Abstract
Increasing heat shock protein 70 (HSP70) in aged and/or insulin-resistant animal models confers benefits to healthspan and lifespan. Heat application to increase core temperature induces HSPs in metabolically important tissues, and preliminary human and animal data suggest that heated hydrotherapy is an effective method to achieve increased HSPs. However, safety concerns exist, particularly in geriatric medicine where organ and cardiovascular disease commonly will preexist. We evaluated young vervet monkeys compared to old, insulin-resistant vervet monkeys (Chlorocebus aethiops sabaeus) in their core temperatures, glucose tolerance, muscle HSP70 level, and selected safety biomarkers after 10 sessions of hot water immersions administered twice weekly. Hot water immersion robustly induced the heat shock response in muscles. We observed that heat-treated old and young monkeys have significantly higher muscle HSP70 than control monkeys and treatment was without significant adverse effects on organ or cardiovascular health. Heat therapy improved pancreatic responses to glucose challenge and tended to normalize glucose excursions. A trend for worsened blood pressure and glucose values in the control monkeys and improved values in heat-treated monkeys were seen to support further investigation into the safety and efficacy of this intervention for metabolic syndrome or diabetes in young or old persons unable to exercise.
Keywords: Heat shock protein 70, Heat therapy, Muscle, Glucose metabolism
Introduction
Heat shock protein (HSP)70, and its ability to upregulate via heat shock factor (Hsf) activation, is reduced in aged and insulin-resistant people, monkeys, and invertebrates (Bruce et al. 2003; Heydari et al. 1993; Kavanagh et al. 2011, 2012). Multiple high impact studies using C. elegans have demonstrated that increasing HSP70’s homolog or its transcription factor Hsf increases lifespan (Chiang et al. 2012; Seo et al. 2013). In rodent models that overexpress HSP70 constitutively, or have higher HSP70 abundance in response to applied stress, insulin sensitivity is improved (Chung et al. 2008) and the lifespan-extending effect of HSPs has been attributed to this improved insulin signaling (Chiang et al. 2012; Seo et al. 2013). Preservation of normal glucose metabolism with aging is a common theme in augmented healthspan and lifespan demonstrated by insulin sensitivity being preserved in human centenarians, exceptionally long-lived rodents, and invertebrate animal models (Atzmon et al. 2004; Brown-Borg and Bartke 2012; Edrey et al. 2011; Ulgherait et al. 2014). Our laboratory along with others has reported that heat treatment to induce the heat shock response (HSR) in mice increases muscle HSP70 and improves insulin sensitivity (Chung et al. 2008; Silverstein et al. 2015). Pilot human data also suggests that heating may have benefits in clinical diabetes management (Hooper 1999). We have translated HSP70 biology to the monkey model, demonstrating that pharmacologically restoring deficient HSP70 levels in insulin-resistant monkeys improves glucose metabolism and that higher HSP70 preserves insulin sensitivity as monkeys’ age (Chichester et al. 2015; Kavanagh et al. 2009b, 2011). Interventions to increase HSP70 to improve longevity and health are thus of high interest in the biomedical community.
Muscle is an important target organ for health in an aging organism. Muscle metabolizes at least 80 % of circulating glucose, and the “skeletal muscle index” relates to insulin sensitivity in mid-life adults, even after adjusting for obesity measures and age in the Third National Health and Nutrition Examination Survey (DeFronzo and Tripathy 2009; Srikanthan and Karlamangla 2011). There is a body of supportive data that relates HSP70 to muscle mass preservation, which includes data from aged, insulin-resistant rodents with elevated Hsp70 having increased muscle mass and function (Chung et al. 2008; Henstridge et al. 2014; Silverstein et al. 2015).
Exercise has proven multi-systemic health benefits at any age; however, a significant proportion of the middle-aged and older people are unable or unwilling to exercise due to disabling comorbidities. Heat mimics exercise as a mild physiological stressor (increases in core body temperature, blood pressure, and heart rate). Heated hydrotherapy as an intervention is immediately applicable to human clinical trials and is already well-accepted in geriatric medicine (Verhagen et al. 1997). Concerns however exist about the safety of such procedures in older adults which have greater susceptibility to heat stress and often have preexisting cardiovascular disease (Kenney and Munce 2003). The goals of our study were to conduct a controlled preclinical trial of heat therapy in older monkeys with glucose intolerance, to evaluate both the effectiveness of repeated HSR on HSP70 responses, changes in glucose metabolism, and the safety of the intervention.
Methods
Animal procedures
All study procedures were approved by and performed in accordance with the Wake Forest University Institutional Animal Care and Use Committee. The study population was sourced from a multigenerational pedigreed colony of vervet monkeys (Chlorocebus aethiops sabaeus). Seventeen female vervet monkeys were included in study. The age range extended from 7 to 23 years (maximum lifespan ≈ 27 years; Table 1) which corresponds to a human age range of approximately 20–90 years. The mean age of “young” monkeys approximates a 25- to 30-year-old person, and the mean age of the “old” monkeys to that of 60- to 65-year-old person. All monkeys were fed commercial low fat, high carbohydrate primate laboratory chow (Diet 5038, Lab Diet, Purina, St. Louis, MO) supplemented with fresh produce five times weekly for the duration of the study. Old monkeys displayed a range of glycemic dysregulation from normal to overt diabetes. Six of the nine old monkeys required twice daily insulin therapy to manage their diabetes which has been previously described (Kavanagh et al. 2011). Insulin was withheld for 24 h prior to any outcome measure.
