Abstract
Human small heat shock protein HspB6 (Hsp20) was modified by metabolic α-dicarbonyl compound methylglyoxal (MGO). At low MGO/HspB6 molar ratio, Arg13, Arg14, Arg27, and Arg102 were the primary sites of MGO modification. At high MGO/HspB6 ratio, practically, all Arg and Lys residues of HspB6 were modified. Both mild and extensive MGO modification decreased susceptibility of HspB6 to trypsinolysis and prevented its heat-induced aggregation. Modification by MGO was accompanied by formation of small quantities of chemically crosslinked dimers and did not dramatically affect quaternary structure of HspB6. Mild modification by MGO did not affect whereas extensive modification decreased interaction of HspB6 with HspB1. Phosphorylation of HspB6 by cyclic adenosine monophosphate (cAMP)-dependent protein kinase was inhibited after mild modification and completely prevented after extensive modification by MGO. Chaperone-like activity of HspB6 measured with subfragment 1 of skeletal myosin was enhanced after MGO modifications. It is concluded that Arg residues located in the N-terminal domain of HspB6 are easily accessible to MGO modification and that even mild modification by MGO affects susceptibility to trypsinolysis, phosphorylation by cAMP-dependent protein kinase, and chaperone-like activity of HspB6.
Keywords: Small heat shock proteins, Oligomeric structure, Phosphorylation, Methylglyoxal, Diabetes
Introduction
Diabetes is now one of the major severe diseases globally, and according to the International Diabetes Federation, more than 366 million people were in diabetes in 2011 and this is expected to rise up to 552 million by 2030 (Whiting et al. 2011). Diabetes is accompanied by hyperglycemia which is associated with glucose auto-oxidation, increase in polyol pathway, and protein glycation. All these events lead to metabolic stress, inflammation, and apoptosis which are implicated in the development of diabetes complications (Brownlee 2001; Giacco and Brownlee 2010). Methylglyoxal (MGO) is a highly reactive dicarbonyl compound accumulated under conditions of hyperglycemia (Phillips et al. 1993). There is a special system of enzymes (glyoxalases, aldoketoreductases, etc.) involved in glyoxal detoxification (Rabbani and Thornalley 2012; Vander Jagt and Hunsaker 2003). However, under pathological conditions, methylglyoxal is accumulated and non-enzymatically modifies side chains of Arg and Lys forming a number of different adducts and cross-links of Arg and Lys residues (Rabbani and Thornalley 2012). Since DJ-1 deglycase is able to repair only early intermediates formed by MGO (Richarme et al. 2015), many proteins remain modified. These MGO-modified proteins are combined in the so-called dicarbonyl proteome (Rabbani and Thornalley 2012). This group combines different proteins, and among them are albumin, hemoglobin, transcription factors, mitochondrial proteins, and extracellular matrix proteins.
Recently published data indicate that small heat shock proteins can also belong to this group. Small heat shock proteins are involved in keeping cellular homeostasis and protect the cell against different unfavorable conditions (Bakthisaran et al. 2015; Mymrikov and Haslbeck 2015; Mymrikov et al. 2011). In order to avoid unfavorable consequences of diabetes or aging, certain cells increase the expression of small heat shock proteins (for instance, of α-crystallin) (Reddy and Reddy 2015). It is well established that α-crystallin undergoes glycation and can be modified by methylglyoxal (Nagaraj et al. 2015). Upon certain conditions, modification by MGO increases chaperone-like activity with selected model protein substrates and inhibits glycation-induced loss in chaperone-like activity of α-crystallin (Gangadhariah et al. 2010; Puttaiah et al. 2007). MGO-modification enhances degradation and decreases stability of α-crystallin (Mukhopadhyay et al. 2010; Satish Kumar et al. 2004). The sites predominantly modified by MGO in α-crystallin were determined (Nagaraj et al. 2012), and it was shown that glycation or MGO modification can modify acetylation-induced effects (Nahomi et al. 2013). It is also supposed that α-crystallin glycation is one of the processes leading to opaqueness of eye lens and cataract (Sharma and Santhoshkumar 2009).
Similar results were obtained with another small heat shock protein HspB1 (Hsp27). It was shown that HspB1 is the major protein undergoing MGO modification in endothelial cells, mesangial kidney cell, and certain tumors (Oya-Ito et al. 2011; Sakamoto et al. 2002; Schalkwijk et al. 2006; van Heijst et al. 2006). Overexpression of HspB1 protects sensory neurons from diabetes (Korngut et al. 2012), and it is supposed that MGO modification increases antiapoptotic activity of HspB1 (Oya-Ito et al. 2006; Sakamoto et al. 2002).
Thus, the data of literature indicate that certain ubiquitously expressed small heat shock proteins, such as αB-crystallin and HspB1, easily undergo modification by MGO and that this modification affects their structure and function. HspB6 (Hsp20) is the third ubiquitously expressed small heat shock protein (Mymrikov et al. 2011). It is highly expressed in different muscles and participates in the regulation of smooth muscle contraction (Dreiza et al. 2010; Seit-Nebi and Gusev 2010), and it possesses antiapoptotic activity (Fan and Kranias 2011); in addition, it is liberated in the blood flow and participates in the regulation of platelet aggregation (Kanno and Matsuno 2006; Matsuno et al. 2003). Expression level and expression pattern of HspB6 are similar to those of αB-crystallin and HspB1, and therefore, it can be considered as a potential target of MGO modification. This paper deals with in vitro investigation of MGO-induced modification of HspB6 and analysis of effect induced by this modification on the structure and properties of this protein.
Materials and methods
Protein purification
Untagged recombinant human HspB1 and HspB6 were obtained as described earlier (Bukach et al. 2004). Three different lots of recombinant proteins were used in the course of this investigation.
MGO modification
Samples of HspB6 in buffer B (20 mM Tris/acetate pH 7.6, containing 10 mM NaCl and 2 mM DTT) were passed through Nap5 column equilibrated with buffer I (10 mM phosphate buffer (pH 7.4) containing 150 mM NaCl and 0.01 % of sodium azide). Thus, obtained samples were mixed with MGO and incubated under aseptic conditions at 37 °C for 48 h. Two different conditions were used for protein modification. The first set of samples contained high concentration of HspB6 (6 mg/ml or ∼350 μM per HspB6 monomer) and was incubated either in the absence (this sample is marked as sample 1 in the paper) or in the presence of low concentration of methylglyoxal (500 μM) (sample 2). This sample was designated as mildly modified protein. The second set of samples contained 0.8 mg/ml of HspB6 (∼47 μM per HspB6 monomer) and was incubated either in the absence (sample 3) or in the presence of high concentration of MGO (3 mM) (sample 4). This sample was designated as extensively modified protein. Modification was stopped by the addition of 15 mM mercaptoethanol followed by overnight dialysis performed at 4 °C against buffer I containing 1 mM DTT. Protein concentration in dialysates was determined spectrophotometrically or by Bradford method (Bradford 1976) using unmodified HspB6 as a standard. The procedure of modification was repeated eight times each time providing protein samples with identical properties.
