Abstract
Danger-associated molecular patterns (DAMPs) are activated by endogenous signals that originate from stressed, injured, or necrotic cells, signifying “danger” to the host. In this study, we evaluated the expression of the DAMP heat shock protein 70 (HSP70) in trauma patients with and without secondary infections. Levels of glucose (GLU), procalcitonin (PCT), total cholesterol (T-Chol), and white blood cell (WBC) counts were also evaluated at three time stages after trauma. Our analysis showed that the levels of serum HSP70 in patients with minor, moderate, and severe injuries were significantly higher than in healthy patients at each time point post-injury (P < 0.01), and levels of serum HSP70 in the severe injury group were significantly higher than in the minor injury group at 1–6 h after trauma (P = 0.047). HSP70 was correlated with GLU and was negatively correlated with T-Chol in the period 1–6 h after injury (P = 0.008/0.032). WBC and GLU were elevated after trauma, with mutual positive correlation (P < 0.001). PCT levels increased later than WBC counts and GLU levels; these levels were correlated at the two later time periods, 24–48 h and 60–90 h (P = 0.008/0.041). PCT continued to rise in patients with secondary infection, but PCT dropped at the third time period in patients without secondary infection. In summary, our results suggest that danger and stress theory can be used to predict severity of trauma.
Keywords: Trauma, Infection, DAMPs, HSP70, Biomarkers, Diagnosis
Introduction
Trauma can be thought of as an acute disease caused by physical injury; most deaths due to trauma occur in the acute setting, with around 50 % of in-hospital deaths occurring within the first 72 h after injury (Masella et al. 2008). The characteristics of trauma are tissue necrosis and cell death as a direct result of injury or due to a delayed inflammatory reaction, sepsis, and multiple organ dysfunction (MODS) (Larsen 2012). Because of the complexity of the disease and the diversity of its complications, diagnosis of severity is relatively difficult.
In this study, we evaluated the immunologic response to trauma using danger-associated molecular patterns (DAMPs) theory (Hwang et al. 2011; Pradeu & Cooper 2012). This theory suggests that the innate and the adaptive immune systems are activated by endogenous signals that originate from stressed, injured, or necrotic cells, signifying “danger” to the host (Di Virgilio 2005). DAMPs are the initiators of immune response; however, the true mediators remain to be elucidated (Bianchi 2007).
Matzinger and colleagues suggest that danger signals are in fact signals of “stress” (Gallucci et al. 1999). When cells suffered stress, even in the absence of any foreign substance, endogenous signals are released that activate antigen-producing cells to initiate an immune response. Although not all of these “damage” signals have been identified, heat shock proteins (HSPs) clearly served as DAMPs (Matzinger 1998; Asea et al. 2000a; Gallucci & Matzinger 2001; Wallin et al. 2002). HSPs can activate APCs in the absence of foreign pathogens to trigger an innate immune response and can act as charperones to participate in the adaptive immune response (Osterloh & Breloer 2008).
Studies have provided evidence that levels of the stress protein HSP70 are elevated in trauma patients (da Rocha et al. 2005). Elevations in procalcitonin (PCT) serum levels are also observed after in major surgical procedures and trauma (Meisner M1 et al. 1998; Schneider et al. 2009). Glucose (GLU) and white blood cell (WBC) counts increase after trauma, and total cholesterol (T-Chol) decreases (Yendamuri S1 et al. 2003; Rovlias & Kotsou 2001; Dunham et al. 2003).
No studies to date have correlated DAMPs with severity of injury or prognosis. An understanding of how these molecules are expressed would enable use as biomarkers for clinical diagnosis and treatment of trauma patients. Here, we investigated the concentration–time relationship of serum HSP70, PCT, T-Chol, and GLU and the WBC counts of trauma patients in the innate immune phase to evaluate the relationship between the levels of laboratory markers and the degree of the stress. We also sought to correlate these markers with clinical prognosis and treatment efficacy.
Materials and methods
Characteristics of patients
Fifty-six trauma patients from the HUNAN Second People’s Hospital, Trauma Surgical Department, were recruited for this study. Exclusion criteria were arrival in the emergency department (ED) more than 6 h after injury or diabetes and liver, kidney, or cardiovascular diseases. Patients were excluded retrospectively if they declined to give consent to use the collected research samples. Thirty healthy adult volunteers who were not receiving any medication at the time of blood sampling were also recruited. There were no significant differences in age or gender among the patients and healthy adult volunteers (Table 1).
Table 1.
Characteristics of patients and healthy controls
| Characteristics | Trauma patients | Healthy controls |
|---|---|---|
| (n = 56) | (n = 30) | |
| Age years | ||
| Mean ± SD (min, max) | 38 ± 14 (15, 75) | 34 ± 13 (18, 65) |
| Gender | ||
| % male (number/total) | 83.9 % (47/56) | 80.0 % (24/30) |
| Primary site of injury, % (number/total) | ||
| Multiple injuries | 30.4 % (17/56) | |
| Head injury | 12.5 % (7/56) | |
| Miscellaneous fracture | 14.3 % (8/56) | |
| Limb injury | 42.9 % (24/56) |
Patient grouping
The degree of injury was assessed by doctors and researchers. Patients were divided into minor injury, moderate injury, and severe injury groups by Injury Severity Score (ISS) after admission to the ED. ISS ≤8 was minor injury, ISS 9–15 was moderate injury, and ISS ≥16 was severe (Table 2). Thirty-three of the 56 patients were further divided into infected and non-infected groups according to microbiological evidence: 15 patients had confirmed infection and 18 were confirmed non-infected by clinical criteria (Table 3). Of the cohort, 23 patients could not be confirmed as infected or non-infected and therefore were not included in the infection portion of the analysis.
Table 2.