Table 1.
Health characteristics of monkeys before and after intervention with twice weekly heat treatment for 5 weeks
| Control | Heat-treated | Effect of heat | |||||
|---|---|---|---|---|---|---|---|
| Control (n = 3) | Old (n = 6) | Young (n = 4) | ANCOVA p value | ANCOVA p value | |||
| Age | y | Baseline | 17.7 (2.36)a | 20.9 (0.81)a | 9.45 (1.83)b | <0.001 | 0.74 |
| Bodyweight | kg | Baseline | 5.72 (0.13) | 5.71 (0.50) | 4.76 (0.37) | ||
| Study end | 5.73 (0.20) | 5.73 (0.47) | 4.66 (0.26) | 0.81 | 0.62 | ||
| Temperature | C | Baseline | 38.0 (0.26) | 36.8 (0.69) | 36.7 (0.58) | ||
| Study end | 37.7 (0.22) | 36.6 (0.80) | 37.6 (0.57) | 0.06 | 0.28 | ||
| Glucose | mg/dL | Baseline | 153 (36) | 183 (70) | 61 (8) | ||
| Study end | 233 (38) | 167 (62) | 54 (27) | 0.08 | 0.02 | ||
| Insulin | μIU/mL | Baseline | 11.0 (4.87) | 15.8 (4.87) | 21.5 (5.88) | ||
| Study end | 18.09 (4.44) | 24.4 (4.39) | 23.7 (2.58) | 0.71 | 0.42 | ||
| Triglycerides | mg/dL | Baseline | 71.7 (14.2) | 83.3 (23.5) | 39.5 (5.95) | ||
| Study end | 91.0 (11.0) | 101 (20.7) | 42.5 (6.38) | 0.29 | 0.62 | ||
| Cholesterol | mg/dL | Baseline | 217 (11.1) | 202 (16.2) | 177 (8.73) | ||
| Study end | 199 (2.52) | 197 (23.8) | 194 (8.67) | 0.35 | 0.33 | ||
| BUN | mg/dL | Baseline | 12.33 (0.67) | 14.0 (1.29) | 11.0 (1.22) | ||
| Study end | 9.67 (0.67) | 11.2 (1.38) | 12.0 (1.22) | 0.35 | 0.36 | ||
| Creatinine | mg/dL | Baseline | 0.50 (0.00) | 0.52 (0.03) | 0.52 (0.07) | ||
| Study end | 0.53 (0.03) | 0.50 (0.04) | 0.62 (0.05) | 0.15 | 0.86 | ||
| ALT | U/L | Baseline | 128 (27.8) | 159 (66.9) | 80 (24.3) | ||
| Study end | 277 (154) | 141 (51.1) | 161 (54.3) | 0.52 | 0.25 | ||
| AST | U/L | Baseline | 54.7 (17.0) | 41.2 (4.67) | 39.2 (6.42) | ||
| Study end | 257 (207) | 43.8 (10.1) | 53.2 (11.9) | 0.43 | 0.19 | ||
| ALP | U/L | Baseline | 96.0 (22.1) | 92.8 (17.0) | 108 (10.5) | ||
| Study end | 118 (33.2) | 133 (22.0) | 115 (13.6) | 0.52 | 0.82 | ||
| Creatine Kinase | U/L | Baseline | 1472 (442) | 701 (175) | 608 (217) | ||
| Study end | 795 (325) | 624 (142) | 725 (167) | 0.74 | 0.58 | ||
Variables were measured before study and between 3 and 5 days after the final heat exposure
BUN blood urea nitrogen, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase
Heat therapy studies
A physical therapy extremity whirlpool (Whitehall Mfg., City of Industry CA) was equipped with a heating unit and control box (Process Technology, Mentor OH). The whirlpool action ensured that the water was uniformly heated to 40 °C. Prior to water immersion, each monkey was sedated, and body temperature monitored continuously by rectal thermometer, with data recorded at 5-min intervals. Monkeys were submerged to neck level and secured to the bath. A flotation device was placed around the neck to prevent immersion of the head. Monkeys were maintained at an elevated core temperature (39–41 °C) for 30 min before removal and cooling. The average total immersion time was 43 min.
Acute study
Three young female monkeys were evaluated by muscle biopsies collected from the vastus lateralis before immersion, immediately following 30 min of elevated core temperature, then following 1 and 4 h during recovery to ensure that the HSR was induced.
Chronic study
Thirteen monkeys were included in a small clinical trial. Monkeys were divided into three groups: a control (n = 3), who were aged monkeys that were sedated in the same room, for the same period and frequency, and kept thermoneutral by use of blankets. Heat-treated monkeys were old (n = 6) or young (n = 4) and exposed to repeated heated hydrotherapy, two immersions per week for 5 weeks. The frequency was determined from our mouse trial which had shown HSP70 induction and improvements in insulin sensitivity (Silverstein et al. 2015). Muscle biopsies from the vastus lateralis were collected between 3 and 5 days after the final heat exposure to ensure that sustained protein changes were being reported. Blood pressure was measured indirectly, in triplicate, before study and between 3 and 5 days after the final heat exposure. We included a delay after the final heat exposure prior to study end data collections to ensure we were not measuring acute effects. The 13 monkeys were randomly assigned to be assessed on post-study day 3, 4, or 5.
