Abstract
In vitro fertilized (IVF) embryos show both cell cycle and developmental arrest. We previously showed oxidative damage activates the ATM → Chk1 → Cdc25B/Cdc25C cascade to mediate G2/M cell cycle arrest for repair of hydrogen peroxide (H2O2)-induced oxidative damage in sperm. However, the mechanisms underlying the developmental delay of zygotes are unknown. To develop a model of oxidative-damaged zygotes, we treated mouse zygotes with different concentrations of H2O2 (0, 0.01, 0.02, 0.03, 0.04, 0.05 mM), and evaluated in vitro zygote development, BrdU incorporation to detect the duration of S phase. We also examined reactive oxygen species level and used immunofluorescence to detect activation of γH2AX, Cdc2, and Cdc25. Oxidatively damaged zygotes showed a delay in G2/M phase and produced a higher level of ROS. At the same time, γH2AX was detected in oxidatively damaged zygotes as well as phospho-Cdc25B (Ser323), phospho-Cdc25C (Ser216), and phospho-Cdc2 (Tyr15). Our study indicates that oxidative stress-induced DNA damage of mouse zygotes triggers the cell cycle checkpoint, which results in G2/M cell cycle arrest, and that phospho-Cdc25B (Ser323), phospho-Cdc25C (Ser216), and phospho-Cdc2 (Tyr15) participate in activating the G2/M checkpoint.
Keywords: Oxidative stress, DNA damage, G2/M checkpoint, Cdc25, Cdc2, Zygote
Introduction
In vitro fertilization and embryo transfer (IVF-ET), just as its name implies, involves transfer of embryos, from the outside world to the uterus, after fertilization in vitro. The environment, which includes culture medium, light, temperature, and the gas phase, plays an important role in supporting the growth and development of the zygote (Latham 2016). Alteration of these factors will lead to oxidative stress for producing excessive reactive oxygen species (ROS) (Agarwal et al. 2014; Ménézo et al. 2010). In most mouse strains, IVF embryos always show developmental arrest due to deterioration during culture (Kimura et al. 2010). Among the factors that affect the developmental competence of IVF embryos, oxidative stress (OS) is a well-known inducer of developmental arrest (Orsi and Leese 2001; Takahashi 2012). We found that embryos in clinic are apparently normal in Day 3 (D3), but many do not develop to the blastocyst stage (Ventura-Junca et al. 2015). This may be related to OS (Meuter et al. 2014). However, because of ethics limitations, we cannot test this in clinic. So, we used hydrogen peroxide (H2O2) to treat mouse zygotes to simulate the clinical phenomenon (Radak et al. 2013).
The general consensus is that cell cycle checkpoints, including G1/S, intra-S, and G2/M, are involved in DNA damage response reactions (Luft et al. 2001; Cho et al. 2013). In addition, γH2AX, a marker for DNA damage, is an early indicator of DNA double strand breaks and plays an important role in DNA damage response (Benzina et al. 2015). Our previous studies found that mouse embryos fertilized with H2O2-treated sperm show the appearance of γH2AX and a delay in the first cleavage (Wang et al. 2013). In addition, checkpoint proteins ATM, Chk1, and Cdc25 are phosphorylated and activated in zygotes fertilized with H2O2-treated sperm (Wang et al. 2013; Song et al. 2014), which indicates that embryos fertilized with treated sperm might be arrested at the G2/M checkpoint, through the ATM → Chk1 → Cdc25B/Cdc25C pathway (Song et al. 2014), to give time to repair damaged sperm DNA. Studies in fibroblasts have shown that oxidative damage leads to G1/S arrest (Chien et al. 2000). Interestingly, H2O2-induced damage leads to G2/M growth arrest in normal uroepithelial cells (Chien et al. 2000). Thus, cell signaling can differ in response to oxidative damage in various cells (Shaltiel et al. 2015; Yoshiyama et al. 2013).
However, it remains elusive whether oxidative damage triggers cell cycle checkpoints in zygotes, and the potential oxidative damage repair mechanisms are unknown. Available literature suggests that maternal genes are expressed in the embryo until zygotic genes become activated (Xu et al. 2014). In the mouse, activation of zygotic genes begins at the one-cell stage and peaks at the two-cell stage (Hamazaki et al. 2015). Some studies do not consider that zygotic genes have an effect at the one-cell stage (Hamazaki et al. 2015; Shi et al. 2015), leading to the possibility that oxidative damage repair mechanisms of one-celled embryos might differ from other types of cells. The Cdc25 family of dual-specificity phosphatases, which can regulate cell cycle progression by controlling cyclin dependent kinases, is composed by Cdc25A, Cdc25B, and Cdc25C (Sur and Agrawal 2016). M-phase promoting factor (MPF), composed of Cdc2 and Cyclin B, is a master regulator in cell cycle of mitosis and Cdc2 is inactive via phosphorylation on tyrosine 15 (Tyr15) and threonine 14(Thr14) (Shaltiel et al. 2015). Microinjection of Cdc25B-S321A-mRNA into mouse zygotes in S phase results in premature MPF activity by dephosphorylating Cdc2-Tyr15 (Xiao et al. 2011), and mutant of Cdc25B-S323 causes delayed progression from G2 into mitosis (Lyons et al. 2013), indicating that phosphorylation of Cdc25B plays a vital role in regulating G2/M transition in mouse zygotes (Depamphilis et al. 2012; Meng et al. 2013) and that phosphorylation of Cdc25B and Cdc2 is an important regulatory mechanism for Cdc25B and Cdc2 activity to regulate MPF activity and cell mitosis (Cui et al. 2014; Xiao et al. 2011). This also suggests that oxidative damage in fertilized eggs may lead to a fluctuation of the phosphorylation level of the Cdc25 family and Cdc2, ultimately regulating the activity of MPF and resulting in a retardation of progression through G2/M phase (Bouldin and Kimelman 2014). Studies identified that the Cdc2-Tyr15 is a critical protein involved in G2/M checkpoint control in response to DNA damage (Yan et al. 2012). Moreover, oxidative damage is also associated with the deactivation of Cdc25C by phosphorylation at Ser216 (Le et al. 2006; Savitsky and Finkel 2002). We speculate that the Cdc25 family and Cdc2 can be important signaling molecules in mouse fertilized egg cell cycle checkpoints.
H2O2 has been used as a model for oxidative damage due to its ability to increase intracellular reactive oxygen species levels, as well as its long physiological half-life (8 h–20 days) and involvement in oxidative damage and signaling-related pathways (Bain et al. 2011; Chien et al. 2000; Yang et al. 2012). We here establish a model for fertilized eggs with oxidative damage, using H2O2, and show the effects of oxidative damage on early embryonic development, cell cycle checkpoints, expression of ROS, γH2AX, Cdc25 family members, and Cdc2 to reveal oxidative damage repair mechanisms in fertilized eggs.
