Abstract
Heat shock proteins including the major stress protein HSP70 support intracellular homeostasis and prevent protein damage after a temperature increase and other stressful environmental stimuli, as well as during aging. We have shown earlier that prolonged administration of recombinant human HSP70 to mice exhibiting Alzheimer’s-like neurodegeneration as well as during sepsis reduces the clinical manifestations of these pathologies. Herein, we studied the action of recombinant human HSP70 on young and aged mouse mesenchymal stem cells (MSCs) in culture. The results obtained indicate that HSP70 at concentrations of 2 μg/ml and higher significantly stimulates growth of aged but not young MSCs. A similar effect is produced by application of a mild heat shock (42 °C 5 min) to the cells. Importantly, responses of young and aged MSCs to heat shock treatment of various durations differed drastically, and aged MSCs were significantly more sensitive to higher heat stress exposures than the young cells. Western blotting and protein labeling experiments demonstrated that neither mild heat shock nor exogenous HSP70 administration resulted in significant endogenous HSP70 induction in young and aged MSCs, whereas mild heat shock increased HSC70 levels in aged MSCs. The results of this study suggest that the administration of exogenous HSP70 and the application of mild heat stress may produce a certain “rejuvenating” effect on MSCs and possibly other cell types in vivo, and these interventions may potentially be used for life extension by delaying various manifestations of aging at the molecular and cellular level.
Keyword: Mesenchymal stem cells, Cell growth, Cell senescence, Aging, Heat shock, Recombinant human HSP70
Introduction
Adult stem cells in vertebrates are responsible for maintaining cellular homeostasis of organs and tissues and for their regeneration after damage or various pathological processes. Numerous data accumulated in the last few years indicate that in the course of normal organismal aging, a substantial decrease in stem cell number and/or function is observed (Signer and Morrison 2013). This is considered as one of the most fundamental causes leading to progressive dysfunction of various organs and, finally, to death.
One of the currently best studied adult stem/progenitor cell types, mesenchymal stem cells (MSCs), were identified initially as fibroblastoid cells in bone marrow with plastic adherence, high self-renewal capacity, and ability to form fibroblast-like colonies in vitro and to serve as ossification centers in vivo (reviewed in Phinney and Sensebé 2013). Later on, cells with similar biological properties and surface phenotype were identified in a number of other organs and tissues. The majority of MSCs are thought to be associated with the vascular network, either as pericytes or as vessel wall cells, which explains their ubiquitous occurrence (Murray et al. 2014).
On the basis of existing experimental evidence, four major functions can currently be attributed to MSCs in vivo: (1) maintenance of bone and cartilage homeostasis; (2) participation in the hematopoietic stem cell niche and support of hematopoiesis; (3) function as “sentinels” during pathological processes and as activators of regeneration; and (4) immune regulation, in particular anti-inflammatory action including M2 macrophage polarization (Caplan 2015; Fontaine et al. 2016; Bernardo and Fibbe 2013). Of these, the first two functions are thought to be specific to MSCs residing in bone marrow, whereas the last two may possibly be performed by all tissue-resident MSCs. Since adverse effects of aging are generally associated with increased and widespread chronic inflammation, decline in MSC function with age may not only harm the skeletal system but deleteriously affect the whole organism as well.
MSCs in vitro have extensive proliferative potential, but during long-term culture, adverse changes of their properties are observed. In particular, they undergo a gradual decrease of their proliferation rate and diminution of their differentiation capacity in osteogenic and, to a lesser extent, adipogenic and chondrogenic directions (Wagner et al. 2010). These changes, projected to the organismal level, would lead to stem cell aging, and the progressive adverse shift in MSC properties under long-term cultivation can be thus considered as an in vitro analog and a convenient model for organismal aging studies.