Electrophoretic methods
SDS gel electrophoresis was performed by Laemmli method (Laemmli 1970). Urea polyacrylamide gel electrophoresis was performed as described earlier (Hayes et al. 2009; Perrie and Perry 1970).
Spectroscopic measurements
All fluorescent measurements were performed in buffer I at protein concentration equal to 0.05–0.10 mg/ml on Varian Cary Eclipse spectrofluorometer. Intrinsic Trp fluorescence was excited at 297 nm and recorded in the range of 300–400 nm (slit width 5 nm). Fluorescence of advanced glycation end products (AGEs) was excited at 335 nm and recorded in the range of 350–600 nm (slit width 10 nm). Heat-induced changes in Trp fluorescence (excited at 295 nm) were recorded at 320 and 360 nm upon heating of protein sample (0.2 mg/ml) with a constant rate of 1 °C/min in the range of 20–90 °C and cooling back with the same rate. Heat-induced aggregation was measured by recording light scattering at 340 nm. In this case, protein sample (0.3 mg/ml) in buffer I was heated with a constant rate of 1 °C/min in the range of 20–90 °C and cooled back with the same rate. The cell was illuminated at 340 nm, and the signal was recorded at 340 nm (slit width 2.5 nm) on Varian Cary Eclipse spectrofluorometer.
Limited proteolysis
Unmodified and modified HspB6 (0.25 mg/ml) in buffer I were subjected to limited trypsinolysis by Nα-tosyl-l-phenylalanine chloromethyl ketone (TPCK)-treated trypsin at a weight ratio HspB6/trypsin equal to 500/1 at 30 °C for 0–120 min. Reaction was stopped by the addition of phenylmethane sulfonyl fluoride (PMSF), and thus, obtained samples were analyzed by SDS gel electrophoresis (Laemmli 1970).
Size-exclusion chromatography
Isolated unmodified and modified HspB6 samples and their complexes with HspB1 were subjected to size-exclusion chromatography on Superdex200 HR 10/30 column equilibrated with buffer S (20 mM Tris/acetate pH 7.6, containing 150 mM NaCl, 0.1 mM EDTA, and 15 mM ME) at room temperature. One hundred fifty microliter samples were loaded on the columns and eluted at a flow rate of 0.5 ml/min. The column was calibrated with the protein standards thyroglobulin (669 kDa), ferritin (440 kDa), rabbit skeletal muscle glyceraldehydes-3-phosphate dehydrogenase (136 kDa), bovine serum albumin (68 kDa), ovalbumin (43 kDa), and chymotrypsinogen (25 kDa). Before formation of hetero-oligomeric complexes, HspB1 and HspB6 samples were reduced by incubation in the presence of 15 mM DTT for 30 min at 37 °C. Reduced proteins were mixed in buffer S so that the final concentration of each protein was equal to 0.5 mg/ml and incubated for 1 h at 42 °C providing for complete subunit exchange and formation of hetero-oligomeric complexes (Bukach et al. 2009).
Phosphorylation
Unmodified or modified HspB6 (0.5 mg/ml) was incubated with catalytic subunit of cyclic adenosine monophosphate (cAMP)-dependent protein kinase in buffer P containing 5 mM Tris, 10 mM phosphate (pH 7.5), 1 mM MgCl2, 3 mM mercaptoethanol (ME), 0.03 mM PMSF, and 40 μM of ATP containing trace amounts of γ-32P-ATP for different times (0–60 min) at 37 °C. Aliquots collected at different times of incubation were either spotted on Whatman 3MM filters or mixed with EDTA (up to the final concentration 20 mM) to stop protein kinase reaction. Whatman 3 MM filters were washed in 10 % trichloroacetic acid containing 10 mM of sodium phosphate and 10 mM sodium pyrophosphate, and the extent of HspB6 phosphorylation was determined by liquid scintillation counting. The samples containing EDTA were subjected to urea polyacrylamide gel electrophoresis (Hayes et al. 2009; Perrie and Perry 1970). Autoradiography of stained gel was performed as described earlier (Vorotnikov et al. 1988).
Chaperone-like activity
Porcine mitochondrial malate dehydrogenase (MDH) and skeletal rabbit myosin subfragment 1 (S1) were used as model substrates for estimation of chaperone-like activity of unmodified and modified HspB6. In the first case, MDH (Serva) (0.2 mg/ml) in buffer M (10 mM sodium phosphate pH 7.4, 2 mM DTT) was heated at 45 °C in the absence or in the presence of different quantities of HspB6. Heat-induced aggregation of MDH was followed by measuring optical density at 340 nm.
In the second case, rabbit skeletal muscle myosin subfragment 1 (obtained as described earlier (Weeds and Taylor 1975)) (0.4 mg/ml) was heated at 42 °C in buffer N (20 mM HEPES/NaOH pH 7.0, 115 mM NaCl, 20 mM DTT) in the absence or in the presence of different quantities of HspB6. Heat-induced aggregation of S1 was followed by the increase of optical density at 340 nm. All experiments were performed in 300-μl microcell on Ultrospec 3100 Pro spectrophotometer.
Results
Modification of HspB6 by methylglyoxal
Isolated unmodified HspB6 migrated on SDS electrophoresis as a band with apparent molecular weight of about 20 kDa (Fig. 1a, tracks 1 and 3). Incubation of HspB6 with low concentration of MGO at molar ratio MGO/monomer of HspB6 equal to about 1.5/1 was accompanied by the appearance of a weak diffuse band just above the band of unmodified monomer of HspB6 as well as by appearance of three closely separated protein bands with apparent molecular weight of ∼37 kDa (Fig. 1a, track 2). We suppose that these bands correspond to differently crosslinked dimers of either unmodified or mildly modified monomer of HspB6. Incubation with high concentration of MGO at a molar ratio MGO/HspB6 monomer equal to 60/1 was accompanied by complete disappearance of the band with apparent molecular weight of 20 kDa and appearance of diffuse band with apparent molecular weight of about 25 kDa (Fig. 1a, track 4) that corresponds to extensively modified monomer of HspB6. In addition, incubation with high concentration of MGO was accompanied by appearance of a new band with apparent molecular weight of 43 kDa (Fig. 1a, track 4). This band probably corresponds to the chemically crosslinked modified dimers of HspB6.