Patients grouped by ISS at admission
| Characteristic | Minor injury | Moderate injury | Severe injury |
|---|---|---|---|
| (n = 16) | (n = 19) | (n = 21) | |
| Age, years | |||
| Mean ± SD (min, max) | 35 ± 13 (19, 60) | 37 ± 13 (16, 60) | 41 ± 17 (5, 75) |
| Gender | |||
| % male (number/total) | 93.4 (15/16) | 84.2 (16/19) | 76.2 (16/21) |
| Injury Severity Score | |||
| Mean ± SEM (min, max) | 4.8 ± 1.7 (2, 8) | 11.1 ± 1.9 (9, 13) | 33.7 ± 20.0 (16, 75) |
| Primary site of injury, % (number/total) | |||
| Multiple injuries | 6.3 (1/16) | 15.8 (3/19) | 61.9 (13/21) |
| Head injury | 12.5 (2/16) | 5.3 (1/19) | 19.0 (4/21) |
| Miscellaneous fracture | 6.3 (1/16) | 21.1 (4/19) | 14.3 (3/21) |
| Limb injury | 75.0 (12/16) | 57.9 (11/19) | 4.8 (1/21) |
Table 3.
Patients grouped by infection or not
| Characteristic | Infected | Non-infected |
|---|---|---|
| (n = 15) | (n = 18) | |
| Age, years | ||
| Mean ± SD (min, max) | 43 ± 12 (20, 66) | 38 ± 15 (17, 75) |
| Gender | ||
| % male (number/total) | 93.3 (14 of 15) | 66.7 (12 of 18) |
| Injury Severity Score | ||
| Mean ± SD (min, max) | 20.3 ± 18 (4, 75) | 9.5 ± 6.4 (4, 27) |
| Primary site of infection, % (number/total) | ||
| Multiple sites | 33.3 (5/15) | 38.8 (7/18) |
| Head | 20.0 (3/15) | 11.1 (2/18) |
| Fracture | 20.0 (3/15) | 16.7 (3/18) |
| Extremity | 26.7 (4/15) | 33.3 (6/18) |
Blood sample collection
Three 3-tubes blood samples were collected at each time point. To one tube was added EDTA-K2 anti-freeze; this was used for the WBC count. The other two tubes were centrifuged at 1500×g for 10 min at room temperature; one was used for detection of GLU and T-Chol. The other sample was carefully removed from the blood collection tube and transferred into polypropylene tubes and immediately frozen (−80 °C) for later analysis of PCT and HSP70. Samples were collected 1–6, 24–36, and 60–90 h after injury.
Blood cell count and biochemical analysis
The WBC count was performed using a Siemens ADVIA 2120 automatic blood analyzer. GLU and T-Chol analyses were performed using a Siemens ADVIA 1650. PCT was tested using a Roche Cobas e 411. HSP70 quantitative analysis was by an automatic enzyme immunoassay instrument Labsystems Multiskan MK3 with reagents from Adlitteram Diagnostic Laboratories.
Statistics
Statistical analysis was performed using the Statistical Package for the Social Sciences software package v. 16.0. Comparisons between two independent continuous variables were analyzed by t test. For comparisons among more than two continuous variables, a Kruskal–Wallis H test was performed; non-parametric data comparison tests (Kruskal–Wallis) were performed using the Instat 3.0 program (GraphPad Software). A χ2 test was used in R × 2 contingency table data.
Results
Serum HSP70 levels in patients after trauma
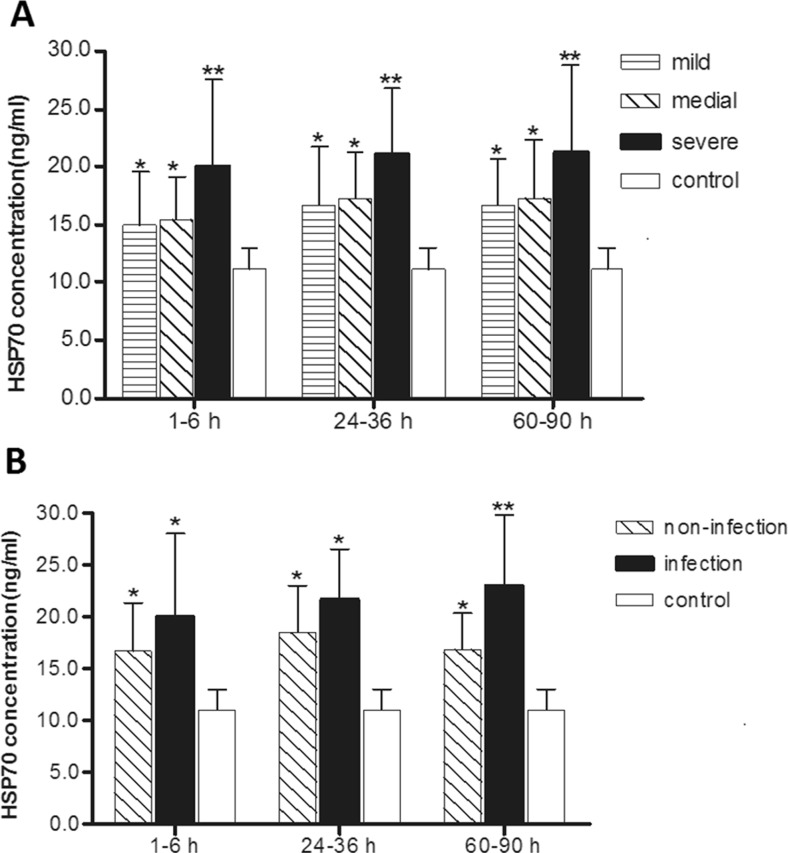
Levels of serum HSP70 in patients divided by injury severity are plotted in Fig. 1 and given in Tables 4 and 5. Levels of serum HSP70 of patients with all severities of injury at each of the three time stages were significantly higher than levels in the healthy group. Levels of serum HSP70 in the severe injure group were significantly higher than in the minor injury group at 1–6 h after trauma, and the degree of elevation was related to the severity of injury (Fig. 1a). In both the 1–6-h period and the 24–48-h period, differences in HSP70 levels were significant when groups were compared (Table 5).
