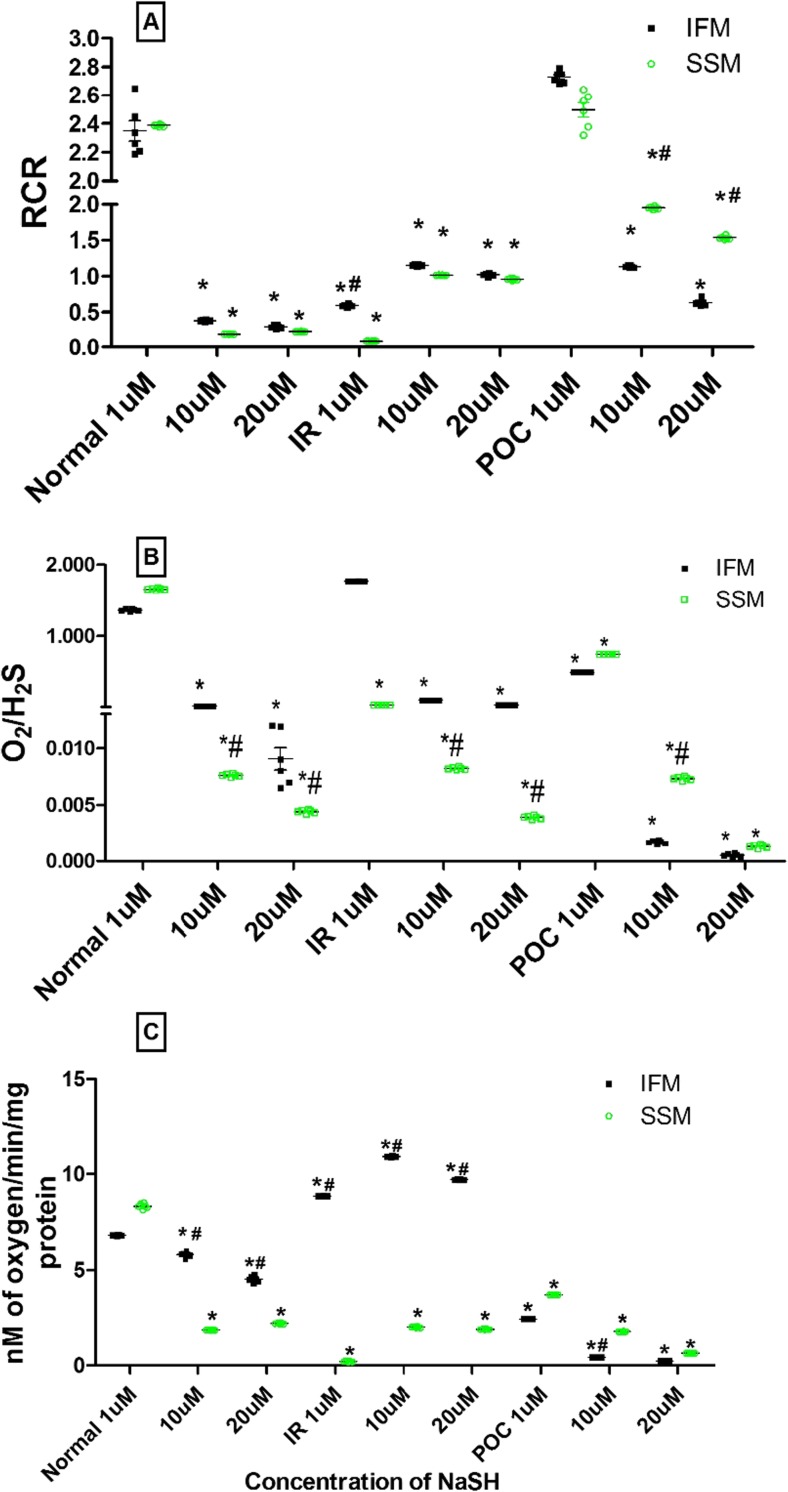
Mitochondria can utilize H2S as substrate for fuel when its concentration is <10 μM, but with >20 μM cellular concentration, it imparts toxic effect through inhibition of complex IV (Szabo et al. 2014). We incubated the mitochondria (SSM and IFM) isolated from normal, I/R, and POC rat hearts with NaHS of different concentrations like 1, 10, and 20 μM concentrations and analyzed RCR, state 3, and O2/H2S respiration rate.