Materials and methods
Experimental animals
Adult Kun-Ming mice (3–6 weeks old), used for our experiments, were purchased from the animal center of Shantou University Medical College. All animals were treated in compliance with the rules of the National Animal Protection of China and The Guide for the Care of Use of Laboratory Animals by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). All experimental protocols were approved by the Laboratory Animal Ethics Committee of our institution (SUMC2014-014). This study was authorized by the Institutional Animal Care and Use Committee of Shantou University Medical College.
Collection of sperm and oocytes, IVF, culture and observation of embryos
According to our previous studies (Xiao et al. 2011), to collect sperm, male mice were euthanized and the caudate epididymis was washed clean in phosphate-buffered saline (PBS) at 37 °C, then minced with scissors and incubated in capacitation medium (HTF medium [Cooper Surgical Inc.] supplemented with 1.5 % BSA) under a 5 % CO2 atmosphere, at 37 °C, for 1 h. Female mice were induced to superovulate by sequential injection of 10 IU pregnant mare serum gonadotropin (PMSG) and 10 IU human chorionic gonadotropin (HCG), 48 h apart. At 13 to 15 h after HCG administration, mice were euthanized and cumulus oocytes were obtained from the oviducts, washed in PBS at 37 °C, and then placed in drops of fertilization liquid (HTF medium containing 0.4 % BSA) under oil. Then, 10 μL sperm, prepared in sperm capacitation liquid, was added to each drop of fertilization liquid, containing denuded oocytes, and incubated in a 5 % CO2 incubator at 37 °C for 6 h. After insemination, embryos were placed in 37 °C HTF medium, washed, and then cultured in embryo culture medium (HTF medium supplemented with 0.4 % BSA and 10 % fetal bovine serum) in a 5 % CO2 incubator at 37 °C. The embryo culture medium was renewed daily. For blastocyst cultures, we performed the same operation as above but used blastocyst medium (Cooper Surgical Inc.) instead of embryo culture medium.
Mouse zygote model for oxidative damage
To develop a mouse zygote model for oxidative damage, we selected two-pronuclear embryos in G1 phase, at 7-h post insemination (hpi), then exposed zygotes to embryo culture medium containing different concentrations of H2O2 (0, 0.01, 0.02, 0.03, 0.04, 0.05 mM), for 30 min, selected on the basis of prior references (Bain et al. 2011; Liu and Keefe 2000), and our preliminary experiments. Embryos in control and treated groups were then washed in fresh embryo culture medium extensively and cultured in embryo culture medium at 37 °C and 5 % CO2 until day 4. To evaluate the model, cleavage and embryo development were examined with an inverted microscope (Olympus Inc., Japan) every 24 h for 5 days. In order to guarantee the reliability of the experimental data, groups of zygotes were treated with various concentrations of H2O2. The total number of zygotes, used at 0, 0.01, 0.02, 0.03, 0.04, and 0.05 mM H2O2, was 443, 363, 316, 393, 365, and 326, respectively.
Onset and endpoint of S phase and the endpoint of M phase of mouse zygotes and mouse 2-cell embryos
The onset and endpoint of S phase was determined by BrdU incorporation according to our previous studies (Wang et al. 2013). Mouse zygotes were divided into H2O2-treated and untreated groups of 15–25 zygotes per group and incubated in embryo culture medium at 37 °C in a 5 % CO2 incubator. Beginning at 8 h and continuing through 19-h post insemination, BrdU incorporation was measured every other hour by incubating zygotes in 1 mM BrdU-containing embryo culture medium for half an hour. Zygotes were then fixed in 2.5 % paraformaldehyde for 15 min, placed on polylysine slides, and washed three times with PBS containing 10 % FBS and 0.2 % Triton X-100, followed by incubation in 1 mM HCl for half an hour, and washing in 0.1 mM borate buffer solution for 20 min. Cells were permeabilized by washing three times with PBS containing 10 % FBS and 0.2 % Triton X-100, then blocked in the same solution for half an hour at 37 °C. Zygotes were subsequently incubated with 6 μg/mL anti-BrdU antibody (Sigma) for 1 h at 37 °C, washed three times with PBS containing 2 % FBS and 0.1 % Triton X-100, then treated with secondary FITC-conjugated goat anti-mouse IgG antibody (Sigma) for 1 h at 37 °C. Embryos were washed three times with PBS containing 2 % FBS and 0.1 % Triton X-100, counterstained with 10 μg/mL propidium iodide (PI) (Beyotime) overnight at 4 °C, washed again, and coverslipped with mounting medium (Beyotime). A confocal microscope (Olympus FluoView FV 1000, Japan) was used to observe the immunocytochemical staining. The number of total zygotes and BrdU-positive zygotes were counted to assess the frequency of BrdU-positive zygotes (number of BrdU-positive zygotes/total number of zygotes scored). The time point when frequency of BrdU-positive ≥10 % was accepted as the starting time of S phase, after 100 % BrdU-positive, it began to fade away, and the time when 90 % BrdU-positive vanishing was accepted as the end of S phase. Six hundred forty-one zygotes from the untreated group and 653 zygotes from the treated group were used for the BrdU incorporation experiments to assess S phase. We observed early embryonic development and calculated the zygotic embryo cleavage rate half an hour a time from 17 to 26 hpi then calculated the 2-cell embryo cleavage rate an hour a time from 45 to 55 hpi. The time point when 95 % of the zygotic embryos and 2-cell embryos cleaved was assessed as the endpoint of M phase. Two hundred thirty-four zygotes from the treated group and 230 zygotes from the untreated control group were used for determination of the endpoint of M phase.
Determination of ROS products
To determine ROS levels, zygotes in 0.03 mM H2O2 treated and untreated group were incubated in 2 μM dichlorodihydrofluorescein diacetate (Sigma, USA) (DCFH-DA)-containing HTF medium for 30 min at 37 °C after exposing to H2O2. Zygotes were then washed three times with HTF medium and mounted on glass slides. Fluorescence was measured using a confocal microscope (Olympus FluoView FV 1000, Japan) with exciting light of 495 nm and emissive light of 520 nm. Image-Pro Plus 5.0 software was used to analyze DCF fluorescence intensity in zygotes. Fifty zygotes from the treated group and 47 zygotes from the untreated group were used for determination of ROS products.