Fig. 1.
a Serum HSP70 in minor injury group (n = 17), moderate injury group (n = 22), and severe injury group (n = 17) over time compared with healthy control group (n = 30). b Serum HSP70 in infection group (n = 15) and non-infection group (n = 18) over time periods compared with healthy control group (n = 30)
Table 4.
Levels of HSP70 and other biomarkers in injury groups as a function of time data are presented as median (min- max), in comparison with one-way ANOVA
| Variables | HSP70 | WBC | GLU | T-Chol | PCT |
|---|---|---|---|---|---|
| (ng/ml) | (*109) | (mmol/L) | (mmol/L) | (ng/ml) | |
| Minor injury groups | |||||
| 1–6 h after trauma | 15.01 (9.63–27.68) | 9.53 (3.30–15.7) | 5.61 (4.25–10.06) | 4.77 (3.45–6.04) | 0.89 (0.68–1.56) |
| 24–48 h after trauma | 16.66 (9.63–21.36) | 7.63 (4.50–13.1) | 4.87 (4.22–5.89) | 4.34 (3.02–5.63) | 2.33 (0.92–4.05) |
| 60–90 h after trauma | 16.75 (10.13–21.68) | 6.08 (4.20–8.9) | 4.52 (3.99–5.13) | 4.13 (3.22–5.13) | 1.80 (1.23–4.08) |
| P value | 0.397 | 0.004 | 0.004 | 0.111 | 0.000 |
| Moderate injury groups | |||||
| 1–6 h after trauma | 15.49 (9.47–22.12) | 11.28 (5.20–19.7) | 5.89 (4.01–10.19) | 4.56 (2.70–6.91) | 0.89 (0.62–1.36) |
| 24–48 h after trauma | 17.22 (11.97–24.16) | 8.16 (4.20–12.6) | 5.39 (4.24–7.12) | 4.02 (2.51–5.80) | 3.83 (1.49–8.44) |
| 60–90 h after trauma | 17.36 (13.01–26.91) | 6.45 (3.70–9.00) | 4.97 (4.47–5.47) | 3.97 (2.82–5.74) | 4.03 (1.17–6.59) |
| P value | 0.343 | 0.000 | 0.393 | 0.176 | 0.000 |
| Severe injury groups | |||||
| 1–6 h after trauma | 20.33 (13.36–43.54) | 16.58 (3.00–34.3) | 10.11 (4.46–20.62) | 3.73 (1.80–5.26) | 0.94 (0.63–2.51) |
| 24–48 h after trauma | 21.16 (14.10–33.30) | 12.81 (3.72–43.4) | 6.70 (4.79–14.14) | 3.55 (2.23–5.13) | 4.24 (1.95–8.56) |
| 60–90 h after trauma | 21.34 (14.78–3946) | 8.74 (3.38–12.9) | 5.59 (4.40–7.04) | 3.74 (2.50–5.14) | 4.43 (1.33–9.28) |
| P value | 0.903 | 0.011 | 0.001 | 0.840 | 0.000 |
Table 5.
Levels of HSP70 and other biomarkers at time intervals after injury as a function of injury severity data are presented as medians (min-max); comparisons were made using one-way ANOVA
| Variables | HSP70 | WBC | GLU | T-Chol | PCT |
|---|---|---|---|---|---|
| (ng/ml) | (*109) | (mmol/L) | (mmol/L) | (ng/ml) | |
| 1–6 h after trauma | |||||
| Minor injury | 15.01 (9.63–27.68) | 9.53 (3.30–15.7) | 5.61 (4.25–10.06) | 4.77 (3.45–6.04) | 0.89 (0.68–1.56) |
| Moderate injury | 15.49 (9.47–22.12) | 11.28 (5.20–19.7) | 5.89 (4.01–10.19) | 4.56 (2.70–6.91) | 0.89 (0.62–1.36) |
| Severe injury | 20.33 (13.36–43.54) | 16.58 (3.00–34.3) | 10.11 (4.46–20.62) | 3.73 (1.80–5.26) | 0.94 (0.63–2.51) |
| P value | 0.007 | 0.000 | 0.000 | 0.005 | 0.865 |
| 24–48 h after trauma | |||||
| Minor injury | 16.66 (9.63–21.36) | 7.63 (4.50–13.1) | 4.87 (4.22–5.89) | 4.34 (3.02–5.63) | 2.33 (0.92–4.05) |
| Moderate injury | 17.22 (11.97–24.16) | 8.16 (4.20–12.6) | 5.39 (4.24–7.12) | 4.02 (2.51–5.80) | 3.83 (1.49–8.44) |
| Severe injury | 21.16 (14.10–33.30) | 12.81 (3.72–43.4) | 6.70 (4.79–14.14) | 3.55 (2.23–5.13) | 4.24 (1.95–8.56) |
| P value | 0.022 | 0.029 | 0.005 | 0.101 | 0.005 |
| 60–90 h after trauma | |||||
| Minor injury | 16.75 (10.13–21.68) | 6.08 (4.20–8.9) | 4.52 (3.99–5.13) | 4.13 (3.22–5.13) | 1.80 (1.23–4.08) |
| Moderate injury | 17.36 (13.01–26.91) | 6.45 (3.70–9.00) | 4.97 (4.47–5.47) | 3.97 (2.82–5.74) | 4.03 (1.17–6.59) |
| Severe injury | 21.34 (14.78–3946) | 8.74 (3.38–12.9) | 5.59 (4.40–7.04) | 3.74 (2.50–5.14) | 4.43 (1.33–9.28) |
| P value | 0.077 | 0.005 | 0.029 | 0.516 | 0.002 |
Levels of HSP70 in infection and non-infection groups were significantly higher than in the healthy group in each of the three time periods. During the third time period (60–90 h after trauma), serum HSP70 in the infection group was significantly higher than in the non-infection group (Fig. 1b and Table 6).
Table 6.
