Abstract
Key points
Luminal short‐chain fatty acids (SCFAs) influence gut physiological function via SCFA receptors and transporters.
The contribution of an SCFA receptor, free fatty acid receptor (FFA)3, to the enteric nervous system is unknown.
FFA3 is expressed in enteric cholinergic neurons.
Activation of neural FFA3 suppresses Cl− secretion induced by nicotinic ACh receptor activation via a Gi/o pathway.
Neural FFA3 may have an anti‐secretory function by modulating cholinergic neural reflexes in the enteric nervous system.
Abstract
The proximal colonic mucosa is constantly exposed to high concentrations of microbially‐produced short‐chain fatty acids (SCFAs). Although luminal SCFAs evoke electrogenic anion secretion and smooth muscle contractility via neural and non‐neural cholinergic pathways in the colon, the involvement of the SCFA receptor free fatty acid receptor (FFA)3, one of the free fatty acid receptor family members, has not been clarified. We investigated the contribution of FFA3 to cholinergic‐mediated secretory responses in rat proximal colon. FFA3 was immunolocalized to enteroendocrine cells and to the enteric neural plexuses. Most FFA3‐immunoreactive nerve fibres and nerve endings were cholinergic, colocalized with protein gene product (PGP)9.5, the vesicular ACh transporter, and the high‐affinity choline transporter CHT1. In Ussing chambered mucosa–submucosa preparations (including the submucosal plexus) of rat proximal colon, carbachol (CCh)‐induced Cl− secretion was decreased by TTX, hexamethonium, and the serosal FFA3 agonists acetate or propionate, although not by an inactive analogue 3‐chloropropionate. Serosal application of a selective FFA3 agonist (N‐[2‐methylphenyl]‐[4‐furan‐3‐yl]‐2‐methyl‐5‐oxo‐1,4,5,6,7,8‐hexahydro‐quinoline‐3‐carboxamide; MQC) dose‐dependently suppressed the response to CCh but not to forskolin, with an IC50 of 13 μm. Pretreatment with MQC inhibited nicotine‐evoked but not bethanechol‐evoked secretion. The inhibitory effect of MQC was reversed by pretreatment with pertussis toxin, indicating that FFA3 acts via the Gi/o pathway. Luminal propionate induced Cl− secretion via the cholinergic pathway, which was reduced by MQC, as well as by TTX, hexamethonium or removal of the submucosal plexus. These results suggest that the SCFA‐FFA3 pathway has a novel anti‐secretory function in that it inhibits cholinergic neural reflexes in the enteric nervous system.
Key points
Luminal short‐chain fatty acids (SCFAs) influence gut physiological function via SCFA receptors and transporters.
The contribution of an SCFA receptor, free fatty acid receptor (FFA)3, to the enteric nervous system is unknown.
FFA3 is expressed in enteric cholinergic neurons.
Activation of neural FFA3 suppresses Cl− secretion induced by nicotinic ACh receptor activation via a Gi/o pathway.
Neural FFA3 may have an anti‐secretory function by modulating cholinergic neural reflexes in the enteric nervous system.
