Abstract
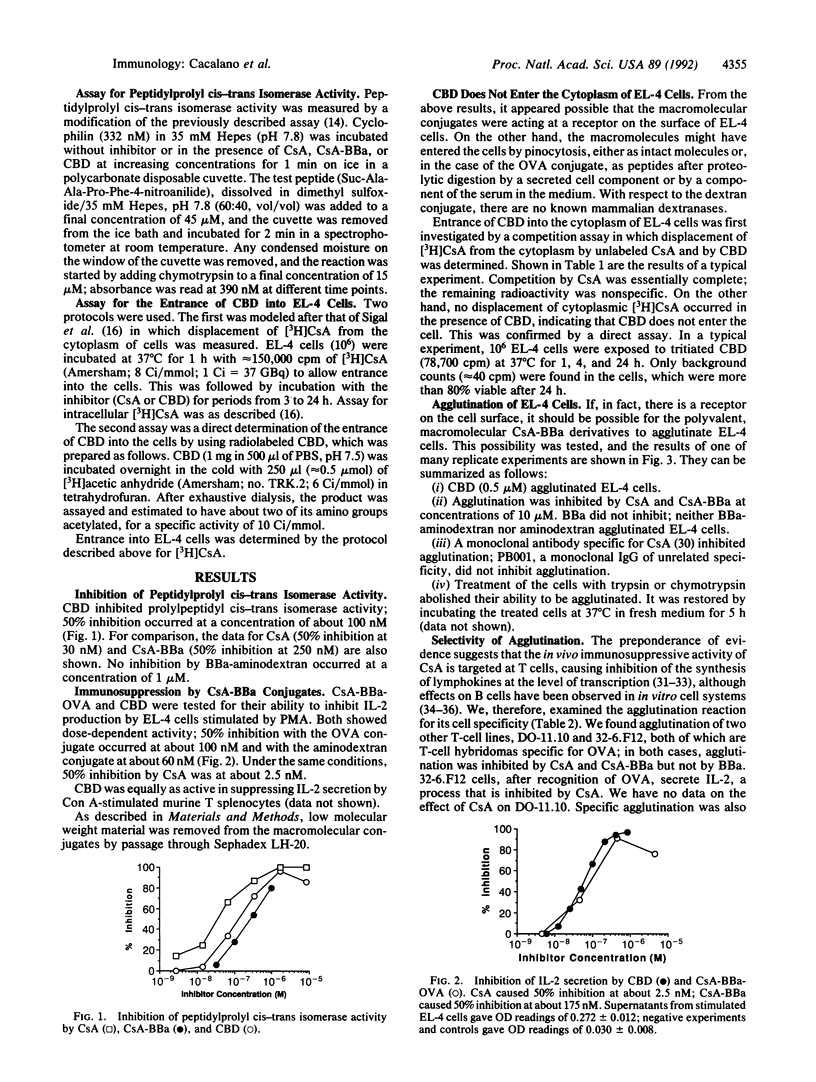
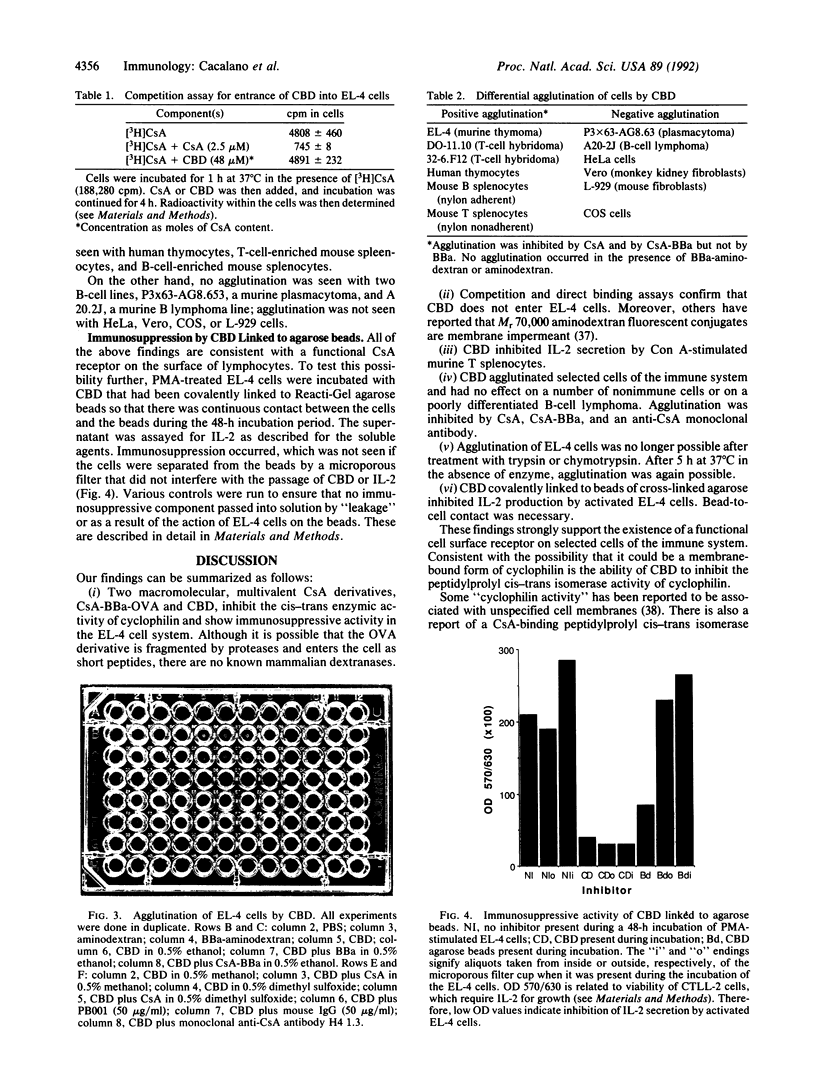
Cyclosporin A (CsA) is an immunosuppressive agent that inhibits the synthesis of lymphokines by T lymphocytes at the level of transcription. A cytoplasmic protein, cyclophilin, is the most thoroughly studied CsA-binding protein, but its ubiquitous presence in cells of all types raises questions about its role in immunosuppression. In an attempt to ascertain the presence of a cell surface receptor, we synthesized two polyvalent macromolecular CsA derivatives, CsA-BBa-ovalbumin and CsA-BBa-aminodextran (CBD), from the product of the photochemical reaction of CsA and 4-benzoylbenzoic acid (CsA-BBa). (i) They inhibited the peptidylprolyl cis-trans isomerase activity of cyclophilin and the synthesis of interleukin 2 by phorbol ester-activated EL-4 cells. (ii) CBD also inhibited interleukin 2 secretion by Con A-activated T-cell-enriched mouse splenocytes. 4-Benzoylbenzoic acid (BBa)-aminodextran and aminodextran were inactive. (iii) Direct binding and competition studies with [3H]CsA indicated that CBD does not enter EL-4 cells (i.e., it acted at the surface). (iv) CBD caused agglutination of EL-4 cells, murine B and T lymphocytes, human thymocytes, and two T-cell hybridomas. Agglutination was inhibited by a monoclonal antibody to CsA and by CsA and CsA-BBa, but not by BBa. No agglutination was seen with BBa-aminodextran or aminodextran. HeLa cells, Vero (monkey kidney) cells, a mouse plasmacytoma, COS cells, and a poorly differentiated B-cell lymphoma were not agglutinated. (v) EL-4 cells failed to be agglutinated after treatment with trypsin or chymotrypsin. Specific agglutination was again possible after incubation for 5 h at 37 degrees C in the absence of enzyme. (vi) CBD covalently linked to crosslinked agarose beads inhibited interleukin 2 production by phorbol ester-stimulated EL-4 cells. No activity was seen if cell-to-bead contact was prevented by a 0.02-microns microporous filter that did not interfere with the passage of CBD. Our findings support the presence of a functional receptor on the surface of selected cells of the immune system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Hexham J. M., Crumpton M. J. The association of type 1, type 2A and type 2B phosphatases with the human T lymphocyte plasma membrane. Biochem J. 1988 Dec 15;256(3):885–892. doi: 10.1042/bj2560885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel J. F. Comparative study of in vitro and in vivo drug effects on cell-mediated cytotoxicity. Immunology. 1976 Oct;31(4):631–641. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F. Cyclosporin-A--present experimental status. Transplant Proc. 1981 Mar;13(1 Pt 1):344–348. [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Cacalano N. A., Aggarwal R., Quesniaux V. F., Cleveland W. L., Erlanger B. F. Novel monoclonal antibodies to cyclosporine A: characterization and epitope mapping with cyclosporine analogs and cyclophilin. Mol Immunol. 1992 Jan;29(1):107–118. doi: 10.1016/0161-5890(92)90162-q. [DOI] [PubMed] [Google Scholar]