Levels of HSP70 and other biomarkers in infection and non-infection groups as a function of time data are presented as median (min–max) and were compared using t test
| Variables | HSP70 | WBC | GLU | CHOL | PCT |
|---|---|---|---|---|---|
| (ng/ml) | (*109) | (mmol/L) | (mmol/L) | (ng/ml) | |
| 1–6 h after trauma | |||||
| Infected | 20.01 (13.36–43.54) | 13.46 (3–25.4) | 7.97 (4.01–20.62) | 4.18 (1.8–6.91) | 1.05 (0.63–2.51) |
| Non-infected | 16.79 (9.47–26.36) | 11.23 (5.2–17.2) | 6.32 (4.26–10.06) | 4.36 (2.7–6.02) | 0.88 (0.62–1.36) |
| P value | 0.169 | 0.297 | 0.128 | 0.676 | 0.222 |
| 24–48 h after trauma | |||||
| Infected | 21.74 (16.26–33.3) | 10.98 (7.6–15.4) | 5.94 (4.47–8.5) | 3.83 (1.57–5.17) | 3.92 (3.3–8.44) |
| Non-infected | 18.54 (13.49–29.11) | 7.84 (4.20–11.5) | 5.13 (4.24–7.27) | 3.94 (2.82–5.74) | 4.04 (1.49–8.56) |
| P value | 0.092 | 0.003 | 0.052 | 0.794 | 0.871 |
| 60–90 h after trauma | |||||
| Infected | 23.07 (14.78–39.46) | 8.45 (5.0–12.9) | 5.44 (4.51–7.04) | 3.81 (2.5–5.8) | 5.05 (3.1–9.28) |
| Non-infected | 16.93 (13.16–24.69) | 5.37 (3.70–6.50) | 4.89 (4.4–5.47) | 3.94 (2.51–5.21) | 2.20 (1.17–3.56) |
| P value | 0.019 | 0.000 | 0.084 | 0.765 | 0.000 |
WBC, GLU, and T-Chol of patients after trauma
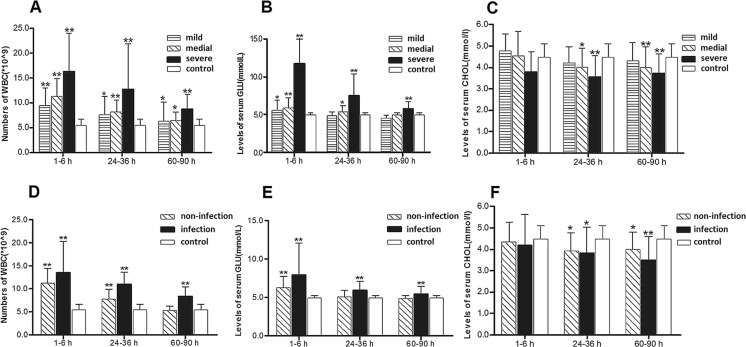
Data on WBC counts and GLU and T-Chol levels in patients divided by injury severity are plotted in Fig 2a, b, c. At 1–6 h after trauma, GLU levels and WBC counts in the three injured groups were significantly higher than in healthy group. At 24–48 h after trauma, WBC counts in the three injured groups were significantly higher than in the healthy group, and GLU levels were higher in the moderate injury and severe injury patient groups than in healthy group. WBC counts and GLU levels decreased over time but increased with the severity of the injury. The T-Chol levels in the minor injury group were not statistically different from levels in healthy volunteers at any time point, but levels in the moderate injury and severe injury patient groups were significantly lower than in healthy group at 24–28 and 60–90 h after trauma.
Fig. 2.
a WBC counts, b GLU levels, and c T-Chol levels of minor injury group (n = 17), moderate injury group (n = 22), and severe injury group (n = 17) over time compared with healthy control group (n = 30). d WBC counts, e levels of GLU, and f levels of T-Chol of infection group (n = 15), non-infection group (n = 18), and controls (n = 30) over time
At 1–6 h after trauma, WBC counts and GLU levels in the infection and the non-infection groups were significantly higher than in healthy group (Table 6 and Table 7). At 24–48 h after trauma, WBC counts in the infection and the non-infection groups were significantly higher than in healthy group. GLU levels were higher in the infection group than in healthy group but were similar in the non-infection group and in the healthy controls. T-Chol levels in the infection and the non-infection groups were significantly lower than in healthy group at 24–36 and at 60–90 h after trauma. In both groups, WBC and GLU decreased significantly decrease with the passage of time. At both 24–48 and 60–90 h, WBC counts in the infection group were significantly higher than in non-infection group.
Table 7.