Immunofluorescence staining for checkpoint pathway activation
To investigate the oxidative damage checkpoint pathway underlying the possible cell cycle arrest in oxidative-damaged mouse zygotes, we collected zygotes in the pronuclear stage to detect the activations of relevant regulatory proteins: γH2AX, phospho-Cdc25A (Ser76), phospho-Cdc25B (Ser323), phospho-Cdc25C (Ser216), and phospho-Cdc2 (Tyr15). Zygotes were initially washed with TPBS (PBS containing 0.05 % Tween-20). To remove the zona pellucidae, embryos were digested with 0.1 % pancreatin, then fixed in 4 % paraformaldehyde for half an hour and mounted on polylysine-coated slides, washed with TPBS three times, 5 min each time, then permeabilized with TPBS supplemented with 0.5 % Triton X-100 at room temperature for half an hour. Permeabilized zygotes were washed with TPBS as above and blocked for 1 h at room temperature in blocking solution (TPBS containing 3 % BSA and 10 % goat serum) then incubated 1 h at 37 °C with antibody against γH2AX (1:500 dilution, Abcam) or incubated overnight at 4 °C with antibodies against phospho-Cdc25A (Ser76) (1:200 dilution, Santa Cruz Biotechnology), phospho-Cdc25B (Ser323) (1:200 dilution, Biorbyt), phospho-Cdc25C (Ser216) (1:200 dilution, Biorbyt), and phospho-Cdc2 (Tyr15) (1:500 dilution, Abcam). Embryos were then washed with TBS and incubated with secondary antibody at room temperature for 1 h (For γH2AX: goat anti-mouse IgG-Alexa Fluor 488, 1:500 dilution, Sigma; for Cdc25 and Cdc2: goat anti-rabbit IgG-FITC, 1:500 dilution, Santa Cruz Biotechnology), washed with TPBS and counterstained with PI (Beyotime) (for γH2AX) or 4,6-diamidino-2-phenylindole (DAPI) (Sigma) (for Cdc25 and Cdc2) at room temperature for half an hour. Zygotes were then washed three times and coverslipped with mounting medium (Beyotime). A confocal microscope (Olympus FluoView FV 1000, Japan) was used to observe the signal.
Statistical analysis
Data are shown as mean ± SD. To identify the differences between the treated and untreated groups, we applied a t test to analyze the data, including the onset and the endpoint of S phase, the onset of M phase, the percentage of embryos at different developmental stages, and quantification of relative fluorescence intensity of ROS, by using SPSS 17.0 software (SPSS Inc., USA). A p < 0.05 was considered statistically significant.
Results
Development of zygotes treated with different concentrations of H2O2
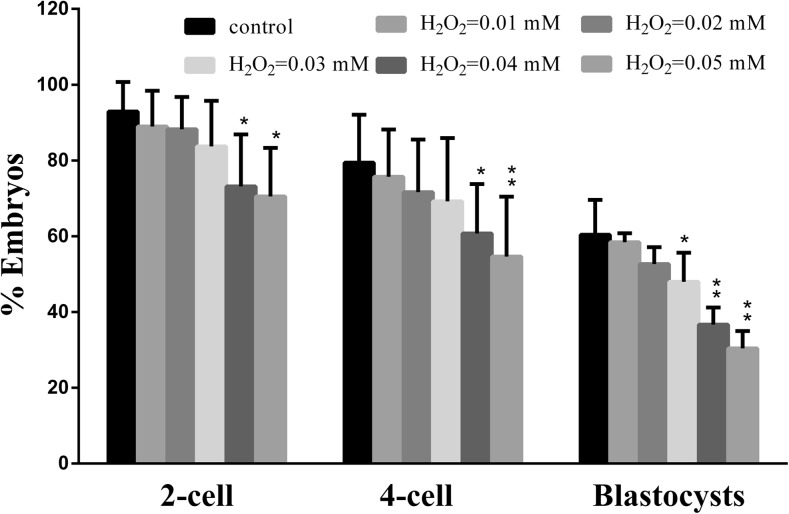
To induce oxidative stress, different concentrations (0, 0.01, 0.02, 0.03, 0.04, 0.05 mM) of H2O2 were used to treat mouse zygotes, then the dose-effect of H2O2 on embryonic development was investigated. H2O2 at 0.01 and 0.02 mM had no significant effect on the embryonic development (Fig. 1). However, exposure of mouse zygotes to 0.03 mM H2O2 reduced the rate of blastocyst formation but had no significant reduction in the rates of 2- or 4-cell formation. Exposure to H2O2 at 0.04 and 0.05 mM produced a reduction in the rates of 2-cell, 4-cell, and blastocyst formation. As embryos in clinic are apparently normal in D3, but some do not develop to the blastocyst stage which may be related to oxidative stress, so we assume that the concentration of H2O2 in 0.03 mM may be the minimum value which induced oxidative damage in the zygote.
Fig. 1.
The developmental profiles of zygotes treated with a titration of H2O2. Zygotes exposed to H2O2 at 0.03 mM had no significant reduction in the rates of 2-cell, 4-cell formation, while produced a reduction in the rates of blastocyst formation. Treatment during the zygote stage resulted in a significant decrease in the proportion of both 2-cell embryos, 4-cell embryos, and blastocyst formation with the concentration of 0.04 and 0.05 mM. H2O2 at 0.01 and 0.02 mM had no significant effect on the embryonic development. Significant (p < 0.05) differences are noted by different asterisks above bars
Onset and endpoint of S phase and the endpoint of M phase of mouse zygotes and mouse 2-cell embryos in the treated vs. untreated groups
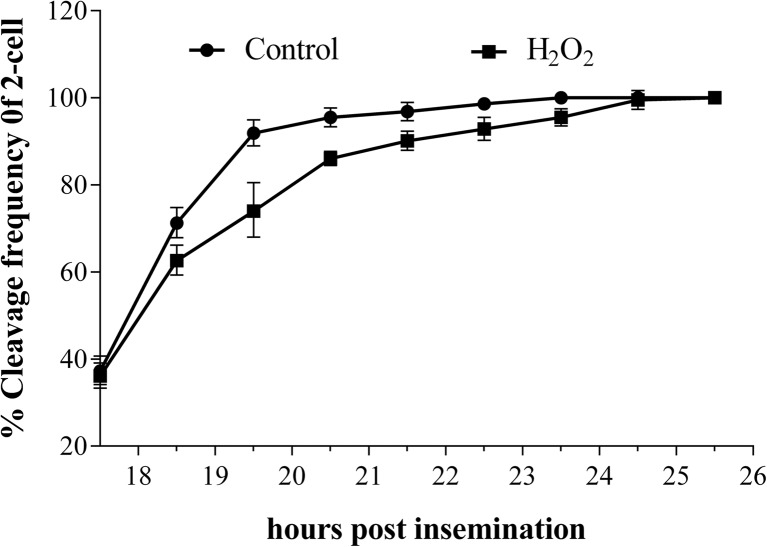
The onset and endpoint of S phase were determined by BrdU incorporation (Fig. 2). At 0.03 mM H2O2, onset of S phase of the zygotes was nearly identical for the treated (9.86 ± 0.26 hpi) and control (9.67 ± 0.42 hpi) groups (p > 0.05) (Table 1). Similarly, the S phase endpoints of the treated and control groups were 17.42 ± 0.37 hpi and 17.14 ± 0.26 hpi, respectively (p > 0.05). However, the zygotes in the treated and control groups displayed a significant difference in M phase endpoint, with the treated group exhibiting a 3-h delay (23.43 ± 0.40 hpi vs. 20.10 ± 0.36 hpi, p < 0.05) (Table 1, Fig. 3). For 2-cell embryos, the endpoints of M phase of the treated and the control groups were similar at 49.47 ± 1.45 hpi and 48.37 ± 0.57 hpi (p > 0.05), respectively. These results suggest that the reduction in the rate of blastocyst formation, observed above, was due to a delay in G2/M phase.
