Abstract
Tumor necrosis factor receptor-associated protein 1 (TRAP1), a member of the HSP90 family, controls a variety of physiological functions, including cell proliferation, differentiation, and survival. Most studies have been devoted to understanding the anti-apoptotic roles of TRAP1 in cancer and targeting it for tumor control in clinical settings. Additionally, we have identified a new role for TRAP1 in regulation of liver regeneration after partial hepatectomy in TRAP1 transgenic mice and cellular proliferation in TRAP1-overexpressing cells, via mitochondrial alterations. Moreover, recent works have indicated a role for TRAP1 in the regulation of cancer stem cells (CSCs) as well as a metabolic switch between mitochondrial respiration and aerobic glycolysis called as “Warburg effect.” This review discusses the implications of TRAP1 action for both metabolism and the regulation of CSCs.
Keywords: TRAP1, Apoptosis, Fatty liver, Cancer stem cells, Warburg effect
Introduction
Cells use a variety of mechanisms to defend themselves against extracellular and intracellular stresses and to maintain homeostasis. When proteins are newly synthesized or misfolded, molecular chaperones act to prevent their aggregation and maintain protein homeostasis. The most well-studied molecular chaperones are the heat shock protein, 70 kDa (HSP70s) (Li et al. 2000; Shim et al. 2002) and their co-chaperones, such as DnaJ/HSP40 (Laufen et al. 1999) and BCL-2-associated athanogene 3 (BAG3)/BCL2-interacting cell death suppressor (BIS), which regulate stress-induced cell death (Lee et al. 1999; Yoo et al. 2014). Another molecular chaperone, HSP90, is a global cellular regulator critical for the folding and regulation of a variety of proteins within cells. There are different homologs of Hsp90 in mammalian cells: cytosolic HSP90, tumor necrosis factor receptor-associated protein 1 (TRAP1, also known as mitochondrial chaperone HSP75 (Felts et al. 2000)), and endoplasmic glucose-regulated protein, 94 kDa (GRP94) (Johnson 2012). Like HSP70, HSP90 also functions with several co-chaperones, such as FK506 binding protein (FKBP) and Hsp70-Hsp90 organizing protein (HOP). In addition, cooperation has been well documented between HSP70 and HSP90 and their co-chaperones in the regulation of client proteins (Riggs et al. 2004).
In 1995, TRAP1 was first identified by Song et al. as an Hsp90 family member, in a yeast two-hybrid screen for proteins associated with the type 1 tumor necrosis factor receptor-1 (TNFR1) (Song et al. 1995). That study revealed that TRAP1 mRNA was expressed variably in skeletal muscle, liver, heart, brain, kidney, pancreas, lung, and placenta, as well as in a transformed cell line, suggesting that TRAP1 is involved in a variety of cellular functions. Since then, TRAP1 has been investigated extensively, in roles associated with apoptosis, cancer, and neuronal diseases such as Parkinson’s disease (PD). In addition, we recently suggested a new role for TRAP1 in regulating both liver regeneration after partial hepatectomy (Im et al. 2013) and cellular proliferation via mitochondrial alteration (Im et al. 2007; Im and Seo 2014). TRAP1 interacts with several proteins, including retinoblastoma protein (Chen et al. 1996), cyclophilin D (CypD) (Kang et al. 2009), the calcium-binding protein Sorcin (Landriscina et al. 2010b), and the proteasome regulatory particle TBP7 (Amoroso et al. 2012). In this review, we summarize the roles of TRAP1 identified to date and provide insight into emerging roles for TRAP1 in metabolism and in the regulation of cancer stem cells.
Roles of TRAP1
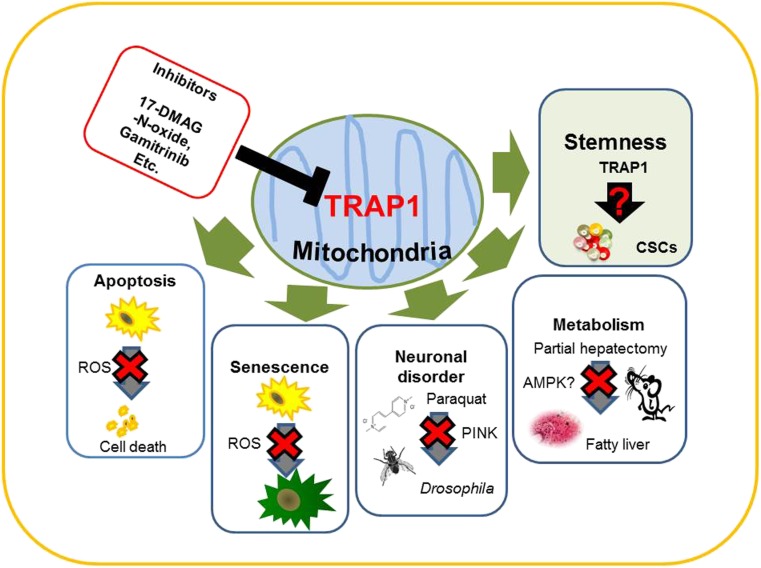
Previously, TRAP1 was found to be involved in diverse cellular processes, particularly those associated with apoptosis. Several functions of TRAP1 are summarized in Fig. 1. As for other HSPs, the involvement of TRAP1 in the proliferation and apoptosis of cancer cells has also been widely investigated. However, recently presented evidence has illustrated other aspects of TRAP1 function involved in regulation of neuronal disease, metabolism, and stemness.
Fig. 1.
Emerging roles of TRAP1 in metabolism and stemness. TRAP1 prevents apoptosis induced by various stressors. TRAP1 downregulation by iron chelation leads to a cellular senescence-like phenotype. In Drosophila, TRAP1 increases survival of paraquat-induced toxicity. In TRAP1-transgenic mice, partial hepatectomy leads to fatty liver. The role of TRAP1 in stemness is not yet clear. Current mitochondria-targeting HSP90 inhibitors suppress both HSP90 and TRAP1, requiring the development of specific TRAP1 inhibitors
Proliferation and senescence
After careful investigation, HSP75 (an alternate name for TRAP1) was shown to interact with retinoblastoma protein (RB) during M phase and after heat shock, suggesting that it chaperones RB and regulates the cell cycle (Chen et al. 1996). Moreover, expression analysis with microarrays showed that MYC regulates genes involved in growth and cell cycle including TRAP1 during differentiation (Coller et al. 2000). Liu et al. analyzed differentially expressed genes and found high levels of cell proliferation-promoting genes coding for G protein-coupled receptors, cell adhesion genes, and genes associated with Rho-kinase pathways (Liu et al. 2010). TRAP1 is overexpressed in specimens of esophageal squamous cell carcinoma (ESCC), particularly in poorly differentiated tumors (Tian et al. 2014), as well as in human colorectal carcinomas (CRC) (Condelli et al. 2015). Moreover, TRAP1 knockdown induced increased reactive oxygen species (ROS) and mitochondrial depolarization, together with arrest in G2/M phase (Tian et al. 2014). The above findings indicate that targeting TRAP1 has the potential to regulate proliferation of cancer cells via regulating ROS generation as well as cell cycle-related proteins such as RB and MYC.