Levels of HSP70 and other biomarkers in infected and non-infected groups over time data are presented as median (min–max) and were compared with one-way ANOVA
| Variables | HSP70 | WBC | GLU | CHOL | PCT |
|---|---|---|---|---|---|
| (ng/ml) | (*109) | (mmol/L) | (mmol/L) | (ng/ml) | |
| Infected | |||||
| 1–6 h after trauma | 20.01 (13.36–43.54) | 13.46 (3.00–25.4) | 7.97 (4.01–20.62) | 4.18 (1.80–6.91) | 1.05 (0.63–2.51) |
| 24–48 h after trauma | 21.74 (16.26–33.3) | 10.98 (7.60–15.4) | 5.94 (4.47–8.50) | 3.83 (1.57–5.17) | 3.92 (3.30–8.44) |
| 60–90 h after trauma | 23.07 (14.78–39.46) | 8.45 (5.00–12.90) | 5.44 (4.51–7.04) | 3.81 (2.50–5.8) | 5.05 (3.10–9.28) |
| P value | 0.486 | 0.016 | 0.039 | 0.689 | 0.000 |
| Non-infected | |||||
| 1–6 h after trauma | 16.79 (9.47–26.36) | 11.23 (5.20–17.2) | 6.32 (4.26–10.06) | 4.36 (2.70–6.02) | 0.88 (0.62–1.36) |
| 24–48 h after trauma | 18.54 (13.49–29.11) | 7.84 (4.20–11.5) | 5.13 (4.24–7.27) | 3.94 (2.82–5.74) | 4.04 (1.49–8.56) |
| 60–90 h after trauma | 16.93 (13.16–24.69) | 5.37 (3.70–6.50) | 4.89 (4.40–5.47) | 3.94 (2.51–5.21) | 2.20 (1.17–3.56) |
| P value | 0.516 | 0.000 | 0.003 | 0.317 | 0.000 |
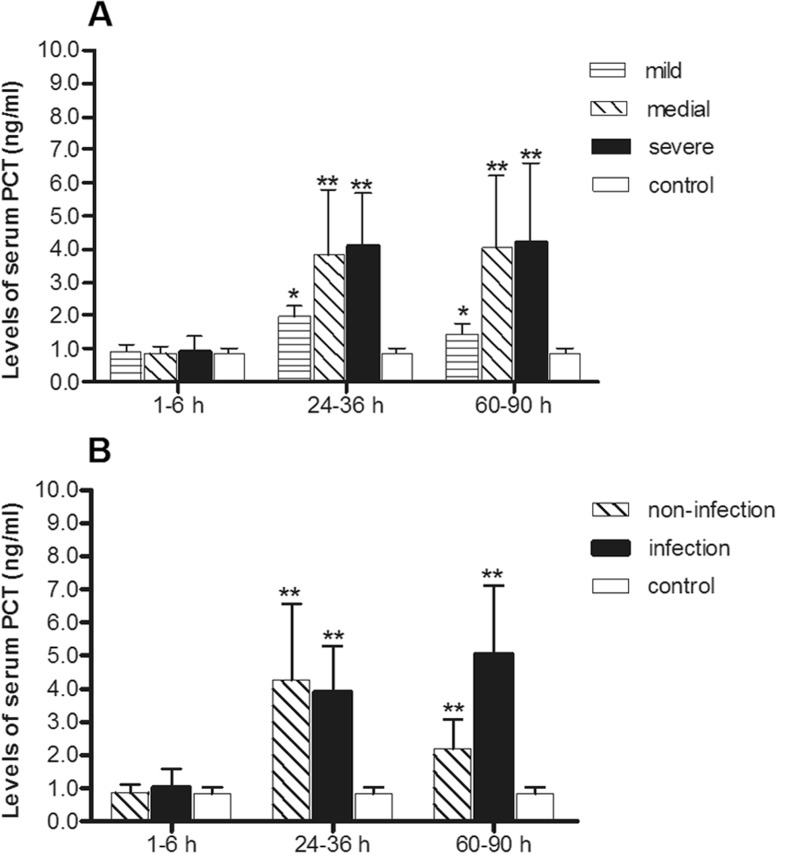
Serum PCT of patients after trauma
Levels of serum PCT in three injury groups significantly higher than in healthy control groups in the second time period (24–48 h after trauma) and in the third time period (60–90 h after trauma) are plotted in Fig. 3a. Differences were significant among groups divided on the basis of injury severity as Table 4 showed.
Fig. 3.
a Serum PCT levels of minor injury group (n = 17), moderate injury group (n = 22), severe injury group (n = 17) over time compared with healthy control group (n = 30). b Serum PCT levels of infection group (n = 15) and non-infection group (n = 18) over time periods compared with healthy control group (n = 30)
Levels of serum PCT in the infection and non-infection groups were also significantly higher than in the healthy group at 24–48 h after trauma and at 60–90 h after trauma (Fig 3b). There were also differences over time. Especially at 60–90 h, levels of serum PCT in the infection group were significantly higher than in the non-infection group (Table 6 and Table 7).
Correlation analysis
When all injury patients were considered, at 1–6 h after trauma, HSP70 and GLU were positively correlated, and HSP70 and T-Chol were negatively correlated. GLU and WBC were also positively correlated. The correlation coefficients for correlations in the 1–6-h period are given in Table 8. At 24–48 h after trauma, PCT was positively correlated with GLU and WBC count and GLU and WBC were positively correlated. The correlation coefficients for correlations in the 24–48-h period are given in Table 9. At 60–90 h after trauma, PCT remained positively correlated with GLU and WBC and GLU and CHOL were negatively correlated. The correlation coefficients for correlations in the 60–90-h period are given in Table 10.
Table 8.
Correlation analysis of parameters in the period 1–6 h after injury
| WBC | GLU | CHOL | PCT | HSP70 | |||
|---|---|---|---|---|---|---|---|
| Spearman’s rho | WBC | Correlation coefficient | 1.000 | 0.498** | −0.037 | −0.040 | 0.188 |
| Sig. (2-tailed) | . | 0.000 | 0.799 | 0.782 | 0.192 | ||
| N | 50 | 50 | 50 | 50 | 50 | ||
| GLU | Correlation coefficient | 0.498** | 1.000 | 0.008 | −0.082 | 0.373** | |
| Sig. (2-tailed) | 0.000 | . | 0.958 | 0.571 | 0.008 | ||
| N | 50 | 50 | 50 | 50 | 50 | ||
| CHOL | Correlation coefficient | −0.037 | 0.008 | 1.000 | −0.021 | −0.304* | |
| Sig. (2-tailed) | 0.799 | 0.958 | . | 0.887 | 0.032 | ||
| N | 50 | 50 | 50 | 50 | 50 | ||
| PCT | Correlation coefficient | −0.040 | −0.082 | −0.021 | 1.000 | 0.033 | |
| Sig. (2-tailed) | 0.782 | 0.571 | 0.887 | . | 0.821 | ||
| N | 50 | 50 | 50 | 50 | 50 | ||
| HSP70 | Correlation coefficient | 0.188 | 0.373** | −0.304* | 0.033 | 1.000 | |
| Sig. (2-tailed) | 0.192 | 0.008 | 0.032 | 0.821 | . | ||
| N | 50 | 50 | 50 | 50 | 50 |
*Correlation is significant at the 0.05 level (2-tailed); **Correlation is significant at the 0.01 level (2-tailed)
Table 9.