Stress proteins that are commonly termed heat shock proteins (HSPs) normally perform multiple protective functions, especially under various stressful environmental conditions (Lindquist 1980; Feder and Krebs 1997; Evgen’ev et al. 2014). It has been previously demonstrated that induction of HSP70 or its experimental administration by different routes may have a protective effect in various animal and cellular neurodegeneration models (Latchman 2004; Leak 2014; Rozhkova et al. 2010; Bobkova et al. 2014). In addition, in a wide variety of organisms ranging from nematode and Drosophila to humans, a protective role of HSP70 in aging was demonstrated (Calderwood et al. 2009; Morimoto and Cuervo 2014; Sarup et al. 2014). The protective role of HSP70 in aging and neurodegeneration is mediated by various properties of these proteins including, but not limited to, apoptosis inhibition, reduction in the levels of reactive oxygen species, and suppression of protein aggregate formation (Latchman 2004; Evans et al. 2006; Calabrese et al. 2011; Morimoto and Cuervo 2014).
Based on these data, we studied in this work the effects of recombinant HSP70 as well as mild heat shock on growth of MSCs of various ages. The obtained data demonstrate drastically different responses of young and aged MSCs to these interventions and are compatible with the notion of geroprotective effects of controlled heat shock treatment in general and HSP70 in particular.
Materials and methods
Isolation of recombinant HSP70
Recombinant N-terminal His-tagged human HSP70 protein was expressed in Escherichia coli and purified as described earlier (Guzhova et al. 2011). Briefly, the E. coli BL21 strain was used for protein expression. The cells were grown to an OD600 of 0.6 at 37 °C, induced by adding of 0.3 mM IPTG, additionally grown for 3 h at 37 °C, and harvested by centrifugation. His-HSP70 was purified using a Ni-NTA Agarose (Qiagen) in native conditions according to the manufacturer’s recommendations. Purification of the isolated HSP70 from LPS was performed by double passage through polymyxin B-agarose columns (Sigma). The purity of the resulting HSP70 preparation was higher than 95 %, and endotoxin levels were less than 0.1 U/ml.
Isolation and growing of mouse MSCs
All animal experiments were performed in accordance with the guidance of the National Institutes of Health for Care and Use of Laboratory Animals, NIH Publications No. 8023, revised 1978.
Isolation of MSCs from adipose tissue of С57ВL/6 female mice (6 to 10 weeks old) was performed as described (Andreeva et al. 2015). Adipose tissue (1 to 3 g) was finely minced and treated with 10 ml of collagenase type IA (Sigma) 2 mg/ml in DMEM at 37 °C for 30 to 40 min at constant agitation. Cells were filtered through a 100-μm nylon filter (BD Biosciences) and washed from adipocytes three times in DMEM-low glucose by centrifugation at 367×g for 7 min. Following initial isolation, MSCs were cultured in DMEM-low glucose (1000 mg/L glucose) containing 10 % fetal bovine serum, 2 mM glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml) at 37 °C at hypoxia conditions (5 % CO2, 5 % O2). Cells were cultured for 4 to 6 days to approximately 80 % confluency. For passaging, cells were detached by treatment with 0.25 % trypsin (Sigma) in 1× phosphate-buffered saline (PBS) with 0.02 % EDTA at 37 °C for 5 to 7 min, washed once with DMEM-low glucose, and split at a 1:4 ratio. Medium changes were performed every 3 days. Freezing of cells after various numbers of passages was performed in medium containing 0.4× DMEM-low glucose, 50 % fetal bovine serum, and 10 % DMSO. Three independent MSC isolations were performed for the experiments described in this study.
Characterization of the surface phenotype of viable (propidium iodide-negative) cells was performed by flow cytometry using fluorescently labeled antibodies against positive surface markers CD29-FITC (clone HMβ1-1), CD44-PE (clone IM7) (both BioLegend), and CD-105-PE (clone MJ7/18, Miltenyi Biotech) and negative markers CD45-PE (clone 30 F11), CD-11b-PE (clone M1/70.15.11.5) (both Miltenyi Biotech), and CD34-Alexa Fluor 700 (clone RAM34, eBioscience). After passage 2, the MSC populations were about 99 % positive for CD29 and CD44 markers and about 99 % negative for CD11b and CD45.