Muscle HSP70
Samples from the acute study were evaluated for HSP70 and HSP90 gene expression using standard methods previously described (Kavanagh et al. 2009a). HSP70 and HSP90 primer sequence pairs used were 5′CTCCGACCTGTTCCGAAG3′, 5′TTCTGCACCTTGGGGATG3′ and 5′CACGAAGACTCTCAAAATCGGAAG3′, 5′TGACAGAAACCATCTCATCACCAG3′, respectively. Samples from the chronic study had HSP70 protein levels quantitated by ELISA as described (Chichester et al. 2015).
Circulating endpoints and glucose metabolism
For blood collections, monkeys were sedated with ketamine (15 mg/kg intramuscularly) to enable femoral venipuncture. Plasma was processed and stored at −80 °C. Samples were sent to a commercial veterinary laboratory for serum biochemistry panels. Plasma HSP70 (StressMarq, Victoria BC), C-reactive protein (CRP; Alpco, Salem NH), brain-derived natriuretic peptide-1 (BNP-1), and cardiac creatine kinase (CK-MB; MyBioscource Inc., San Diego CA) were measured by ELISA according to the manufacturer’s recommendations. Troponin and fibrinogen concentrations were measured by the Wake Forest Clinical Chemistry Laboratory. Angiotensin II (AngII) and angiotensin 1–7 (Ang1–7) concentrations were measured by radioimmunoassay by methods previously described (Nakamoto et al. 1995; Senanayake et al. 1994). Intravenous glucose tolerance tests were performed in old monkeys (n = 9) between 3 and 5 days after the final sedation following an overnight fast (<12 h). Monkeys were sedated prior to infusing 50 % dextrose (750 mg/kg) via the saphenous vein, with blood samples collected at 0, 2, 3, 5, 8, 10,15, 20, 30, and 60 min. Samples were centrifuged, and plasma stored at −80 °C until analysis for glucose and insulin concentrations. Acute insulin and glucose responses were calculated as the positive area under the first phase curve (0–10 min) and total (0–60 min) by the trapezoidal method.
Data analysis
Data are presented as mean ± standard error of mean (SEM) for each group. Data was analyzed for normality and logarithmically transformed where necessary (AngII, CK-MB, BNP-1) before analysis of variance or covariance (ANOVA/ANCOVA) was performed to assess for group differences (control, old, and young heat-treated monkeys). ANCOVA analyses were also conducted to compare control monkeys (n = 3) to heat therapy monkeys (n = 10), and results are presented in data tables as separate p values. ANCOVA was used when baseline values were available to use as continuous predictors of post-study data. Post hoc determinations of group differences were done using Tukey’s honestly significant difference tests. Associations were assessed by Pearson’s correlation coefficients. Statistical analysis was performed using Statistica v10 (StatSoft Inc. Tulsa, OK). Significance was set at α ≤ 0.05 for group differences and α ≤ 0.10 for trends.
Results
Aside from age, no significant differences were observed at baseline between groups of monkeys. A trend for metabolic health to be worsened with advanced age (Control and Old) was observed, as glucose, systolic blood pressure (SBP), and triglyceride (TG) values were higher (p = 0.08 for comparisons of control and old vs. young; Table 1 shows data separated by study group). Resting core temperature did not change significantly with repeated heat exposures, although a trend for young animals to increase temperature over the study period was seen. Temperature changes during heat therapy did not differ by group and were reliably increased (Fig. 1a). The desired 1 °C increase was achieved on average following 12 min of submersion in hot water.
Fig. 1.
a Core temperature pattern following heat therapy with 41 °C water immersion (white circle; n = 10) in monkeys. Control monkeys (black circle; n = 3) tended to reduce temperature, while treated monkeys were significantly warmer (asterisk) after 10 min of heating. No differences in the response to heat were seen between young and old monkeys. b A robust heat shock response is seen following core temperature elevation in monkeys (n = 3) with heat shock protein 70 (HSP70) gene expression in skeletal muscle increasing more than 10-fold and remaining elevated beyond 4 h post-heat exposure
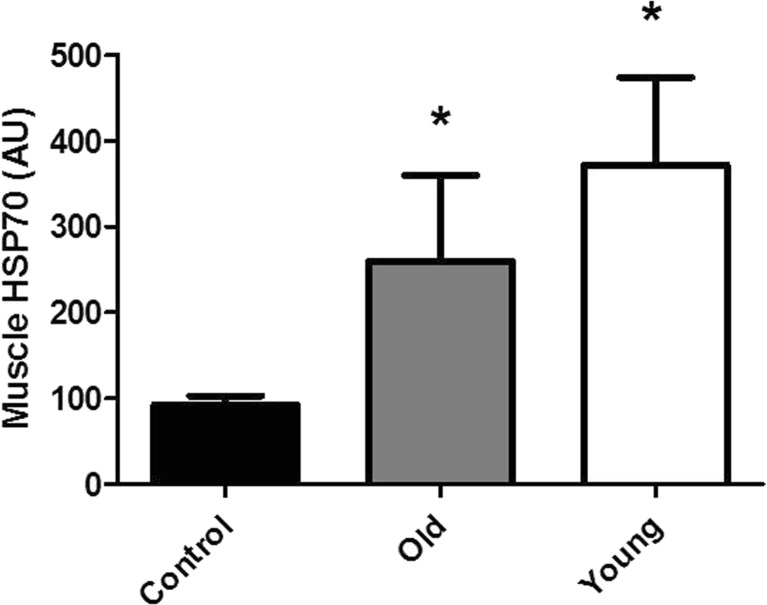
Robust induction of the HSR was induced with the heat therapy regimen. HSP70 (Fig. 1b) and HSP90 (data not shown) gene expression was significantly increased during the heating period and remained high for at least 4 h after the procedure. HSP70 was highly inducible with greater than 10-fold induction. After repeated HSR (10 sessions over 5 weeks), HSP70 protein levels in muscle were significantly increased in young and old heat-treated monkeys (Fig. 2). Plasma levels of HSP70 (Table 2) were not different at baseline or post-study (Table 2). A trend to increase plasma HSP70 with heat therapy was seen (p = 0.08) with young monkeys having a more robust increase in levels. However, plasma HSP70 concentrations or the change in concentrations across study were not related to muscle HSP70 concentrations (data not shown), consistent with monkey data we have previously described (Kavanagh et al. 2011).