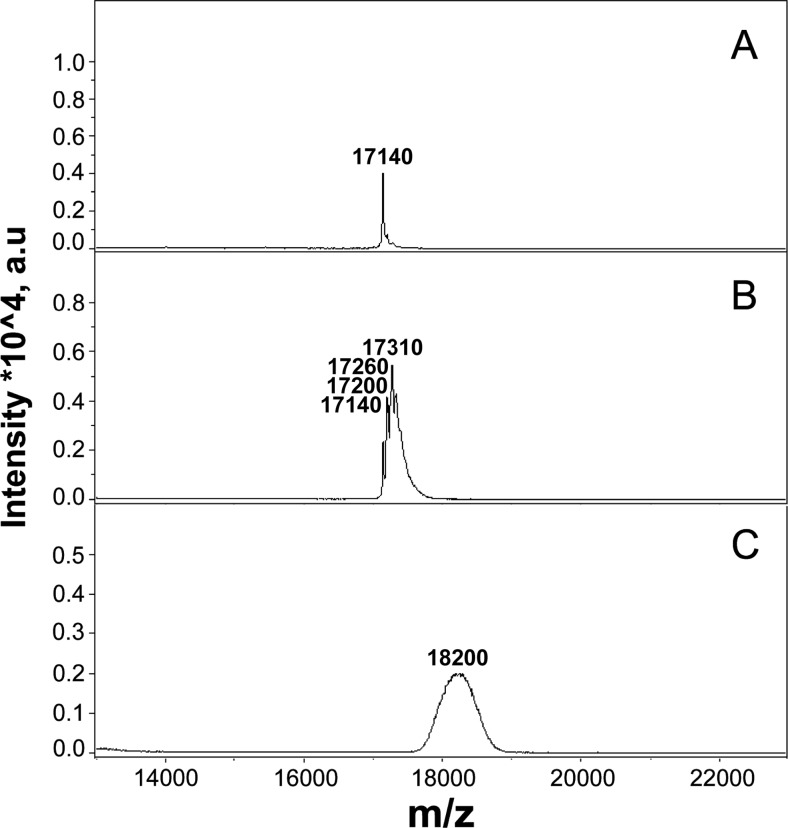
Fig. 1.
a SDS-gel electrophoresis of unmodified and MGO-modified samples of HspB6. The samples with high concentration of HspB6 (6 mg/ml, 350 μM per HspB6 monomer) were incubated either in the absence (1) or in the presence (2) of 500 μM MGO. The samples with low concentration of HspB6 (0.8 mg/ml, 47 μM per HspB6 monomer) were also incubated either in the absence (3) or in the presence (4) of 3 mM MGO. Molar ratio MGO/HspB6 monomer was equal to 1.5 (sample 2) or 60 (sample 4). Equal quantity of protein was loaded on each track. b Urea gel electrophoresis of control unmodified HspB6 preincubated at high (1) or low (3) protein concentration and the corresponding samples modified by low (2) or high (4) concentration of MGO. Unmodified HspB6 was loaded on tracks 1 and 3, and mildly and extensively modified samples of HspB6 were loaded on tracks 2 and 4, respectively. Arrows indicate positions of unmodified HspB6 monomer
In order to determine approximate extent of modification, the bands corresponding to unmodified and modified monomers of HspB6 were cut off and subjected to mass spectroscopy (Fig. 2). Molecular weight determined for unmodified HspB6 was equal to 17,140 Da and was close to expected calculated molecular weight of human HspB6 equal to 17,135 (UniProt O14558). In the case of mildly modified HspB6, the method of mass spectroscopy revealed at least three peaks with molecular weights of 17,200, 17,260, and 17,310 (Fig. 2). These peaks probably correspond to HspB6 modified by MGO at one, two, or three residues, respectively. Molecular weight of extensively modified HspB6 was equal to 18,200, and the peak of this protein was very broad thus indicating that it is represented by several different species having slightly different molecular weights. MGO adducts have different molecular weights varying in the range of 51 up to 81 Da. Therefore, it is difficult to determine the exact stoichiometry of HspB6 modification. However, taking an approximate molecular weight of MGO adduct equal to 66, we can assume that in the extensively modified HspB6, about 16 residues were modified by MGO. This value is close to the sum of Lys (3) and Arg (11) residues in HspB6. Thus, under conditions of extensive modification, practically, all Arg and Lys residues were modified by MGO.
Fig. 2.
Mass spectroscopy of unmodified (a), mildly (b), or extensively (c) MGO-modified HspB6
Mapping of MGO-modified residues of HspB6
Trying to map MGO-modified residues, we subjected unmodified, mildly, and extensively modified HspB6 to limited trypsinolysis (Fig. 3). Under conditions, used unmodified HspB6 was rapidly cleaved by trypsin leading to the accumulation of a series of fragments with apparent molecular weights of 18.5, 17.0, and 16.0 kDa (Fig. 3a, c). Taking into account that the apparent molecular weight of unhydrolyzed HspB6 was equal to 20 kDa, accumulation of a large fragment with the abovementioned apparent molecular weights indicates that predominant sites of trypsinolysis are located close to either N- or C-terminal ends of HspB6. There are several sites of trypsin cleavage located close to the N-terminal end of HspB6 (Arg13, Arg14, Arg27, Arg31), whereas the C-terminal end contains the only one site of trypsin cleavage (Arg122) located 38 residues from the C-terminal end of HspB6. Since limited trypsinolysis was accompanied by the accumulation of a rather large fragment of HspB6, we can suppose that the sites highly susceptible to trypsinolysis are predominantly located in the N-terminal part of HspB6.
Fig. 3.
Limited trypsinolysis of unmodified HspB6 (6.0 mg/ml) preincunbated for 48 h in the absence (a) or in the presence (b) of 0.5 mM MGO, or of unmodified HspB6 (0.8 mg/ml) preincubated for 48 h in the absence (c) or in the presence (d) of 3.0 mM MGO. After preincubation, all samples were diluted so that the concentration of HspB6 was equal to 0.25 mg/ml and subjected to limited trypsinolysis (weight ratio HspB6/trypsin was equal to 500/1). Time of trypsinolysis (min) is indicated above each track. The position of protein markers (M) and their molecular weights are indicated by lines
Mildly modified by MGO, HspB6 (Fig. 3b) was more resistant to limited trypsinolysis than the unmodified protein (Fig. 3a). Indeed, incubation for 60 min was accompanied by practically complete disappearance of the band of unmodified HspB6 (Fig. 3a), whereas even after incubation for 120 min, significant quantities of uncleaved mildly modified HspB6 were detected on the gel (Fig. 3b). The data presented indicate that the sites most susceptible to trypsinolysis and at the same time accessible to MGO modification are predominantly located in the N-terminal part of HspB6.
Extensively modified HspB6 was practically completely resistant to trypsinolysis (Fig. 3d). Even long incubation with trypsin was accompanied by very weak diminishing of the bands corresponding to modified HspB6 monomers or crosslinked dimers (Fig. 3d). This agrees with the fact that practically all Arg and Lys residues of extensively modified HspB6 were blocked by methylglyoxal.