Analysis of the effects of HSP70 and heat shock treatment on murine MSCs
Aged and young MSCs at 50 to 70 % confluency were detached by trypsin treatment as described above, stained with 0.2 % trypan blue for live cells, counted in a hemocytometer and seeded at a density of 10 × 103 live cells onto 3.5-cm cultural dishes. Heat shock was performed by placing dishes on solid-state thermostat set at 42 °C. Thermal contact of dishes with the solid-state block was mediated by the water layer. After recombinant, HSP70 addition or heat shock treatment cells were cultured for 1 day under hypoxia conditions (5 % СО2, 5 % О2), detached by trypsin, stained with trypan blue, and counted in a hemocytometer. All experimental variants were performed in triplicates; cells in each sample were counted in the hemocytometer twice and mean values were used for calculations and statistical analysis.
The cell growth rate of cultures was estimated by calculating the integral number of population doublings normalized to 24 h (INPD/24), according to the formula: INPD/24 = 24/T × log2(NT/N0), where NT is a number of harvested live cells, N0 is a number of seeded live cells, and T is cultivation time (h). This parameter depends both on cell proliferation and cell death occurring during cell growth.
Western blot analysis
Cells growing on 3.5-cm dishes were subjected to heat shock treatment (42 °C for 5 min or 43 °C for 30 min) on a solid-state thermostat, and then incubated for 4 h at 37 °C in a CO2 incubator under hypoxia conditions (5 % CO2, 5 % O2). Cells were then detached by 0.25 % trypsin (Sigma) in 1× PBS, centrifuged, washed by 1× PBS and lysed in Laemmli sample buffer followed by heat treatment at 98 °C for 5 min. Proteins were separated on 8 % (w/v) polyacrylamide gels by SDS-PAGE and blotted onto nitrocellulose membrane using a Bio-Rad Trans-Blot Cell. Blocked membranes were incubated for 12 h with 1:1000 dilutions of specific primary antibodies: mouse monoclonal anti-Hsp70 (ADI-SPA-810, Enzo), rat monoclonal anti-Hsc70 (ADI-SPA-815, Enzo), rat monoclonal anti HSP90 (ADI-SPA-835, Enzo), and mouse monoclonal anti-actin 5C ( clone C4, Millipore). Blots were then washed and incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies: goat anti-rat IgG (AP136P, Millipore) or goat anti-mouse IgG (12–349, Millipore). Immune complexes were detected via chemiluminescence (ECL kit, Amersham). Blots were imaged with ChemiDoc XRS+ imaging system (Bio-Rad). Blots were stained with Ponceau S reagent (Sigma) after detection to verify that proteins have been transferred.
[35S]-labeled protein analysis
Cells growing on 3.5-cm dishes were subjected to heat shock treatment (42 °C for 5 min or 43 °C for 30 min) on a solid-state thermostat or incubated with recombinant HSP70 10 μg/ml for 2 h at 37 °C in a CO2 incubator under hypoxia conditions (5 % CO2, 5 % O2). Following removal of culture medium and washing cells with 1× Hanks’ balanced salt solution (HBSS), cell proteins were labeled with 1 MBq of [35S] methionine (Amersham Biosciences) in 200 μl of 1× HBSS for 1 h at 37 °C in a CO2 incubator under hypoxia conditions. Cells were then detached with trypsin, centrifuged, washed with 1× HBSS, and lysed in Laemmli sample buffer followed by heat treatment at 98 °C for 5 min. Proteins were separated on 10 % (w/v) polyacrylamide gels by SDS-PAGE. Gels were dried and autoradiographed using a Cyclone phosphorimager (PerkinElmer).
Statistical analysis
For statistical analysis (calculations of mean values, standard deviations, and Student’s t tests), statistical functions of Microsoft Excel were used.