- Cacalano N. A., Cleveland W. L., Erlanger B. F. Antibodies to cyclosporine A (CsA) by a novel route and their use to monitor cyclosporine levels by radioimmunoassay (RIA). J Immunol Methods. 1989 Mar 31;118(2):257–263. doi: 10.1016/0022-1759(89)90014-8. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Fuller-Farrar J., Simon P. L., Hilfiker M. L., Stadler B. M., Farrar W. L. Thymoma production of T cell growth factor (Interleukin 2). J Immunol. 1980 Dec;125(6):2555–2558. [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Frey J. L., Bino T., Kantor R. R., Segal D. M., Giardina S. L., Roder J., Anderson S., Ortaldo J. R. Mechanism of target cell recognition by natural killer cells: characterization of a novel triggering molecule restricted to CD3- large granular lymphocytes. J Exp Med. 1991 Dec 1;174(6):1527–1536. doi: 10.1084/jem.174.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E., Speicher D. W. Isolation and amino acid sequence of cyclophilin. J Biol Chem. 1986 Jun 25;261(18):8547–8555. [PubMed] [Google Scholar]
- Hasel K. W., Glass J. R., Godbout M., Sutcliffe J. G. An endoplasmic reticulum-specific cyclophilin. Mol Cell Biol. 1991 Jul;11(7):3484–3491. doi: 10.1128/mcb.11.7.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kappler J., White J., Wegmann D., Mustain E., Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Differential localization of calmodulin-dependent enzymes in rat brain: evidence for selective expression of cyclic nucleotide phosphodiesterase in specific neurons. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1118–1122. doi: 10.1073/pnas.84.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus G. G., Hawrylowicz C. M. Activation and proliferation signals in mouse B cells. II. Evidence for activation (G0 to G1) signals differing in sensitivity to cyclosporine. Eur J Immunol. 1984 Mar;14(3):250–254. doi: 10.1002/eji.1830140309. [DOI] [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- London R. E., Davis D. G., Vavrek R. J., Stewart J. M., Handschumacher R. E. Bradykinin and its Gly6 analogue are substrates of cyclophilin: a fluorine-19 magnetization transfer study. Biochemistry. 1990 Nov 13;29(45):10298–10302. doi: 10.1021/bi00497a002. [DOI] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker M. P., Merker M. M., Handschumacher R. E. Uptake and nature of the intracellular binding of cyclosporin A in a murine thymoma cell line, BW5147. J Immunol. 