In contrast to cellular proliferation, senescence is a state of irreversible cell cycle arrest in which cells display characteristic and drastic morphological and metabolic changes. It is reasonable to assume that when chaperones or co-chaperones no longer function, many proteins remain misfolded, leading to a variety of dysfunctional processes within cells. For example, silencing BIS, a known co-chaperone of HSP70, induced a senescence-like phenotype in glioblastoma cells (Lee et al. 2014). We previously reported that iron chelation using deferoxamine led to decreased TRAP1 in human Chang liver cells, together with increased ROS generation, caveolin-1 (CAV1) expression, and senescence-associated beta-galactosidase (SA beta-gal) activity, the representative phenotypes of cellular senescence (Fig. 1). Overexpression of TRAP1 ameliorated the SA beta-gal activity, CAV1 expression, and ROS-related proteins such as manganese superoxide dismutase induced by deferoxamine as well as ROS generation (Im et al. 2007), indicating that TRAP1 might be associated with the upstream pathway of ROS regulation. In addition, we found that ROS levels were increased in TRAP1-overexpressing mouse NIH3T3 fibroblasts, together with activation of extracellular signal-regulated kinase (ERK) and proliferation as shown in Fig. 2 (Im and Seo 2014). These cells also display distinct morphology as well as the dramatic decrease of PGC1-α levels, indicating that TRAP1 is linked to mitochondrial biogenesis. TRAP1 plays a key role in protecting mitochondria against damaging stimuli by reducing ROS generation. For instance, granzyme M, an orphan granzyme, can cause mitochondrial swelling and mitochondrial depolarization and directly cleaves TRAP1, abolishing its antagonistic function against ROS and resulting in intracellular ROS accumulation (Hua et al. 2007). ROS seem to be a double-edged sword; they can accelerate cellular proliferation, but higher levels of ROS lead to apoptosis. This may give rise to this discrepancy between our finding and observations from Tian group, although there are also differences in cellular context and methodology. A recent review also highlighted the role of mitochondrial homeostatic mechanisms, mitochondrial metabolites, and ROS generation in signaling pathways leading to the induction and maintenance of cellular senescence and contributing to the aging process (Correia-Melo and Passos 2015). Therefore, TRAP1 may influence these intracellular processes via regulation of ROS generation.
Fig. 2.
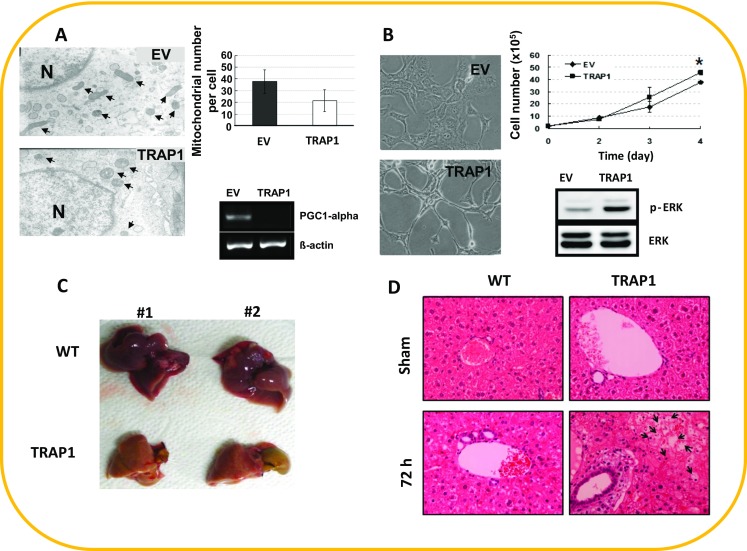
Effects of TRAP1 overexpression on in vitro and in vivo models. a Mitochondrial morphology by transmission electron microscopy illustrating decreased mitochondrial numbers in stable TRAP1-expressing cell lines (upper panel). Decreased PGC-1α mRNA levels in TRAP1 cells (lower panel). b Altered morphology, enhanced proliferation, and ERK phosphorylation in TRAP1 cells. c Fat accumulation in the liver at 72 h after partial hepatectomy (PH). d Pathological analysis of liver tissues at 72 h after PH in wild-type (WT) and TRAP1 transgenic mice; hematoxylin and eosin (H&E) staining. (a, b reprinted by permission from Im and Seo ( 2014 ); c, d from Im et al. ( 2013 )
Apoptosis
Apoptosis is the process of programmed cell death that is important for homeostasis of cells and tissues in multicellular organisms. During apoptosis, biochemical events lead to morphological and molecular alterations and finally to cell death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation. Mitochondria play a critical role in this process, in which Bcl-2 family members such as Bcl-2 and BAX interact reciprocally (Li and Dewson 2015). As a chaperone in mitochondria, it is not surprising that TRAP1 might play a role in apoptosis. Moreover, TRAP1 is overexpressed in cancers and implicated in drug resistance (Costantino et al. 2009; Maddalena et al. 2013). In 2007, Hua et al. first showed a link between TRAP1 and apoptosis (Hua et al. 2007). They demonstrated that it antagonizes ROS generation and protects cells from granzyme M-mediated apoptosis (Fig. 1). This is consistent with our own work in which we found that overexpression of TRAP1 ameliorated iron chelation-induced senescence via ROS in Chang cells (Im et al. 2007) or cardiomyocytes (Zhang et al. 2015b). Interestingly, Takemoto et al. suggested a relationship between TRAP1 in mitochondria and the unfolded protein response (UPR) in the endoplasmic reticulum (ER) (Takemoto et al. 2011). They found that TRAP1 knockdown activated the ER-resident caspase-4, which is activated by ER stress; it also increased the basal level of GRP78 expression, which protects cells, and decreased the basal level of C/EBP homologous protein (CHOP), which induces cell death, even under ER stress. This study indicated that the mitochondria could be a potential regulator of the UPR in the ER via mitochondrial TRAP1. Subsequently, increasing evidence indicates that TRAP1 is a critical regulator of apoptosis (Masuda et al. 2004; Tian et al. 2014). Amoroso et al. demonstrated that TRAP1 and the proteasome regulatory particle TBP7 interact in the ER and control cellular ubiquitination of specific mitochondrial proteins. TRAP1 and/or TBP7 interference enhanced stress-induced cell death and increased intracellular protein ubiquitination. These data confirmed that TRAP1 functions not only in the mitochondria but also in the ER to help refold damaged proteins and prevent apoptosis (Amoroso et al. 2012). Another study in human breast cancer cells showed that TRAP1 is found in the ER, where it plays a role in protecting tumor cells against DNA-damaging agents such as anthracyclin by modulating the protein kinase RNA-like ER kinase (PERK) pathway (Sisinni et al. 2014).