Results
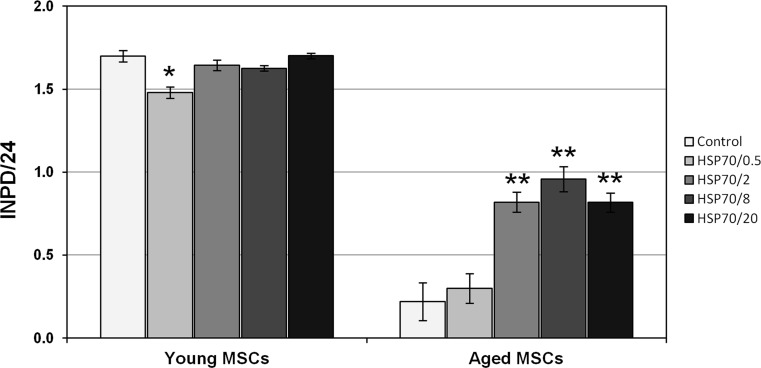
To compare the effects of recombinant HSP70 on aged and young mouse MSCs, cells of the same batch but of different biological ages were prepared by using cells frozen at later passages and cultured longer for the aged population (passages 10 to 15, 50 to 70 days in culture) as compared to the young one (passages 3 to 6, 19 to 35 days in culture). In total, four separate experiments with three independently prepared MSC batches were performed. Figure 1 depicts the results of analysis of HSP70 effects on a representative young cell population as compared with the most aged cell population tested. In all the experiments performed, the growth rate of untreated aged cells was significantly lower than that of the young cells and decreased progressively with time in culture. Thus, if the number of integral population doublings per day for young cells in the experiment on Fig. 1 was above 1.7, the same parameter for the aged cells was only about 0.22, which corresponds to about 7.7-fold lower growth rate for the aged compared to the young cells. The calculated population doubling times were about 14 and 109 h for the young and aged cells, respectively. Addition of HSP70 to the young cells did not change significantly their growth rate. At the same time, addition of HSP70 to aged cells resulted in drastic enhancement of their growth. This effect was dose-dependent, with insignificant increase at a concentration 0.5 μg/ml, and reaching the highest level at a concentration of 8 μg/ml. Under optimal HSP70 concentration, the growth rate of aged cells increased about 4.4-fold compared to that of untreated cells (calculated population doubling times about 25 h for MSCs treated with HSP70 at 8 μg/ml compared to 109 h for the control cells). In the other three experiments, a significant enhancement of aged MSC growth rate by HSP70 was also observed, and the magnitude of this effect correlated with MSC age, being smaller for the cells of less advanced age.
Fig. 1.
Effects of recombinant human HSP70 on growth of young and aged mouse MSCs. Young MSCs of the passage 6 (35 days in culture), and aged MSCs of the passage 15 (61 days in culture) were used in this experiment. The Y-axis depicts the integral number of population doublings of the cell culture normalized to a 24-h period (INPD/24). Experimental groups included control without HSP70 addition and variants with HSP70 added to the culture medium to final concentrations of 0.5 μg/ml to 20 μg/ml (HSP70/0.5 to HSP70/20). All groups were performed in triplicates. Four experiments of this type with three independently isolated MSC batches were performed. The diagram shows mean values and standard deviations. Comparisons between groups were performed using Student’s t test. The probability is less than 0.01 (*) or 0.005 (**) relative to control
To test the potential connection of the observed HSP70 effect on aged cells with a heat shock response, we subjected young and aged cells to mild heat stress conditions (42 °C for 5, 10, or 30 min). Essentially as in the case of HSP70 treatment, four experiments with three independent MSC batches were performed, which demonstrated qualitatively similar patterns of cell response to heat shock treatment. The results of one of these experiments, with the most aged cells among those tested, are depicted in Fig. 2. The 5-min heat shock had little if any effect on young cells, whereas the same treatment induced about a 4.1-fold increase in growth rate for the aged cells (calculated population doubling times about 38 h for MSCs subjected to mild heat shock compared to 158 h for untreated cells). Thus, the effects of the 5-min heat shock on young and old MSCs were similar to those of the HSP70 treatment.