Unmodified and modified HspB6 were subjected to urea gel electrophoresis (Fig. 1b). Unmodified HspB6 migrated as a single band having moderate electrophoretic mobility (Fig. 1b, tracks 1 and 3). Mildly modified HspB6 formed one band with electrophoretic mobility slightly lower than unmodified protein and a series of closely separated bands with electrophoretic mobility higher than unmodified protein (Fig. 1b, track 2). The band with lower electrophoretic mobility probably corresponds to crosslinked species of HspB6, whereas the bands with higher electrophoretic mobility correspond to differently modified monomers of HspB6. Indeed, modification of Arg or Lys residues by MGO will lead to the decrease of positive charge and will be accompanied by the increase of electrophoretic mobility of modified protein. Multiple bands detected in the case of mildly modified protein probably correspond to HspB6 modified by MGO at one, two, or three sites. Extensively modified HspB6 formed two badly stained bands (Fig.1b, track 4). The upper band probably corresponds to crosslinked modified HspB6 species, whereas the lower band possessing the highest electrophoretic mobility probably corresponds to extensively modified HspB6 monomer (Fig.1b, track 4).
The bands possessing different electrophoretic mobilities in the case of mildly modified HspB6 were cut out and subjected to trypsinolysis followed by mass spectroscopy. Two upper bands corresponding to the singly and doubly modified protein contained HspB6 modified at Arg13, Arg14, Arg27, and Arg102. This means that modification by MGO is nonspecific, and the four abovementioned Arg residues are more or less equally accessible for modification by MGO. The third band corresponding to triply modified HspB6 contained the same set of modified residues supplemented by Arg106 (Fig. 4). In all of these cases, modified peptides have an extra weight of 54 kDa thus indicating formation of arginine-derived hydroimidazolone (Fig. 4).
Fig. 4.
MS-MS spectra of tryptic peptides of HspB6 unmodified by MGO (a) or modified by MGO at one/two (b) or three (c) sites. Modified residues are marked as m and the sequence of modified peptides and their location are marked by arrows. The scheme of HspB6 primary structure, position of modified Arg residues, and the structure of hydroimidazolone (H) (d)
Fluorescent properties and thermal stability of unmodified and modified HspB6
HspB6 contains the single Trp residue (Trp11). The fluorescent spectrum of unmodified HspB6 is characterized by broad maximum located at about 350 nm (Fig. 5a) thus indicating that Trp is located in a hydrophilic environment. Mild modification of HspB6 by MGO does not dramatically affect either position of maximum or amplitude of intrinsic Trp fluorescence (Fig. 5a, curves 1 and 2). At the same time, extensive modification of HspB6 by MGO is accompanied by significant decrease of amplitude of Trp fluorescence and shifting of the maximum of fluorescence towards higher wavelength (Fig. 5a, curves 3 and 4).
Fig. 5.
Fluorescent properties of unmodified (1 and 3) and mildly (2) or extensively (4) modified HspB6. a Intrinsic Trp fluorescence excited at 295 nm and recorded in the range of 300–400 nm. Protein concentration of all samples 0.1 mg/ml. b Fluorescence of advanced glycation products excited at 335 nm and recorded in the range of 350–600 nm. Protein concentration of all samples 0.2 mg/ml
Glycation is accompanied by accumulation of advanced glycation end products (AGEs), some of them being fluorescent. Fluorescence of AGE was excited at 335 and recorded in the range of 350–600 nm (Fig. 5b). Under these conditions, fluorescence of unmodified protein was negligible low, whereas modification by MGO was accompanied by the appearance of a new peak of fluorescence at about 400–410 nm (Fig. 5b). This peak was especially pronounced in the case of extensively modified HspB6 (Fig. 5b, curve 4).
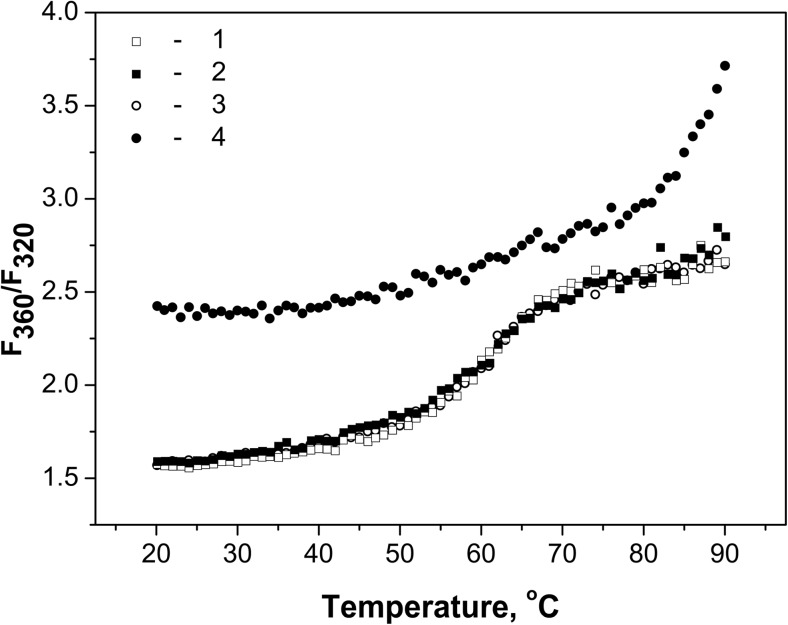
Thermal stability of unmodified and modified HspB6 was analyzed by measuring temperature-depending changes of Trp fluorescence and temperature-induced changes of light scattering. In the first case, we measured dependence of the ratio F360/F320 (where F320 and F360 are intensities of fluorescence at 360 and 320 nm, respectively) upon temperature in the range of 20–90 °C (Fig. 6). The ratio F360/F320 was lower for unmodified and mildly modified protein than in the case of extensively modified HspB6. This is due to the fact that the maximum of fluorescence of extensively modified protein is shifted towards higher wavelength. The half-maximal thermal transition for unmodified and mildly modified HspB6 was observed at about 65 °C (Fig. 6, curves 1–3), and this thermal transition was irreversible (data not shown). At the same time, we were unable to achieve complete thermal transition even at 90 °C for extensively modified protein (Fig. 6, curve 4).
Fig. 6.
Heat-induced changes of fluorescence of unmodified (1 and 3) and mildly (2) or extensively (4) modified HspB6. Final protein concentration of all samples was 0.2 mg/ml
Heating of unmodified HspB6 was accompanied by slow and small increase of light scattering at 340 nm (Fig. 7a, b, curves 1 and 3). At the beginning of cooling, the light scattering slightly falls down and afterwards was significantly increased so that the final value of light scattering after cooling was three to four times higher than before heating (Fig. 7a, b, curves 1 and 3). Heating of MGO-modified HspB6 is accompanied by minimal changes of light scattering and slightly falls down at high temperatures (Fig. 7a, b, curves 2 and 4). Upon cooling, the light scatter of modified HspB6 was not practically changed (Fig. 7a, b, curves 2 and 4). Qualitatively, similar results were obtained for both mildly (Fig. 7a) and extensively (Fig. 7b) modified HspB6. Thus, even mild modification by MGO significantly affects aggregation occurring on the cooling of heated samples of HspB6.
Fig. 7.