IFM and SSM from normal hearts exhibit higher O2/H2S ratio with low concentration of H2S (1 μM). But, when the concentration increased, magnitude of O2/H2S ratio decreased progressively and, the extent of the decline was more prominent in SSM. A similar pattern of changes was observed in the subpopulations from both I/R and POC hearts (Fig. 6). However, RCR value showed a gradual decrease with increasing concentration of H2S in the case of subpopulations from normal hearts, whereas with I/R hearts, with increasing concentration of H2S, RCR was increased. These observations were well correlated with the P/O ratio and RCR values described earlier (Fig. 4), where IFM activity was intact throughout the experimental group.
Fig. 6.
In vitro effect of NaHS on mitochondrial respiration. a Respiratory control ratio. b O2 to H2S consumption. c State 3 respiration rate. Hearts from normal control, ischemic control, and POC control were subjected to NaHS at three different concentrations (1, 10, and 20 μM) * p < 0.05, statistically different from the normal controls; values were expressed as mean ± SE of n = 3 independent assays. # p < 0.05 statistically different from SSM
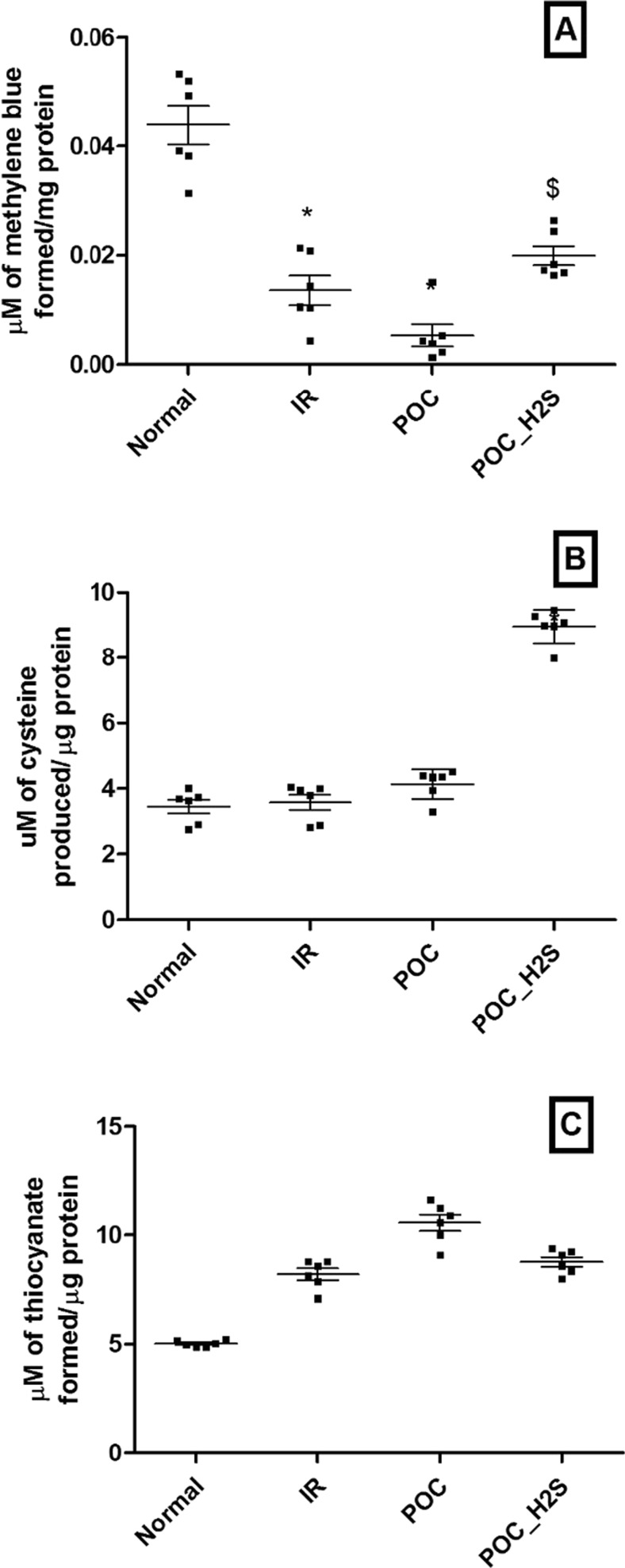
Levels of H2S and its metabolizing enzymes
As shown in Fig. 7, H2S level was significantly decreased in I/R rat heart and was not recovered in POC heart, but improved with H2S post-treatment. The corresponding elevated activity of rhodanase with no change in CBS activity indicates the rapid utilization of H2S during reperfusion and this observation was sustained with POC hearts.