Abbreviations
- CCh
carbachol
- CF3‐MQC
N‐(2‐methylphenyl)‐4‐[5‐(2‐trifluoromethoxy‐phenyl)‐furan‐2‐yl]‐2‐methyl‐5‐oxo‐1,4,5,6,7,8‐hexahydro‐quinoline‐3‐carboxamide
- CGRP
calcitonin gene‐related peptide
- ChAT
choline acetyltransferase
- CHT1
high‐affinity choline transporter‐1
- CSMG
celiac superior mesenteric ganglia
- DAPI
4′,6‐diamidino‐2‐phenylindole
- DMSO
dimethyl sulfoxide
- FK
forskolin
- GI
gastrointestinal
- GLP
glucagon‐like peptide
- GPCR
G protein‐coupled receptor
- Gt
tissue conductance
- Isc
short‐circuit current
- IR
immunoreactivity
- FFA3
free fatty acid receptor 3
- MQC
N‐[2‐methylphenyl]‐[4‐furan‐3‐yl]‐2‐methyl‐5‐oxo‐1,4,5,6,7,8‐hexahydro‐quinoline‐3‐carboxamide
- NaAc
sodium acetate
- NaP
sodium propionate
- NPY
neuropeptide Y
- PGP
protein gene product
- PTX
pertussis toxin
- PYY
peptide YY
- SCFA
short‐chain fatty acid
- VAChT
vesicular ACh transporter
- VIP
vasoactive intestinal peptide
Introduction
Short‐chain fatty acids (SCFAs) are 1–6 carbon monocarboxylic acids. Acetic, propionic and butyric acids are gut microbial fermentation products of dietary fibre. Intracellular SCFAs are the major energy source for colonocytes, although luminal SCFAs are also an indicator of luminal microbial fermentative activity. Alteration of gut microbial populations is implicated in the pathogenesis of gastrointestinal (GI) or metabolic disorders, including obesity, inflammatory bowel disease and irritable bowel syndrome (Schwartz et al. 2001; Cani & Delzenne, 2011; Soldavini & Kaunitz, 2013). Therefore, clarifying the sensory mechanisms for and physiological responses to SCFAs advances medical knowledge, potentially leading to SCFA‐based therapies. The de‐orphanization and identification of the transmembrane SCFA receptors, namely free fatty acid receptor (FFA)2 and FFA3, has facilitated the accumulation of data regarding their expression, localization and function. FFA3 is a Gi/o‐coupled G protein‐coupled receptor (GPCR), whereas FFA2 activates the Gq/11 and Gi/o pathways (Brown et al. 2003; Le et al. 2003). Other FFA family members, FFA1 and FFA4, comprise long‐chain fatty acid receptors not activated by SCFAs. FFA2 and FFA3 are implicated in the regulation and function of the gut immune system, in energy metabolism, and in the enteroendocrine system, which is consistent with their broad expression (Nohr et al. 2013). In the GI tract, FFA2 is abundantly expressed in neutrophils and adipocytes, whereas FFA3 is detected in enteroendocrine cells and in enteric neurons (Nohr et al. 2013). Although gut hormones are synthesized within enteroendocrine cells, which differ between the small and large intestine, FFA3 is consistently co‐expressed with glucagon‐like peptide (GLP)‐1 throughout the intestine (Kaji et al. 2012 a; Nohr et al. 2013). To date, the only reported effect of FFA3 activation in the GI mucosa is an increase in GLP‐1 release from L cells in response to perfused SCFAs (Tolhurst et al. 2012). This is consistent with the first report of FFA3‐deficient mice that have a low plasma concentration of peptide YY (PYY), which is also produced in L cells (Samuel et al. 2008).
Luminally applied SCFAs acutely increase intestinal motility and the transmural potential difference in vivo and in vitro in rat small and large intestine (Wall et al. 1976; Yajima & Sakata, 1986; Richardson et al. 1991; Grider & Piland, 2007). From studies of isolated colonic segments, the contractile and secretory effects of SCFAs are primarily mediated by neural and non‐neural cholinergic pathways (Yajima, 1985; Yajima et al. 2011 a). Nevertheless, repeated luminal infusion of SCFAs inhibits colonic motility and fluid secretion via PYY release in rat colon (Squires et al. 1992; Cherbut et al. 1998). Furthermore, SCFAs reduce electrical stimulation‐evoked neurally‐mediated contractions of isolated rat colon, which are independent of the presence of mucosa (Dass et al. 2007), indicating that SCFAs can inversely regulate enteric neural activity. High concentrations (∼100 mm) of mixed SCFAs are generated in the cecal lumen as a result of microbial metabolism (Bergman, 1990). Accordingly, the proximal colon absorbs SCFAs via monocarboxylate transporters (MCTs) as a primary colonocyte energy source and as substrates of hepatocyte metabolism (Iwanaga & Kishimoto, 2015). Thus, SCFA sensing in the proximal colon probably reduces motility and secretion, which facilitates fermentation of the luminal content and the absorption of luminal SCFAs. The mechanisms by which luminal SCFAs evoke gut physiological responses are confounded by the presence not only of membrane receptors, but also epithelial cell SCFA transporters and metabolic enzymes. We have recently reported that the rat proximal duodenum possesses an active SCFA absorption mechanism (Kaji et al. 2015 b) and that luminal FFA2 and FFA3 agonists activate duodenal bicarbonate secretion via gut hormone‐ and SCFA transporter‐dependent pathways (Akiba et al. 2015). Because the absorption‐dependent secretory pathway involves activation of capsaicin‐sensitive afferent nerves (Akiba et al. 2015), we hypothesize that SCFA receptors are functionally expressed in neurons innervating the intestinal mucosa. Despite the ubiquitous expression of FFA3 in autonomic and sensory neurons (Kimura et al. 2011; Won et al. 2013; Nohr et al. 2015), the means by which neuronal FFA3 activation affects GI function have not been clarified.