Temperature-dependent changes of light scattering at 340 nm for unmodified (1, 3) and mildly (2) or extensively modified (4) HspB6. Light scattering was measured at 340 nm upon heating from 20 up to 100 °C (indicated by filled symbols) and cooling back from 100 to 20 °C (indicated by empty symbols). a Protein samples were preincubated for 48 h at high (6.0 mg/ml) protein concentration in the absence (1) or in the presence of 0.5 mM MGO (2). b Protein samples were preincubated for 48 h at low (0.8 mg/ml) protein concentration in the absence (3) or in the presence of 3.0 mM MGO (4). Final protein concentration of all samples was 0.3 mg/ml
Effect of MGO modification on quaternary structure of HspB6 and its interaction with HspB1
Size-exclusion chromatography (SEC) was used for the investigation of MGO modification on the quaternary structure of HspB6. Unmodified HspB6 was eluted on size-exclusion chromatography as symmetrical peak with apparent molecular weight of ∼58 kDa (Fig. 8, curve 1). Two peaks with apparent molecular weights of 58 and 120 kDa were detected on SEC of mildly modified HspB6 (Fig. 8, curve 2). The first peak contained mildly modified uncrosslinked HspB6 (Fig. 8, insert track IV), whereas the second peak contained small quantities of crosslinked dimers as well as monomers of modified HspB6 (Fig. 8, insert track I). Thus, even mild modification of HspB6 was accompanied by formation of small quantities of crosslinked dimers tending to associate with modified monomers and to form oligomers with apparent molecular weight of 120 kDa on SEC.
Fig. 8.
Size-exclusion chromatography of unmodified (1), mildly (2), and extensively (3) modified HspB6 on a Superdex 200 column. The protein composition of samples collected in the first and in the second peaks is presented in the insert. Position of molecular weight of protein markers are marked by lines
Extensively modified HspB6 also migrated as two peaks with apparent molecular weights of ∼56 and ∼120 kDa (Fig. 8, curve 3). According to the data of SDS gel electrophoresis, the peak with apparent molecular weight of 56 kDa contained uncrosslinked modified monomers of HspB6 (Fig. 8, insert track V) which even after modification were able to form dimers and therefore migrated on size-exclusion chromatography with apparent molecular weight of 56 kDa. The peak eluted on SEC with apparent molecular weight of 120 kDa contained crosslinked dimers of modified HspB6 which migrated on the SDS electrophoresis as a band with apparent molecular weight of 43 kDa (Fig. 8, insert track II). We suppose that under conditions of SEC, extensively modified crosslinked dimers of HspB6 can associate forming complexes with apparent molecular weight of ∼120 kDa. Rather unexpectedly, the size of the peak with apparent molecular weight of 120 kDa was similar both in the case of extensively and mildly modified HspB6 (Fig. 8). Thus, even mild MGO modification is accompanied by the accumulation of large self-associated complexes of HspB6 which are not a characteristic for unmodified HspB6.
Unmodified HspB6 forms two types of hetero-oligomeric complexes with another human small heat shock protein, HspB1 (Bukach et al. 2009). These complexes can be detected on SEC as two peaks with apparent molecular weights of ∼100 and 300 kDa (Fig. 9a). Mildly modified HspB6 effectively interacted with HspB1, and this interaction was practically indistinguishable from that of unmodified HspB6 (Fig. 9b). Extensively modified by MGO, HspB6 was much less effective in the formation of hetero-oligomeric complexes with HspB1. In this case, only minor part of HspB1 and modified HspB6 was involved in the formation of hetero-oligomeric complex with apparent molecular weight of ∼100 kDa (Fig. 9c) and the largest part of both proteins remained in the form of homo-oligomers.
Fig. 9.
Interaction of HspB1 with unmodified (a), mildly (b), and extensively (c) modified HspB6. Size-exclusion chromatography of isolated HspB6 (1), of the equimolar mixture of HspB6 and HspB1 (2), and of isolated HspB1 (3)
MGO modification and HspB6 phosphorylation
Phosphorylation of Ser16 catalyzed by cyclic nucleotide-dependent protein kinases (Beall et al. 1999; Rembold et al. 2000) and by protein kinase D1 (Sin and Baillie 2015) affects the structure and physiologically important properties of HspB6 (Dreiza et al. 2010). Therefore, it was reasonable to analyze the effect of MGO modification on HspB6 phosphorylation by catalytic subunit of cAMP-dependent protein kinase. Under conditions used about 0.8 mol of phosphate were transferred per mole of unmodified HspB6 (Fig. 10a, curves 1 and 3). Mild modification by MGO induced 35–40 % decrease of the level of phosphorylation (Fig. 10a, curve 2), whereas extensive modification completely prevented phosphorylation of HspB6 by cyclic AMP-dependent protein kinase (Fig. 10a, curve 4).
Fig. 10.
Phosphorylation of unmodified and MGO-modified HspB6 samples by catalytic subunit of cAMP-dependent protein kinase. a Kinetics of phosphorylation of unmodified (1 and 3), mildly (2), and extensively (4) modified HspB6 by cAMP-dependent protein kinase. b Urea gel electrophoresis (panels labeled C) and their autoradiography (panels labeled R) of unmodified (panels 1 and 3), mildly modified (panel 2), or extensively modified (panel 4) HspB6. Time of incubation (in min) is indicated above each panel. Positions of unphosphorylated and phosphorylated unmodified HspB6 are marked as P 0 MGO 0 and P 1 MGO 0, respectively. Positions of unphosphorylated and phosphorylated HspB6 containing n residues of MGO are marked as P 0 MGO 0 and P 1 MGO n, respectively
The samples of mildly and extensively MGO-modified HspB6 after phosphorylation were subjected to urea gel electrophoresis followed by autoradiography. In the case of mild MGO modification, radioactive phosphate was detected only in the bands corresponding to the samples containing one or two modified residues (Fig. 10b, panels 2C and 2R). As already mentioned under these conditions, Arg13, Arg14, Arg27, and Arg102 are predominantly modified in HspB6. Singly or doubly modified HspB6 can contain different sets of modified Arg residues and not all of them will affect HspB6 phosphorylation. In the samples containing three or more residues of MGO, the probability of simultaneous modification of Arg13, Arg14, and Arg27 located in the vicinity of Ser16 is increased. Therefore, the level of phosphorylation of unmodified (or weakly modified) HspB6 is higher than in the case of HspB6 containing large number of MGO residues. As expected, we were unable to detect any phosphorylated bands in the case of extensively modified HspB6 (Fig. 10c, panels 4C and 4R).
Chaperone-like activity of unmodified and MGO-modified HspB6
Chaperone-like activity of HspB6 was analyzed by using two model protein substrates, i.e. subfragment 1 (S1) of rabbit skeletal myosin and porcine liver malate dehydrogenase (MDH). Unmodified HspB6 retarded heat-induced aggregation of S1 (Fig. 11, curves 1 and 3). With this model substrate, the chaperone-like activity of mildly modified HspB6 was slightly larger than that of unmodified protein (Fig. 11, curve 2). At the same time, the chaperone-like activity of extensively modified protein was markedly higher than that of unmodified protein (Fig. 11, curve 4), and extensively modified HspB6 effectively retarded aggregation of S1.