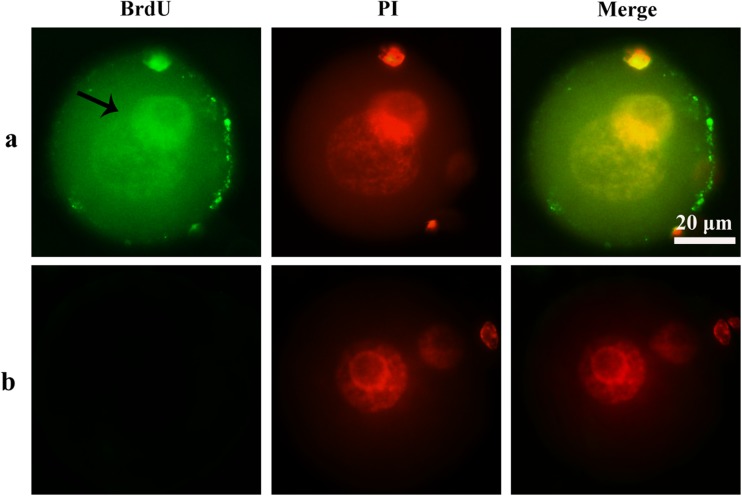
Fig. 2.
The signal of BrdU. a BrdU-positive zygotes. b BrdU-negetive zygotes. Nuclei were stained with PI (red fluorescent), PI propidium iodide. In BrdU-positive zygotes, BrdU fluorescent (green fluorescent) was detected in the nucleus. No staining was detected in BrdU-negative zygotes
Table 1.
Onset and endpoint of S phase and the endpoint of M phase of mouse embryos in the untreated control and the treated groups
| Groups | S phase onset of zygotes | S phase endpoint of zygotes | M phase endpoint of zygotes | The M phase endpoint of 2-cell embryos |
|---|---|---|---|---|
| Control | 9.67 ± 0.42 hpi | 17.14 ± 0.26 hpi | 20.10 ± 0.36 hpi | 48.37 ± 0.57 hpi |
| H2O2-treated | 9.86 ± 0.26 hpi | 17.42 ± 0.37 hpi | 23.43 ± 0.40 hpi | 49.47 ± 1.45 hpi |
| p | >0.05 | >0.05 | <0.05 | >0.05 |
Data are expressed as mean ± SD. p values were determined by t test. The concentration of H2O2 = 0.03 mM
hpi hours post insemination
Fig. 3.
The time of first cleavage in control and treated group. Zygotes cultured under H2O2 at 0.03 mM underwent their first cleavage division after those without H2O2. This difference in cleavage was significant (p < 0.05) during 19 and 20 hpi between the two groups. When time came to 24 hpi, difference in cleavage was not significant (p > 0.05)
The ROS level in treated and untreated zygotes
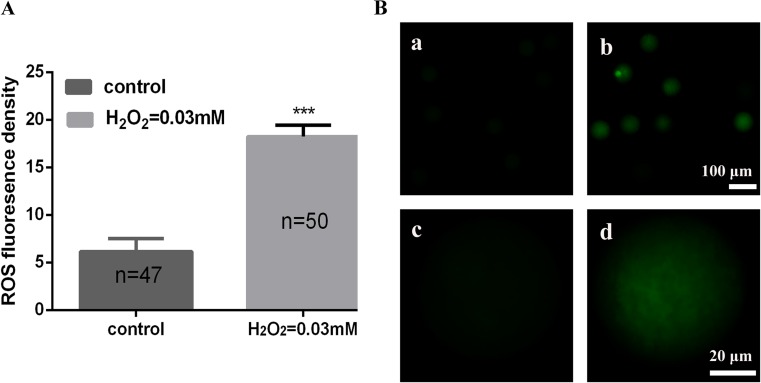
To evaluate OS in zygotes, we tested the level of intracellular ROS using the fluorescent probe DCFH-DA. The DCF fluorescence intensity in zygotes of 0.03 mM H2O2 group was 18.23 ± 1.20, significantly higher than untreated group (6.14 ± 1.38) which indicated an augmented production of ROS (p < 0.001) (Fig. 4a, b).
Fig. 4.
The ROS level in control and treated group. a Quantification of relative fluorescence intensity of ROS in control and treated group. Data are presented as mean ± SD. b Representative images of ROS levels in zygotes: (a) control group, (b) H2O2 treated group, (c) a magnified view of one zygote from a, (d) a magnified view of one zygote from b. Intracellular ROS were measured by the DCF fluorescence (green)
Activation of γH2AX of mouse zygotes in treated and untreated groups
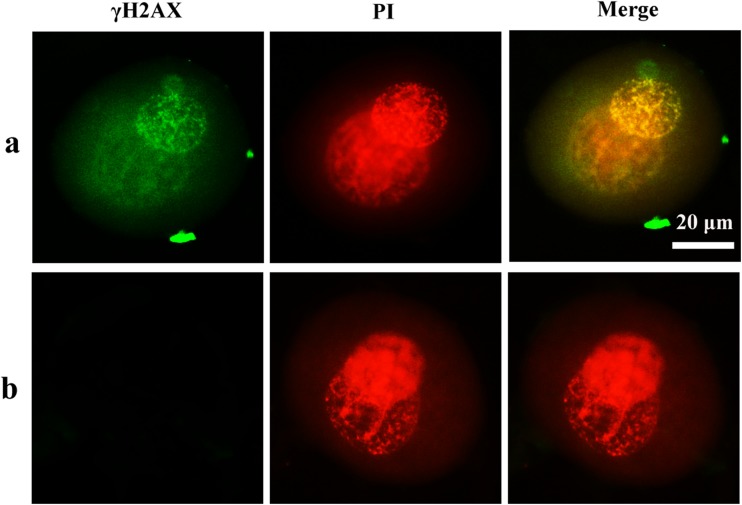
In order to assess whether OS causes DNA damage, we used immunofluorescence microscopy to monitor the presence of γH2AX staining, a sensitive and early marker of DNA damage. As reported in Fig. 5, we detected γH2AX staining in pronucleus of zygotes in treated groups with 0.03 mM H2O2. In contrast to the H2O2-treated group, γH2AX was not detected in control zygotes.
Fig. 5.
