Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by a chronic relapsing-remitting joint inflammation. Perturbations in the balance between CD4 + T cells producing IL-17 and CD4 + CD25highFoxP3 + Tregs correlate with irreversible bone and cartilage destruction in RA. APL1 is an altered peptide ligand derived from a CD4+ T-cell epitope of human HSP60, an autoantigen expressed in the inflamed synovium, which increases the frequency of CD4 + CD25highFoxP3+ Tregs in peripheral blood mononuclear cells from RA patients. The aim of this study was to evaluate the suppressive capacity of Tregs induced by APL1 on proliferation of effector CD4+ T cells using co-culture experiments. Enhanced Treg-mediated suppression was observed in APL1-treated cultures compared with cells cultured only with media. Subsequent analyses using autologous cross-over experiments showed that the enhanced Treg suppression in APL1-treated cultures could reflect increased suppressive function of Tregs against APL1-responsive T cells. On the other hand, APL1-treatment had a significant effect reducing IL-17 levels produced by effector CD4+ T cells. Hence, this peptide has the ability to increase the frequency of Tregs and their suppressive properties whereas effector T cells produce less IL-17. Thus, we propose that APL1 therapy could help to ameliorate the pathogenic Th17/Treg balance in RA patients.
Keywords: Rheumatoid arthritis, Heat shock protein 60, Altered peptide ligands, Regulatory T cells
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disorder that eventually leads to the functional impairment of peripheral joints. A subset of CD4 effector T helper cells that produce IL-17, termed T helper 17 (Th17) cells, has been implicated as the pivotal driving force of autoimmune inflammation in several human autoimmune diseases (Waite and Skokos 2012). A novel hypothesis suggests that a Th17/regulatory T-cell (Treg) imbalance may be responsible for the development and progression of RA (Wang et al. 2012; AlFadhli 2013). Expression levels of IL-17 have been found increased in RA synovium (Kotake et al. 1999), as well as in peripheral blood mononuclear cells (PBMCs) supernatants from patients compared with healthy controls (Shen et al. 2010). Moreover, the frequencies of Th17 cells and levels of IL-17 strongly correlated with systemic disease activity at both the onset and the progression of RA (Leipe et al. 2010). The cytokine is involved in different pathogenic processes, for instance in increasing the production of pro-inflammatory cytokines, chemokines, and matrix-degrading enzymes in a range of cell types (Park et al. 2005). On the other hand, CD4 + CD25high forkhead box P3-expressing (FoxP3+) Tregs are among the most important cells in the regulation of the immune system. Tregs prevent the development of various inflammatory diseases by suppressing autoaggressive T and B cell responses (Sakaguchi et al. 2009). Intensive research has focused on Tregs in RA and other chronic rheumatic diseases (Prakken et al. 2001; Wehrens et al. 2013). Some studies have shown reduced numbers and/or suppressive function of Tregs in peripheral blood of RA patients compared to healthy controls (Ehrenstein et al. 2004; Valencia et al. 2006; Jiao et al. 2007; Sempere-Ortells et al. 2009; Lina et al. 2011; Nie et al. 2013). Tregs from RA patients seem unable to inhibit the secretion of pro-inflammatory cytokines such as IFNγ and TNFα even though they are competent at suppressing the proliferation of autologous CD4+ CD25- T cells (Cao et al. 2003; Ehrenstein et al. 2004). This phenomenon is perhaps due to an inhibition of their functions by pro-inflammatory cytokines, increased number of activated effector T (Teff) cells and/or the resistance of Teff cells to suppression (Pasare and Medzhitov 2003; Valencia et al. 2006; Bettelli et al. 2006; Buckner 2010). Altogether, it seems that the predominance of pathogenic Th17 cells and pro-inflammatory cytokines together with a deficiency in the mechanisms that control the immune response have deleterious effects in the persistence of inflammatory conditions (Cope et al. 2007; McInnes and Schett 2011). Therefore, the balance between Tregs and pathogenic Teff cells is crucial for immune regulation in RA.
Antigen-specific approaches can manipulate in a more specific way such balance avoiding the generalized immune suppression. However, the selection of a specific autoantigen is a crucial point in this approach. Heat shock protein 60 kDa (HSP60) has been successfully used in the induction of tolerance in autoimmune arthritis (van Eden et al. 2005; Zonneveld-Huijssoon et al. 2013). We have predicted two novel CD4+ T-cell epitopes (E18-3 and E18-12) from human HSP60 by the use of bioinformatics (Domínguez et al. 2011; Barberá et al. 2013; Lorenzo et al. 2012). In particular, the wild-type peptide E18-3 was modified in one amino acid residue for increasing its affinity to RA associated HLA class II molecules. According to previous results, stimulation with this new peptide, called APL1, increases the frequency of CD4 + CD25highFoxP3+ Tregs from PBMCs of RA patients and inhibits the course of arthritis in an animal model for RA (Domínguez et al. 2011).
In the present study, we investigated the suppressive capacity of Tregs induced by APL1 on proliferation of effector CD4+ T cells using autologous co-culture experiments. We also explored its effects on activation and function of highly purified Tregs and Teff cells isolated from PBMCs of RA patients.