Fig. 11.
Chaperone-like activity of unmodified (1 and 3) and mildly (2) or extensively (4) modified HspB6 with rabbit skeletal muscle myosin subfragment (S1). Heat-induced aggregation of isolated model protein substrates is marked by dotted line. Representative results of not less than three experiments are presented
HspB6 was rather ineffective in preventing heat-induced aggregation of MDH, and unmodified protein as well as mildly or extensively modified HspB6 only moderately decreased the rate of MDH aggregation (data not presented).
Discussion
Many proteins and among them are histone H2A (Mir et al. 2014), serum albumin (Lo et al. 1994), hemoglobin (Bose et al. 2013; Gao and Wang 2006), α-crystallin (Gangadhariah et al. 2010; Nagaraj et al. 2003, 2012; Nahomi et al. 2013; Satish Kumar et al. 2004), HspB1 (Hsp27) (Nagaraj et al. 2012), and many others were subjected to MGO modification in vitro. However, to our knowledge, MGO modification of HspB6 was not analyzed up to now.
The intracellular concentration of MGO is estimated to be in the range of 2–4 μM (reviewed by (Rabbani and Thornalley 2012)). However, these concentrations could be underestimated, as MGO is a highly reactive molecule and may not remain in a steady-state concentration. In addition, this value can be cell type and tissue dependent. Efficiency of protein modification is also dependent not only on MGO and protein concentration but also on the duration of incubation (Mukhopadhyay et al. 2010). Therefore, it is very complicated to select proper concentration and conditions of incubation of MGO with analyzed protein. The data of literature indicate that the conditions of modification (i.e., protein and MGO concentration) were varied in a very wide range. For instance, concentration of MGO was varied between 2–10 μM (Nagaraj et al. 2012) and 2.5–10 mM (Mir et al. 2014), and protein concentration was varied in between 40–50 μM (Mir et al. 2014; Mukhopadhyay et al. 2010) and 250 μM (Gangadhariah et al. 2010). Although in some publications, the authors used very small MGO concentrations (2–100 μM) and rather long (3–7 days) incubation (Nagaraj et al. 2012; Nahomi et al. 2013); the majority of investigations on small heat shock proteins were performed at rather high (0.1–10 mM) MGO concentration (Bento et al. 2010; Kumar et al. 2007; Mukhopadhyay et al. 2010; Nagaraj et al. 2003; Puttaiah et al. 2007) or at the molar ratio Arg of HspB1/MGO equal to 1/10 (Oya-Ito et al. 2006). Therefore, as a first approach, we choose intermediate (500 μM) and high (3 mM) concentrations of MGO. It is worthwhile to mention that under conditions used, the molar ratio Arg of HspB6/MGO was in the range 7.7/1 and 1/5, i.e., was much smaller than the corresponding ratio used in the case of HspB1. As expected, the extent of protein modification is strongly increased with the increase of MGO concentration (Gangadhariah et al. 2010). Similar results were obtained in our investigation. At low MGO concentration, less than three residues of HspB6 underwent modification, whereas at high MGO concentration, practically, all Arg and Lys residues of HspB6 became modified leading to the formation of modified protein having higher molecular weight than the unmodified protein (Figs. 1 and 2).
Under mild conditions and at very early stages of modification, Arg residues are predominant sites of MGO modification (Gangadhariah et al. 2010; Westwood and Thornalley 1995) and hydroimidazolone MG-H1 (Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine) accounts for more than 90 % of adducts formed (Rabbani and Thornalley 2012). In order to map modified sites of HspB6, we subjected unmodified and modified protein to limited trypsinolysis (Fig. 3). Earlier, Satish Kumar et al. (Satish Kumar et al. 2004) analyzed the effect of MGO modification on the proteolysis of α-crystallin by trypsin and chymotrypsin and found that this type of modification enhances the rate of degradation of this small heat shock protein. In our case, MGO modification induced opposite effect and decreased the rate of HspB6 proteolysis (Fig. 3). In agreement with the earlier published data (Heirbaut et al. 2014), we supposed that the most susceptible sites of trypsinolysis are located in the N-terminal domain of HspB6 and that this part of the molecule also contains the sites predominantly modified by MGO. This suggestion was confirmed by means of mass spectroscopy. The data of tandem mass spectrometry (MS-MS) indicate that Arg13, Arg14, Arg27, and Arg102 are the predominant sites modified by MGO in mildly modified HspB6 (Fig. 4). It is worthwhile to mention that Arg14 of HspB6 is homologous to Arg12 of αB-crystallin (HspB5) and Arg12 of HspB1, i.e., the sites predominantly modified by MGO in these two proteins (Nagaraj et al. 2012). This can be due to the fact that the N-terminal end of all small heat shock proteins is very flexible and poorly ordered and therefore easily accessible to chemical modifications and proteolysis.
Modification by MGO affects the structure and some properties of HspB6. Extensive MGO modification was accompanied by dramatic decrease of Trp fluorescence and shifting of the maximum of fluorescence towards higher wavelength (Fig. 5). In addition, modification by MGO was accompanied by the appearance of new peak of fluorescence at about 400 nm. In the case of mildly modified HspB6, this peak of fluorescence can correspond to 5-methylimidazol-4-one which can be formed by the oxidation of 5-hydro-5-methylimidazol-4-one (Westwood and Thornalley 1995). In the case of extensively modified HspB6, this peak can correspond to the abovementioned adduct as well as to the formation of argpyrimidines or pentosidines which can be accumulated at high concentration of MGO (Miyata et al. 1996).
Slow heating followed by cooling was accompanied by aggregation of unmodified HspB6 which was registered by significant increase of light scattering (Fig. 7). Both mild and extensive MGO modification prevented aggregation of HspB6 indicating that modification even of restricted number of Arg residues significantly affects self-association of this protein (Fig. 7).
MGO can be considered as a crosslinking reagent. However, even at high MGO concentration, we observed only small quantities of crosslinked HspB6 dimer (Figs. 1a and 8). This indicates that under conditions used, HspB6 predominantly forms dimers and does not tend to form large oligomers, a characteristic for many other small heat shock proteins like HspB1 or HspB5. This conclusion agrees with our earlier published results (Sluchanko et al. 2015; Weeks et al. 2014). It is worthwhile to mention that MGO modification does not lead to dissociation of HspB6 dimers, and modified monomers were still able to form dimers. Crosslinked dimers tend to self-association, and this leads to the formation of complexes with apparent molecular weight of 120 kDa on size exclusion chromatography. Although the quaternary structure of even extensively modified HspB6 was not dramatically changed, extensively modified HspB6 was unable to form hetero-oligomeric complexes with HspB1 (Fig. 9). At the same time, mild MGO modification of HspB6 had no effect on its interaction with HspB1 (Fig. 9).