Fig. 7.
H2S level and its metabolizing enzymes. a H2S level. b Cystathione beta synthase (CBS) enzyme activity. c Rhodanase activity. Values were expressed as mean ± SE of n = 3 independent assays. *p < 0.05 vs normal control; $ p < 0.05, POC_H2S vs POC
Discussion
In the present study, we have identified IFM-based cardioprotective mechanism of hydrogen sulfide in ameliorating reperfusion injury in isolated rat heart. It is well established that H2S protects hearts from reperfusion injury by preserving cardiac mitochondria (Ji et al. 2008). But in the heart, two distinct mitochondrial subpopulations have been identified (Geng et al. 2004), namely interfibrillar and subsarcolemmal mitochondria and has been reported to exhibit a distinct behavior towards ischemia- and reperfusion-associated changes (Kurian et al. 2012a, b). It is reasonable to believe that H2S-mediated protection of reperfusion injury should involve mitochondrial subpopulation. The importance of our finding not only provide some insight on few failures of clinical trials with H2S and cardioprotection as compared to the 100 % success rate in preclinical trials, but also necessitate the importance of developing mitochondria subpopulation targeted drugs.
Effect of H2S on the functional enzyme activities of IFM & SSM
Mitochondrial energetic dysfunction is the key pathologic event in cardiac ischemia–reperfusion injury and several studies have attributed this to complex I damage, considering its ability to release ROS and subsequent oxidation of cardiolipin. Both IFM and SSM exhibit low complex I activity during reperfusion with parallel change in reduced state 3 respiration and subsequent decline in P/O ratio, substantiate the mitochondrial damage (Fig. 4). This observation was further strengthened by the altered mitochondrial membrane potential and swelling behavior (Fig. 5) in the sub population. H2S is considered to be an endogenous modulator of mitochondria function by acting as a substrate for the ETC activity (Szabo et al. 2014 and Katalin et al. 2013) and can also activate cardioprotective signaling pathways like SAFE (Luan et al. 2012) and RISK (Jonathan et al. 2014). In the present study, both POC heart and H2S post-conditioning hearts significantly improved the complex I activity to near normal level. But a prominent elevated complex I activity was observed with POC_ H2S in SSM, suggesting its efficient H2S utilization. With a physiological level of H2S, cytochrome c oxidase, the target for H2S toxicity, is not inhibited (Fig. 3d), and sulfide oxidation likely contributes to mitochondrial ATP production (Fig. 4b) Ming Fu and his coworkers (2012) has shown that H2S may function as an energy substrate (Goubern et al. 2007) to sustain ATP production under stress conditions. With reperfusion, the electrons from sulfide can be injected into the mitochondrial electron transport chain, especially in the ubiquinone pool (Tatjana 2011), thereby reversing the activities of either complex I and II (Emilie et al. 2010). However, why SSM is more sensitive towards the increased complex I activity is yet to be explored.
During ischemia, complex II activity was found to be distinct among the subpopulation, where SSM showed significant elevated activity than IFM. This may a compensatory mechanism for the low complex I activity in SSM. Early studies have supported the mitochondrial respiration compensatory mechanism which is responsible for the development of urgent adaptive responses and resistance to ischemia (Chen et al. 2008). Ischemic heart also showed a prominent decline in the activity of complex IV (threefold) in both IFM and SSM with a relative low magnitude of decrease in complex III activity. This makes an accumulation of electron that may leak through complex III and could be the source of free radical in irreversible ischemic injury.
Revascularizing the ischemic heart aggravated the ischemic injury and the impact was reflected in complex I and IV, where its activities was markedly reduced, with prominent difference between the subpopulations. Also, earlier studies had shown that complex I and IV are the major sites of damage during reperfusion (Andrew et al. 2006). Considering the fact that ROS-mediated damage underlines the complex I and IV impairment during reperfusion (Yeong-Renn and Jay 2014), in particular, with SSM provides enough evidence for the vulnerability of SSM during reperfusion, perhaps due to its cellular location.
Given that post-conditioning the heart by repeated cycles of short ischemia and reperfusion can render cardioprotection by modulating mitochondria either as an initial triggering phase of the process, or as an end effector, or both (Michel et al. 2010). The upstream and downstream electron transport chain complex activities were significantly improved in both IFM and SSM except with complex II. In agreement with this observation, complex II + III measurement that reflects the respirosome activities with FADH2-linked respiration was low in both IFM and SSM. In our previous studies, we have shown that the distal part of the ETC, say, complex IV and V, were severely affected by both ischemia and reperfusion (Kurian et al. 2012a, b).