Moreover, inhibiting both TRAP1 and related HSPs may be an effective strategy to manipulate cancers, although this has only been demonstrated at the cellular level (Zhang et al. 2011). Zhang’s group administered green tea extract (GTE) to human pancreatic ductal adenocarcinoma HPAF-II cells and performed two-dimensional gel electrophoresis of the cell lysates. HSP90 and TRAP1, as well as HSP27, were identified among 32 proteins with significantly altered expression. These findings suggested that GTE inhibited multiple molecular targets and provided further evidence of green tea activity against pancreatic cancer. Another recent report demonstrated that TRAP1 targeted by the mitochondria-directed HSP90 chaperone inhibitor gamitrinib induced apoptosis and inhibited colony formation in BRAF-driven CRC cells (Condelli et al. 2015). Similarly, TRAP1 has recently been shown to be aberrantly upregulated in breast tumors relative to control tissues. TRAP1 knockdown downregulates mitochondrial aerobic respiration, sensitizes cells to lethal stimuli, and inhibits tumor growth in representative breast cancer cells in vivo, suggesting that TRAP1 is required for tumorigenesis. Moreover, this study showed that TRAP1 regulates mitochondrial morphology, demonstrating that lower TRAP1 levels were associated with rod-shaped mitochondrial phenotypes in invasive and metastatic MDA-MB-231 breast cancer cells. Conversely, higher TRAP1 levels were associated with a tubular network mitochondrial phenotype in non-invasive MCF-7 cells, linking TRAP1-regulated mitochondrial dynamics and function with tumorigenesis in breast cancer (Zhang et al. 2015a).
Neurodegenerative disorders: PD
Parkinson’s disease (PD) is a degenerative disorder of the central nervous system mainly affecting the motor system. The motor symptoms of PD result from the death of dopamine-generating cells in the substantia nigra, but the causes of this cell death are poorly understood. The most obvious symptoms are movement-related, including shaking, rigidity, slowness of movement, and difficulty with walking and gait. Later, cognitive and behavioral problems may arise, with dementia commonly occurring in the advanced stages of the disease; depression is the most common psychiatric symptom. Other symptoms include sensory, sleep, and emotional problems. PD is more common in older people, with most cases occurring after the age of 50 years. The genetic etiology of PD is associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes (Nuytemans et al. 2010). Among them, pivotal roles for PINK1 and autophagy in mitochondrial quality control have been implicated in PD (Chu 2010). PINK1 is a mitochondrial serine/threonine kinase. TRAP1 has been shown to interact with PINK1 affecting PD. Pridgeon et al. identified TRAP1 as a cellular substrate for PINK1, which interacts and colocalizes with TRAP1 in the mitochondria, to phosphorylate it. The ability of PINK1 to promote both TRAP1 phosphorylation and cell survival is impaired by the PD-associated PINK1 mutations G309D, L347P, and W437X, indicating that PINK1 is upstream of TRAP1 and mutation of PINK1 might affect TRAP1 function. The ability of PINK1 to protect cells against oxidative stress depends on its kinase activity (Pridgeon et al. 2007). Drosophila TRAP1 also protects against mitochondrial dysfunction in a PINK1 model of PD (Costa et al. 2013). As shown in Fig. 1, loss of TRAP1 results in decreased mitochondrial function and increased sensitivity to stress (paraquat, rotenone, and antimycin) and its upregulation in neurons of PINK1 mutants rescues mitochondrial impairment. Moreover, the expression of TRAP1 partially rescued mitochondrial impairment in PARKIN mutant flies, and conversely, expression of PARKIN rescued mitochondrial impairment in TRAP1 mutants, indicating that TRAP1 works downstream of PINK1 and in parallel with PARKIN in Drosophila. In another study, however, Zhang et al. reported that overexpression of human TRAP1 was able to mitigate PINK1 but not PARKIN loss-of-function phenotypes in Drosophila. In human neuronal SH-SY5Y cells, TRAP1 also rescued mitochondrial fragmentation and dysfunction after siRNA-induced silencing of PINK1 but not PARKIN (Zhang et al. 2013). This discrepancy in the relationships between PARKIN and TRAP1 remains poorly understood. Recently, Fallaize et al. found that PINK1 resides in the cristae membrane and intracristae space but not on the outer mitochondrial membrane (OMM) of healthy mitochondria. This localization seems to be altered by stimuli. Under normal physiological conditions, PINK1 is colocalized with its substrate TRAP1 in the cristae membrane and intracristae space. In response to mitochondrial depolarization, PINK1, but not TRAP1, translocates to the OMM, suggesting that differential submitochondrial localization of PINK1 serves as a molecular switch mediating two distinct mitochondrial signaling pathways to maintain mitochondrial homeostasis (Fallaize et al. 2015). Following this observation, it may be informative to define whether mutations in PINK1 affect the function of TRAP1 in stress-induced neuronal death.