HspB6 is phosphorylated at Ser16 by a number of different protein kinases (Beall et al. 1999; Rembold et al. 2000; Sin and Baillie 2015), and phosphorylation of this site affects cardioprotective activity of HspB6 (Fan and Kranias 2011) and its ability to regulate smooth muscle contraction (Dreiza et al. 2010). Certain sites accessible to MGO modification (Arg13, Arg14, Arg27) are located in the vicinity of Ser16 undergoing phosphorylation. The primary structure at Ser16 (13RRA16SA) ideally corresponds to the consensus sequences (RXS, RRXS, RXXS) recognized by cAMP-dependent protein kinase (Pearson and Kemp 1991). Therefore, we supposed that MGO modification of Arg13 and/or Arg14 will affect phosphorylation of HspB6. Indeed, the data presented on Fig. 10 indicate that even mild MGO modification significantly inhibited phosphorylation of HspB6 by cyclic AMP-dependent protein kinase.
The chaperone-like activity is considered as one of the most important properties of small heat shock proteins. We compared chaperone-like activity of unmodified and MGO-modified HspB6 using subfragment 1 of rabbit skeletal muscle myosin and porcine liver malate dehydrogenase. Mildly and especially extensively modified HspB6 possessed higher chaperone-like activity than the unmodified protein when S1 fragment of myosin was used as a model protein substrate (Fig. 11a). Earlier, Gangadhariah et al. (Gangadhariah et al. 2010) reported that MGO modification increases chaperone-like activity of α-crystallin measured with different protein substrates (βL-crystallin, γ-crystallin, lactate dehydrogenase), but does not change α-crystallin ability to assist in refolding of denatured proteins. Chaperone-like activity of HspB6 with MDH was rather low and was not changed after mild MGO modification (data not presented). Thus, modification by MGO differently affects chaperone-like activity of HspB6 with different substrates.
We suppose that the data presented indicate that HspB6 can be a potential substrate for MGO modification and that this modification extensively affects many of its properties. Further investigations are required for understanding whether HspB6 is modified in vivo and if so what effect of this modification on the cell is.
Acknowledgments
Investigation of physico-chemical properties was supported by the Russian Science Foundation (RSF) (grant no. 14-35-00026). Investigation of protein-protein interaction was supported by the Russian Foundation for Basic Research (grant no. 16-04-00016). MALDI MS facility became available in the framework of the Moscow State University Development Program PNG 5.13. The authors are grateful to Dr. O.P. Nikolaeva and Prof. D.I. Levitsky (A.N. Bach Institute of Biochemistry, Russian Academy of Sciences) for providing subfragment-1 of rabbit skeletal myosin.
Abbreviations
- DTT
Dithiotreitol
- MDH
Malate dehydrogenase
- ME
β-Mercaptoethanol
- MGO
Methylglyoxal
- PMSF
Phenylmethanesulfonyl fluoride
- S1
Subfragment 1 of rabbit skeletal muscle myosin
- SEC
Size-exclusion chromatography
- TPCK
Nα-tosyl-l-phenylalanine chloromethyl ketone
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Beall A, et al. The small heat shock-related protein, HSP20, is phosphorylated on serine 16 during cyclic nucleotide-dependent relaxation. J Biol Chem. 1999;274:11344–11351. doi: 10.1074/jbc.274.16.11344. [DOI] [PubMed] [Google Scholar]
- Bento CF, Marques F, Fernandes R, Pereira P. Methylglyoxal alters the function and stability of critical components of the protein quality control. PLoS One. 2010;5:e13007. doi: 10.1371/journal.pone.0013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose T, Bhattacherjee A, Banerjee S, Chakraborti AS. Methylglyoxal-induced modifications of hemoglobin: structural and functional characteristics. Arch Biochem Biophys. 2013;529:99–104. doi: 10.1016/j.abb.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Bukach OV, Seit-Nebi AS, Marston SB, Gusev NB. Some properties of human small heat shock protein Hsp20 (HspB6) Eur J Biochem. 2004;271:291–302. doi: 10.1046/j.1432-1033.2003.03928.x. [DOI] [PubMed] [Google Scholar]
- Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB. Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20) Biochim Biophys Acta. 2009;1794:486–495. doi: 10.1016/j.bbapap.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Dreiza CM, et al. The small heat shock protein, HSPB6, in muscle function and disease. Cell Stress Chaperones. 2010;15:1–11. doi: 10.1007/s12192-009-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GC, Kranias EG. Small heat shock protein 20 (HspB6) in cardiac hypertrophy and failure. J Mol Cell Cardiol. 2011;51:574–577. doi: 10.1016/j.yjmcc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadhariah MH, Wang B, Linetsky M, Henning C, Spanneberg R, Glomb MA, Nagaraj RH. Hydroimidazolone modification of human alphaA-crystallin: effect on the chaperone function and protein refolding ability. Biochim Biophys Acta. 2010;1802:432–441. doi: 10.1016/j.bbadis.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Wang Y. Site-selective modifications of arginine residues in human hemoglobin induced by methylglyoxal. Biochemistry. 2006;45:15654–15660. doi: 10.1021/bi061410o. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D, Napoli V, Mazurkie A, Stafford WF, Graceffa P. Phosphorylation dependence of hsp27 multimeric size and molecular chaperone function. J Biol Chem. 2009;284:18801–18807. doi: 10.1074/jbc.M109.011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirbaut M, Beelen S, Strelkov SV, Weeks SD. Dissecting the functional role of the N-terminal domain of the human small heat shock protein HSPB6. PLoS ONE. 2014;9:e105892. doi: 10.1371/journal.pone.0105892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y, Matsuno H. The possibility of novel antiplatelet peptides: the physiological effects of low molecular weight HSPs on platelets. Curr Pharm Des. 2006;12:887–892. doi: 10.2174/138161206776056047. [DOI] [PubMed] [Google Scholar]
- Korngut L, et al. Overexpression of human HSP27 protects sensory neurons from diabetes. Neurobiol Dis. 2012;47:436–443. doi: 10.1016/j.nbd.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PA, Kumar MS, Reddy GB. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem J. 2007;408:251–258. doi: 10.1042/BJ20070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem. 1994;269:32299–32305. [PubMed] [Google Scholar]
- Matsuno H, Ishisaki A, Nakajima K, Kato K, Kozawa O. A peptide isolated from alpha B-crystallin is a novel and potent inhibitor of platelet aggregation via dual prevention of PAR-1 and GPIb/V/IX. J Thromb Haemost. 2003;1:2636–2642. doi: 10.1111/j.1538-7836.2003.00502.x. [DOI] [PubMed] [Google Scholar]
- Mir AR, uddin M, Alam K, Ali A (2014) Methylglyoxal mediated conformational changes in histone H2A-generation of carboxyethylated advanced glycation end products Int J Biol Macromol 69:260–266 doi:10.1016/j.ijbiomac.2014.05.057 [DOI] [PubMed]