Fig. 2.
Effects of heat shock treatments of various duration on growth of young and aged MSCs. Young MSCs of the passage 5 (27 days in culture) and aged MSCs of the passage 13 (58 days in culture) were used in this experiment. Heat shock treatments were performed at 42 °C for 5 to 30 min (HS 42/5 to HS 42/30). Other conditions and designations are the same as in Fig. 1. Four experiments of this type with three independently isolated MSC batches were performed. Negative INPD/24 values reflect the fact that due to cell death fewer cells were collected after culturing than seeded
The differences between the responses of young and aged cells were also observed at longer heat shock exposures. However, the latter treatments turned out to be significantly more harmful to the aged cells, which was especially prominent for the longest exposure. Thus, while the young cells responded to this treatment by a decrease of growth rate from 1.5 to about 1.0 population doublings per day, aged cells demonstrated a significant cytotoxic response since about twofold fewer cells were collected after cultivation than seeded. Therefore, the aged MSCs were substantially more sensitive to the deleterious effects of prolonged heat shock treatments than were the young ones.
In order to study the potential involvement of heat shock proteins in the observed effects of HSP70 and mild heat shock treatments on aged MSCs, Western blot and in vitro protein labeling experiments were performed. Since Western blotting does not allow to discriminate between endogenous and exogenous HSP70 proteins, effects of HSP70 treatments were not tested in blotting experiments.
The results, depicted in Fig. 3a, indicate that the basal HSP70 levels were similar in young and aged cells. Standard heat shock treatment (43 °C for 30 min) produced strong HSP70 induction in young MSCs and a less pronounced HSP70 response in the aged MSCs. However, no HSP70 induction, either in young or aged cells, was observed after mild heat shock (42 °C for 5 min) treatment that produced significant growth effects in the aged MSCs. At the same time, probing the blots with antibodies to other heat shock proteins revealed a distinct elevation of constitutive HSC70 protein levels after mild heat shock specifically in the old cells, whereas the standard heat shock treatment reduced HSC70 levels compared to controls (Fig. 3a). Thus, HSC70 levels seem to correlate with positive or negative effects of heat shock treatments on aged MSCs. A certain elevation of HSP90 levels was also observed after mild heat shock treatment in both old and young cells.
Fig. 3.
Analysis of heat shock protein expression following heat shock or recombinant HSP70 treatments. a. Analysis of selected heat shock protein cellular levels following mild or standard heat shock treatments by Western blotting. Proteins were separated on 8 % gels by SDS-PAGE and electrophoretically transferred onto nitrocellulose membrane. Control—untreated cells, HS 42/5—mild heat shock treatment (42 °C for 5 min), HS 43/30—standard heat shock treatment (43 °C for 30 min); y—young cells, o—old cells. Young MSCs of the passage 5 (34 days in culture) and aged MSCs of the passage 11 (70 days in culture) were used in this experiment. Blots were probed for indicated proteins using respective antibodies. b Analysis of proteins labeled by [35S] methionine after mild or standard heat shock treatments or incubation with recombinant HSP70. After treatment, proteins were labeled by incubation of cells with [35S] methionine in HBSS for 1 h at 37 °C in the CO2 incubator, separated on 10 % gels by SDS-PAGE, and autoradiographed using Cyclone phosphorimager (PerkinElmer). C—untreated control, ms—mild heat shock (42 °C for 5 min), ss—standard heat shock (43 °C for 30 min), hsp—incubation with recombinant HSP70 10 μg/ml for 2 h at 37 °C. Position of HSP70 according to molecular size markers is indicated by an arrow. Young MSCs of the passage 3 (27 days in culture) and aged MSCs of the passage 11 (55 days in culture) were used in this experiment
To study the effects of both heat shock and HSP70 administration on protein synthesis, we performed [35S] methionine in vitro labeling experiments. The young and aged MSCs were subjected to heat shock or HSP70 treatments followed by labeling and protein electrophoresis analysis. The results of this experiment are provided in Fig. 3b. Heavy labeling of HSP70 in young cells and weak labeling in aged cells were observed upon standard heat shock treatment (43 °C for 30 min). However, no detectable labeling of HSP70 was observed after mild heat shock (42 °C for 5 min) or HSP70 administration. Interestingly, recombinant HSP70 addition resulted in a labeling of at least one protein band in the HSP70 region, which was not present in the control. The identity of this band remains unclear.