Signal of γH2AX in mouse zygotes. a H2O2-treated group. b Control group. Nuclei are stained with PI (red fluorescent). In H2O2-treated group, γH2AX was detected in the pronucleus. No staining was detected in the control group
Activation of Cdc25 isoforms and Cdc2 of mouse zygotes in treated and untreated groups
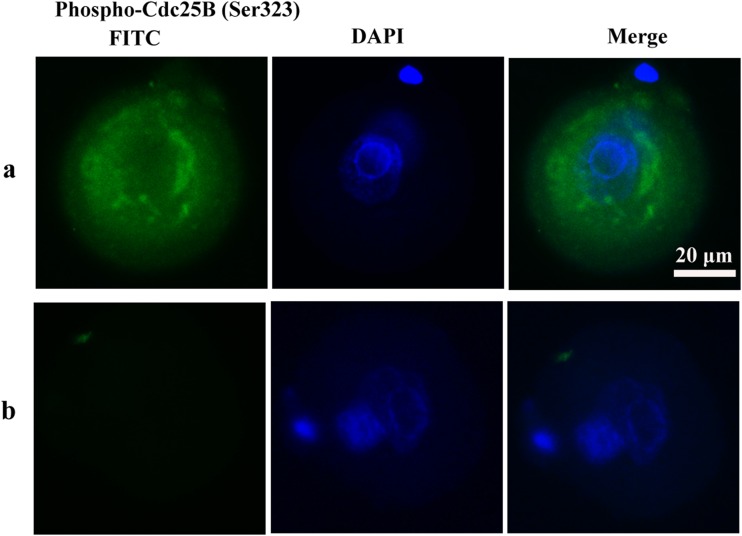
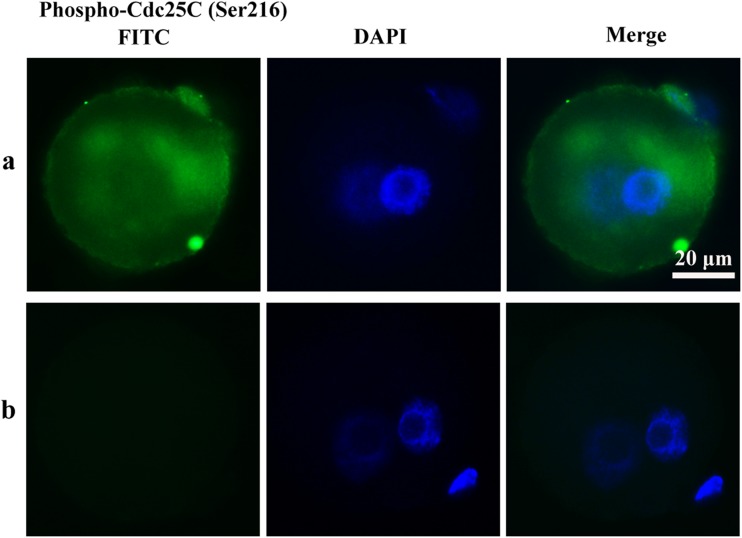
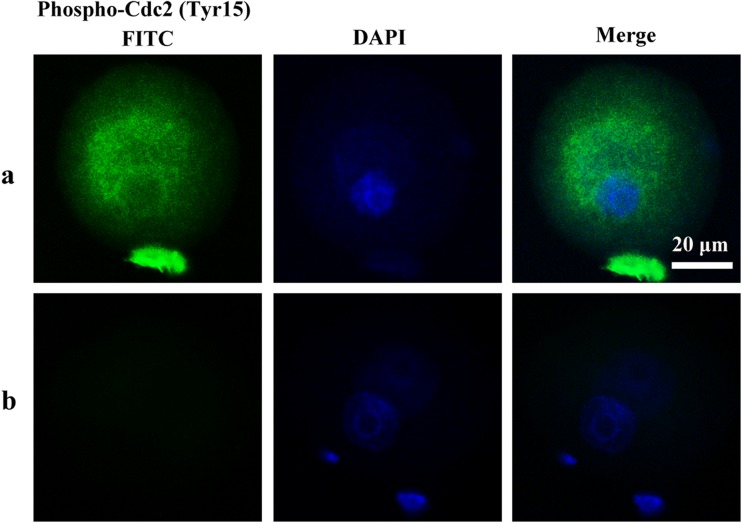
To determine whether cell cycle checkpoint activation was responsible for the delay of M phase, the phosphorylation of Cdc25A (Ser76), Cdc25B (Ser323), Cdc25C (Ser216), and Cdc2 (Tyr15) of mouse zygotes in the treated and the untreated control groups was determined by immunocytochemistry. In the H2O2-treated group, phospho-Cdc25C (Ser216), Cdc25B (Ser323), and Cdc2 (Tyr15) were detected in the zygote cytoplasm. Phospho-Cdc25A (Ser76) activation was not observed in the H2O2-treated group in zygotes. In contrast to the H2O2-treated group, phosphorylation of the Cdc25 isoforms and Cdc2 was not detected in control zygotes (Figs. 6, 7, and 8). These results suggest oxidative stress-induced DNA Damage of mouse zygotes triggers G2/M checkpoint and phosphorylates Cdc25 and Cdc2.
Fig. 6.
Phosphorylation of Cdc25B in mouse zygotes. a H2O2-treated group. b Control group. Nuclei are stained with DAPI (blue fluorescent), DAPI: 40′6-diamidino-2-phenylindole. In H2O2-treated group, Phospho-Cdc25B (Ser323) was detected in the cytoplasm diffusely surrounding the pronucleus. No staining was detected in control group
Fig. 7.
Phosphorylation of Cdc25C in mouse zygotes. a H2O2-treated group. b Control group. Nuclei are stained with DAPI (blue fluorescent), DAPI: 4′6-diamidino-2-phenylindole. In H2O2-treated group, Phospho-Cdc25C (Ser216) was detected in the cytoplasm diffusely surrounding the pronucleus. No staining was detected in the control group
Fig. 8.
Phosphorylation of Cdc2 in mouse zygotes. a H2O2-treated group. b Control group. Nuclei are stained with DAPI (blue fluorescent), DAPI: 4′6-diamidino-2-phenylindole. In H2O2-treated group, Phospho-Cdc2 (Tyr15) was detected in the cytoplasm diffusely surrounding the pronucleus. No staining was detected in the control group
Discussion
Gametes and embryos are sensitive to culture conditions and the surrounding environment. During embryo collection and manipulation in vitro, the fertilizability and subsequent developmental competence are negatively impacted (Kimura et al. 2010; Wale and Gardner 2016), in particular by oxidative stress (Wang et al. 2009; Orsi and Leese 2001). Also, others have reported that cell cycle arrest may be the result of oxidative damage (Houtgraaf et al. 2006), and oxidative damage ultimately may affect viability or long-term development (Wale and Gardner 2016). Although numerous studies have examined the early development of DNA-damaged zygotes caused by ROS (Bain et al. 2011; Liu and Keefe 2000), it is not clear how mammalian zygotes respond to oxidative damage (Gawecka et al. 2013).