Fig. 2.
HSP70 protein levels from muscles of old control monkeys (n = 3), heat-treated old monkeys (n = 6), and young heat-treated monkeys (n = 4). Protein levels were quantitated multiple days following their final HSR. Heat treatment increases muscle levels of HSP70 (*p < 0.05)
Table 2.
Selected cardiovascular biomarkers measured from monkeys before and after intervention with twice weekly heat treatment for 5 weeks
| Control | Heat-treated | ||||||
|---|---|---|---|---|---|---|---|
| Control | Old | Young | ANCOVA p value | ANCOVA p value | |||
| SBP | mmHg | Baseline | 124 (9.56) | 147 (13.8) | 123 (8.24) | ||
| Study end | 134 (7.90) | 123 (7.51) | 119 (9.23) | 0.48 | 0.11 | ||
| DBP | mmHg | Baseline | 64.6 (1.27) | 78 (6.64) | 67.2 (2.98) | ||
| Study end | 66.5 (5.74) | 61.5 (5.27) | 61.0 (3.58) | 0.65 | 0.18 | ||
| MAP | mmHg | Baseline | 85.7 (3.97) | 97.9 (9.61) | 84.2 (4.46) | ||
| Study end | 90.5 (6.25) | 82.9 (4.56) | 79.9 (4.76) | 0.36 | 0.07 | ||
| HR | bpm | Baseline | 163 (5.28) | 165 (8.76) | 166 (8.47) | ||
| Study end | 164 (9.97) | 144 (9.32) | 164 (6.09) | 0.12 | 0.13 | ||
| Plasma HSP70 | ng/mL | Baseline | 0.70 (0.62) | 3.86 (2.61) | 3.22 (2.42) | ||
| Study end | 3.73 (2.12) | 11.21 (3.29) | 16.93 (5.93) | 0.20 | 0.08 | ||
| Troponin | ng/mL | Baseline | 0.009 (0.004) | 0.008 (0.003) | 0.003 (0.001) | ||
| Study end | 0.011 (0.002) | 0.008 (0.002) | 0.024 (0.007) | 0.05 | 0.37 | ||
| Fibronogen | mg/mL | Baseline | 265 (96.0) | 230 (29.8) | 219 (42.9) | ||
| Study end | 163 (43.5) | 332 (103) | 158 (15.7) | 0.22 | 0.15 | ||
| CRP | ng/mL | Baseline | 22.1 (12.3) | 1.06 (0.38) | 12.1 (3.70) | ||
| Study end | 2.38 (0.67) | 1.38 (0.14) | 2.11 (0.93) | 0.10 | 0.24 | ||
| BNP-1 | ng/mL | Baseline | 7.44 (6.11) | 3.36 (1.75) | 2.24 (2.23) | ||
| Study end | 0.91 (0.76) | 6.52 (3.33) | 3.29 (1.11) | 0.55 | 0.18 | ||
| CK-MB | U/L | Baseline | 1.69 (1.69) | 2.25 (1.40) | 26.2 (14.4) | ||
| Study end | 0.14 (0.14) | 6.22 (2.12) | 14.7 (7.22) | 0.41 | 0.12 | ||
| AngII | pg/mL | Baseline | 506 (146) | 576 (386) | 215 (57.4) | ||
| Study end | 2101 (1338) | 722 (435) | 627 (389) | 0.88 | 0.30 | ||
| Ang1–7 | pg/mL | Baseline | 84.8 (24.8) | 81.8 (34.0) | 53.0 (7.08) | ||
| Study end | 129 (61.3) | 97.7 (41.1) | 66.9 (22.8) | 0.58 | 0.14 | ||
Variables were measured before study and between 3 and 5 days after the final heat exposure
SBP systolic blood pressure, DBP diastolic BP, MAP mean arterial pressure, CRP c-reactive protein, BNP brain natriuretic peptide, CK-MB creatine kinase–myocardial isoform, Ang angiotensin
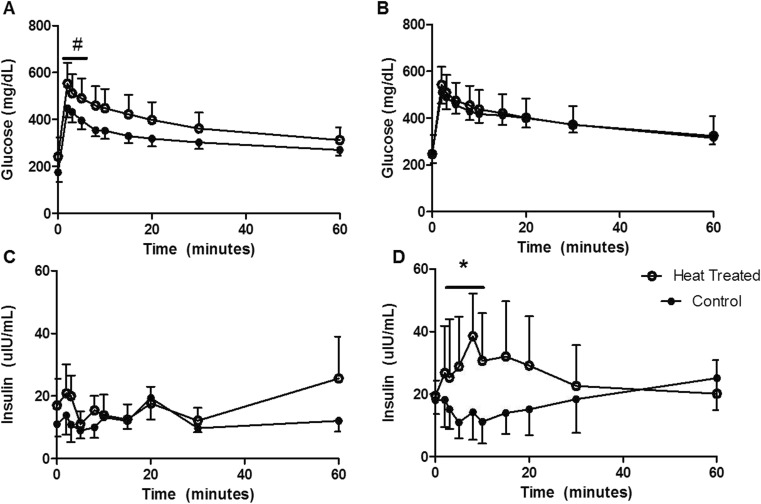
Glycemic responses were assessed in the old monkeys (control and old groups), as these animals had evidence of prediabetes or overt diabetes (Table 1). Heat treatment resulted in a modest 10 % average reduction in fasting glucose concentrations, whereas the old, unhealthy, control monkeys increased glucose over the course of study which suggests that heat therapy has a net positive effect (p = 0.02). Glucose changes were seen in the absence of changes in fasting insulin concentrations. Heat treatment resulted in significantly improved first phase insulin secretion and normalization of early glucose disposal (Fig. 3). First phase insulin secretion was absent from old monkeys with glucose dysregulation (Fig. 3c), which depicts beta cell dysfunction that is considered a hallmark of insulin resistance. Inadequate insulin release fails to drive circulating glucose into cells, of which the majority is metabolized by muscle (Fig. 3a). Heat therapy restores both phenotypes in old monkeys. Overall, AUCs for insulin were not different despite being doubled on average (Table 3; p = 0.28). Fasting glucose had a trend toward reduction in heat-treated monkeys, driven mainly by increases in fasting glucose seen in control monkeys (Table 1). The increase in glucose seen in control monkeys is not unexpected as significant stress is associated with twice weekly sedation. The levels of HSP70 measured from muscle at study end were significantly associated with fasting glucose levels (r = −0.73, p = 0.005; Fig. 4) and with the change in fasting glucose levels over the course of the study (r = −0.65, p = 0.02).