Hydrogen sulfide post-conditioning mechanism was explained as the ROS scavenging ability of H2S and its potential to activate RISK pathway and may act through S-sulfhydration of proteins (Paul and Snyder 2012) especially some of ETC enzyme subunits. Accumulating evidences indicate that sulfhydration is a physiologic post-translational modification, often associated with mitochondrial proteins, that regulates its function. Changes in the mitochondrial ROS/RNS levels are frequently linked with the oxidation/nitrosation of highly redox-sensitive cysteine residues present in the mitochondrial iron–sulfur (Fe–S) assembly and heme (Mailloux et al. 2014 and Lesnefsky et al. 2001). The reciprocal regulation of mitochondrial protein through nitrosation and sulfhydration of the same protein determine the extent of H2S-mediated mitochondrial preservation (Chen et al. 2007). The complex I to IV enzyme activities was recovered significantly to near normal level by H2S treatment in both IFM and SSM. In fact, complex I activity of SSM showed approximately 3 fold increase by H2S post-conditioning. Barring the latter, we have clearly shown that H2S post-conditioning could protect both mitochondrial subpopulations effectively, suggesting the efficacy of volatile drug to target spatially distinct cell organelles.
Effect of H2S on the mitochondrial respiration, swelling and membrane permeability transition pore of IFM and SSM
The respiratory control ratio, an index to determine the coupling efficiency of mitochondria was measured and expressed as a ratio of rate of ATP consumption in the cell to the rate of ATP production by oxidative phosphorylation. Low RCR value in IFM and SSM during ischemia and reperfusion confirms the mitochondrial dysfunction (Fig. 4a). In agreement with the previously published data, POC preserves the mitochondrial coupling efficiency (measured by P/O ratio and RCR). The RCR (State 3/state 4) value is strongly influenced by almost every functional aspect of oxidative phosphorylation, making them good indicators of dysfunction. Post-conditioning the ischemic myocardium either by mechanical means or by H2S, imparts protection towards mitochondrial integrity as well.
In general, mitochondrial dysfunction site could be understood well with absolute respiration rates. The measured oxygen consumption rate (State 3) was significantly lower in both SSM and IFM during I/R, in the presence of glutamate/malate as the respiratory substrate. Oxygen consumption rate of both IFM and SSM were significantly improved by H2S post-conditioning procedure (Fig. 4c). Sulfide is known to stimulate oxygen consumption resulting in the energization of mitochondria (Raymond et al. 1996), which explains the improved respiratory rate in both subpopulations.
The magnitude of mitochondrial inner membrane permeability can be measured by State 4 respiration, found to be increased in both SSM and IFM following ischemia and reperfusion (Fig. 4d). Consistent with this result, mitochondrial permeability transition index and swelling behavior were altered and exhibited higher swelling during ischemia and ischemia reperfusion. Importantly, both POC and H2S post-conditioning restore the mitochondrial membrane physiology, in particular with IFM (Fig. 5).
Direct impacts of different concentration of NaHS on IFM and SSM
In order to reconfirm the distinct effect of H2S on IFM and SSM and further understand the impact of H2S donor (NaHS) directly on mitochondria subpopulation, we incubated the isolated IFM and SSM with varying concentrations of NaHS. By using oxygen to H2S ratio (O2/H2S), RCR, and state 3 respiration, we assessed the ability of IFM and SSM to utilize H2S as an energy fuel. O2/H2S was significantly declined in SSM (approximately 83 %) with no change in IFM from the reperfusion heart compared to the normal heart, when the concentration of NaHS was 1 μM. Evidences from the previous reports suggest that sulfide-rich environment may be utilized by invertebrates as well as by some vertebrates, as an alternative source of energy and it involves oxidation of sulfide at the mitochondrial level, perhaps coupled to mitochondrial bioenergetics (Szabo et al. 2014). Thus the effective NaHS utilization of IFM from I/R heart indicates the sturdiness of IFM in pathological conditions, compared to the vulnerable nature of SSM. The capacity for sulfide oxidation will depend upon the presence of the sulfide oxidizing unit (dioxygenase, rhodanase) and downstream mitochondrial activities of complex III and complex IV (cytochrome oxidase), both of which pump protons to energize the mitochondrial inner membrane. However, with POC hearts, both subpopulations could utilize H2S effectively with 1 μM concentration of H2S, indicating the protective effect of POC on subpopulations. Interestingly, the subpopulations from I/R hearts being able to utilize H2S, when the concentration was more than 10 μM, without any distinction between the subpopulations, agreed with the previous notion that H2S could be effectively utilized by the mitochondria during stress conditions (Katalin et al. 2013).