Methods
Peptides
Peptides were manually synthetized by the Fmoc/tBu strategy in syringes using the Fmoc-AM-MBHA resin (0.54 mmol/g). After treatment with TFA, the peptides were lyophilized and analyzed by reverse phase HPLC and mass spectrometry. The sequences of APL1 and E18-3 peptides were previously published (Domínguez et al. 2011; Barberá et al. 2013). The adenoviral peptide 475C489 (A5, Ansynth Service B.V., Roosendaal, The Netherlands) was used as control for suppression assays.
Patients and healthy subjects
RA patients were diagnosed according to the European League against Rheumatism criteria (Aletaha et al. 2010). Eleven patients were recruited from the University Medical Center, Utrecht, The Netherlands, and ten patients from the National Institute of Rheumatology, Havana, Cuba. Approval for this study was obtained from both institutional medical ethics review boards.
Patients have an average age of 53 ± 13 years (range 23–69) and a mean duration of disease of 14 years ± 9 years (range 3–38 years). Most patients were taking steroidal anti-inflammatory drugs and disease-modifying anti-rheumatic drugs like methotrexate.
Ten healthy subjects participated in the study with an age range of 25–55 years.
Informed consent was obtained from all patients and healthy subjects.
Isolation and culture of CD4 + T-cell subsets
PBMCs were isolated by density gradient centrifugation (Ficoll-Paque PLUS, GE Healthcare Europe). CD4 + CD25-CD127+ T cells (Teff) and CD4 + CD25highCD127- (Tregs) were isolated directly from PBMCs using a BD influx™ cell sorter (BD Biosciences) after staining using PerCP-conjugated anti-CD4, FITC-conjugated anti-CD25, and PE-conjugated anti-CD127 (purities more than 95 %). Teff were cultured with irradiated (3000 rads) autologous PBMCs as antigen-presenting cells (APC) in a T:APC ratio of 1:5, with APL1 (40 μg/mL), A5 (40 μg/mL) or media for 4 days in 6-well plates. Tregs isolated from PBMCs were cultured with autologous APC (1:5 ratio), with APL1 (40 μg/mL), A5 (40 μg/mL) or media for 4 days in 96-well plates. Cells were cultured in RPMI 1640 Glutamax medium supplemented with 1 % penicillin/streptomycin and 10 % heat inactivated pooled human AB serum. Table 1 summarizes the CD4+ T cell subsets, cell culture treatments, and terms used in this study. After culture, cell viability was monitored by trypan blue exclusion. The culture of cells with APL1 or A5 peptides did not affect cell viability when compared to cells cultured only with media. All cells were washed in PBS1×, counted, resuspended in fresh supplemented RPMI 1640 Glutamax medium, and used for phenotypic, proliferation, suppression, and cytokine detection assays as described below. Total PBMCs from RA patients and healthy controls were used in some experiments.
Table 1.
CD4+ T cell subsets, cell culture treatments, and terms used in this study
| Cells | Treatment | Term |
|---|---|---|
| CD4 + CD25-CD127 + (Teff) | Irradiated PBMC(1:5) plus media | Tc- |
| Irradiated PBMC(1:5) plus APL1 | TAPL1 | |
| Irradiated PBMC(1:5) plus A5 | Tcp | |
| Tregs | Irradiated PBMC(1:5) plus media | Tregc- |
| CD4 + CD25 + CD127-(Treg) | Irradiated PBMC(1:5) plus APL1 | TregAPL1 |
| Irradiated PBMC(1:5) plus A5 | Tregcp |
Phenotypic analysis of Tregs and CD4+ effector T cells
To determine the percentage of Tregs in PBMCs from RA and healthy controls, 1 × 106 PBMCs were cultured in triplicate with or without 40 μg/mL of APL1 or wild-type peptide E18-3 for 5 days. Afterwards, cells were stained using the Human Regulatory T cell Staining Kit (eBioscience) according to the manufacturer’s instructions. Samples were acquired on a FACS Partec flow cytometer (Partec, Germany) and analyzed using the Partec Flomax software version 2.81. One hundred thousand cells were acquired for analysis. CD4 + T cells were gated according to their expression of CD25. FoxP3 expression was then analyzed on CD4 + CD25high gated T cells. Then, the numbers of FoxP3+ cells were used to calculate the percentage of CD4 + CD25high FoxP3+ Tregs among total CD4 + T cells.
Tregs and Teff cells cultured with media or APL1 (5 × 104) were used for phenotypic analyses. Cells were stained using a PerCP-conjugated anti-CD4, an APC-conjugated anti-CD25, and a PE-conjugated anti-FoxP3. Phosphorylation of STAT-5 was determined in Tregs using a Pacific blue-conjugated anti-pSTAT5 (Y694). Samples were acquired on Becton Dickson FACS Canto machine and data were analyzed using FlowJo software (Treestar Inc., Ashland, OR, USA). A minimum of 30–50,000 events were acquired for analysis.
Proliferation and suppression assays
After the initial cultures, Tc-, TAPL1, and Tcp cells (5 × 104) were washed and cultured for 4 days in the absence or presence of anti-human CD3/CD28-coated magnetic beads (bead/cell ratio, 1:15). During the last 18 h of culture, 3H-thymidine was added at 1 μCi/well. Proliferative responses upon CD3/CD28 stimulation were expressed as the mean +/−SD counts per minute (cpm) of triplicate wells. For suppression assays, Tregc-, TregAPL1, or Tregcp were added accordingly to cultures in suppressor/effector ratios of 1:2 and 1:4. Percentage of suppression was calculated as 100*1-(cpm of co-cultures/cpm of Teff alone). In other experiments, proliferation was assessed by CFSE method. The suppression of the proliferation was calculated using the formula: 100*1-(% CFSElow co-culture/%CFSElow Teff alone). Samples were acquired on Becton Dickson FACs Canto machine as described above.