Fig. 3.
Intravenous glucose tolerance testing in old monkeys (control black circle; n = 3, and heat-treated white circle; n = 6) demonstrate that heat treatment normalizes early response to glucose challenge (a, b are baseline and post-heat, respectively; #p = 0.10) with coincident restoration of first phase insulin secretion (c, d are baseline and post-heat, respectively; *p < 0.05)
Table 3.
Calculated areas under the curves (AUC, 0–10 and 0–60 min) from glucose tolerance testing in old monkeys with impaired fasting glucose before and after and after intervention with twice weekly heat treatment for 5 weeks
| Old control | Old heat therapy | ANCOVA p value |
||
|---|---|---|---|---|
| AUCglu (0–60) | Baseline | 18,730 (2090) | 22,799 (4822) | |
| Study end | 22,650 (2509) | 23,080 (5619) | 0.44 | |
| AUCglu (0–10) | Baseline | 3720 (427) | 4662 (960) | |
| Study end | 4378 (502) | 4582 (918) | 0.10 | |
| AuCins (0–60) | Baseline | 490 (241) | 833 (266) | |
| Study end | 407 (162) | 948 (558) | 0.28 | |
| AUCins (0–10) | Baseline | 108 (43.4) | 134 (75.3) | |
| Study end | 158 (66.0) | 289 (153) | 0.04 | |
Fig. 4.
Levels of HSP70 in muscle of monkeys were significantly associated with fasting glucose concentrations and were similarly associated with the change in fasting glucose from prestudy (r = −0.65, p = 0.02) such that the higher HSP70 was coupled with lower blood glucose and larger reductions in blood glucose over time
There are many concerns with the potential adverse effects of heat stress. We saw no changes in biochemical markers of liver and kidney function (Table 1), organs that are susceptible to hyperthermic damage. An outlying individual in the control group had evidence post-study of hepatocellular damage (ALT, AST), but no changes with repeated hyperthermia was detected. Cardiovascular damage is a frequent finding and a cause for death in heat stress. Table 2 presents a comprehensive panel of outcomes related to cardiac health. A trend for reduced MAP was seen post-heat therapy, driven primarily by reductions in SBP and changes in pressure measured in old monkeys. We saw no changes in heart damage aside from young monkey’s troponin levels increasing post-heat treatment.
Discussion
We demonstrate in a relevant animal model of spontaneous age-related metabolic disease that repeated induction of the HSR creates sustained changes in tissue HSP70 levels and positively changes glucose handling. Further, we provide data that heated hydrotherapy is a safe and reliable method to increase core temperature in primates. Based on our and other studies, we suggest that twice weekly therapy for as little as 10–15 min is likely to be effective in causing desired protein changes in metabolically important tissues (Ohori et al. 2012). It has been demonstrated that aged muscles do not increase HSPs in adaptation to normal exercise the way that younger muscle tissue does (Vasilaki et al. 2003) and thus alternative methods for increasing HSPs should be investigated for aging. It is also common that aged human and nonhuman primates have co-morbidities such as arthritis or sarcopenia that prevent exercise, so development of exercise-mimetics is important additions to geriatric therapy.