Metabolism
Cells acquire energy in the form of ATP via two main pathways: glycolytic conversion of glucose into pyruvate in the cytosol and the tricarboxylic acid (TCA) cycle in mitochondria. Yoshida et al. first explored the metabolic and phenotypic consequences of TRAP1 gene disruption/knockdown or overexpression in fibroblast cell lines established from adult WT and TRAP1-null mice and in human tumor cells transiently transfected with either TRAP1-specific siRNA or TRAP1 expression plasmids. They demonstrated that loss of TRAP1 increased mitochondrial oxygen consumption, elevated levels of TCA cycle intermediates, and increased ATP and ROS levels, with concomitant suppression of aerobic glycolysis, together with strikingly enhanced invasiveness. Overexpression of TRAP1 has the opposite effect. Loss of c-Src expression abrogates the ability of TRAP1 to modulate mitochondrial respiration and ATP levels, and TRAP1 and c-Src colocalize and interact within mitochondria. Interestingly, TRAP1 expression is also inversely correlated with tumor grade in several cancers. The findings of the Yoshida group suggest that TRAP1 acts as a tumor suppressor (Yoshida et al. 2013). This is in contrast to current opinion that TRAP1 functions as an oncoprotein, based on its elevated expression in various cancers and on our previous finding that overexpression of TRAP1 leads to increased basal ROS levels and enhanced proliferation (Im and Seo 2014). This discrepancy may arise from cell-type-specific and/or gene expression methodology differences among studies and requires further clarification.
Meanwhile, in another study, we established TRAP1 transgenic mice and found that TRAP1 overexpression led to fatty liver and increased inflammation after partial hepatectomy (PH) (Im et al. 2013). The incidence of fatty liver in TRAP1 transgenic mice was significantly higher (∼50 %) than that in WT control mice (0 %) at 48 and 72 h after PH (Figs. 1 and 2). Although the mechanism remains unknown, these findings suggest that TRAP1 plays a critical role in balancing energy substrates during liver regeneration by regulating lipid accumulation. The most common liver pathology is nonalcoholic fatty liver disease (NAFLD), which is characterized by intrahepatic accumulation of lipids. NAFLD can evolve into nonalcoholic steatohepatitis (NASH) in the presence of oxidative stress and inflammation, and NASH is a serious risk factor for liver diseases such as cirrhosis and hepatocellular carcinoma. Among the patients who develop NASH, up to 20 % may advance to cirrhosis and are at risk for complications of end-stage liver disease. One of the major complications observed in patients with NASH-related cirrhosis is hepatocellular carcinoma (HCC), which has emerged as the sixth most common cancer and second leading cause of cancer-related deaths worldwide (Khan et al. 2015). Little is known about the role of TRAP1 in the liver, but it is highly expressed in normal liver and has been suggested as a novel biomarker for HCC (Megger et al. 2013). TRAP1 has been found to dampen activation of the nutrient-sensing AMP-activated protein kinase (AMPK) and to overcome metabolic stress and promote tumor cell metastasis by limiting the ability of AMPK to trigger autophagy (Caino et al. 2013), indicating that TRAP1 is involved in AMPK-related energy sensing. Therefore, elucidation of the role of TRAP1 in the liver may contribute significantly to the prevention of metabolic diseases, including HCC, and to providing therapeutic strategies. In addition, TRAP1 transgenic mice may be a good in vivo model to study its role in metabolic regulation such as AMPK activation and how its malfunction may affect carcinogenesis (Fig. 1).
Emerging role: stemness in CSCs
Stem cells have self-renewal activity and the ability to differentiate into multiple cell types during development and tissue repair after damage, maintaining cellular and tissue homeostasis. Unfortunately, the roles for TRAP1 in stem cells during development remain undefined. Involvement of the TRAP-1 homologue Dd-TRAP1 in spore differentiation during Dictyostelium development has been reported (Morita et al. 2005). Dd-TRAP1 synthesized at the vegetative growth phase is retained during the entire course of Dictyostelium discoideum development; at the multicellular slug stage, it is located in prespore-specific vacuoles (PSVs) of prespore cells, as well as in the cell membrane and mitochondria. Dd-TRAP1 in PSVs was exocytosed during sporulation to constitute the outermost layer of the spore cell wall. In RNAi-mediated TRAP1-silenced cells, PSV formation and, therefore, prespore differentiation were impaired significantly, particularly under heat-stress conditions. This suggested that Dd-TRAP1 might be involved in late development, including spore differentiation, as well as in early development.
Tumors comprise heterogeneous groups of cells, in which a specific subpopulation of cancer stem cells (CSCs) is proposed to be responsible for tumorigenesis, resistance to therapy, and recurrence, leading to metastasis from primary sites to distant organs via epithelial-to-mesenchymal transition (EMT) (Kapoor and Kumar 2014). This behavior has been attributed to the extensive self-renewal and multipotent differentiation abilities of CSCs, characteristics similar to those of other stem cells (Jordan et al. 2006). Specifically, CSCs express a signature series of proteins, including SOX-2, OCT-4, and CD133 (Chen et al. 2007; Sahlberg et al. 2014; Tam and Ng 2014); in vitro, they display enhanced levels of several stemness-related transcription factors, such as SOX-2, under specific culture conditions (Im et al. 2015).