In the present study, we demonstrate for the first time that FFA3 is expressed in enteric neurons and in mucosal and submucosal varicosities expressing cholinergic markers. We further show that neural FFA3 activation suppresses carbachol (CCh)‐ or luminal propionate‐induced Cl− secretion via the Gi/o pathway in rat proximal colon.
Methods
Animals
Male Sprague–Dawley rats weighing 200–250 g (Harlan, San Diego, CA, USA) were fed a pellet diet and water ad libitum. All studies were performed with approval of the Veterans Affairs Institutional Animal Care and Use Committee. Rats were fasted overnight with free access to water before the experiments. Animals were killed by terminal exsanguination under deep isoflurane anaesthesia, followed by thoracotomy.
Antibody production
Antibody was produced in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Hokkaido University School of Medicine. An antibody against α‐calcitonin gene‐related peptide (CGRP) was raised in guinea‐pigs using CGRP1–37 of mature mice and rats (i.e. SCNTATCVTHRLAGLLSRSGGVVKDNFVPTNVGSEAF; 83–119 amino acid residues; GenBank AF330212), which was fused to glutathione S‐transferase (GST). Immunization and affinity‐purification of antibodies have been reported previously (Fukaya et al. 2006). The specificity of the α‐CGRP antibody was confirmed by absorption with rat CGRP1–37. An antibody against rat FFA3 (RK1103) was raised in accordance with our previous method for FFA2 antibody production (Akiba et al. 2015). Briefly, cDNA fragment encoding C‐terminal 28 amino acids of rat FFA3 (296‐319 amino acid residues; GenBank NM 001108912) was cloned using a pGEM‐T Easy Vector System I kit (Promega, Madison, WI, USA). The cDNA fragment was subcloned into pGEX4T‐2 plasmid and Escherichia coli BL21 for expression of GST fusion proteins in accordance with the manufacturer's instructions (Pharmacia Biotech AB, Uppsala, Sweden). Fusion proteins, emulsified with Freund's complete or incomplete adjuvant (Difco, Detroit, MI, USA), were injected s.c. into a female New Zealand white rabbit at 2 week intervals. Anti‐serum sampled 2 weeks after the sixth injection was affinity‐purified using CNBr‐activated Sepharose 4B coupled with GST‐free polypeptides that were obtained by in‐column thrombin digestion of fusion proteins. The FFA3 antibody RK1103 was characterized by immunostaining of rat FFA2‐ or rat FFA3‐expressing HeLa and HEK298T cells and by western blotting of rat colonic samples as described previously (Akiba et al. 2015). The protein samples were extracted from the colonic submucosal layer, which was detached from the mucosa after meticulously removing the muscle layers using fine surgical forceps (Natsume Seisakusho Co., Ltd, Tokyo, Japan) under a stereomicroscope. All procedures were performed in ice‐cold Krebs buffer.
Chemicals
N‐(2‐methylphenyl)‐4‐(furan‐3‐yl)‐2‐methyl‐5‐oxo‐1,4,5,6,7,8‐hexahydro‐quinoline‐3‐carboxamine (MQC) and N‐(2‐methylphenyl)‐4‐[5‐(2‐trifluoromethoxy‐phenyl)‐furan‐2‐yl]‐2‐methyl‐5‐oxo‐1,4,5,6,7,8‐hexahydro‐quinoline‐3‐carboxamide (CF3‐MQC) were synthesized, purified and verified in Laboratory of Organic Chemistry, School of Pharmaceutical Sciences, University of Shizuoka, Japan, in accordance with the published chemical structures (Ulven, 2012). AR420626 was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). Pertussis toxin (PTX) and rat CGRP1–‐37 was obtained from Tocris Bioscience (Ellisville, MO, USA). TTX was purchased from Calbiochem (EMD Millipore, Billerica, MA, USA). CCh, sodium propionate (NaP), sodium acetate (NaAc), 3‐chloropropionic acid, forskolin (FK), capsaicin, hexamethonium, atropine and other chemicals were purchased from Sigma Chemical (St Louis, MO, USA). TTX was dissolved in citrate buffer (pH 4.5). Indomethacin and capsaicin were dissolved in ethanol. MQC, CF3‐MQC and FK were dissolved in dimethyl sulfoxide (DMSO). Other chemicals were dissolved in distilled water.