Discussion
Our results demonstrate that the addition of recombinant human HSP70 significantly stimulates growth of aged mouse MSCs, and this effect is dose-dependent since it is practically absent at the protein concentration 0.5 μg/ml but significantly increases at 2 μg/ml and attains the highest level at 8 μg/ml.
It is important to note that HSP70 addition enhanced growth of the aged cells only, whereas for young cells, this effect was practically absent, and the same cellular response was observed following application of mild heat shock (42 °C, 5 min). The HSP70 addition tended to produce a somewhat higher stimulation of aged cell growth compared to the mild heat shock treatment, but this may well be attributed to the insufficiently optimized mild heat shock conditions.
Similar results were reported in a recent paper (Choudhery et al. 2015), wherein a mild heat stress (41 °C, 60 min) was demonstrated to significantly activate proliferation of aged human MSCs, whereas the effect on young cells was negligible. According to the authors’ data, mild heat stress suppressed expression of negative regulators of proliferation p16 and p21 and induced expression of superoxide dismutase and SIRT-1, which is involved in deceleration of aging processes.
Interestingly, stressful conditions such as reduced temperature during cell culture, have been shown, in contrast to our results, to produce certain positive effects (mainly, protection from oxidative stress) in young, but not aged, MSCs (Stolzing et al. 2006).
It has been previously demonstrated that heat shock treatment under certain conditions may increase lifespan of various organisms such as Drosophila, nematodes, and certain vertebrate species (Calderwood et al. 2009; Sarup et al. 2014; Calabrese et al. 2012). On the other hand, it has been shown that heat shock may slow down proliferation of various cells in culture (Roti Roti et al. 1992), and sublethal heat shock induces premature in vitro senescence of MSCs (Alekseenko et al. 2014). Our data indicate that under conditions of mild heat shock, an opposite effect is observed, namely the growth of aged cells is stimulated, which can be considered, to some extent, as a “rejuvenation” effect. However, the increase of heat shock severity first leads to inhibition of growth and eventually to overt cell death.
It has been earlier demonstrated that normal cellular response to heat shock during organismal aging is substantially weakened (Calderwood et al. 2009; Murshid et al. 2013; Morimoto and Cuervo 2014; Leak 2014). Our results are in agreement with this notion as far as MSCs aged in vitro subjected to relatively severe stress are concerned, both in terms of cell survival and HSP70 induction. Our data, however, demonstrate that MSCs aged in vitro mount robust growth response both to exogenous HSP70 and controlled mild heat shock. Therefore, the effects of heat stress on growth of aged MSC, depending on its magnitude, may have opposite directions. This is in full agreement with classification of heat stress as one of the manifestations of hormesis, a phenomenon of biphasic dose response with low dose stimulation or beneficial effect and a high dose inhibitory or toxic effect (Calabrese et al. 2012).
One of the most striking results of our study is the virtual absence of effect of HSP70 addition and mild heat shock treatment on young cells. This can be explained by suggesting that exogenous HSP70 or endogenous stress proteins induced by mild heat shock treatment are able to diminish pathological manifestations of cell aging, such as denaturation and aggregation of cellular proteins. This would lead to activation of cellular processes, including proliferation, in the aged cells but not in the young ones, since these processes are not compromised in the latter. The results of blotting experiments suggest that one of possible candidates mediating the observed effects is HSC70, whose cellular levels correlated with the observed effects of mild and more severe heat shock treatments. However, more experiments are definitely needed to justify or reject this hypothesis.