Cytokine detection assays
Tc- and TAPL1 cells (5 × 104) were washed and stimulated with anti-human CD3/CD28 coated magnetic beads (bead: cell ratio, 1:15) for 4 days. Cytokines were measured in supernatants using a multiplex immunoassay based on Luminex technology (xMAP, Luminex Austin TX USA) and analyzed as described previously (de Jager et al. 2005; de Jager et al. 2007). In other experiments, Tc- and TAPL1 (5 × 104) were washed and stimulated with PMA (50 ng/mL) and ionomycin (2000 ng/mL) for 5 h. BFA (5 μg/mL) was added for the last 3 h. Then, the staining of CD4 surface expression was performed. Intracellular cytokines were stained using PE-conjugated anti-IFNγ and APC-conjugated anti-IL-10. In other experiments, PBMCs (1 × 106) from RA patients were cultured in triplicate with or without 40 μg/mL of APL1 for 4 days. IL-17 levels were evaluated in supernatants using the Human IL-17 Quantikine ELISA Kit (R&D Systems).
Statistical analysis
All data analyses were performed using GraphPad Prism version 5.00 (GraphPad Software, San Diego California, USA). Samples were examined for normality with Kolmogorov–Smirnov test. Results were expressed as mean ± standard deviation (SD), and differences were analyzed with Student’s t test, Mann–Whitney test or ANOVA with a Tukey’s post-test, accordingly. P < 0.05 was considered statistically significant.
Results
Treg-mediated suppression is enhanced in APL1-treated cultures
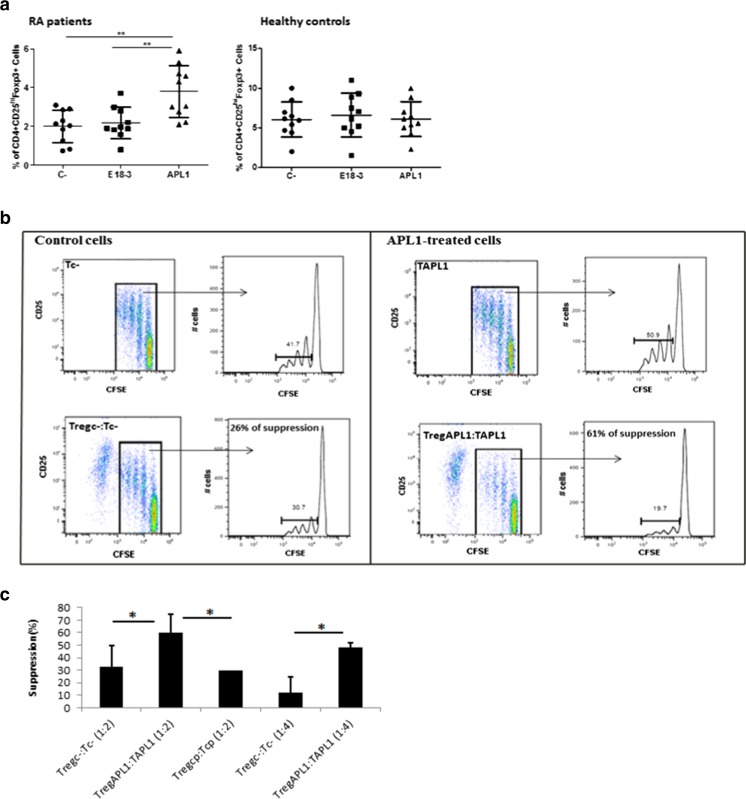
In previous studies, we have demonstrated that APL1 increases the frequency of CD4 + CD25highFoxP3+ Tregs in PBMCs of RA patients. In contrast, the wild-type peptide E18-3 had not such effect (Domínguez et al. 2011). Here, we confirmed these earlier results and showed that APL1 did not induce an increase of Tregs when the PBMCs were isolated from healthy subjects (Fig. 1a). Furthermore, we observed a reduced circulating Tregs percentages in RA compared to healthy individuals as previously reported (Jiao et al. 2007; Sempere-Ortells et al. 2009; Lina et al. 2011).
Fig. 1.