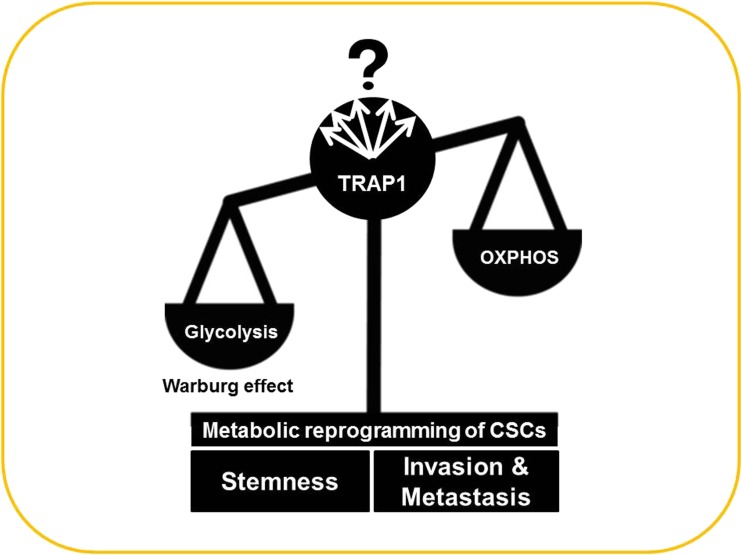
Several lines of evidence suggest that TRAP1 may be involved in the maintenance or regulation of stemness in CSCs; while CSCs are a small subpopulation within tumors, HSP90 family members, including TRAP1, are associated with tumorigenesis. First, several reports have demonstrated that TRAP1 is associated with drug resistance, migration, and/or invasion (Matassa et al. 2014; Agliarulo et al. 2015; Wu et al. 2015; Zhang et al. 2015a), in some colon and ovarian cancers (Costantino et al. 2009; Landriscina et al. 2010a; Maddalena et al. 2013), a characteristic of CSCs. Second, most tumors experience severe hypoxia and adapt to these harsh conditions via mitochondrial alterations. TRAP1 is a mitochondrial HSP that is highly expressed in many tumors and cancer cells. Third, TRAP1 transgenic mice and TRAP1-overexpressing cells display both aberrant mitochondria and mitochondrial dysfunction (Im et al. 2007, 2013; Im and Seo 2014). Recently, Kadye et al. hypothesized that TRAP1 serves to modulate mitochondrial activity in stem cell maintenance (Kadye et al. 2014), although evidence for this remains elusive. In agreement with that hypothesis, Lettini et al. reported that TRAP1 represents a key mediator of stemness and glycolytic metabolism in colorectal cancer cells (Lettini et al. 2014). A recent paper also provides important evidence that TRAP1 is involved in the regulation of CSCs. Wu et al. demonstrated that TRAP1 is crucial for the Warburg effect (Alfarouk et al. 2014) in human glioblastoma multiforme (GBM). In contrast to normal brain, TRAP1 is highly expressed in GBM. TRAP1 depletion strongly decreased GBM cell proliferation and migration, inhibited neurosphere recovery, secondary neurosphere formation, and enhanced the therapeutic effect of temozolomide in neurosphere cultures. Since cancer cells preferentially use aerobic glycolysis to support growth, a metabolic alteration commonly referred to as the “Warburg effect,” downregulation of TRAP1 seems to inhibit tumor growth and migration through its regulatory effects on metabolic reprogramming in GBM (Wu et al. 2015). In addition, Yoshida et al. also demonstrated that TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis (Yoshida et al. 2013). Considering that Warburg effect is a crucial metabolic switch and a metabolic reprogramming of CSCs is considered as another cancer hallmark (Menendez et al. 2013), TRAP1 might contribute to this progress (Fig. 3). Although TRAP1 associated with tumor specimens is clear, its involvement in the initiation of tumors is not yet convincing. Hence, it will be interesting to determine whether TRAP1 knockdown or its overexpression affects stemness-related genes such as SOX-2 and/or sphere-forming activity, which are representative properties of CSCs. Furthermore, since CSCs display metastasis and drug resistance, it will also be important to evaluate whether manipulating TRAP1 levels alters these properties. In parallel, it is an open question as to whether TRAP1-positive or TRAP1-negative cells in general display stemness properties as described above. Hence, future studies should address the role of TRAP1 in stemness of other types of stem cells, as well as CSCs.
Fig. 3.
TRAP1 as a switch from oxidative metabolism (oxidative phosphorylation, OXPHOS) to glycolysis in metabolic reprogramming of CSCs. TRAP1 levels alter mitochondrial respiration and fatty acid oxidation, together with cellular accumulation of tricarboxylic acid cycle intermediates, ATP, and ROS. At the same time, TRAP1 deficiency affects glucose metabolism as well as invasiveness (Yoshida et al. 2013)
Inhibitors targeting TRAP1
As described above, mitochondrial TRAP1, like HSP90 in the cytoplasm, is highly expressed in various cancers. Several studies have examined organelle-specific inhibitors of targets including HSP90 (Seo 2015). Considering that mitochondria play crucial roles in structural integrity and oxidative stress, as well as in apoptosis, it is reasonable that TRAP1 is a good target for inducing apoptosis in cancer. As shown in Fig. 1, Tsutsumi et al. first identified dimethylaminoethylamino-17-demethoxygeldanamycin (DMAG)-N-oxide as a cell-impermeable HSP90 inhibitor, after analyzing a number of geldanamycin-derived molecules for membrane permeability and binding affinity to HSP90 (Tsutsumi et al. 2008); this inhibitor may act on both HSP90 and TRAP1 since several reports suggested extracellular HSP90 and TRAP1 localization. For instance, immunoelectron microscopy revealed that TRAP1 localizes at specific extramitochondrial sites such as the cell surface of blood vessel endothelial cells (Cechetto and Gupta 2000). Based those observations, it will be intriguing elucidation of their roles and targeting them in extracellular matrix (Seo 2015). Kang et al. designed gamitrinibs, mitochondria-targeted small molecules that inhibit both TRAP1 and HSP90 inside the mitochondria (Kang et al. 2009; Kang 2012) (Fig. 1). Conventional HSP90 inhibitors do not affect the function of mitochondrial HSP90 or TRAP1 directly, nor do they induce mitochondrial dysfunction, probably owing to their inability to penetrate the mitochondrial membrane. However, gamitrinibs inhibit mitochondrial HSP90 and TRAP1 function directly. TRAP1 has been shown to play a critical role in regulating the opening of the mitochondrial transition pore induced by excessive ROS production. Kang et al. demonstrated that gamitrinibs promoted activation of the peptidyl-prolyl cis-trans isomerase CypD, opening of the mitochondrial permeability transition pore, release of cytochrome c, and induction of cell death in cancer cells. Although TRAP1 and HSP90 seem to work independently and be functionally redundant in mitochondria, use of current inhibitors containing gamitrinibs has not been successful in distinguishing the two. Targeting multiple proteins that are overexpressed in various cancer cells, such as HSPs and TRAP1, may be another strategy for cancer therapy; while not specific, it can be effective, as has been seen with GTEs (Zhang et al. 2011). Lee et al. tried to develop mitochondrial TRAP1 inhibitors and replaced the isopropyl amine of the Hsp90 inhibitor PU-H71 with the mitochondria-targeting moiety triphenylphosphonium to produce SMTIN-P01, which showed TRAP1-specific activity and had improved cytotoxicity to cancer cells (Lee et al. 2015). Recent advances in delivery systems that have combined specific polymers and siRNA (An et al. 2015) suggest that it will be possible to specifically target TRAP1 by this method. In the near future, it should also be helpful to utilize organoids (Gjorevski et al. 2014; Ranga et al. 2014) in vitro and an avatar mouse model (Ohman et al. 2014) in vivo to explore the therapeutic potential of TRAP1 inhibitors for diseases such as cancer and PD.