Correlation analysis of parameters in the period 24–48 h after injury
| WBC | GLU | CHOL | PCT | HSP70 | |||
|---|---|---|---|---|---|---|---|
| Spearman’s rho | WBC | Correlation coefficient | 1.000 | 0.343* | 0.076 | 0.376* | 0.183 |
| Sig. (2-tailed) | . | 0.024 | 0.627 | 0.013 | 0.239 | ||
| N | 43 | 43 | 43 | 43 | 43 | ||
| GLU | Correlation coefficient | 0.343* | 1.000 | −0.158 | 0.399** | −0.144 | |
| Sig. (2-tailed) | 0.024 | . | 0.311 | 0.008 | 0.356 | ||
| N | 43 | 43 | 43 | 43 | 43 | ||
| CHOL | Correlation coefficient | 0.076 | −0.158 | 1.000 | −0.071 | −0.282 | |
| Sig. (2-tailed) | 0.627 | 0.311 | . | 0.653 | 0.067 | ||
| N | 43 | 43 | 43 | 43 | 43 | ||
| PCT | Correlation coefficient | 0.376* | 0.399** | −0.071 | 1.000 | 0.136 | |
| Sig. (2-tailed) | 0.013 | 0.008 | 0.653 | . | 0.386 | ||
| N | 43 | 43 | 43 | 43 | 43 | ||
| HSP70 | Correlation coefficient | 0.183 | −0.144 | −0.282 | 0.136 | 1.000 | |
| Sig. (2-tailed) | 0.239 | 0.356 | 0.067 | 0.386 | . | ||
| N | 43 | 43 | 43 | 43 | 43 |
*Correlation is significant at the 0.05 level (2-tailed); **Correlation is significant at the 0.01 level (2-tailed)
Table 10.
Correlation analysis of parameters in the period 60–90 h after injury
| WBC | GLU | CHOL | PCT | HSP70 | |||
|---|---|---|---|---|---|---|---|
| Spearman’s rho | WBC | Correlation coefficient | 1.000 | 0.271 | 0.071 | 0.411** | 0.152 |
| Sig. (2-tailed) | . | 0.091 | 0.665 | 0.008 | 0.351 | ||
| N | 40 | 40 | 40 | 40 | 40 | ||
| GLU | Correlation coefficient | 0.271 | 1.000 | −0.318* | 0.325* | 0.176 | |
| Sig. (2-tailed) | 0.091 | . | 0.046 | 0.041 | 0.277 | ||
| N | 40 | 40 | 40 | 40 | 40 | ||
| CHOL | Correlation coefficient | 0.071 | −0.318* | 1.000 | 0.064 | −0.285 | |
| Sig. (2-tailed) | 0.665 | 0.046 | . | 0.693 | 0.075 | ||
| N | 40 | 40 | 40 | 40 | 40 | ||
| PCT | Correlation coefficient | 0.411** | 0.325* | 0.064 | 1.000 | 0.213 | |
| Sig. (2-tailed) | 0.008 | 0.041 | 0.693 | . | 0.186 | ||
| N | 40 | 40 | 40 | 40 | 40 | ||
| HSP70 | Correlation coefficient | 0.152 | 0.176 | −0.285 | 0.213 | 1.000 | |
| Sig. (2-tailed) | 0.351 | 0.277 | 0.075 | 0.186 | . | ||
| N | 40 | 40 | 40 | 40 | 40 |
*Correlation is significant at the 0.05 level (2-tailed); **Correlation is significant at the 0.01 level (2-tailed)
Discussion
HSP70 is expressed at low or undetectable levels in plasma of unstressed, healthy individuals; it is produced under various stress conditions. HSP70 is released into the extracellular environment and binds to Toll-like receptors (TLR2 and TRL4) to trigger pro-inflammatory responses by upregulation of adhesion molecules, co-stimulatory molecules, and cytokine and chemokine secretion (Prohászka et al. 2002; Asea et al. 2000b). “Danger theory” has significantly extended our understanding of these responses (de Haan et al. 2013; Pacheco-Tena & González-Chávez 2015; LeRoux et al. 2015; Heil & Land 2014). Endogenous danger signals such as HSP70 released from necrotic or stressed cells, called DAMPs (Bianchi 2007; Harris & Raucci 2006), share structural and functional similarities with molecules released from invading microorganisms, called pathogen-associated molecular patterns (PAMPs). Both DAMPs and PAMPs are recognized by a number of receptors termed pathogen-recognition receptors (PRRs) (Seong & Matzinger 2004; Hirsiger et al. 2012).