An alternative albeit partially overlapping explanation is that the young cells already proliferate at a maximal rate under the culture conditions used, whereas aged cells retain significant potential of accelerated proliferation that remains unrealized due to the action of intrinsic or extrinsic factors. One of the leading candidates of such extrinsic factors is the production by cells aged in vitro of senescence-associated secretory phenotype (SASP) factors that are mostly proinflammatory in nature (Davalos et al. 2010). The SASP factors tend to significantly slow down proliferation of aged cells, and HSP70 or heat shock treatment might inhibit production or action of SASP factors.
The observed stimulating action of HSP70 and mild heat shock on aged MSCs in culture suggests that a similar phenomenon may take place in vivo as well. The results of our in vivo studies (Bobkova et al. 2015) indicate that in the mouse model of aging, administration of recombinant HSP70 acts differently on young and aged animals. Given that adult stem cells play a critical role in maintenance of homeostasis of various organs and systems of organism and that organismal aging is accompanied by the decrease of functional activity of stem cells, it seems likely that the activation of signaling pathways involved in the heat shock response may decelerate processes involved in aging.
As a cautionary note, our results demonstrate that the positive effect of mild heat shock on aged MSCs is confined to a narrow window of treatment conditions, beyond which the effect is negated or even changes the direction. This conclusion is in full agreement with the findings of recent meta-analysis of the effects of heat shock on life extension (Lagisz et al. 2013), postulating that life extension via heat shock treatment is likely constrained to a narrow window of experimental conditions. In this respect, the recombinant HSP70 administration, which does not produce deleterious effects in a wide range of concentrations, might be considered as a safer and more promising strategy of life extension as compared to heat shock treatment.
Acknowledgments
This work was supported by the Russian Science Foundation grant No. 14-50-00060.
References
- Alekseenko LL, Zemelko VI, Domnina AP, Lyublinskaya OG, Zenin VV, Pugovkina NA, Kozhukharova IV, Borodkina AV, Grinchuk TM, Fridlyanskaya II, Nikolsky NN. Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells. Cell Stress Chaperones. 2014;19:355–366. doi: 10.1007/s12192-013-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva NV, Bonartsev AP, Zharkova II, Makhina TK, Myshkina VL, Kharitonova EP, Voinova VV, Bonartseva GA, Shaitan KV, Belyavsky AV. Culturing of mouse mesenchymal stem cells on poly-3-hydroxybutyrate scaffolds. Bull Exp Biol Med. 2015 doi: 10.1007/s10517-015-3015-5. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Bobkova NV, Garbuz DG, Nesterova I, Medvinskaya N, Samokhin A, Alexandrova I, Yashin V, Karpov V, Kukharsky MS, Ninkina NN, Smirnov AA, Nudler E, Evgen’ev M. Therapeutic effect of exogenous hsp70 in mouse models of Alzheimer’s disease. J Alzheimers Dis. 2014;38:425–435. doi: 10.3233/JAD-130779. [DOI] [PubMed] [Google Scholar]
- Bobkova NV, Evgen’ev M, Garbuz DG, Kulikov AM, Morozov A, Samokhin A, Velmeshev D, Medvinskaya N, Nesterova I, Pollock A, Nudler E. Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc Natl Acad Sci U S A. 2015;112:16006–16011. doi: 10.1073/pnas.1516131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E, Calabrese EJ. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med. 2011;32:279–304. doi: 10.1016/j.mam.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, Cuzzocrea S, Rizzarelli E, Calabrese EJ. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim Biophys Acta. 2012;1822:753–83. doi: 10.1016/j.bbadis.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging--a mini-review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. MSCs: The sentinel and safe-guards of injury. J Cell Physiol. 2015 doi: 10.1002/jcp.25255. [DOI] [PubMed] [Google Scholar]