APL1 increases the frequency and suppressive properties of Tregs isolated from rheumatoid arthritis patients. a APL1 increases the frequency of CD4 + CD25highFoxP3+ Tregs in PBMCs of RA patients but not in PBMCs of healthy subjects. PBMCs were cultured with or without peptide (40 μg/mL) for 5 days. PBMCs in absence of peptide were used as control of basal frequency in each sample (C-). Data were analyzed using ANOVA and Tukey’s post-test (**p < 0.01). b, c Treg-mediated suppression is enhanced in APL1-treated cultures from RA patients. b Tregc- or TregAPL1 were added to Tc- or TAPL1 cells, respectively, in a ratio suppressor/effector of 1:2 (c) Tregc-, TregAPL1, or Tregcp were added to Tc-, TAPL1, or Tcp cells, respectively, in ratios suppressor/effector of 1:2 and 1:4. Proliferation was assessed by CFSE in (b) and [3H]-thymidine method (c), n = 5. Data were analyzed using Student’s t test for each ratio (*p < 0.05)
The hallmark of Tregs is their ability to suppress immune responses by inhibiting the proliferation of Teff cells. Whether APL1 influences the suppressive function of Tregs from peripheral blood of RA patients was next investigated. To test this approach, CD4 + CD25highCD127- Tregs and CD4 + CD25-CD127+ Teff cells were isolated from PBMCs of RA patients by cell sorting and were cultured with APC and with medium alone or APL1 for 4 days (see Table 1 and Methods section for details). The suppressive capacity of TregAPL1 on proliferation of Teff cells was first assessed on TAPL1 cells and compared with control cells, trying to simulate an in vivo administration of APL1, in which it would be in contact with APC, Tregs but also with Teff cells. A representative example of the suppressive activity of Tregc- and TregAPL1 against Tc- and TAPL1 cells, respectively, in a ratio suppressor/effector of 1:2, is shown in Fig. 1b. TregAPL1 showed a twice higher suppressive activity on proliferation of TAPL1 cells compared with Tregc- against Tc- for all suppressor/effector ratios tested (Fig. 1c). However, Treg-mediated suppression was not increased when cells were cultured with a control peptide (A5) compared to control cells. Thus, the increased suppressive function of Tregs in APL1-treated cells seems to be specific to this peptide rather than broadly applicable to antigen specific in vitro experiments of this nature.
Enhanced Treg suppression in APL1-treated cultures seems to reflect increased suppressive function of Tregs against APL1 responsive T cells
We next determined whether enhanced suppression observed in APL1-treated cultures reflects increased sensitivity of TAPL1 cells to suppression or increased Treg cell suppressive functions. This was examined using autologous cross-over suppression assays.
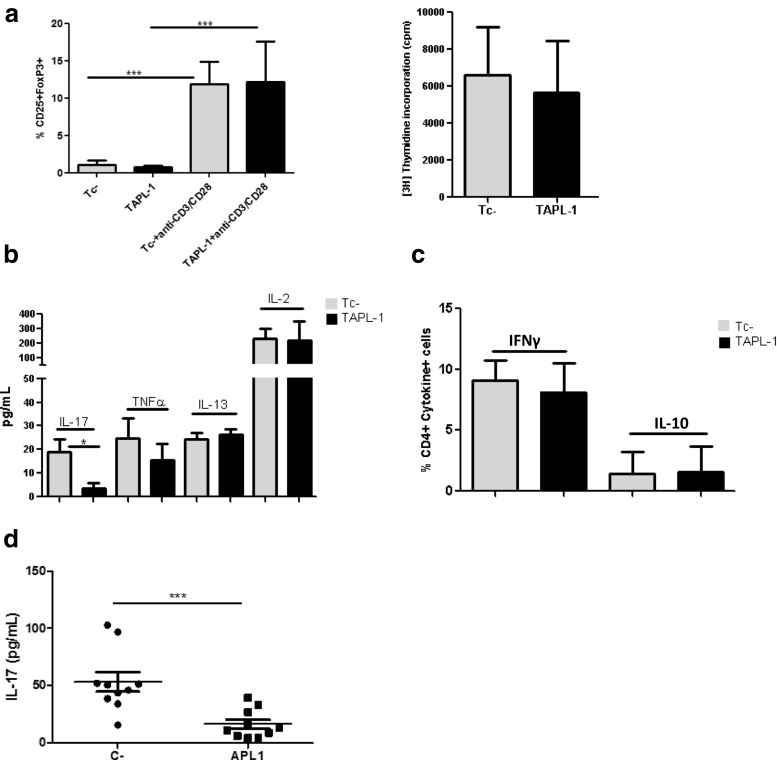
The sensitivity of TAPL1 cells to Treg suppression was assessed by comparing the capacity of Tregc- cells to decrease the proliferation of Tc- and TAPL1 cells. First, it was confirmed that effector cells cultured with APL1 for 4 days displayed comparable phenotypic and proliferative properties to cells cultured with media at the CD3/CD28 bead: cell ratio used for all the assays. Figure 2a (left hand panel) shows that the expression levels of CD25 and FoxP3 were similarly low in Tc- and TAPL1 cells before the mitogenic stimulation. Human CD4 + CD25- T cells upregulate transiently the expression of CD25 and FoxP3 after activation (Allan et al. 2005; Gavin et al. 2006). As expected, the expression of both markers was induced in Teff cells after CD3/CD28 stimulation, but again, comparable levels were detected between Tc- and TAPL1 cells (Fig. 2a, left hand panel). Furthermore, Tc- and TAPL1 cells exhibited similar levels of proliferation upon mitogenic stimulation (Fig. 2a, right hand panel). To better characterize the effector T cell function of Tc- and TAPL1 cells, their production of cytokines upon CD3/CD28 stimulation was analyzed using a Luminex multiparameter immunoassay. As shown in Fig. 2b, TAPL1 cells produced four times less IL-17 than Tc-, while both displayed similar levels of TNFα, IL-13, and IL-2. The latter is in accordance with the fact that Tc- and TAPL1 cells have the same ability to proliferate after CD3/CD28 stimulation. In this assay, IFNγ and IL-10 levels were under the limits of detection, so we measured the frequencies of CD4 + T cells producing IFNγ and IL-10 upon PMA/ionomycin stimulation but no differences were detected between Tc- and TAPL1 cells (Fig. 2c). Taking into consideration that APL1 reduced only the secretion of IL-17 produced by Teff cells, we proceeded to confirm this effect in ex vivo assays using PBMCs from RA patients. As shown in Fig. 2d, APL1 strongly reduced IL-17 secretion in theses cultures. Thus, TAPL1 cells show a classic phenotype of activated T cells upon a mitogenic stimulus, but with a decreased production of IL-17 compared to Tc- cells. As immunosuppressive Treg-cell function shows a non-reciprocal relationship with some pro-inflammatory cytokines like TNFα (Valencia et al. 2006), we wondered whether an impaired IL-17 production by TAPL1 cells could render these cells more susceptible to Treg-mediated suppression. However, although some increase in the suppressive activity of Tregc- against TAPL1 compared to Tc- cells was seen, no significant differences were found (Fig. 3a). Thus, TAPL1 cells do not appear to be more susceptible to Treg-mediated suppression than Tc- cells.