To study the mechanism of how the zygote responds to oxidative damage, and identify the related signaling pathways, and to simulate clinical in vitro fertilization, we developed an oxidative damage zygote model using H2O2 as the oxidative damaging agent. We show that H2O2 at 0.01 and 0.02 mM had no significant effect on embryonic development. However, although mouse zygotes exposed to H2O2 at 0.03 mM had no significant reduction in the rates of 2- and 4-cell formation, the zygotes showed a reduction in the rate of blastocyst formation. At 0.04 and 0.05 mM, H2O2 produced a reduction in the rates of 2-cell, 4-cell, and blastocyst formation. These results suggest that zygotes exposed to H2O2 at 0.03 mM produced a reduction in the rates of blastocyst formation that may be the result of the oxidative damage. We assume that the concentration of H2O2 in 0.03 mM may be the minimum value which induced oxidative damage in the zygote, so is the most similar situation to the phenomenon in the clinic.
Based on our established oxidative-damaged mouse zygote model, a single minimum concentration of H2O2 (0.03 mM) which induced oxidative damage in the zygote was elected as the treated group. We compared duration of S phase and G2/M phase between the control group and treated group. No significant difference was found in S phase (p > 0.05) between treated and untreated control groups. However, treatment of zygotes resulted in a statistically significant 3-h delay in M phase entry (p < 0.05). In addition, our data show that exposure to H2O2 at 0.03 mM produced a higher level of ROS than untreated group, and γH2AX was detected in mouse zygotes exposed to H2O2 at 0.03 mM. Although several cellular components will be oxidized upon H2O2 exposure, including lipids, proteins, and DNA, because γ-H2AX was only detected in H2O2 treated zygotes; this would suggest that DNA damage is the likely cause for the delay. Consistent with this, H2O2-mediated oxidative stress induced G2/M cell cycle arrest, a cell cycle checkpoint, although cells did not arrest in G1/S and S phase. However, it has been reported that embryonic stem cells lack a G1/S checkpoint due to degradation of p21 protein after γ-irradiation or X-irradiation (Shimura et al. 2002). A similar mechanism may also be responsible for the lack of a G1/S checkpoint in oxidative-damaged mouse zygotes. Cdc25A is related to the intra-S checkpoint (Sur and Agrawal 2016). However, phospho-Cdc25A (Ser76) was not detected in our experiment. This would also explain why oxidative-damaged zygotes go through S phase without any delay. As our results show that the activation of the G2/M checkpoint may result from phosphorylation of Cdc25B and Cdc25C. Moreover, the 3-h delay in G2/M, induced with 0.03 mM H2O2, is longer than the 2.5-h delay observed in mouse embryos following fertilization with oxygen-stressed sperm (2.5 h) (Wang et al. 2013), indicating a requirement for more time for the zygote to repair oxidative damage induced in G1 phase than introduction of oxidative by sperm. DNA repair in the embryo relies entirely on maternal mRNAs and proteins (Derijck et al. 2008). Based on data presented here, we speculate that when oxidative damage occurs in the fertilized egg, maternal genes may also be damaged, which will affect DNA repair. Thus, more time is needed for zygotes to repair oxidative damage induced in G1 phase. The involvement of maternal genes in the mechanism of DNA repair in zygotes will be our research direction in the future. In addition, M phase entry of zygotes in the treated group is delayed, but the difference in cleavage is not significant between two groups at 24 hpi. One possible reason might be that the 2-cell cleavage stage has a pause, i.e., when control zygotes in the 2-cell cleavage stage reach 95 % at 20 hpi, cells arrest without significant changes in 2-cell cleavage during 20 hpi to 24 hpi, enabling the H2O2-treated zygotes to catch up. Another possibility is that the speed of the 2-cell cleavage in the treated group is faster as a result of embryo mitochondrial energy consumption in advance after oxidative damage recovery (Bain et al. 2011). This may also result in a decline of embryo development potential (Van et al. 2006).
At a concentration of 0.03 mM, we observed a delay of G2/M phase and a decline in the rate of blastocyst formation in the treated group, without a difference in the 2-cell cleavage rate and 4-cell cleavage rate, compared with the control group, which is consistent with clinical phenomena (Johnson and Nasr-Esfahani 1994). We assume that DNA repair functions are activated in mouse embryos once DNA is damaged, which helps embryos reach the 4-cell stage. However, the function of the G2/M checkpoint is limited in mouse zygotes because it depends on the limited oocyte-derived transcripts and proteins (Yukawa et al. 2007). The results in our experiment indicate that the G2/M checkpoint in mouse zygotes is only partially able to cope with oxidative DNA damage. Several studies have shown that after DNA damage sensing, cell cycle checkpoints would proceed to apoptosis if the damage overwhelms the repair mechanisms, is incorrectly repaired, or is partially repaired (Cho et al. 2013; Kastan and Bartek 2004). Our study suggests that residual DNA damage prevents embryo entry into the blastocyst stage. As mentioned above, DNA damage repair may just be partial, limiting the developmental potential of the embryo to reach the blastocyst stage. Indeed, this phenomenon corresponds with clinical trials (Nasr-Esfahan et al. 1992), yet most of the embryo transfer occurs within a 3-day period, ahead of blastocyst formation. Thus, some apparently normal embryos are transplanted, despite the oxidative damage-mediated decline in the success rate of transplantation.
Consistent with our previous research (Song et al. 2014), we found that in the H2O2-treated group, phospho-Cdc25C (Ser216) and phospho-Cdc25B (Ser323), but not phospho-Cdc25A (Ser76), are detected. Savitsky and Finkel also demonstrated that exposure to H2O2 impacts Cdc25C rather than Cdc25A levels (Savitsky and Finkel 2002). Moreover, Cdc25B is also a key regulator of G2/M transition (Astuti et al. 2010; Lammer et al. 1998). We assume that Cdc25B and Cdc25C are concomitant with G2/M cell cycle arrest in oxidatively damaged zygotes. In our previous research, phospho-Cdc25C (Ser216) was found in and around one of the pronucleus in mouse zygotes fertilized with H2O2-treated sperm (Song et al. 2014). Phospho-Cdc25C (Ser216) has been reported expression mainly in nucleus in vulvar carcinomas and expression in cytoplasm in normal vulvar squamous epithelium. Phospho-Cdc25C (Ser 216) may stay in the nucleus and activate MPF, thus triggering G2/M transition (Wang et al. 2010). In our research, Phospho-Cdc25C (Ser 216) only expressed in cytoplasm in H2O2 treated zygotes, which may lose its access to nuclear MPF, thus inhibiting mitotic entry. It indicates that Phospho-Cdc25C (Ser 216) can be located in the nucleus as well as the cytoplasm, but different position may result in different function (Wang et al. 2010). In addition, phospho-Cdc25C (Ser216), phospho-Cdc25B (Ser323), and Cdc2-Tyr15 are detected surrounding one of the pronuclei, possibly the maternal pronucleus, because DNA damage in the zygote stage is repaired by maternal gene expression (Derijck et al. 2008). In agreement with our study, γ-irradiation (IR) and Jaridonin induce G2/M arrest and concomitant diminution of activation of Cdc25C-Ser216 and Cdc2-Tyr15 (Yan et al. 2012; Ma et al. 2015).