Immunofluorescence staining and real‐time PCR
Rats were deeply anaesthetized by isoflurane inhalation and perfused through the left ventricle of the heart with saline and subsequently with Zamboni's fixative containing 2% paraformaldehyde and 0.2% picric acid in 0.1 m phosphate buffer (pH 7.4). The colonic segments were taken and immersed in the same fixative overnight for 4°C. For frozen sections, small pieces of colon were then submerged in 20% sucrose solution overnight at 4°C, embedded in OCT compound and cut to 8 μm‐thickness on amino silane‐coated glass slides (Matsunami Glass USA Inc., Bellingham, WA, USA). For whole‐mount preparations, the fixed colon was separated into mucosa, submucosa and muscle layers with fine forceps under a stereomicroscope.
Sections and whole‐mount preparations were pretreated with 5% normal donkey serum in phosphate‐buffered saline containing 0.3% Triton X‐100 for 1 h. Pre‐blocked tissues were incubated with primary antibodies anti‐FFA3 (RK1103; 1 μg ml−1), guinea‐pig anti‐α‐CGRP (1 μg ml−1), mouse anti‐protein gene product (PGP)9.5 (dilution 1:500; ab8189, Abcam, Cambridge, MA, USA), guinea‐pig anti‐vesicular acetylcholine transporter (VAChT; dilution 1:500; AB1588, EMD Millipore, Temecula, CA, USA), goat anti‐CHT1 (high‐affinity choline transporter‐1; 0.5 μg ml−1; Frontier Institute Co., Ltd, Sapporo, Japan), goat anti‐calbindin (CB‐28 kDa; 0.25 μg ml−1; Frontier Institute Co.) or rabbit anti‐VIP (CURE Antibody Core, Los Angeles, CA, USA) for 24–72 h at 4°C. For the pre‐absorption test, RK1103 was pre‐incubated with GST‐free antigen peptide (10 μg ml−1) overnight at 4°C. After rinsing in phosphate‐buffered saline, fluorescence‐conjugated antibodies were reacted for 2 h at room temperature. The tissues were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI) and covered with EverBrite mounting medium (Biotium, Hayward, CA, USA). Immunofluorescence was imaged and captured using a confocal laser microscope (LSM710; Carl Zeiss GmbH, Jena, Germany).
Real‐time RT‐PCR was performed as described previously (Akiba et al. 2015) with primers for rat FFA3 (sense: 5′‐CTACAACGTGTCCCATGTCG‐3′ and anti‐sense: 5′‐TTCTGCTCCTTCAGCTCCAT‐3′) giving rise to a 219 bp product and for β‐actin. The FFA3 expression level was presented as the fold induction per 103 copies of β‐actin by the ΔCt method.
Short‐circuit current measurements in Ussing chambered preparations
Mucosa–submucosa preparations were fashioned from the proximal colon as described previously (Kaji et al. 2012 b). Submucosal layers were further removed using fine surgical forceps for some of the experiments. Two preparations were prepared from each segment by dividing each longitudinally, and these were then mounted between two hemichambers with an aperture = 0.3 cm2 (Physiologic Instruments, San Diego, CA, USA). Measurements of short‐circuit current (I sc) and tissue conductance (G t) were conducted as described previously (Kaji et al. 2015 a). Positive values for I sc indicate a negative electrical charge flux from serosal → luminal bath as a result of anion secretion or cation absorption. Indomethacin (10 μm) was added to the luminal and serosal baths to eliminate the effects of endogenous PG production. The tissues were stabilized for ∼50 min before the effects of CCh and other drugs were investigated.
Statistical analysis
Values are expressed as the mean ± SEM. The number of animals in each experimental group was ≥5. Statistical analysis was performed using Prism, version 6 (GraphPad Software Inc., La Jolla, CA, USA) using Student's t test or one‐way ANOVA followed by Fischer's least significant difference test depending on the number of experimental groups. P < 0.05 was considered statistically significant.