Fig. 2.
TAPL1 cells show a classic phenotype of activated T cells upon a mitogenic stimulus, but with a decreased production of IL-17. a TAPL1 cells exhibit similar phenotype (left hand panel) and proliferative properties (right hand panel) as Tc- cells. CD25 and FoxP3 expressions were determined in Tc- and TAPL1 cells before and after (left hand panel) CD3/CD28 stimulation, n = 5. Proliferation was assessed by 3H-thymidine method (right hand panel), n = 5. Data were analyzed using ANOVA and Tukey’s post-test (***p < 0.0001). b APL1 decreases IL-17 produced by activated CD4 + T cells from RA patients. Tc- and TAPL1 cells were cultured with anti-CD3/CD28 beads for 4 days. Cytokine levels were measured in supernatants using an in-house developed and validated multiplex immunoassay based on Luminex technology, n = 3. Data were analyzed using Mann–Whitney test (*p < 0.05). c The frequencies of CD4 + T cells producing IFNγ and IL-10 were determining in Tc- and TAPL1 cells by intracellular staining after PMA/ionomycin stimulation, n = 5. d APL1 inhibits IL-17 secretion in PBMCs from RA patients. PBMCs were cultured with or without peptide (40 μg/mL) for 4 days. PBMCs in absence of peptide were used as control of basal levels in each sample (C-). Levels of this cytokine were detected in supernatants using the Human IL-17 Quantikine ELISA Kit (R&D Systems). These results are representative of three similar experiments, n = 10. Data were analyzed using ANOVA and Tukey’s post-test (***p < 0.001)
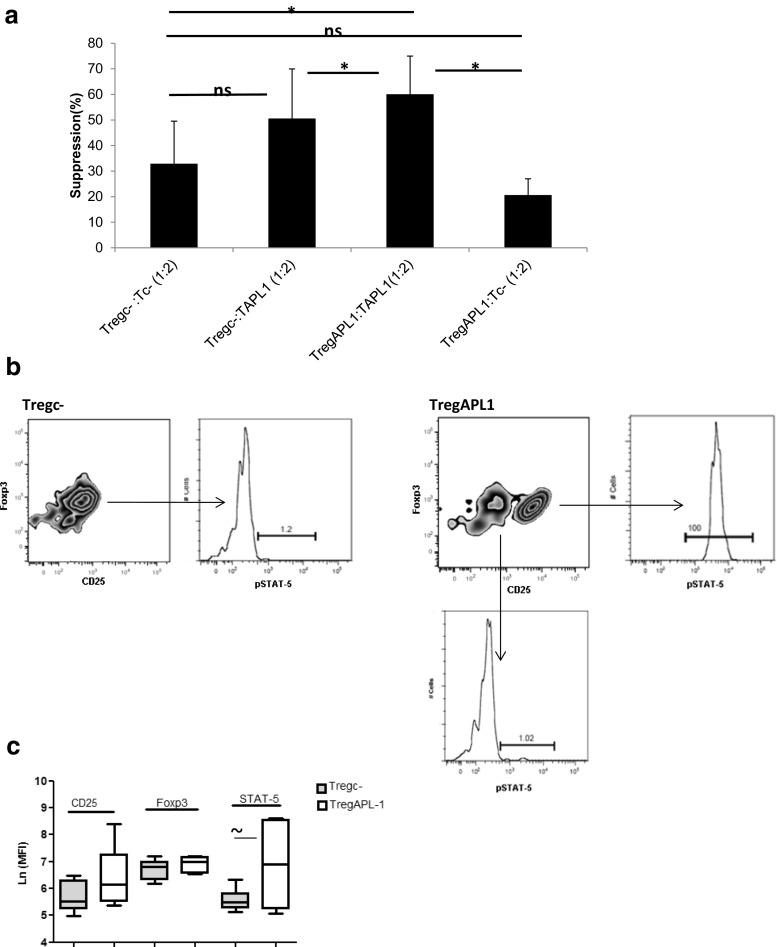
Fig. 3.