Conclusions and perspectives
Mitochondrial TRAP1 plays important roles in regulating mitochondrial integrity, protecting against oxidative stress, and inhibiting cell death. Hence, pharmacological activation or inactivation of TRAP1 will alter mitochondrial function and concomitant cellular fate selectively in aging, unhealthy, or cancer cells, including CSCs, suggesting that it may be a good therapeutic target. A small number of studies have attempted to determine the mechanism of action of TRAP1 (Masuda et al. 2004). Several drug candidates targeting TRAP1 have been developed that have shown strong cytotoxic activity against many cancers, but not against normal cells, in vitro and in vivo (Kang 2012). However, they are only able to inhibit TRAP1; further development would be required to induce dysfunctional TRAP1 to function normally. Elucidation of TRAP1 functions in a variety of processes should guide new approaches to TRAP1 modulation for therapy. The establishment of appropriate model systems in vitro and in vivo is also necessary to elucidate the mechanisms and develop specific inhibitors.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2014R1A1A1006961). I am grateful to Jeong-Hwa Lee, Jeong-Sun Seo, and Jung-Sook Im for their inspiration and support.
References
- Agliarulo I, Matassa DS, Amoroso MR, Maddalena F, Sisinni L, Sepe L, Ferrari MC, Arzeni D, Avolio R, Paolella G, Landriscina M, Esposito F. TRAP1 controls cell migration of cancer cells in metabolic stress conditions: correlations with AKT/p70S6K pathways. Biochim Biophys Acta. 2015;1853:2570–2579. doi: 10.1016/j.bbamcr.2015.05.034. [DOI] [PubMed] [Google Scholar]
- Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HH, Elhassan GO, Ibrahim ME, David Polo Orozco J, Cardone RA, Reshkin SJ, Harguindey S. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience. 2014;1:777–802. doi: 10.18632/oncoscience.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso MR, Matassa DS, Laudiero G, Egorova AV, Polishchuk RS, Maddalena F, Piscazzi A, Paladino S, Sarnataro D, Garbi C, Landriscina M, Esposito F. TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins. Cell Death Differ. 2012;19:592–604. doi: 10.1038/cdd.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, He D, Wagner E, Jiang C. Peptide-like polymers exerting effective glioma-targeted siRNA delivery and release for therapeutic application. Small. 2015;11:5142–5150. doi: 10.1002/smll.201501167. [DOI] [PubMed] [Google Scholar]
- Caino MC, Chae YC, Vaira V, Ferrero S, Nosotti M, Martin NM, Weeraratna A, O’Connell M, Jernigan D, Fatatis A, Languino LR, Bosari S, Altieri DC. Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J Clin Invest. 2013;123:2907–2920. doi: 10.1172/JCI67841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto JD, Gupta RS. Immunoelectron microscopy provides evidence that tumor necrosis factor receptor-associated protein 1 (TRAP-1) is a mitochondrial protein which also localizes at specific extramitochondrial sites. Exp Cell Res. 2000;260:30–39. doi: 10.1006/excr.2000.4983. [DOI] [PubMed] [Google Scholar]
- Chen CF, Chen Y, Dai K, Chen PL, Riley DJ, Lee WH. A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock. Mol Cell Biol. 1996;16:4691–4699. doi: 10.1128/MCB.16.9.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Choo AB, Nai-Dy W, Heng-Phon T, Oh SK. Knockdown of Oct-4 or Sox-2 attenuates neurogenesis of mouse embryonic stem cells. Stem Cells Dev. 2007;16:413–420. doi: 10.1089/scd.2006.0099. [DOI] [PubMed] [Google Scholar]
- Chu CT. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Hum Mol Genet. 2010;19:R28–R37. doi: 10.1093/hmg/ddq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller HA, Grandori C, Tamayo P, Colbert T, Lander ES, Eisenman RN, Golub TR. Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc Natl Acad Sci U S A. 2000;97:3260–3265. doi: 10.1073/pnas.97.7.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condelli V, Maddalena F, Sisinni L, Lettini G, Matassa DS, Piscazzi A, Palladino G, Amoroso MR, Esposito F, Landriscina M. Targeting TRAP1 as a downstream effector of BRAF cytoprotective pathway: a novel strategy for human BRAF-driven colorectal carcinoma. Oncotarget. 2015;6:22298–22309. doi: 10.18632/oncotarget.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-Melo C, Passos JF. Mitochondria: are they causal players in cellular senescence? Biochim Biophys Acta. 2015;1847:1373–1379. doi: 10.1016/j.bbabio.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Costa AC, Loh SH, Martins LM. Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson’s disease. Cell Death Dis. 2013;4:e467. doi: 10.1038/cddis.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino E, Maddalena F, Calise S, Piscazzi A, Tirino V, Fersini A, Ambrosi A, Neri V, Esposito F, Landriscina M. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells. Cancer Lett. 2009;279:39–46. doi: 10.1016/j.canlet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Fallaize D, Chin LS, Li L. Differential submitochondrial localization of PINK1 as a molecular switch for mediating distinct mitochondrial signaling pathways. Cell Signal. 2015;27:2543–2554. doi: 10.1016/j.cellsig.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Ranga A, Lutolf MP. Bioengineering approaches to guide stem cell-based organogenesis. Development. 2014;141:1794–1804. doi: 10.1242/dev.101048. [DOI] [PubMed] [Google Scholar]
- Hua G, Zhang Q, Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J Biol Chem. 2007;282:20553–20560. doi: 10.1074/jbc.M703196200. [DOI] [PubMed] [Google Scholar]