- Choudhery MS, Badowski M, Muise A, Harris DT. Effect of mild heat stress on the proliferative and differentiative ability of human mesenchymal stromal cells. Cytotherapy. 2015;17:359–368. doi: 10.1016/j.jcyt.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29:273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Wisén S, Gestwicki JE. Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1–42) aggregation in vitro. J Biol Chem. 2006;281:33182–33191. doi: 10.1074/jbc.M606192200. [DOI] [PubMed] [Google Scholar]
- Evgen’ev MB, Garbus DG, Zatsepina OG. Heat shock proteins and whole body adaptation to extreme environments. Berlin: Springer; 2014. [Google Scholar]
- Feder ME, Krebs RA. Ecological and evolutionary physiology of heat shock proteins and the stress response in Drosophila: complementary insights from genetic engineering and natural variation. EXS. 1997;83:155–173. doi: 10.1007/978-3-0348-8882-0_9. [DOI] [PubMed] [Google Scholar]
- Fontaine MJ, Shih H, Schäfer R, Pittenger MF. Unraveling the mesenchymal stromal cells’ paracrine immunomodulatory effects. Transfus Med Rev. 2016;30:37–43. doi: 10.1016/j.tmrv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Lazarev VF, Kaznacheeva AV, Ippolitova MV, Muronetz VI, Kinev AV, Margulis BA. Novel mechanism of Hsp70 chaperone-mediated prevention of polyglutamine aggregates in a cellular model of huntington disease. Hum Mol Genet. 2011;20:3953–3963. doi: 10.1093/hmg/ddr314. [DOI] [PubMed] [Google Scholar]
- Lagisz M, Hector KL, Nakagawa S. Life extension after heat shock exposure: assessing meta-analytic evidence for hormesis. Ageing Res Rev. 2013;12:653–660. doi: 10.1016/j.arr.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Protective effect of heat shock proteins in the nervous system. Curr Neurovasc Res. 2004;1:21–27. doi: 10.2174/1567202043480206. [DOI] [PubMed] [Google Scholar]
- Leak RK. Heat shock proteins in neurodegenerative disorders and aging. J Cell Commun Signal. 2014;8:293–310. doi: 10.1007/s12079-014-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980;77:463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Cuervo AM. Proteostasis and the aging proteome in health and disease. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S33–8. doi: 10.1093/gerona/glu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Péault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshid A, Eguchi T, Calderwood SK. Stress proteins in aging and life span. Int J Hyperthermia. 2013;29:442–447. doi: 10.3109/02656736.2013.798873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG, Sensebé L. Mesenchymal stromal cells: misconceptions and evolving concepts. Cytotherapy. 2013;15:140–145. doi: 10.1016/j.jcyt.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Roti Roti JL, Mackey MA, Higashikubo R. The effects of heat shock on cell proliferation. Cell Prolif. 1992;25:89–99. doi: 10.1111/j.1365-2184.1992.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Rozhkova E, Yurinskaya M, Zatsepina O, Garbuz D, Karpov V, Surkov S, Murashev A, Ostrov V, Margulis B, Evgen’ev M, Vinokurov M. Exogenous mammalian extracellular HSP70 reduces endotoxin manifestations at the cellular and organism levels. Ann N Y Acad Sci. 2010;1197:94–107. doi: 10.1111/j.1749-6632.2009.05375.x. [DOI] [PubMed] [Google Scholar]
- Sarup P, Sørensen P, Loeschcke V. The long-term effects of a life-prolonging heat treatment on the Drosophila melanogaster transcriptome suggest that heat shock proteins extend lifespan. Exp Gerontol. 2014;50:34–39. doi: 10.1016/j.exger.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzing A, Sethe S, Scutt AM. Stressed stem cells: temperature response in aged mesenchymal stem cells. Stem Cells Dev. 2006;15:478–487. doi: 10.1089/scd.2006.15.478. [DOI] [PubMed] [Google Scholar]
- Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev. 2010;16:445–453. doi: 10.1089/ten.teb.2009.0825. [DOI] [PubMed] [Google Scholar]