Aged muscle had 30 % lower HSP70 protein levels as compared to young muscle after 5 weeks of heat therapy. However, the aged animals exposed to heat therapy had 2.8-fold greater levels than age-matched untreated control monkeys. These old monkeys had metabolic disease and >50 % lower tissue HSP70 which is typical of what has been seen in diabetic or insulin-resistant primates (Bruce et al. 2003; Kavanagh et al. 2011, 2012). Thus, our therapy restored muscle HSP70 to levels we have seen in healthy aged monkeys and to levels that approached the values seen in heat-treated young monkeys. Advanced age has been shown to reduce the HSR (Heydari et al. 1993) which may explain why a 30 % lower HSP70 level resulted in the old animals; however, this difference was not statistically significant.
We report an improvement in HSP70 protein levels in muscle tissue, which is a biologically important outcome as it predicts future metabolic health (Chichester et al. 2015). Using heat to elevate HSP70 in muscle tissue in young rodents is successful in preventing diet-induced obesity and insulin resistance (Chung et al. 2008). Consistent with this study of younger rodents, aged rodent muscle exposed to heat has improved ex vivo insulin-stimulated glucose uptake through a mechanism that involves enhanced insulin signaling (Gupte et al. 2011). Metabolic improvements in muscle exposed to repeated heat stress have also been attributed to greater oxidative capacity and mitochondrial biogenesis (Henstridge et al. 2014; Tamura et al. 2014); concordantly, loss of HSP70 in rodent muscle decreases its oxidative capacity (Drew et al. 2014). It is generally believed that the first change prior to the development of T2DM involves peripheral (e.g., muscle) insulin resistance and a compensatory hyperinsulinemia (DeFronzo and Tripathy 2009), which is why attention to muscle HSP70 is important and predictive of future metabolic health (Chichester et al. 2015). Ultimately, the beta cells of the pancreas begin failing, resulting in declining insulin production and sustained hyperglycemia. Our old monkeys clearly show a deficient first-phase insulin secretory response prior to heat therapy. After therapy, there is evidence of a beta cell response and improvement in glucose disposal during the initial hyperglycemic excursion. Heat-treated rodents actually increase beta cell mass over the course of months and have improved insulin sensitivity (Kondo et al. 2012). Rodents have a greater beta cell regeneration rate than that seen in humans or vervet monkeys sourced from the same familial colony as the ones studied in this report (Saisho et al. 2011). Therefore, we believe that the improvement is unlikely to reflect changes in beta cell mass but in function. Beta cells are of neuroendocrine origin, and the neuroprotective effects of HSP70 have been well-documented (Gifondorwa et al. 2007; Kern et al. 2010), so improved function relating to reduced inflammatory signaling and endoplasmic reticulum stress could help restore normal excitation-exocytosis in these cells.
Another possible explanation for improved insulin sensitivity could relate to enhanced tissue perfusion. Vascular function declines with age and prematurely declines with insulin resistance (Skilton et al. 2005). Normal arterial function is mediated by endothelial nitric oxide synthase (eNOS) which produces vasorelaxant nitric oxide (NO). We detected increased HSP70 and HSP90 as a result of the HSR in muscle tissue. Induction of HSP90 in older patients with heat therapy improves vascular reactivity as HSP90 has a specific role in eNOS activity, and NO production is doubled when these proteins are associated (Harris et al. 2003). HSP70 and HSP90 may collectively influence insulin resistance on two levels. Enhanced HSPs in aged muscles improve glucose uptake through JNK activation inhibition and greater insulin signaling efficiency (Gupte et al. 2011). Enhanced vasorelaxation will increase tissue perfusion and mediate improvements in insulin sensitivity as insulin is exposed to greater cellular surface area (Baron et al. 1994). This has a feed-forward effect as insulin itself is a modest eNOS activator (Clerk et al. 2004) and NO can stimulate glucose uptake by insulin-independent mechanisms (Young et al. 1997). By improving vascular reactivity, insulin and HSP90 enhance NO production further down the vascular tree and recruits more capillaries within muscle, which is so important in insulin action and glucose disposal.
Despite repeated heat therapy being effective in improving cardiovascular function in heart failure patients and reducing cardiovascular-related deaths (Laukkanen et al. 2015; Ohori et al. 2012), concerns regarding cardiovascular strain and adverse effects, including sudden cardiac death, on aged persons persist (Eshel et al. 1998; Satoh et al. 2013). We conducted an extensive panel of cardiovascular health biomarkers, including emerging targets such as Ang1–7. Ang1–7 has been shown to increase signaling effectiveness coincidently with HSP70 levels in young rats with just once weekly heat therapy (Karpe and Tikoo 2014). We saw no adverse or beneficial effects on cardiac biomarkers in the old monkeys exposed to heat.
In contrast to the old monkeys, young monkeys increased troponin levels following heat therapy. All post-heat concentrations of troponin fell in the concentration range that is associated with the lowest risk of mortality (Antman et al. 1996), but the small change in young monkeys does suggest a low level of myocardial damage. Increases in troponin have also been reported as a normal response to repeated ketamine sedation, and we observed 9 of 13 animals increasing troponin concentrations over the experimental period which consisted of 10 sedations (Franco et al. 2009). Young animals have the ability to increase heart rate more than old animals, and a small amount of tachycardia related-damage with heat stress is a possible explanation for the greater levels of post-treatment troponin in this age group.