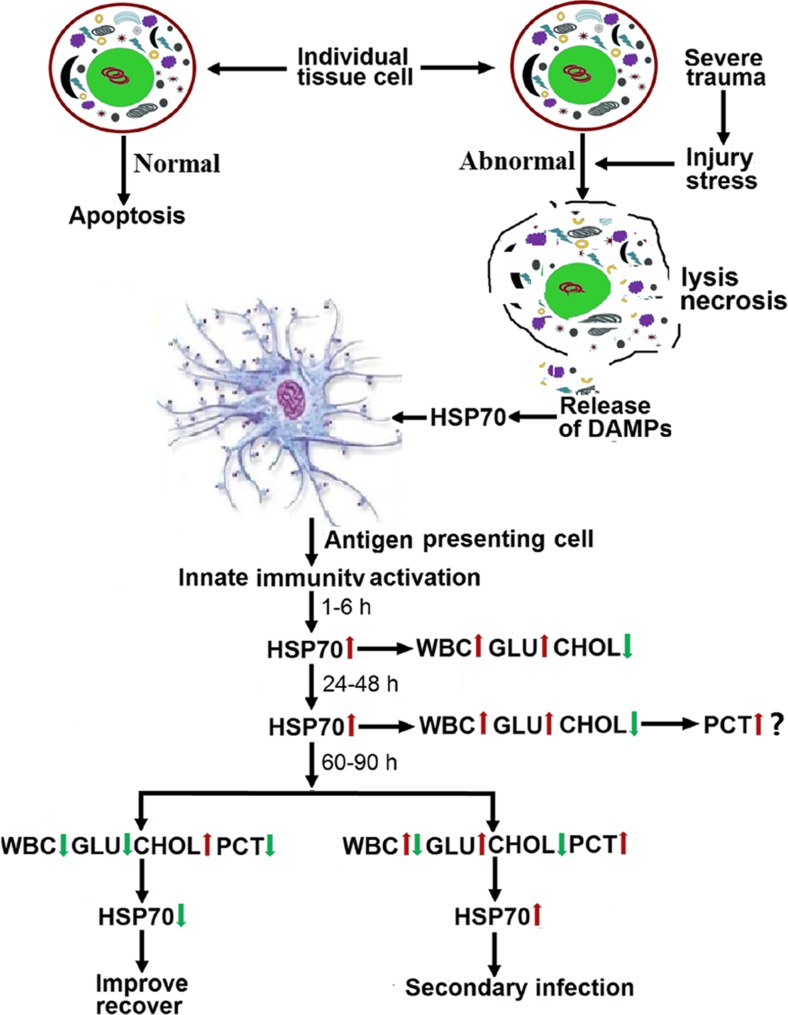
In this study, the levels of serum HSP70 in trauma patients were elevated at 1–6 h after injury, and the magnitude of the increase was related to the severity of the injury. These findings suggest that HSP70 is produced as a danger signal to stimulate the immune systems of trauma patients (Hietbrink et al. 2006). Our data indicate that HSP70 can serve as a marker of the degree of injury suffered by trauma patients and for prediction of secondary infection. HSP70 levels rose quickly, were of long duration, and were related to the severity of the injury. We observed that if HSP70 levels decreased in the period from 60 to 90 h post-injury, the patient had a better outcome than if levels did not decrease. An increase in HSP70 levels between the 24–48-h period and the 60–90-h period suggested infection (Fig. 4).
Fig. 4.
DAMP HSP70 in trauma patients and the concentration–time relationship of serum HSP70, PCT, T-Chol, GLU and the WBC counts of trauma patients in the innate immune phase, and the relationship of the laboratory marker levels with clinical prognosis and treatment efficacy
WBCs are the earliest immune cells observed in the innate immune phase after trauma, and WBC counts increased over time post-injury. Serum GLU increase is protective, and levels were also related to the severity of the injury. WBC was positively correlated with GLU at 1–6 h after trauma indicating that the innate immune response is consistent with the neuroendocrine regulation. The levels of serum GLU were also positively correlated with serum HSP70. The levels of T-Chol in serum were reduced in patients compared to healthy controls. T-Chol levels are regulated by the hypothalamic pituitary adrenal axis, and T-Chol was negatively correlated with HSP70 at 1–6 h after trauma. GLU and T-Chol may be affected by treatments given injured patients, such as insulin and fat emulsion treatments. WBC increased after trauma, and then gradually decreased, but in patients with infection patients, WBC will rise again suggesting that trauma and infection result in the same immune response (Table 7).
Researchers have compared PCT levels with other several markers of inflammation, such as C-reactive protein (CRP). PCT has a higher positive predictive value than CRP; thus, the clinical value of PCT appears to be promising (Koivula et al. 2011). A growing body of evidence supports the use of PCT to improve the diagnosis of bacterial infections and to guide antibiotic therapy (Schuetz P1 et al. 2011). Elevations in PCT levels are sometimes observed in the absence of a bacterial infection such as situations of massive cell death, for example, after major surgical procedures, trauma, pancreatitis, or renal impairment (Fritz HG1 et al. 2003; Rau BM1 et al. 2007). Our results indicated that PCT levels increased after injury, but PCT levels rose later than did levels of HSP70, GLU, and WBC. We observed that PCT levels were similar to those of healthy volunteers in the 1–6-h period but were higher at 24–48 h after trauma. PCT levels decreased from 60 to 90 h if there was no infection. This phenomenon explains the non-specific increases reported in the literature reports and indicates that PCT is also a stress protein produced by the immune system. That PCT continued to rise in cases of infection after injury illustrates that the infection is another stress stimulus. The delay in PCT increase is a mystery. A report in the literature shows that PCT rises with an early peak within 36 h (Kibe et al. 2011); this was consistent with our study. PCT was positively correlated with WBC and GLU at 24–48 h after trauma and also positively correlated at 60–90 h after trauma.
As acquisition of patient specimens in a trauma situation was very difficult, we could not observe the variation of each parameter on a more accurate time course after injury. Furthermore, we only measured parameters of the innate immunity system, not the acquired immune system, and did not perform a systematic study of the cytokine, receptors, and other factors known to be involved in the innate immune system. A further study will be required to consider these aspects.
In summary, levels of the danger signal HSP70 increased significantly after trauma, and the magnitude of the increase was related to the severity of the injury, Furthermore, HSP70 levels continued to rise in cases of infection. WBC, GLU, and T-Chol reflected the response of body to the trauma; PCT levels increased at later times than did HSP70, WBC, and GLU. This type of comprehensive analysis can assist clinical diagnosis and monitoring of trauma. Danger and stress theory can explain the occurrence and development of trauma disease through detection of serum HSP70 and other laboratory biomarkers.
Acknowledgments
This work was supported by a research grant from the Health Department of Hunan Province Foundation.
Abbreviations
- DAMPs
Danger-associated molecular patterns
- HSP70
Heat shock protein 70
- PCT
Procalcitonin
- GLU
Glucose
- WBC
White blood cell
- T-Chol
Total cholesterol
- ED
Emergency department
- ISS
Injury Severity Score
- PAMPs
Pathogen-associated molecular patterns
- PRRs
Pathogen-recognition receptors
Compliance with ethical standards
Ethics approval
The study complied with the Declaration of Helsinki and was reviewed and granted ethical approval by the Ethics Committee of the Second People’s Hospital of Hunan Province.