Enhanced Treg suppression in APL1-treated cultures could to be due to increased suppressive function of Tregs against APL1-responsive T cells. a Cross-over experiments showing the potency of Tregs on proliferation of Teff cells in different experimental conditions. TAPL1 or Tc- cells were cultured with anti-CD3/CD28 beads for 4 days in the presence or absence of TregAPL1 or Tregc- in a suppressor: responder ratio of 1:2, n = 5. Proliferation was assessed by [3H]- thymidine method. Data were analyzed using Student’s t test (*p < 0.05, ns: not significant). b APL1 treatment leads to the activation of STAT-5 in Tregs. The expression of CD25 and FoxP3 within CD4+ cells in Tregc- and TregAPL1 is shown. pSTAT-5 expression was analyzed in pointed populations. c Mean fluorescence intensity (MFI) values of CD25, FoxP3, and pSTAT-5 in Tregc- and TregAPL1, n = 6. Data were expressed as Ln of MFI values and analyzed using Student’s t test for each pair of data (∼p = 0.0)
Then, we compared the capacity of TregAPL1 and Tregc- to decrease the proliferation of TAPL1 cells. A significant difference between the suppressive function of TregAPL1 compared with Tregc- against TAPL1 cells was observed (Fig. 3a). The suppressive function of TregAPL1 was also analyzed on proliferation of Tc- cells but no differences were detected with respect to Tregc-. All these results might suggest that the enhanced Treg suppression observed in APL1-treated cultures appears to reflect higher potency of TregAPL1 cells against APL1 responsive Teff cells.
To gain some insights into the effect of APL1 on Tregs, we investigated their phenotype after culture with this peptide for 4 days by assessing their expression of CD25, FoxP3, and pSTAT-5. The activation of STAT-5 pathway, through phosphorylation at residue Y694, has been involved in the development of Tregs with higher reactivity to self-antigens through direct up-regulation of CD25 and FoxP3 expression (Moran et al. 2011; Mahmud et al. 2012). APL1 induced a population with higher expression of CD25 in patients. A representative experiment is shown in Fig. 3b. The increased expression of CD25 was associated with the expression of pSTAT-5 in this subpopulation. A trend toward increased MFI values of pSTAT-5 was observed in TregAPL1 cells compared to Tregc- (Fig. 3c). These data could suggest that APL1 is able to activate pathways involved in the expansion and survival of Tregs.
Discussion
We have reported that APL1 has two major effects in PBMCs from RA patients. This peptide increases the frequency of CD4 + CD25highFoxP3+ Tregs (Domínguez et al. 2011) and induces apoptosis of activated CD4 + T cells presumably through a Treg-dependent mechanism (Barberá et al. 2013). Both effects could help to restore the balance between Tregs and Teff cells which is essential for immune regulation in RA. In addition, APL1 increased Treg frequencies in PBMCs isolated from patients with Crohn’s disease and Juvenile idiopathic arthritis (Domínguez et al. 2014). Here, we investigated the specificity of this mechanism by exploring such effects in healthy subjects. APL1 did not increase the proportions of the CD4 + CD25highFoxP3+ Treg cells from healthy individuals. Similar results to those seen in healthy subjects were found in assays using PBMCs from patients with osteoarthritis (OA) (data not shown), which is the most common form of arthritis, but is not considered as an autoimmune disease (Berenbaum 2013). Given all these facts, we think that APL1 is able to expand Tregs within an inflammatory context, associated with autoimmune conditions.
In last decades, a consistent number of studies investigated the number, phenotype, and function of Tregs in the peripheral blood, synovial fluid, and synovial membrane of RA patients. In agreement with our results, most studies observed reduced circulating Tregs percentages in RA compared to healthy individuals (Jiao et al. 2007; Sempere-Ortells et al. 2009; Lina et al. 2011). One fact that could explain the low frequency of circulating Tregs in RA patients is the finding that natural Tregs can convert into Th17 cells and other effector T cells in certain environments (Zheng 2013). For instance, IL-6, which is highly expressed in RA, favors the conversion of natural Tregs into Th17 cells (Zheng et al. 2008). The increased frequency of Th17 cells as well as low circulating levels of Tregs have been found to correlate with the disease activity of RA patients (Sempere-Ortells et al. 2009; Leipe et al. 2010). On the other hand, there is a clear evidence that the frequencies of Tregs in the synovial fluid of patients with RA are elevated compared with those in the peripheral blood (Cao et al. 2003; Jiao et al. 2007). A selective migration of Tregs from peripheral blood to the inflamed joint involving interactions through CXCR4 has been proposed as one of the plausible mechanisms to explain these differences (Zou et al. 2004). Thus, the last mentioned could also help to explain low numbers of Tregs in peripheral blood from patients with rheumatic diseases compared to healthy controls.
In addition to Treg frequency, others have also reported that the functional activity of Tregs is altered in RA (Ehrenstein et al. 2004; Leipe et al. 2005; Valencia et al. 2006). Failures in the function/numbers of Tregs can be responsible for the development of autoimmune diseases, and enhancing their function may be a treatment strategy. In this study, we analyzed whether in addition to increasing the frequency of Tregs, APL1 influenced the suppressive function of Tregs from peripheral blood of RA patients. Elevated Treg-mediated suppression on proliferation was observed in APL1-treated cells in contrast to control cells. This comparison was possible because cells cultured with APL1 had the same ability to proliferate than cells cultured with media. This fact could seem contradictory considering our previous reports in which APL1 induced apoptosis of activated CD4+ T cells (Barberá et al. 2013). However, it is important to notice that here APL1 was not present during the stimulation of cells with anti-CD3/CD28-coated magnetic beads. APL1 was cultured with CD4 + CD25- Teff cells for 4 days and then cells were washed and stimulated with anti-CD3/CD28 beads as it was described in Methods section. Thus, APL1 was absent in cultures when T cells became active. Similarly to our previous findings, APL1 had no effect on cell viability in CD4 + CD25- T cell cultures (data not shown). Due to the same reasons, differences seen in cytokine production of TAPL1 and Tc- cells cannot be associated with the pro-apoptotic effect of APL1 to T effector cells. In this study, APL1 treatment only decreased the production of IL-17 by Teff cells. This effect was confirmed in ex vivo assays using PBMCs from RA patients. IL-17 production and defects in Treg function/numbers in RA patients have been correlated with irreversible bone and cartilage destruction (AlFadhli 2013). Thus, the fact that APL1 decreased the production of IL-17 by Teff cells together with the increased frequency/function of Tregs in peripheral blood could represent a beneficial effect in the control of the inflammatory process in RA.