1984 Jun;132(6):3064–3070. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nussenblatt R. B., Rodrigues M. M., Salinas-Carmona M. C., Gery I., Cevario S., Wacker W. Modulation of experimental autoimmune uveitis with cyclosporin A. Arch Ophthalmol. 1982 Jul;100(7):1146–1149. doi: 10.1001/archopht.1982.01030040124022. [DOI] [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randak C., Brabletz T., Hergenröther M., Sobotta I., Serfling E. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 1990 Aug;9(8):2529–2536. doi: 10.1002/j.1460-2075.1990.tb07433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Shieh B. H., Stamnes M. A., Seavello S., Harris G. L., Zuker C. S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989 Mar 2;338(6210):67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F., Durette P., Siekierka J. J., Peterson L., Rich D. H., Dunlap B. E., Staruch M. J., Melino M. R., Koprak S. L. Is cyclophilin involved in the immunosuppressive and nephrotoxic mechanism of action of cyclosporin A? J Exp Med. 1991 Mar 1;173(3):619–628. doi: 10.1084/jem.173.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller C. R., Dupré J., Gent M., Jenner M. R., Keown P. A., Laupacis A., Martell R., Rodger N. W., von Graffenried B., Wolfe B. M. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984 Mar 30;223(4643):1362–1367. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Barthelmess I. B., Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989 Dec 21;342(6252):953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- White J., Haskins K. M., Marrack P., Kappler J. Use of I region-restricted, antigen-specific T cell hybridomas to produce idiotypically specific anti-receptor antibodies. J Immunol. 1983 Mar;130(3):1033–1037. [PubMed] [Google Scholar]
- Wiesinger D., Borel J. F. Studies on the mechanism of action of cyclosporin A. Immunobiology. 1980 Jan;156(4-5):454–463. doi: 10.1016/S0171-2985(80)80078-7. [DOI] [PubMed] [Google Scholar]
- ZAJAC I., CROWELL R. L. EFFECT OF ENZYMES ON THE INTERACTION OF ENTEROVIRUSES WITH LIVING HELA CELLS. J Bacteriol. 1965 Mar;89:574–582. doi: 10.1128/jb.89.3.574-582.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Frimmer M. Identification of cyclosporin binding sites in rat liver plasma membranes, isolated hepatocytes, and hepatoma cells by photoaffinity labeling using [3H]cyclosporin-diaziridine. Biochim Biophys Acta. 1986 Feb 13;855(1):147–156. doi: 10.1016/0005-2736(86)90199-9. [DOI] [PubMed] [Google Scholar]
- Ziegler K., Frimmer M., Koepsell H. Photoaffinity labeling of membrane proteins from rat liver and pig kidney with cyclosporine diazirine. Involvement of binding to plasma membranes cytotoxic effects. Transplantation. 1988 Aug;46(2 Suppl):15S–20S. [PubMed] [Google Scholar]