Results
Antibody specificity
Celiac superior mesenteric ganglia (CSMG) neurons, used as an FFA3‐positive control (Won et al. 2013), were immunoreactive for FFA3 antibody RK1103 (Fig. 1 A–C), which was abolished by preincubation of antibody with antigen peptide (Fig. 1 D). FFA3‐immunoeactivity (IR) was absent in the liver (Fig. 1 E), which is consistent with low mRNA expression in the liver (Fig. 1 I). In HeLa‐rFFA3 cells, abundant FFA3‐IR was detected in the plasma membrane, whereas mock‐transfected or HeLa‐rFFA2 cells were faintly stained (Fig. 1 F–H), indicating specificity of the antibody and lack of cross‐reaction with FFA2. Western blot was performed with proteins extracted from rat colonic submucosa, which was obtained by meticulous removal of the mucosa and the muscle layers using fine forceps. FFA3 antibody RK1103 detected a unique band with apparent size of ∼38 kDa, close to the predicted molecular mass of FFA3 (36 kDa), which was absorbed by antigen peptide (Fig. 1 J).
Figure 1. Antibody characterization .
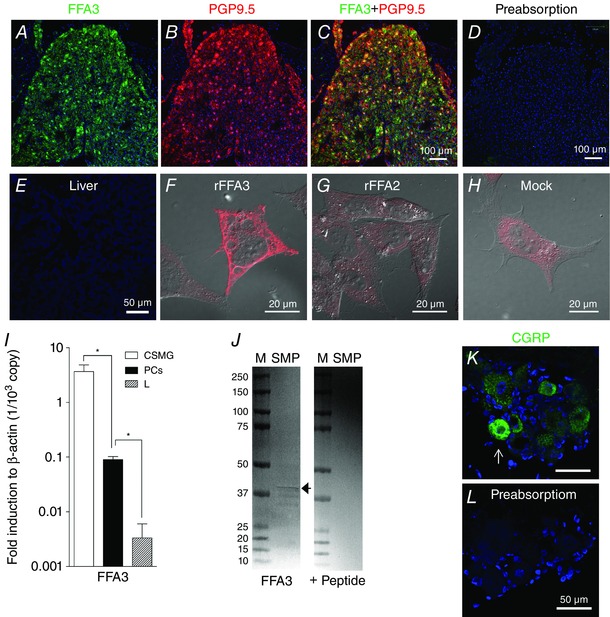
A and B, frozen sections of CSMG were double‐stained with antibodies to FFA3 (A) (green) and PGP9.5 (B) (red). C, merged image showing FFA3 and PGP9.5 colocalization. D, preabsorption of FFA3 antibody in the section of CSMG. E, liver section as a negative control had no FFA3‐IR. Nuclei were counterstained with DAPI (blue). F–H, FFA3‐IR (red) in rat FFA3‐ (F), rat FFA2‐ (G) or mock‐(H) transfected HeLa cells. After a 48 h culture, the transfected cells were fixed by 4% paraformaldehyde for 15 min and reacted with FFA3 antibody. I, FFA3 mRNA expression assessed by real‐time RT‐PCR in the CSMG, in the muscle layer of proximal colon (PCs) and in the liver (L). *P < 0.05. J, western blot using FFA3 antibody (1 μg ml−1) detected a ∼38 kDa band (arrow) in the extracted protein from rat colonic submucosa (SM) and absorbed by antigen peptide (10 μg ml−1). M, standard proteins with molecular size (kDa) on the left. K and L, immunohistochemistry with α‐CGRP antibody in serial sections of rat dorsal root ganglia. α‐CGRP‐IR (arrow, K) was absorbed by antigen peptide (5 μg ml−1, L).
The specificity of the anti‐α‐CGRP antibody was tested with adjacent sections of dorsal root ganglia. α‐CGRP antibody labelled the cell bodies of small‐sized ganglion cells (Fig. 1 K), whereas such immunohistochemical labelling disappeared after preincubation of the α‐CGRP antibody with rat CGRP1–37 peptide (Fig. 1 L).