- Im CN, Seo JS. Overexpression of tumor necrosis factor receptor-associated protein 1 (TRAP1), leads to mitochondrial aberrations in mouse fibroblast NIH/3T3 cells. BMB Rep. 2014;47:280–285. doi: 10.5483/BMBRep.2014.47.5.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im CN, Lee JS, Zheng Y, Seo JS. Iron chelation study in a normal human hepatocyte cell line suggests that tumor necrosis factor receptor-associated protein 1 (TRAP1) regulates production of reactive oxygen species. J Cell Biochem. 2007;100:474–486. doi: 10.1002/jcb.21064. [DOI] [PubMed] [Google Scholar]
- Im CN, Zheng Y, Kim SH, Huang TQ, Cho DH, Seo JS (2013) The establishment of tumor necrosis factor receptor-associated protein1 (TRAP1) transgenic mice and severe fat accumulation in the liver of TRAP1 mice during liver regeneration. Interdisciplinary Bio Central 5:1–7. doi:10.4051/ibc.2013.5.4.0009
- Im CN, Yun HH, Yoo HJ, Park MJ, Lee JH. Enhancement of SOX-2 expression and ROS accumulation by culture of A172 glioblastoma cells under non-adherent culture conditions. Oncol Rep. 2015;34:920–928. doi: 10.3892/or.2015.4021. [DOI] [PubMed] [Google Scholar]
- Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta. 2012;1823:607–613. doi: 10.1016/j.bbamcr.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- Kadye R, Kramer AH, Joos-Vandewalle J, Parsons M, Njengele Z, Hoppe H, Prinsloo E. Guardian of the furnace: mitochondria, TRAP1, ROS and stem cell maintenance. IUBMB Life. 2014;66:42–45. doi: 10.1002/iub.1234. [DOI] [PubMed] [Google Scholar]
- Kang BH. TRAP1 regulation of mitochondrial life or death decision in cancer cells and mitochondria-targeted TRAP1 inhibitors. BMB Rep. 2012;45:1–6. doi: 10.5483/BMBRep.2012.45.1.1. [DOI] [PubMed] [Google Scholar]
- Kang BH, Plescia J, Song HY, Meli M, Colombo G, Beebe K, Scroggins B, Neckers L, Altieri DC. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J Clin Invest. 2009;119:454–464. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Kumar S. Cancer stem cell: a rogue responsible for tumor development and metastasis. Indian J Cancer. 2014;51:282–289. doi: 10.4103/0019-509X.175364. [DOI] [PubMed] [Google Scholar]
- Khan FZ, Perumpail RB, Wong RJ, Ahmed A. Advances in hepatocellular carcinoma: nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol. 2015;7:2155–2161. doi: 10.4254/wjh.v7.i18.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landriscina M, Amoroso MR, Piscazzi A, Esposito F. Heat shock proteins, cell survival and drug resistance: the mitochondrial chaperone TRAP1, a potential novel target for ovarian cancer therapy. Gynecol Oncol. 2010;117:177–182. doi: 10.1016/j.ygyno.2009.10.078. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Laudiero G, Maddalena F, Amoroso MR, Piscazzi A, Cozzolino F, Monti M, Garbi C, Fersini A, Pucci P, Esposito F. Mitochondrial chaperone Trap1 and the calcium binding protein Sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res. 2010;70:6577–6586. doi: 10.1158/0008-5472.CAN-10-1256. [DOI] [PubMed] [Google Scholar]
- Laufen T, Mayer MP, Beisel C, Klostermeier D, Mogk A, Reinstein J, Bukau B. Mechanism of regulation of hsp70 chaperones by DnaJ cochaperones. Proc Natl Acad Sci U S A. 1999;96:5452–5457. doi: 10.1073/pnas.96.10.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Takahashi T, Yasuhara N, Inazawa J, Kamada S, Tsujimoto Y. Bis, a Bcl-2-binding protein that synergizes with Bcl-2 in preventing cell death. Oncogene. 1999;18:6183–6190. doi: 10.1038/sj.onc.1203043. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Lee JS, Cui MN, Yun HH, Kim HY, Lee SH, Lee JH. BIS targeting induces cellular senescence through the regulation of 14-3-3 zeta/STAT3/SKP2/p27 in glioblastoma cells. Cell Death Dis. 2014;5:e1537. doi: 10.1038/cddis.2014.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Park HK, Jeong H, Lim J, Lee AJ, Cheon KY, Kim CS, Thomas AP, Bae B, Kim ND, Kim SH, Suh PG, Ryu JH, Kang BH. Development of a mitochondria-targeted Hsp90 inhibitor based on the crystal structures of human TRAP1. J Am Chem Soc. 2015;137:4358–4367. doi: 10.1021/ja511893n. [DOI] [PubMed] [Google Scholar]
- Lettini G, Maddalena F, Sisinni L, Condelli V, Del Vecchio L, Gemei M, Notarangelo T, Landriscina M. TRAP1 represents a key mediator of stemness and glycolytic metabolism in colorectal cancer cells. Eur J Cancer. 2014;50:60–60. doi: 10.1016/S0959-8049(14)70308-9. [DOI] [Google Scholar]
- Li MX, Dewson G. Mitochondria and apoptosis: emerging concepts. F1000Prime Rep. 2015;7:42. doi: 10.12703/P7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- Liu D, Hu J, Agorreta J, Cesario A, Zhang Y, Harris AL, Gatter K, Pezzella F. Tumor necrosis factor receptor-associated protein 1(TRAP1) regulates genes involved in cell cycle and metastases. Cancer Lett. 2010;296:194–205. doi: 10.1016/j.canlet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Maddalena F, Sisinni L, Lettini G, Condelli V, Matassa DS, Piscazzi A, Amoroso MR, La Torre G, Esposito F, Landriscina M. Resistance to paclitxel in breast carcinoma cells requires a quality control of mitochondrial antiapoptotic proteins by TRAP1. Mol Oncol. 2013;7:895–906. doi: 10.1016/j.molonc.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Shima G, Aiuchi T, Horie M, Hori K, Nakajo S, Kajimoto S, Shibayama-Imazu T, Nakaya K. Involvement of tumor necrosis factor receptor-associated protein 1 (TRAP1) in apoptosis induced by beta-hydroxyisovalerylshikonin. J Biol Chem. 2004;279:42503–42515. doi: 10.1074/jbc.M404256200. [DOI] [PubMed] [Google Scholar]