Conclusion
Based on the present study results, we conclude that H2S post-conditioning deliver significant cardioprotection against I/R injury in agreement with the previous reports. The distinct contributory role of cardiac mitochondrial subpopulation towards H2S post-infarction treatment was evaluated in this present study. In fact, much preserved IFM provide enough support for the overall function of mitochondria, playing a significant role in H2S-mediated cardioprotection. Further studies are required to examine the impact of modulating the SSM functional aspects might pave the way for a novel therapy against I/R injury.
References
- Andrew JT, Lindsay SB, Stanley BD, Corinne Z, William LH, Paul SB. Mitochondrial dysfunction in cardiac ischemia–reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2006;1762(2):223–231. doi: 10.1016/j.bbadis.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Ang AD, Konigstorfer A, Giles GI, Bhatia M. Measuring free tissue sulfide. Adv Biol Chem. 2012;2:360–365. doi: 10.4236/abc.2012.24044. [DOI] [Google Scholar]
- Biase FH, Franco MM, Goulart LR, Antunes RC. Protocol for extraction of genomic DNA from swine solid tissues. Genet Mol Biol. 2002;25(3):3313–3315. doi: 10.1590/S1415-47572002000300011. [DOI] [Google Scholar]
- Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Chen YR, Chen CL, Pfeiffer DR, Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- Chen CL, Chen J, Rawale S, Varadharaj S, Kaumaya PP, Zweier JL, Chen YR. Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J Biol Chem. 2008;283:27991–28003. doi: 10.1074/jbc.M802691200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevion M, Jiang Y, Har-El R, Berenshtein E, Uretzky G, Kitrossky N. Copper and iron are mobilized following myocardial ischemia: possible predictive criteria for tissue injury. Proc Natl Acad Sci U S A. 1993;90(3):1102–1106. doi: 10.1073/pnas.90.3.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek M, Knutelska J, Bednarski M, Nowinski L, Zygmunt M, Bilska-Wilkosz A, Iciek M, Otto M, Zytka I, Sapa J, Włodek L, Filipek B. Alpha lipoic acid protects the heart against myocardial post ischemia–reperfusion arrhythmias via KATP channel activation in isolated rat hearts. Pharmacol Rep. 2014;66(3):499–504. doi: 10.1016/j.pharep.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Emilie L, Sabria M, Mireille A, Catherine C, Francois B, Frederic B. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta (BBA) Bioenerg. 2010;1797(8):1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Frazier AE, Thorburn DR. Biochemical analyses of the electron transport chain complexes by spectrophotometry Methods. Mol Biol. 2012;837:49–62. doi: 10.1007/978-1-61779-504-6_4. [DOI] [PubMed] [Google Scholar]
- Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004;313(2):362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- Goubern M, Andriamihaja M, Nubel T, Blachier F, Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21(8):1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- Ji Y, Pang QF, Xu G, Wang L, Wang JK, Zeng YM. Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol. 2008;587(1-3):1–7. doi: 10.1016/j.ejphar.2008.03.044. [DOI] [PubMed] [Google Scholar]
- John WE, John WC, Joanna M, Jeannette ED, David WK, Ling T, Xiangying J, Rosario S, Levente K, Csaba S, Hideo K, Chi-Wing C, David JL. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104(39):15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonathan PL, Chad KN, Hena A, Sana A, John WC. Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med Gas Res. 2014;4:20. doi: 10.1186/s13618-014-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katalin M, Ciro C, Katalin E, Andreas P, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27(2):601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- Kurian GA, Sachu, Thomas V. Effect of aqueous extract of Desmodium Gangeticum root in the severity of isoproterenol induced Myocardial infarcted rats. J Ethnapharmacol. 2005;97(3):457–461. doi: 10.1016/j.jep.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Kurian GA, Berenshtein E, Saada A, Chevion M. Rat cardiac mitochondrial sub-population show distinct features of oxidative phosphorylation during ischemia, reperfusion and ischemic preconditioning. Cell Physiol Biochem. 2012;30(1):83–94. doi: 10.1159/000339043. [DOI] [PubMed] [Google Scholar]