Localization of FFA3 in rat colon
FFA3‐IR was detected in the myenteric and submucosal plexuses, as well as intramuscular and mucosal nerve‐like fibres, in frozen sections (Fig. 2 A). FFA3‐IR was present in fibres surrounding crypts and the fibres were double‐stained with PGP9.5 to confirm their identity as nerve fibres (Fig. 2 B–C). All of the FFA3‐IR in proximal colon was abolished by pre‐absorption with antigen peptide (Fig. 2 D). We further separately stained whole‐mount preparations of mucosa, submucosa or circular muscle‐stripped longitudinal muscle layers, which includes mucosal, submucosal (Meissner's) or myenteric (Auerbach's) plexus, respectively (Fig. 2 E–G). FFA3‐IR was detected in the mucosal and submucosal plexuses, located in the nerve fibres and endings rather than in the neuronal cell bodies. In the myenteric plexus, a subset of myenteric neurons expressed cytoplasmic FFA3.
Figure 2. Localization of FFA3‐IR in rat proximal colon .
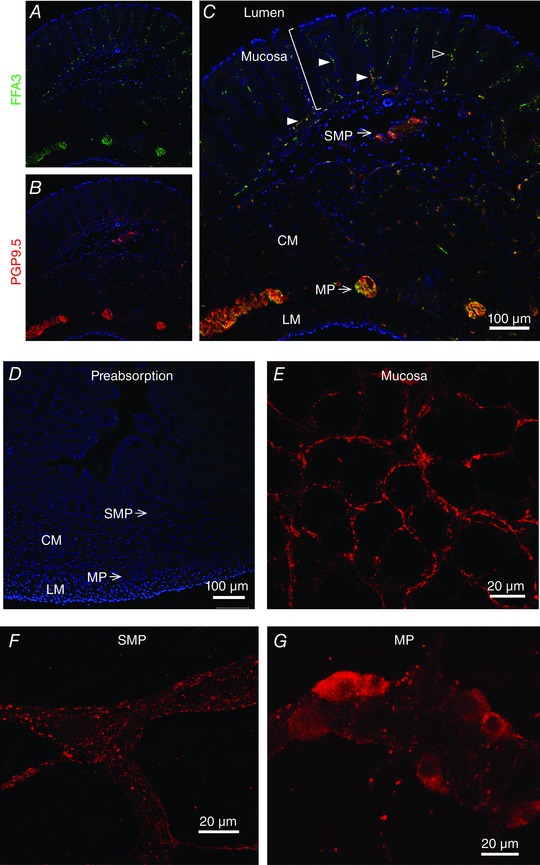
A–C, frozen sections were double‐stained with FFA3 (A) and with PGP9.5 (B). The merged image (C) showed that FFA3 colocalized with PGP9.5 in subepithelial nerve fibres (solid arrowheads), in the submucosal plexus (SMP), in intramuscular nerves in circular (CM) and longitudinal (LM) muscle, and in myenteric plexus (MP). Scattered FFA3‐positive epithelial cells had morphology resembling enteroendocrine cells (open arrowhead). D, preabsorption abolished FFA3‐IR. Nuclei were counterstained with DAPI (blue). E–G, whole mounts of mucosa (E), submucosa (F) and myenteric plexus (G) of proximal colon were stained with FFA3 antibody (red).
Because choline acetyltransferase (ChAT) has two variants (Chiocchetti et al. 2003), we used VAChT and CHT1 as cholinergic markers, as previously reported in the rat enteric nervous system (Harrington et al. 2007). Most of the FFA3‐IR nerves colocalized with VAChT and CHT1 in the mucosal and submucosal plexuses, indicating that FFA3 was expressed in cholinergic nerves (Fig. 3 A–F). CHT1 did not colocalize with vasoactive intestinal peptide (VIP) in the mucosal plexus (Fig. 3 G), although both nerves were closely approximated, suggesting that FFA3‐IR cholinergic nerves and VIP‐ergic nerves are distinct populations. We detected α‐CGRP‐IR, a marker of afferent neurons, in varicosities and nerve fibres but not in submucosal neuronal cell bodies in the proximal colon. α‐CGRP‐IR did not colocalize with FFA3‐IR (Fig. 3 H). Calbindin, which labels neuropeptide Y(NPY)/VIP‐ergic secretomotor neurons in the submucosal plexus of rat small intestine (Buchan, 1991), did not colocalize with FFA3‐IR in the submucosal ganglia (Fig. 3 I).
Figure 3. Localization of FFA3‐IR in cholinergic nerves in whole mounts of mucosa and in submucosal plexus .