In conclusion, our study suggests that cell cycle arrest mediated by G2/M checkpoint activation occurs in mouse zygotes treated with H2O2. Phospho-Cdc25 and phospho-Cdc2 correlate with activating the G2/M checkpoint in mouse zygotes treated with H2O2.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81471522, No. 81070542, and No. 30872771) and the Natural Science Foundation of Guangdong Province of China (No. 2060203, No. 10151503102000020, and No. 81515031102000010).
References
- Agarwal A, Durairajanayagam D, du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol. 2014;12:112. doi: 10.1186/1477-7827-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti P, Boutros R, Ducommun B, Gabrielli B. Mitotic phosphorylation of Cdc25B Ser321 disrupts 14-3-3 binding to the high affinity Ser323 site. J Biol Chem. 2010;285:34364–70. doi: 10.1074/jbc.M110.138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain NT, Madan P, Betts DH. The early embryo response to intracellular reactive oxygen species is developmentally regulated. Reprod Fertil Dev. 2011;23:561–75. doi: 10.1071/RD10148. [DOI] [PubMed] [Google Scholar]
- Benzina S, Pitaval A, Lemercier C, Lustremant C, Frouin V, Wu N, Papine A, Soussaline F, Romeo PH, Gidrol X. A kinome-targeted RNAi-based screen links FGF signaling to H2AX phosphorylation in response to radiation. Cell Mol Life Sci. 2015;72:3559–73. doi: 10.1007/s00018-015-1901-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouldin CM, Kimelman D. Cdc25 and the importance of G2 control: insights from developmental biology. Cell Cycle. 2014;13:2165–71. doi: 10.4161/cc.29537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien M, Rinker SC, Stadler WM. A G2/M growth arrest response to low-dose intermittent H2O2 in normal uroepithelial cells. Int J Oncol. 2000;17:425–32. doi: 10.3892/ijo.17.3.425. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Oh YJ, Han SH, Chung HJ, Kim CH, Lee NS, Kim WJ, Choi JM, Kim H. Cdk1 protein-mediated phosphorylation of receptor-associated protein 80 (RAP80) serine 677 modulates DNA damage-induced G2/M checkpoint and cell survival. J Biol Chem. 2013;288:3768–76. doi: 10.1074/jbc.M112.401299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Ren X, Liu D, Deng X, Qin X, Zhao X, Wang E, Yu B. 14-3-3 epsilon prevents G2/M transition of fertilized mouse eggs by binding with Cdc25B. BMC Dev Biol. 2014;14:33. doi: 10.1186/s12861-014-0033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depamphilis ML, de Renty CM, Ullah Z, Lee CY. "The Octet": Eight Protein Kinases that Control Mammalian DNA Replication. Front Physiol. 2012;3:368. doi: 10.3389/fphys.2012.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijck A, van der Heijden G, Giele M, Philippens M, de Boer P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mole Genet. 2008;17:1922–37. doi: 10.1093/hmg/ddn090. [DOI] [PubMed] [Google Scholar]
- Gawecka JE, Marh J, Ortega M, Yamauchi Y, Ward MA, Ward WS. Mouse zygotes respond to severe sperm DNA damage by delaying paternal DNA replication and embryonic development. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki N, Uesaka M, Nakashima K, Agata K, Imamura T. Gene activation-associated long noncoding RNAs function in mouse preimplantation development. Development. 2015;142:910–20. doi: 10.1242/dev.116996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtgraaf JH, Versmissen J, van der Giessen WJ. A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovascular Revascularization Medicine Including Molecular Interventions. 2006;7:165–72. doi: 10.1016/j.carrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–8. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tsunoda S, Iuchi Y, Abe H, Totsukawa K, Fujii J. Intrinsic oxidative stress causes either 2-cell arrest or cell death depending on developmental stage of the embryos from SOD1-deficient mice. Mol Hum Reprod. 2010;16:441–51. doi: 10.1093/molehr/gaq007. [DOI] [PubMed] [Google Scholar]
- Lammer C, Wagerer S, Saffrich R, Mertens D, Ansorge W, Hoffmann I. The Cdc25B phosphatase is essential for the G2/M phase transition in human cells. J Cell Sci. 1998;111:2445–53. doi: 10.1242/jcs.111.16.2445. [DOI] [PubMed] [Google Scholar]
- Latham KE. Stress signaling in mammalian oocytes and embryos: a basis for intervention and improvement of outcomes. Cell Tissue Res. 2016;363:159–67. doi: 10.1007/s00441-015-2124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le GG, Esteve PO, Ferec C, Pradhan S. DNA damage-induced down-regulation of human Cdc25C and Cdc2 is mediated by cooperation between p53 and maintenance DNA (cytosine-5) methyltransferase 1. J Biol Chem. 2006;281:24161–70. doi: 10.1074/jbc.M603724200. [DOI] [PubMed] [Google Scholar]
- Liu L, Keefe DL. Cytoplasm mediates both development and oxidation-induced apoptotic cell death in mouse zygotes. Biol Reprod. 2000;62:1828–34. doi: 10.1095/biolreprod62.6.1828. [DOI] [PubMed] [Google Scholar]
- Luft JC, Benjamin IJ, Mestril R, Dix DJ. Heat shock factor 1-mediated thermotolerance prevents cell death and results in G2/M cell cycle arrest. Cell Stress Chaperones. 2001;6:326–36. doi: 10.1379/1466-1268(2001)006<0326:HSFMTP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J, Bastian BC, McCormick F. MC1R and cAMP signaling inhibit cdc25B activity and delay cell cycle progression in melanoma cells. Proc Natl Acad Sci U S A. 2013;110:13845–50. doi: 10.1073/pnas.1201917110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YC, Su N, Shi XJ, Zhao W, Ke Y, Zi X, Zhao NM, Qin YH, Zhao HW, Liu HM. Jaridonin-induced G2/M phase arrest in human esophageal cancer cells is caused by reactive oxygen species-dependent Cdc2-tyr15 phosphorylation via ATM-Chk1/2-Cdc25C pathway. Toxicol Appl Pharmacol. 2015;282:227–36. doi: 10.1016/j.taap.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménézo Y, Dale B, Cohen M. DNA damage and repair in human oocytes and embryos: a review. Zygote. 2010;18:357–65. doi: 10.1017/S0967199410000286. [DOI] [PubMed] [Google Scholar]