- Miyata T, et al. Identification of pentosidine as a native structure for advanced glycation end products in beta-2-microglobulin-containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc Natl Acad Sci U S A. 1996;93:2353–2358. doi: 10.1073/pnas.93.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Kar M, Das KP. Effect of methylglyoxal modification of human alpha-crystallin on the structure, stability and chaperone function. Protein J. 2010;29:551–556. doi: 10.1007/s10930-010-9289-6. [DOI] [PubMed] [Google Scholar]
- Mymrikov EV, Haslbeck M. Medical implications of understanding the functions of human small heat shock proteins. Expert Rev Proteomics. 2015;12:295–308. doi: 10.1586/14789450.2015.1039993. [DOI] [PubMed] [Google Scholar]
- Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, et al. Enhancement of chaperone function of alpha-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Panda AK, Shanthakumar S, Santhoshkumar P, Pasupuleti N, Wang B, Biswas A. Hydroimidazolone modification of the conserved Arg12 in small heat shock proteins: studies on the structure and chaperone function using mutant mimics. PLoS ONE. 2012;7:e30257. doi: 10.1371/journal.pone.0030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Nahomi RB, Mueller NH, Raghavan CT, Ammar DA, Petrash JM. Therapeutic potential of alpha-crystallin. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbagen.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahomi RB, Oya-Ito T, Nagaraj RH. The combined effect of acetylation and glycation on the chaperone and anti-apoptotic functions of human alpha-crystallin. Biochim Biophys Acta. 2013;1832:195–203. doi: 10.1016/j.bbadis.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya-Ito T, Liu BF, Nagaraj RH. Effect of methylglyoxal modification and phosphorylation on the chaperone and anti-apoptotic properties of heat shock protein 27. J Cell Biochem. 2006;99:279–291. doi: 10.1002/jcb.20781. [DOI] [PubMed] [Google Scholar]
- Oya-Ito T, et al. Heat-shock protein 27 (Hsp27) as a target of methylglyoxal in gastrointestinal cancer. Biochim Biophys Acta. 2011;1812:769–781. doi: 10.1016/j.bbadis.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-I. [DOI] [PubMed] [Google Scholar]
- Perrie WT, Perry SV. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970;119:31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SA, Mirrlees D, Thornalley PJ. Modification of the glyoxalase system in streptozotocin-induced diabetic rats. Effect of the aldose reductase inhibitor. Statil Biochem Pharmacol. 1993;46:805–811. doi: 10.1016/0006-2952(93)90488-I. [DOI] [PubMed] [Google Scholar]
- Puttaiah S, Biswas A, Staniszewska M, Nagaraj RH. Methylglyoxal inhibits glycation-mediated loss in chaperone function and synthesis of pentosidine in alpha-crystallin. Exp Eye Res. 2007;84:914–921. doi: 10.1016/j.exer.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Rabbani N, Thornalley PJ. Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids. 2012;42:1133–1142. doi: 10.1007/s00726-010-0783-0. [DOI] [PubMed] [Google Scholar]
- Reddy VS, Reddy GB. Role of crystallins in diabetic complications. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbagen.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Rembold CM, Foster DB, Strauss JD, Wingard CJ, Eyk JE. cGMP-mediated phosphorylation of heat shock protein 20 may cause smooth muscle relaxation without myosin light chain dephosphorylation in swine carotid artery. J Physiol. 2000;524(Pt 3):865–878. doi: 10.1111/j.1469-7793.2000.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme G, Mihoub M, Dairou J, Bui LC, Leger T, Lamouri A. Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J Biol Chem. 2015;290:1885–1897. doi: 10.1074/jbc.M114.597815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Mashima T, Yamamoto K, Tsuruo T. Modulation of heat-shock protein 27 (Hsp27) anti-apoptotic activity by methylglyoxal modification. J Biol Chem. 2002;277:45770–45775. doi: 10.1074/jbc.M207485200. [DOI] [PubMed] [Google Scholar]
- Satish Kumar M, Mrudula T, Mitra N, Bhanuprakash Reddy G. Enhanced degradation and decreased stability of eye lens alpha-crystallin upon methylglyoxal modification. Exp Eye Res. 2004;79:577–583. doi: 10.1016/j.exer.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, van Bezu J, van der Schors RC, Uchida K, Stehouwer CD, van Hinsbergh VW. Heat-shock protein 27 is a major methylglyoxal-modified protein in endothelial cells. FEBS Lett. 2006;580:1565–1570. doi: 10.1016/j.febslet.2006.01.086. [DOI] [PubMed] [Google Scholar]
- Seit-Nebi AS, Gusev NB. Versatility of the small heat shock protein HSPB6 (Hsp20) Cell Stress Chaperones. 2010;15:233–236. doi: 10.1007/s12192-009-0141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KK, Santhoshkumar P. Lens aging: effects of crystallins. Biochim Biophys Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin YY, Baillie GS. Heat shock protein 20 (HSP20) is a novel substrate for protein kinase D1 (PKD1) Cell Biochem Funct. 2015 doi: 10.1002/cbf.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluchanko NN, Chebotareva NA, Gusev NB. Quaternary structure of human small heat shock protein HSPB6 (Hsp20) in crowded media modeled by trimethylamine N-oxide (TMAO): effect of protein phosphorylation. Biochimie. 2015;108:68–75. doi: 10.1016/j.biochi.2014.11.001. [DOI] [PubMed] [Google Scholar]
- van Heijst JW, Niessen HW, Musters RJ, van Hinsbergh VW, Hoekman K, Schalkwijk CG. Argpyrimidine-modified Heat shock protein 27 in human non-small cell lung cancer: a possible mechanism for evasion of apoptosis. Cancer Lett. 2006;241:309–319. doi: 10.1016/j.canlet.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Vander Jagt DL, Hunsaker LA. Methylglyoxal metabolism and diabetic complications: roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem Biol Interact. 2003;143–144:341–351. doi: 10.1016/S0009-2797(02)00212-0. [DOI] [PubMed] [Google Scholar]
- Vorotnikov AV, Shirinsky VP, Gusev NB. Phosphorylation of smooth muscle caldesmon by three protein kinases: implication for domain mapping. FEBS Lett. 1988;236:321–324. doi: 10.1016/0014-5793(88)80047-4. [DOI] [PubMed] [Google Scholar]
- Weeds AG, Taylor RS. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975;257:54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Weeks SD, Baranova EV, Heirbaut M, Beelen S, Shkumatov AV, Gusev NB, Strelkov SV. Molecular structure and dynamics of the dimeric human small heat shock protein HSPB6. J Struct Biol. 2014;185:342–354. doi: 10.1016/j.jsb.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Westwood ME, Thornalley PJ. Molecular characteristics of methylglyoxal-modified bovine and human serum albumins. Comparison with glucose-derived advanced glycation endproduct-modified serum albumins. J Protein Chem. 1995;14:359–372. doi: 10.1007/BF01886793. [DOI] [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]