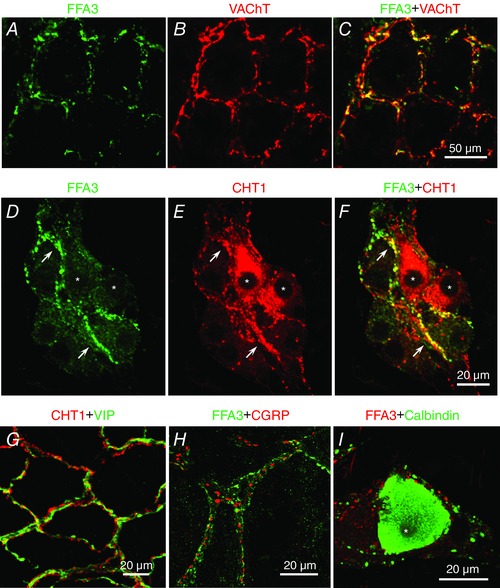
A–C, FFA3 (A) and VAChT (B) in mucosal plexus. Both FFA3‐ and VAChT‐IR nerves (merged image in C) surrounded the crypts. D–F, FFA3 (D) and CHT1 (E) expression in the submucosal plexus. Most FFA3‐IR nerve fibres and endings expressed CHT1 (arrows in F), whereas FFA3‐IR was faint in CHT1‐IR neuron somata (asterisks). G, CHT1 (red) and VIP (green) expressed in mucosal plexus. VIP‐IR and CHT1‐IR were detected in individual nerve fibres. H and I, FFA3 and α‐CGRP (H) or calbindin (I) in submucosal plexus. Calbindin‐IR neuron (asterisk) had no FFA3‐IR.
Effect of FFA3 agonist and CCh on I sc in rat proximal colon
We studied the contribution of neural FFA3 activation with respect to regulation of ion transport in Ussing chambered mucosal–submucosal preparations of rat proximal colon. The serosal application of CCh (10 μm) in the absence of pretreatment rapidly increased I sc, accompanied by G t increase, peaking at ∼2 min, followed by a gradual return to basal levels (Fig. 4 A). The I sc increase evoked by CCh in rat proximal colon exclusively reflects Cl− secretion (Kaji et al. 2015 a). The selective FFA3 agonist MQC itself, when applied into the luminal or serosal bath after the stabilization period, had no effect on basal I sc, whereas pretreatment with MQC decreased the peak value of CCh‐induced ΔI sc but did not change the time course of the response (Fig. 4 A). CCh‐induced ΔI sc was suppressed by serosal MQC in a dose‐dependent manner (Fig. 4 B) with a 65% maximal inhibition and a calculated IC50 = 13 μm by fitting to a sigmoid curve by the least squares method, which is comparable to the previously reported affinity of MQC as measured in a cell expression system (6 μm) (Hudson et al. 2014). These results suggest that activation of FFA3 expressed on the submucosal plexus inhibits cholinergic‐mediated anion secretion.
Figure 4. Effect of FFA3 ligands on CCh‐induced Isc increases in Ussing‐chambered rat proximal colon .
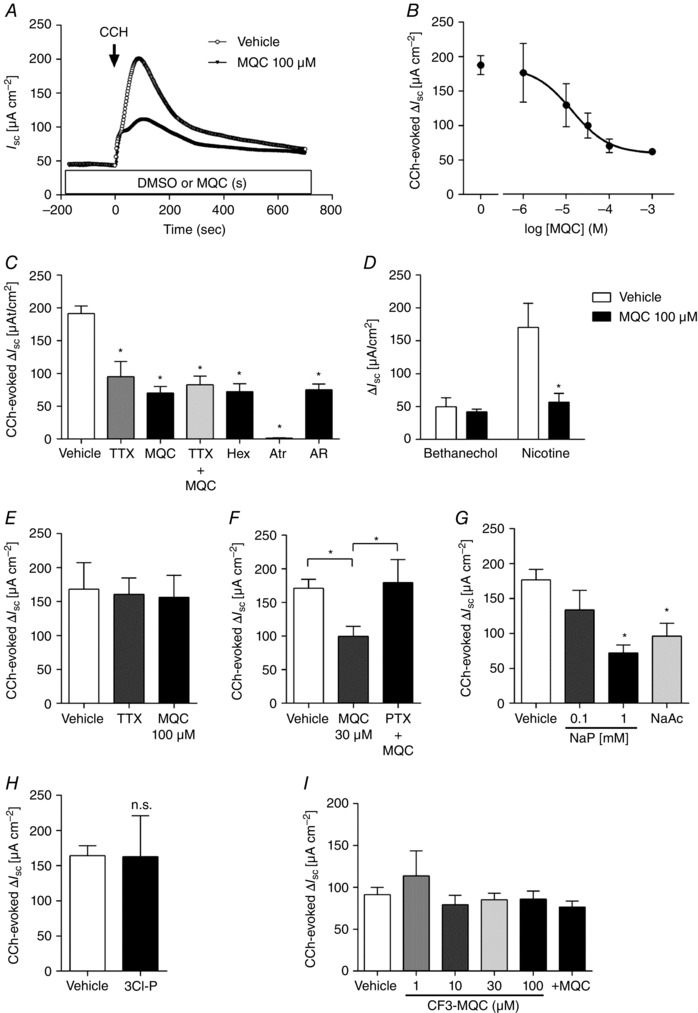
Preparations were incubated with inhibitors during the stabilization period, whereas MQC or AR420626 was added 15 min before CCh application. All chemicals were added into the serosal bath. A, representative I sc traces in mucosa–submucosa preparations in response to CCh (10 μm) in the presence or absence of MQC. B, concentration‐dependent inhibition of MQC on CCh‐evoked ΔI sc in mucosa–submucosa preparations. A single concentration of MQC was applied to each preparation. C, CCh‐evoked ΔI sc