- Matassa DS, Agliarulo I, Amoroso MR, Maddalena F, Sepe L, Ferrari MC, Sagar V, D’Amico S, Loreni F, Paolella G, Landriscina M, Esposito F. TRAP1-dependent regulation of p70S6K is involved in the attenuation of protein synthesis and cell migration: relevance in human colorectal tumors. Mol Oncol. 2014;8:1482–1494. doi: 10.1016/j.molonc.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megger DA, Bracht T, Kohl M, Ahrens M, Naboulsi W, Weber F, Hoffmann AC, Stephan C, Kuhlmann K, Eisenacher M, Schlaak JF, Baba HA, Meyer HE, Sitek B. Proteomic differences between hepatocellular carcinoma and nontumorous liver tissue investigated by a combined gel-based and label-free quantitative proteomics study. Mol Cell Proteomics. 2013;12:2006–2020. doi: 10.1074/mcp.M113.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Joven J, Cufi S, Corominas-Faja B, Oliveras-Ferraros C, Cuyas E, Martin-Castillo B, Lopez-Bonet E, Alarcon T, Vazquez-Martin A. The Warburg effect version 2.0: metabolic reprogramming of cancer stem cells. Cell Cycle. 2013;12:1166–1179. doi: 10.4161/cc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T, Yamaguchi H, Amagai A, Maeda Y. Involvement of the TRAP-1 homologue, Dd-TRAP1, in spore differentiation during Dictyostelium development. Exp Cell Res. 2005;303:425–431. doi: 10.1016/j.yexcr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat. 2010;31:763–780. doi: 10.1002/humu.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman AW, Hasan N, Dinulescu DM. Advances in tumor screening, imaging, and avatar technologies for high-grade serous ovarian cancer. Front Oncol. 2014;4:322. doi: 10.3389/fonc.2014.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga A, Gjorevski N, Lutolf MP. Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 2014;69–70:19–28. doi: 10.1016/j.addr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Cheung-Flynn J, Prapapanich V, Carrigan PE, Smith DF. Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol. 2004;39:279–295. doi: 10.1080/10409230490892513. [DOI] [PubMed] [Google Scholar]
- Sahlberg SH, Spiegelberg D, Glimelius B, Stenerlow B, Nestor M. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PLoS One. 2014;9:e94621. doi: 10.1371/journal.pone.0094621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo YH. Organelle-specific Hsp90 inhibitors. Arch Pharm Res. 2015 doi: 10.1007/s12272-015-0636-1. [DOI] [PubMed] [Google Scholar]
- Shim EH, Kim JI, Bang ES, Heo JS, Lee JS, Kim EY, Lee JE, Park WY, Kim SH, Kim HS, Smithies O, Jang JJ, Jin DI, Seo JS. Targeted disruption of hsp70.1 sensitizes to osmotic stress. EMBO Rep. 2002;3:857–861. doi: 10.1093/embo-reports/kvf175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisinni L, Maddalena F, Lettini G, Condelli V, Matassa DS, Esposito F, Landriscina M. TRAP1 role in endoplasmic reticulum stress protection favors resistance to anthracyclins in breast carcinoma cells. Int J Oncol. 2014;44:573–582. doi: 10.3892/ijo.2013.2199. [DOI] [PubMed] [Google Scholar]
- Song HY, Dunbar JD, Zhang YX, Guo D, Donner DB. Identification of a protein with homology to hsp90 that binds the type 1 tumor necrosis factor receptor. J Biol Chem. 1995;270:3574–3581. doi: 10.1074/jbc.270.8.3574. [DOI] [PubMed] [Google Scholar]
- Takemoto K, Miyata S, Takamura H, Katayama T, Tohyama M. Mitochondrial TRAP1 regulates the unfolded protein response in the endoplasmic reticulum. Neurochem Int. 2011;58:880–887. doi: 10.1016/j.neuint.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Tam WL, Ng HH. Sox2: masterminding the root of cancer. Cancer Cell. 2014;26:3–5. doi: 10.1016/j.ccr.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Tian X, Ma P, Sui CG, Meng FD, Li Y, Fu LY, Jiang T, Wang Y, Jiang YH. Suppression of tumor necrosis factor receptor-associated protein 1 expression induces inhibition of cell proliferation and tumor growth in human esophageal cancer cells. FEBS J. 2014;281:2805–2819. doi: 10.1111/febs.12822. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Scroggins B, Koga F, Lee MJ, Trepel J, Felts S, Carreras C, Neckers L. A small molecule cell-impermeant Hsp90 antagonist inhibits tumor cell motility and invasion. Oncogene. 2008;27:2478–2487. doi: 10.1038/sj.onc.1210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu Y, Cho K, Dong X, Teng L, Han D, Liu H, Chen X, Chen X, Hou X, Peng F, Bi Y, Shen C, Zhao S. Downregulation of TRAP1 sensitizes glioblastoma cells to temozolomide chemotherapy through regulating metabolic reprogramming. Neuroreport. 2015 doi: 10.1097/WNR.0000000000000513. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Im CN, Youn DY, Yun HH, Lee JH. Bis is induced by oxidative stress via activation of HSF1 Korean. J Physiol Pharmacol. 2014;18:403–409. doi: 10.4196/kjpp.2014.18.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Tsutsumi S, Muhlebach G, Sourbier C, Lee MJ, Lee S, Vartholomaiou E, Tatokoro M, Beebe K, Miyajima N, Mohney RP, Chen Y, Hasumi H, Xu W, Fukushima H, Nakamura K, Koga F, Kihara K, Trepel J, Picard D, Neckers L. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc Natl Acad Sci U S A. 2013;110:E1604–E1612. doi: 10.1073/pnas.1220659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Pang E, Loo RR, Rao J, Go VL, Loo JA, Lu QY. Concomitant inhibition of HSP90, its mitochondrial localized homologue TRAP1 and HSP27 by green tea in pancreatic cancer HPAF-II cells. Proteomics. 2011;11:4638–4647. doi: 10.1002/pmic.201100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Karsten P, Hamm S, Pogson JH, Muller-Rischart AK, Exner N, Haass C, Whitworth AJ, Winklhofer KF, Schulz JB, Voigt A. TRAP1 rescues PINK1 loss-of-function phenotypes. Hum Mol Genet. 2013;22:2829–2841. doi: 10.1093/hmg/ddt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang J, Huang Z, Wei P, Liu Y, Hao J, Zhao L, Zhang F, Tu Y, Wei T (2015a) Aberrantly upregulated TRAP1 is required for tumorigenesis of breast cancer. Oncotarget 6:44495–44508. doi:10.18632/oncotarget.6252 [DOI] [PMC free article] [PubMed]
- Zhang P, Lu Y, Yu D, Zhang D, Hu W. TRAP1 provides protection against myocardial ischemia-reperfusion injury by ameliorating mitochondrial dysfunction. Cell Physiol Biochem. 2015;36:2072–2082. doi: 10.1159/000430174. [DOI] [PubMed] [Google Scholar]