The decreased secretion of IL-17 by TAPL1 cells did not render them more susceptible to Treg-mediated suppression. Autologous cross-over experiments suggested that the higher Treg-mediated suppression seen in APL1-treated cultures could be due to higher potency of TregAPL1 cells against APL1-responsive Teff cells. This result could be explained considering that Tregs that share the same antigenic specificity with effector cells are much more suppressive than polyclonal populations (Masteller et al. 2006). Indeed, it is hypothesized that effectiveness of the Treg-based therapy can be improved by using antigen-specific Tregs rather than polyclonal Tregs because of their higher suppressive properties. However, it is necessary to identify relevant antigens in the pathogenesis of the disease. Tregs need to be activated via their T cell receptor for maintaining their suppressive potential; this implicates that their cognate antigen should be abundantly present and presented in the context of MHC class II molecules in the inflamed joints. We and others believe that peptides derived from HSPs could be such antigens (Prakken et al. 2004; van Eden et al. 2005; Koffeman et al. 2009; Zonneveld-Huijssoon et al. 2013; Barberá et al. 2015). HSP60-peptides are considered bystander antigens because they are upregulated at the site of inflammation and are immunologically dominant. Tolerogenic immune responses induced to such antigens could lead to a local downregulation of the ongoing inflammatory response (Zonneveld-Huijssoon et al. 2013). In particular, the response induced to APL1 should be relevant in the context of autoimmune arthritis considering the protective effect of this peptide in the AA model associated with increased proportions of Tregs (Domínguez et al. 2011). In addition, our data seem to support that APL1 is able to activate the STAT-5 pathway in Tregs. This pathway has been involved in the expansion, survival, stabilization of FoxP3 expression in these cells, and it suppresses differentiation into Th17 cells (Wei et al. 2008). The specific mechanism by which APL1 induces the activation of STAT-5 in Tregs remains unclear but it is interesting in the light of earlier gain-of-function studies, in which the expression of a constitutively active STAT-5 resulted in the expansion of Tregs and rescued their numbers in the absence of IL-2 (Burchill et al. 2003; Burchill et al. 2008).
In summary, we have described in this study that APL1, a peptide derived from HSP60, has the ability to increase the frequency of Tregs and its suppressive capacity against APL1 responding Teff cells and on the other hand, decreases IL-17 secretion by activated Teff cells. Thus, we propose that APL1 therapy could help to ameliorate the pathogenic Th17/Treg balance in RA patients.
Acknowledgments
Dr. Ger Arkesteijn, Dr. Irene Ludwig, and Drs. Charlotte de Wolf are acknowledged for their assistance. Dr. Emmerik Leijten and Dr. Yusimy Reyes are acknowledged for their support in the selection of patients.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Funding
This work was supported by the Women in Science Program financed by L’Oreal/UNESCO, grants of Dutch Arthritis Association, and the Innovation Oriented Programme Genomics (IOP) and Biomedical Research Department at Center for Genetic Engineering and Biotechnology.
References
- Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- AlFadhli S. The interleukin 23/Interleukin-17 axis and the role of Treg/Th17 cells in rheumatoid arthritis and joint destruction. OA Arthritis. 2013;1(1):5–11. doi: 10.13172/2052-9554-1-1-494. [DOI] [Google Scholar]
- Allan SE, Passerini L, Bacchetta R, et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberá A, Lorenzo N, Garrido G, et al. APL-1, an altered peptide ligand derived from human heat-shock protein 60, selectively induces apoptosis in activated CD4+ CD25+ T cells from peripheral blood of rheumatoid arthritis patients. Int Immunopharmacol. 2013;17(4):1075–1083. doi: 10.1016/j.intimp.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Barberá A, Broere F, van Eden W. Heat shock proteins as target for the induction of antigen-specific tolerance in rheumatoid arthritis and other chronic inflammatory diseases. J Autoimmune Dis Rheumatol. 2015;3(2):41–52. doi: 10.12970/2310-9874.2015.03.02.3. [DOI] [Google Scholar]
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis) Osteoarthr Cartil. 2013;21(1):16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Buckner JH. Mechanisms of impaired regulation by CD4 + CD25 + FOXP3+ regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Goetz CA, Prlic M, et al. Distinct effects of STAT5 activation on CD4 + and CD8 + T cell homeostasis: development of CD4 + CD25 + regulatory T cells versus CD8 + memory T cells. J Immunol. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vang KB, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Malmstrom V, Baecher-Allan C, et al. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33(1):215–223. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- Cope AP, Schulze-Koops H, Aringer M. The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol. 2007;25(5 Suppl 46):S4–S11. [PubMed] [Google Scholar]