Heat therapy has reduced BNP-1 in heart failure patients (Ohori et al. 2012); however, in our monkeys that did not have clinical evidence of heart failure, no change was observed. We did see decreases in blood pressure parameters in both heat-treated groups of monkeys, whereas the control group had small increases as a group (average 12 % reduction with heat treatment; p = 0.11) which is consistent with the pattern seen with fasting glucose (average 6 % reduction with heat treatment; p = 0.01). Repeated sedation without heat therapy worsened health as would be expected, whereas heat treatment not only abrogated these changes but tended to reduce values toward improved health.
Our small sample sizes limited the ability to detect biologically important differences at the conventionally defined statistical error rate. However, to our knowledge, we are the first human or nonhuman primate study that included an appropriate age- and health-matched control group which experienced the same experimental stressors and environments as those exposed to heat therapy. This control group showed some biological changes over the 5-week study, particularly fasting glucose, that are relevant to the interpretation of study results. Monkeys required sedation twice weekly and for assessments which can be associated with some changes in cardiac biomarkers (Franco et al. 2009). Additionally, our control group remained thermoneutral under sedation, but without exposure to hydrostatic pressure which may have independent effects on cardiac health (Epstein 1992). We also were able to only evaluate female monkeys, as they were sourced from a breeding colony. We have previously determined that no sex differences in muscle HSP70 levels exist in monkeys from this colony (Kavanagh et al. 2012). Older women are described as having better thermoregulatory control and less exaggerated cardiovascular responses to heat stress (Kenney and Munce 2003). Therefore, the safety and responses in male subjects should be evaluated although epidemiologic evidence in a large cohort of men concluded that heat therapy had significant benefits for health and longevity (Laukkanen et al. 2015).
Our study supports the health claims of heat therapy being safe and effective for older subjects and may improve glucose metabolism in insulin-resistant subjects. Improved insulin sensitivity is a consistent feature of populations with increased healthspan and lifespan and data generated under controlled conditions in a relevant primate model has high translatability to human health.
Acknowledgments
Funding for this study was from the National Institutes of Health K01 AG033641, P30 AG021332 (Wake Forest University Claude D. Pepper Older Americans Independence Center), P40 OD010965 (Vervet Research Colony), and Wake Forest School of Medicine Hypertension & Vascular Research Center.
References
- Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, Fischer GA, Fung AY, Thompson C, Wybenga D, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- Atzmon G, Schechter C, Greiner W, Davidson D, Rennert G, Barzilai N. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004;52:274–277. doi: 10.1111/j.1532-5415.2004.52068.x. [DOI] [PubMed] [Google Scholar]
- Baron AD, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol. 1994;266:E248–E253. doi: 10.1152/ajpendo.1994.266.2.E248. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67:652–660. doi: 10.1093/gerona/gls086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148:322–334. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichester L, Wylie AT, Craft S, Kavanagh K. Muscle heat shock protein 70 predicts insulin resistance with aging. J Gerontol A Biol Sci Med Sci. 2015;70:155–162. doi: 10.1093/gerona/glu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev. 2004;20:3–12. doi: 10.1002/dmrr.414. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew BG, Ribas V, Le JA, Henstridge DC, Phun J, Zhou Z, Soleymani T, Daraei P, Sitz D, Vergnes L, et al. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes. 2014;63:1488–1505. doi: 10.2337/db13-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrey YH, Hanes M, Pinto M, Mele J, Buffenstein R. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J Natl Res Counc, Inst Lab Anim Resour. 2011;52:41–53. doi: 10.1093/ilar.52.1.41. [DOI] [PubMed] [Google Scholar]
- Epstein M. Renal effects of head-out water immersion in humans: a 15-year update. Physiol Rev. 1992;72:563–621. doi: 10.1152/physrev.1992.72.3.563. [DOI] [PubMed] [Google Scholar]
- Eshel GM, Safar P, Sassano J, Stezoski SW. Delayed death after uncomplicated hot tub bathing in dogs and monkeys. Resuscitation. 1998;37:189–195. doi: 10.1016/S0300-9572(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Franco LG, Fioravanti MC, Damasceno AD, Borges AC, Soares LK, Rabelo RE, Silva LA. Assessment of serum enzymatic markers of cardiomyocytes injury in female dogs submitted to ketamine S(+), atropin and xylazine association. Acta cirurgica brasileira / Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia. 2009;24:36–42. doi: 10.1590/s0102-86502009000100008. [DOI] [PubMed] [Google Scholar]
- Gifondorwa DJ, Robinson MB, Hayes CD, Taylor AR, Prevette DM, Oppenheim RW, Caress J, Milligan CE. Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2007;27:13173–13180. doi: 10.1523/JNEUROSCI.4057-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol. 2011;110:451–457. doi: 10.1152/japplphysiol.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol. 2003;285:H333–H340. doi: 10.1152/ajpheart.00726.2002. [DOI] [PubMed] [Google Scholar]