- Meng J, Cui C, Liu Y, Jin M, Wu D, Liu C, Wang E, Yu B. The role of 14-3-3epsilon interaction with phosphorylated Cdc25B at its Ser321 in the release of the mouse oocyte from prophase I arrest. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuter A, Rogmann LM, Winterhoff BJ, Tchkonia T, Kirkland JL, Morbeck DE. Markers of cellular senescence are elevated in murine blastocysts cultured in vitro: molecular consequences of culture in atmospheric oxygen. J Assist Reprod Genet. 2014;31:1259–1267. doi: 10.1007/s10815-014-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr-Esfahani MH, Winston NJ, Johnson MH. Effects of glucose, glutamine, ethylenediaminetetraacetic acid and oxygen tension on the concentration of reactive oxygen species and on development of the mouse preimplantation embryo in vitro. J Reprod Fertil. 1992;96:219–31. doi: 10.1530/jrf.0.0960219. [DOI] [PubMed] [Google Scholar]
- Orsi NM, Leese HJ. Protection against reactive oxygen species during mouse preimplantation embryo development: role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Mol Reprod Dev. 2001;59:44–53. doi: 10.1002/mrd.1006. [DOI] [PubMed] [Google Scholar]
- Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18:1208–46. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel IA, Krenning L, Bruinsma W, Medema RH. The same, only different—DNA damage checkpoints and their reversal throughout the cell cycle. J Cell Sci. 2015;128:607–20. doi: 10.1242/jcs.163766. [DOI] [PubMed] [Google Scholar]
- Savitsky PA, Finkel T. Redox regulation of Cdc25C. J Biol Chem. 2002;277:20535–40. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- Shi J, Chen Q, Li X, Zheng X, Zhang Y, Qiao J, Tang F, Tao Y, Zhou Q, Duan E. Dynamic transcriptional symmetry-breaking in pre-implantation mammalian embryo development revealed by single-cell RNA-seq. Development. 2015;142:3468–77. doi: 10.1242/dev.123950. [DOI] [PubMed] [Google Scholar]
- Shimura T, Inoue M, Taga M, Shiraishi K, Uematsu N, Takei N, Yuan ZM, Shinohara T, Niwa O. p53-dependent S-phase damage checkpoint and pronuclear cross talk in mouse zygotes with X-irradiated sperm. Mol Cell Biol. 2002;22:2220–8. doi: 10.1128/MCB.22.7.2220-2228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Li Z, Wang B, Xiao J, Wang X, Huang J. Phospho-Cdc25 correlates with activating G2/M checkpoint in mouse zygotes fertilized with hydrogen peroxide-treated mouse sperm. Mol Cell Biochem. 2014;396:41–8. doi: 10.1007/s11010-014-2140-1. [DOI] [PubMed] [Google Scholar]
- Sur S, Agrawal DK (2016) Phosphatases and kinases regulating CDC25 activity in the cell cycle: clinical implications of CDC25 overexpression and potential treatment strategies. Mol Cell Biochem 10.1186/1756-0500-3-220. [DOI] [PMC free article] [PubMed]
- Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev. 2012;58:1–9. doi: 10.1262/jrd.11-138N. [DOI] [PubMed] [Google Scholar]
- Van BJ, Cox H, Davis P. Regulatory roles for mitochondria in the peri-implantation mouse blastocyst: possible origins and developmental significance of differential DeltaPsim. Reproduction. 2006;131:961–76. doi: 10.1530/rep.1.00458. [DOI] [PubMed] [Google Scholar]
- Ventura-Junca P, Irarrazaval I, Rolle AJ, Gutierrez JI, Moreno RD, Santos MJ. In vitro fertilization (IVF) in mammals: epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol Res. 2015;48:68. doi: 10.1186/s40659-015-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wale PL, Gardner DK. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod Update. 2016;22:2–22. doi: 10.1093/humupd/dmv034. [DOI] [PubMed] [Google Scholar]
- Wang B, Li Z, Wang C, Chen M, Xiao J, Wu X, Xiao W, Song Y, Wang X. Zygotic G2/M cell cycle arrest induced by ATM/Chk1 activation and DNA repair in mouse embryos fertilized with hydrogen peroxide-treated epididymal mouse sperm. PLoS One. 2013;8 doi: 10.1371/journal.pone.0073987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lin C, Shi H, Xie M, Zhang W, Lv J. Correlation of the mitochondrial activity of two-cell embryos produced in vitro and the two-cell block in Kunming and B6C3F1 mice. Anat Rec (Hoboken) 2009;292:661–9. doi: 10.1002/ar.20890. [DOI] [PubMed] [Google Scholar]
- Wang Z, Trope CG, Florenes VA, Suo Z, Nesland JM, Holm R. Overexpression of CDC25B, CDC25C and phospho-CDC25C (Ser216) in vulvar squamous cell carcinomas are associated with malignant features and aggressive cancer phenotypes. BMC Cancer. 2010;10:233. doi: 10.1186/1471-2407-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Liu C, Hou J, Cui C, Wu D, Fan H, Sun X, Meng J, Yang F, Wang E, Yu B. Ser149 is another potential PKA phosphorylation target of Cdc25B in G2/M transition of fertilized mouse eggs. J Biol Chem. 2011;286:10356–66. doi: 10.1074/jbc.M110.150524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Zhu G, Wang Y, Sun J, Sun J, Liu X, Chen YG, Meng A. Maternal Eomesodermin regulates zygotic nodal gene expression for mesendoderm induction in zebrafish embryos. J Mol Cell Biol. 2014;6:272–85. doi: 10.1093/jmcb/mju028. [DOI] [PubMed] [Google Scholar]
- Yang JI, Yeh CC, Lee JC, Yi SC, Huang HW, Tseng CN, Chang HW. Aqueous extracts of the edible Gracilaria tenuistipitata are protective against H2O2-induced DNA damage, growth inhibition, and cell cycle arrest. Molecules. 2012;17:7241–54. doi: 10.3390/molecules17067241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Greer PM, Cao PT, Kolb RH, Cowan KH. RAC1 GTPase plays an important role in gamma-irradiation induced G2/M checkpoint activation. Breast Cancer Res. 2012;14:R60. doi: 10.1186/bcr3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama KO, Sakaguchi K, Kimura S. DNA damage response in plants: conserved and variable response compared to animals. Biol (Basel) 2013;2:1338–56. doi: 10.3390/biology2041338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa M, Oda S, Mitani H, Nagata M, Aoki F. Deficiency in the response to DNA double-strand breaks in mouse early preimplantation embryos. Bioche Biophys Res Commun. 2007;358:578–84. doi: 10.1016/j.bbrc.2007.04.162. [DOI] [PubMed] [Google Scholar]