- de Jager W, Prakken BJ, Bijlsma JW, Kuis W, Rijkers GT. Improved multiplex immunoassay performance in human plasma and synovial fluid following removal of interfering heterophilic antibodies. J Immunol Methods. 2005;300:124–135. doi: 10.1016/j.jim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- de Jager W, Hoppenreijs EP, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66:589–598. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez MC, Lorenzo N, Barberá A, et al. An altered peptide ligand corresponding to a novel epitope from heat-shock protein 60 induces regulatory T cells and suppresses pathogenic response in an animal model of adjuvant induced arthritis. Autoimmunity. 2011;44(6):471–482. doi: 10.3109/08916934.2010.550590. [DOI] [PubMed] [Google Scholar]
- Domínguez MC, Lorenzo N, Cantera D. A peptide as immunomodulator for the treatment of juvenile idiopathic arthritis. Ann Rheum Dis. 2014;73:130. [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J Exp Med. 2004;200(3):277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. PNAS. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Z, Wang W, Jia R, et al. Accumulation of FoxP3-expressing CD4 + CD25+ T cells with distinct chemokine receptors in synovial fluid of patients with active rheumatoid arthritis. Scand J Rheumatol. 2007;36(6):428–433. doi: 10.1080/03009740701482800. [DOI] [PubMed] [Google Scholar]
- Koffeman EC, Genovese M, Amox D, et al. Epitope specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis Rheum. 2009;60:3207–3216. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe J, Skapenko A, Lipsky PE, Schulze-Koops H. Regulatory T cells in rheumatoid arthritis. Arthritis Res Ther. 2005;7:93. doi: 10.1186/ar1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipe J, Grunke M, Dechant C, et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 2010;62(10):2876–2885. doi: 10.1002/art.27622. [DOI] [PubMed] [Google Scholar]
- Lina C, Conghua W, Nan L, Ping Z. Combined treatment of etanercept and MTX reverses Th1/Th2, Th17/Treg imbalance in patients with rheumatoid arthritis. J Clin Immunol. 2011;31(4):596–605. doi: 10.1007/s10875-011-9542-6. [DOI] [PubMed] [Google Scholar]
- Lorenzo N, Barberá A, Domínguez MC, et al. Therapeutic effect of an altered peptide ligand derived from heat-shock protein 60 by suppressing of inflammatory cytokines secretion in two animal models of rheumatoid arthritis. Autoimmunity. 2012;45(6):449–459. doi: 10.3109/08916934.2012.697592. [DOI] [PubMed] [Google Scholar]
- Mahmud SA, Manlove LS, Farrar MA. Interleukin-2 and STAT5 in regulatory T cell development and function. Landes Bioscience. 2012;2(1):e23154–e23156. doi: 10.4161/jkst.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells—ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18(2):103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zheng Y, Li R, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway dependent blockade of CD4 + CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. 2001;377:2138–2149. doi: 10.1016/S0140-6736(11)60244-4. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Samodal R, Le TD, et al. Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. PNAS. 2004;101(12):4228–4233. doi: 10.1073/pnas.0400061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Wing K, Onishi Y, et al. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21(10):1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- Sempere-Ortells JM, Pérez-García V, Marín-Alberca G, et al. Quantification and phenotype of regulatory T cells in rheumatoid arthritis according to disease activity Score-28. Autoimmunity. 2009;42(8):636–645. doi: 10.3109/08916930903061491. [DOI] [PubMed] [Google Scholar]
- Shen H, Xia L, Lu J, Xiao W. Infliximab reduces the frequency of interleukin 17-producing cells and the amounts of interleukin 17 in patients with rheumatoid arthritis. J Investig Med. 2010;58(7):905–908. doi: 10.2310/JIM.0b013e3181eb9895. [DOI] [PubMed] [Google Scholar]
- Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4 + CD25hi T-regulatory cells. Blood. 2006;108(1):253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- Waite JC, Skokos D. Th17 response and inflammatory autoimmune diseases. Int J Inflamm. 2012;2012:819467. doi: 10.1155/2012/819467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Shao S, Jiao Z, Guo M, Xu H, Wang S. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int. 2012;32:887–893. doi: 10.1007/s00296-010-1710-0. [DOI] [PubMed] [Google Scholar]
- Wehrens EJ, Prakken BJ, van Wijk F. T cells out of control—impaired immune regulation in the inflamed joint. Nat Rev Rheumatol. 2013;9:34–42. doi: 10.1038/nrrheum.2012.149. [DOI] [PubMed] [Google Scholar]
- Wei L, Laurence A, O’Shea JJ. New insights into the roles of Stat5a/b and Stat3 in T cell development and differentiation. Semin Cell Dev Biol. 2008;19(4):394–400. doi: 10.1016/j.semcdb.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SG. Regulatory T cells vs Th17: differentiation of Th17 versus Treg, are the mutually exclusive? Am J Clin Exp Immunol. 2013;2(1):94–106. [PMC free article] [PubMed] [Google Scholar]
- Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3 + CD4 + CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- Zonneveld-Huijssoon E, Albani S, Prakken BJ, van Wijk F. Heat shock protein bystander antigens for peptide immunotherapy in autoimmune disease. Clin Exp Immunol. 2013;171(1):20–29. doi: 10.1111/j.1365-2249.2012.04627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Barnett B, Safah H, et al. Bone marrow is a reservoir for CD4 + CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]