- Kurian GA, Berenshtein E, Kakhlon O, Chevion M. Energy status determines the distinct biochemical and physiological behavior of interfibrillar and sub-sarcolemmal mitochondria. Biochem Biophys Res Commun. 2012;428(3):376–382. doi: 10.1016/j.bbrc.2012.10.062. [DOI] [PubMed] [Google Scholar]
- Lacerda L, Somers S, Opie LH, Lecour S. Ischaemic postconditioning protects against reperfusion injury via the SAFE pathway. Cardiovasc Res. 2009;84(2):201–208. doi: 10.1093/cvr/cvp274. [DOI] [PubMed] [Google Scholar]
- Lee CH, Hwang JH, Lee YS, Cho KS. Purification and characterization of mouse liver rhodanese. J Biochem Mol Biol. 1995;28:170–176. [Google Scholar]
- Lesnefsky EJ, Gudz T, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- Li H, Wang Y, Wei C, Bai S, Zhao Y, Li H, Wu B, Wang R, Wu L, Xu C. Mediation of exogenous hydrogen sulfide in recovery of ischemic post-conditioning-induced cardioprotection via down-regulating oxidative stress and up-regulating PI3K/Akt/GSK-3β pathway in isolated aging rat hearts. Cell Biosci. 2015;5:11. doi: 10.1186/s13578-015-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan HF, Zhao ZB, Zhao QH, Zhu P, Xiu MY, Ji Y. Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Braz J Med Biol Res. 2012;45(10):898–905. doi: 10.1590/S0100-879X2012007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux RJ, Jin X, Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2014;2:123–139. doi: 10.1016/j.redox.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria-Giulia P, Pasquale P, Claudia P. Ischemia/reperfusion injury and cardioprotective mechanisms: role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3(6):186–200. doi: 10.4330/wjc.v3.i6.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens ME, Chang CH, Lee CP. Reye’s syndrome: mitochondrial swelling and Ca2+ release induced by Reye’s plasma, allantoin, and salicylate. Arch Biochem Biophys. 1986;244:773–786. doi: 10.1016/0003-9861(86)90646-6. [DOI] [PubMed] [Google Scholar]
- Mensah K, Mocanu MM, Yellon DM. Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: a potential role for phosphatase and tensin homolog deleted on chromosome ten. J Am Coll Cardiol. 2005;45(8):1287–1291. doi: 10.1016/j.jacc.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Michel O, Gary FB, Fabio DL, Peter F, David GD, Derek JH, Gerd H, Jakob VJ, Derek MY, Rainer S. Postconditioning and protection from reperfusion injury: where do we stand? Cardiovasc Res. 2010;87(3):406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- Ming F, Weihua Z, Lingyun W, Guangdong Y, Hongzhu L, Rui W. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc Natl Acad Sci U S A. 2012;109(8):2943–2948. doi: 10.1073/pnas.1115634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis K, Bos EM, Calzia E, van Goor H, Coletta C, Papapetropoulos A, Mark RH, Peter R, Frederic B, Csaba S. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part II. Pathophysiological and therapeutic aspects. Br J Pharmacol. 2014;171(8):2123–2146. doi: 10.1111/bph.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252(23):8731–8739. [PubMed] [Google Scholar]
- Pan TT, Neo KL, Hu LF, Yong QC, Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am J Physiol Cell Physiol. 2008;294(1):C169–C177. doi: 10.1152/ajpcell.00282.2007. [DOI] [PubMed] [Google Scholar]
- Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- Raymond WL, David WK, Jeannette ED. Sulfide-stimulation of oxygen consumption rate and cytochrome reduction in gills of the estuarine mussel Geukensia demissa. Bid Bull. 1996;191:421–430. doi: 10.2307/1543015. [DOI] [PubMed] [Google Scholar]
- Scaduto RC, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien PP, Pieniazek NJ. Use of the L-serine sulfhydrylase assay for the estimation of cystathionine beta-synthase. Anal Biochem. 1973;54:294–299. doi: 10.1016/0003-2697(73)90278-9. [DOI] [PubMed] [Google Scholar]
- Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol. 2014;171(8):2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatjana MH. Modulation of sulfide oxidation and toxicity in rat mitochondria by dehydroascorbic acid. Biochim Biophys Acta (BBA) Bioenerg. 2011;1807(9):1206–1213. doi: 10.1016/j.bbabio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Yeong-Renn C, Jay L. Zweier2 cardiac mitochondria and ROS generation. Circ Res. 2014;114(3):524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]