References
- Masella CA, Pinho VF, Costa Passos AD, Spencer Netto FA, Rizoli S, Scarpelini S. Temporal distribution of trauma deaths: quality of trauma care in a developing country. J Trauma. 2008;65(3):653–8. doi: 10.1097/TA.0b013e3181802077. [DOI] [PubMed] [Google Scholar]
- Larsen AI. Clinical and biomarker profile of trauma-induced secondary cardiac injury. Br J Surg. 2012;99(6):797–8. doi: 10.1002/bjs.8735. [DOI] [PubMed] [Google Scholar]
- Hwang PF, Porterfield N, Pannell D, Davis TA, Elster EA. Trauma is danger. J Transl Med. 2011;9:92. doi: 10.1186/1479-5876-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeu T, Cooper EL. The danger theory: 20 years later. Front Immunol. 2012;3:349. doi: 10.3389/fimmu.2012.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Purinergic mechanism in the immune system: a signal of danger for dendritic cells. Purinergic Signal. 2005;1(3):205–9. doi: 10.1007/s11302-005-6312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5(11):1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- Matzinger P. An innate sense of danger. Semin Immunol. 1998;10(5):399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13(1):114–9. doi: 10.1016/S0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Wallin RP, Lundqvist A, Moré SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23(3):130–5. doi: 10.1016/S1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- Osterloh A, Breloer M. Heat shock proteins: linking danger and pathogen recognition. Med Microbiol Immunol. 2008;197(1):1–8. doi: 10.1007/s00430-007-0055-0. [DOI] [PubMed] [Google Scholar]
- da Rocha AB, Zanoni C, de Freitas GR, André C, Himelfarb S, Schneider RF, Grivicich I, Borges L, Schwartsmann G, Kaufmann M, Regner A. Serum Hsp70 as an early predictor of fatal outcome after severe traumatic brain injury in males. J Neurotrauma. 2005;22(9):966–77. doi: 10.1089/neu.2005.22.966. [DOI] [PubMed] [Google Scholar]
- Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schüttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 1998;24(7):680–4. doi: 10.1007/s001340050644. [DOI] [PubMed] [Google Scholar]
- Schneider CP, Yilmaz Y, Kleespies A, Jauch KW, Hartl WH. Accuracy of procalcitonin for outcome prediction in unselected postoperative critically ill patients. Shock. 2009;31(6):568–73. doi: 10.1097/SHK.0b013e318193cb52. [DOI] [PubMed] [Google Scholar]
- Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55(1):33–8. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- Rovlias A, Kotsou S. The blood leukocyte count and its prognostic significance in severe head injury. Surg Neurol. 2001;55(4):190–6. doi: 10.1016/S0090-3019(01)00414-1. [DOI] [PubMed] [Google Scholar]
- Dunham CM, Fealk MH, Sever WE., 3rd Following severe injury, hypocholesterolemia improves with convalescence but persists with organ failure or onset of infection. Crit Care. 2003;7(6):R145–53. doi: 10.1186/cc2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohászka Z, Singh M, Nagy K, Kiss E, Lakos G, Duba J, Füst G. Heat shock protein 70 is a potent activator of the human complement system. Cell Stress Chaperones. 2002;7:17–22. doi: 10.1379/1466-1268(2002)007<0017:HSPIAP>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan JJ, Smeets MB, Pasterkamp G, Arslan F. Danger signals in the initiation of the inflammatory response after myocardial infarction. Mediators Inflamm. 2013;2013:206039. doi: 10.1155/2013/206039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Tena C, González-Chávez SA. The danger model approach to the pathogenesis of the rheumatic diseases. J Immunol Res. 2015;2015:506089. doi: 10.1155/2015/506089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoux M, Kirkpatrick RL, Montauti EI, Tran BQ, Peterson SB, Harding BN, Whitney JC, Russell AB, Traxler B, Goo YA, Goodlett DR, Wiggins PA, Mougous JD. Kin cell lysis is a danger signal that activates antibacterial pathways of Pseudomonas aeruginosa. Elife. 2015;4:e05701. doi: 10.7554/eLife.05701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Land WG. Danger signals—damaged-self recognition across the tree of life. Front Plant Sci. 2014;5:578. doi: 10.3389/fpls.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HE, Raucci A. Alarmin (g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7(8):774–8. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4(6):469–78. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- Hirsiger S, Simmen HP, Werner CM, Wanner GA, Rittirsch D. Danger signals activating the immune response after trauma. Mediators Inflamm. 2012;2012:315941. doi: 10.1155/2012/315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietbrink F, Koenderman L, Rijkers G, Leenen L. Trauma: the role of the innate immune system. World J Emerg Surg. 2006;1:15. doi: 10.1186/1749-7922-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivula I, Hämäläinen S, Jantunen E, Pulkki K, Kuittinen T, Nousiainen T, Juutilainen A. Elevated procalcitonin predicts Gram-negative sepsis in haematological patients with febrile neutropenia. Scand J Infect Dis. 2011;43(6-7):471–8. doi: 10.3109/00365548.2011.554855. [DOI] [PubMed] [Google Scholar]
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9:107. doi: 10.1186/1741-7015-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HG, Brandes H, Bredle DL, Bitterlich A, Vollandt R, Specht M, Franke UF, Wahlers T, Meier-Hellmann A. Post-operative hypoalbuminaemia and procalcitonin elevation for prediction of outcome in cardiopulmonary bypass surgery. Acta Anaesthesiol Scand. 2003;47(10):1276–83. doi: 10.1046/j.1399-6576.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- Rau BM, Frigerio I, Büchler MW, Wegscheider K, Bassi C, Puolakkainen PA, Beger HG, Schilling MK. Evaluation of procalcitonin for predicting septic multiorgan failure and overall prognosis in secondary peritonitis: a prospective, international multicenter study. Arch Surg. 2007;142(2):134–42. doi: 10.1001/archsurg.142.2.134. [DOI] [PubMed] [Google Scholar]
- Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011;66(Suppl 2):ii33–40. doi: 10.1093/jac/dkq523. [DOI] [PubMed] [Google Scholar]