- Henstridge DC, Bruce CR, Drew BG, Tory K, Kolonics A, Estevez E, Chung J, Watson N, Gardner T, Lee-Young RS, et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes. 2014;63:1881–1894. doi: 10.2337/db13-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari AR, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993;13:2909–2918. doi: 10.1128/MCB.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341:924–925. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- Karpe PA, Tikoo K. Heat shock prevents insulin resistance-induced vascular complications by augmenting angiotensin-(1–7) signaling. Diabetes. 2014;63:1124–1139. doi: 10.2337/db13-1267. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Davis MA, Zhang L, Wilson MD, Register TC, Adams MR, Rudel LL, Wagner JD. Estrogen decreases atherosclerosis in part by reducing hepatic acyl-CoA:cholesterol acyltransferase 2 (ACAT2) in monkeys. Arterioscler Thromb Vasc Biol. 2009;29:1471–1477. doi: 10.1161/ATVBAHA.109.191825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14:291–299. doi: 10.1007/s12192-008-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300:E894–E901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh K, Wylie AT, Chavanne TJ, Jorgensen MJ, Voruganti VS, Comuzzie AG, Kaplan JR, McCall CE, Kritchevsky SB. Aging does not reduce heat shock protein 70 in the absence of chronic insulin resistance. J Gerontol A Biol Sci Med Sci. 2012;67:1014–1021. doi: 10.1093/gerona/gls008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol. 2003;95:2598–2603. doi: 10.1152/japplphysiol.00202.2003. [DOI] [PubMed] [Google Scholar]
- Kern A, Ackermann B, Clement AM, Duerk H, Behl C. HSF1-controlled and age-associated chaperone capacity in neurons and muscle cells of C. elegans. PLoS One. 2010;5:e8568. doi: 10.1371/journal.pone.0008568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Sasaki K, Matsuyama R, Morino-Koga S, Adachi H, Suico MA, Kawashima J, Motoshima H, Furukawa N, Kai H, et al. Hyperthermia with mild electrical stimulation protects pancreatic beta-cells from cell stresses and apoptosis. Diabetes. 2012;61:838–847. doi: 10.2337/db11-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med. 2015;175:542–548. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- Nakamoto H, Ferrario CM, Fuller SB, Robaczewski DL, Winicov E, Dean RH. Angiotensin-(1–7) and nitric oxide interaction in renovascular hypertension. Hypertension. 1995;25:796–802. doi: 10.1161/01.HYP.25.4.796. [DOI] [PubMed] [Google Scholar]
- Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, Yasumura S, Inoue H. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol. 2012;109:100–104. doi: 10.1016/j.amjcard.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Saisho Y, Manesso E, Butler AE, Galasso R, Kavanagh K, Flynn M, Zhang L, Clark P, Gurlo T, Toffolo GM, et al. Ongoing beta-cell turnover in adult nonhuman primates is not adaptively increased in streptozotocin-induced diabetes. Diabetes. 2011;60:848–856. doi: 10.2337/db09-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh F, Osawa M, Hasegawa I, Seto Y, Tsuboi A. “Dead in hot bathtub” phenomenon: accidental drowning or natural disease? Am J Forensic Med Pathol. 2013;34:164–168. doi: 10.1097/PAF.0b013e31828d68c7. [DOI] [PubMed] [Google Scholar]
- Senanayake PD, Moriguchi A, Kumagai H, Ganten D, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides. 1994;15:919–926. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Seo K, Choi E, Lee D, Jeong DE, Jang SK, Lee SJ. Heat shock factor 1 mediates the longevity conferred by inhibition of TOR and insulin/IGF-1 signaling pathways in C. elegans. Aging Cell. 2013;12:1073–1081. doi: 10.1111/acel.12140. [DOI] [PubMed] [Google Scholar]
- Silverstein MG, Ordanes D, Wylie AT, Files DC, Milligan C, Presley TD, Kavanagh K. Inducing Muscle Heat Shock Protein 70 Improves Insulin Sensitivity and Muscular Performance in Aged Mice. J Gerontol A Biol Sci Med Sci. 2015;70:800–808. doi: 10.1093/gerona/glu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skilton MR, Lai NT, Griffiths KA, Molyneaux LM, Yue DK, Sullivan DR, Celermajer DS. Meal-related increases in vascular reactivity are impaired in older and diabetic adults: insights into roles of aging and insulin in vascular flow. Am J Physiol Heart Circ Physiol. 2005;288:H1404–H1410. doi: 10.1152/ajpheart.00484.2004. [DOI] [PubMed] [Google Scholar]
- Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96:2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Matsunaga Y, Masuda H, Takahashi Y, Takahashi Y, Terada S, Hoshino D, Hatta H. Postexercise whole body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2014;307:R931–R943. doi: 10.1152/ajpregu.00525.2013. [DOI] [PubMed] [Google Scholar]
- Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilaki A, Iwanejko LM, McArdle F, Broome CS, Jackson MJ, McArdle A. Skeletal muscles of aged male mice fail to adapt following contractile activity. Biochem Soc Trans. 2003;31:455–456. doi: 10.1042/bst0310455. [DOI] [PubMed] [Google Scholar]
- Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Knipschild PG. Taking baths: the efficacy of balneotherapy in patients with arthritis. A systematic review. J Rheumatol. 1997;24:1964–1971. [PubMed] [Google Scholar]
- Young ME, Radda GK, Leighton B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem J. 1997;322(Pt 1):223–228. doi: 10.1042/bj3220223. [DOI] [PMC free article] [PubMed] [Google Scholar]