in the presence or absence of MQC (100 μm), AR420626 (10 μm), TTX (1 μm), hexamethonium (Hex: 10 μm) or atropine (Atr: 10 μm) in mucosa–submucosa preparations. *P < 0.05 vs. vehicle. D, effect of bethanechol (100 μm) or nicotine (100 μm) on I sc in the presence or absence of MQC (100 μm) in mucosa–submucosa preparations. *P < 0.05 vs. vehicle. E, effect of MQC and TTX (1 μm) on CCh‐evoked ΔI sc in mucosal preparations. F, effect of MQC in the presence or absence of PTX (500 ng ml−1) in mucosa–submucosa preparations. *P < 0.05. G and H, effect of NaP, NaAc (1 mm) or 3‐chloropropionate (3Cl‐P, 1 mm) on CCh‐evoked ΔI sc in mucosa–submucosa preparations. *P < 0.05 vs. vehicle. n.s., not significant. SCFA solutions were adjusted to pH 7.4. I, effect of CF3‐MQC with or without MQC on CCh‐evoked ΔI sc in mucosa–submucosa preparations. A single concentration of CF3‐MQC was applied to each preparation. MQC (30 μm) was added 10 min after the application of CF3‐MQC (100 μm).
Effect of TTX and ACh receptor antagonists on CCh‐induced I sc increase
Pretreatment with serosal TTX, hexamethonium, MQC (100 μm) or another FFA3 agonist AR420626 (10 μm) decreased CCh‐induced ΔI sc by 45%, 61%, 59% or 61% respectively (Fig. 4 C). The non‐selective muscarinic receptor antagonist atropine abolished CCh‐induced ΔI sc, indicating that cholinergic‐induced Cl− secretion is mediated by muscarinic receptors, whereas the neural reflex via nicotinic ACh receptors mediates ∼60% of the response. A combination of TTX and MQC had no additional effect on CCh‐induced Cl− secretion, suggesting that FFA3 activation suppresses the response to CCh via a TTX‐sensitive neural pathway. Pretreatment with MQC suppressed nicotine‐induced secretion by 55% but had no effect on bethanechol‐induced secretion (Fig. 4 D). Because bethanechol is a selective muscarinic agonist, these results suggest that the TTX‐inhibitable portion of CCh‐evoked secretion is mediated by nicotinic receptors expressed on cholinergic nerves, which are inhibited by pre‐activation of FFA3.
We confirmed the involvement of the neural pathway in MQC‐induced inhibition using mucosa‐only preparations, in which submucosal neurons have been excluded. In Ussing chambered mucosal preparations, CCh‐induced ΔI sc was unaltered by TTX or MQC treatment (Fig. 4 E), further suggesting that TTX‐sensitive submucosal neurons are involved in the inhibitory effect of MQC on CCh‐induced secretion.
Effect of PTX on FFA3‐mediated cholinergic inhibition
We next studied the inhibitory mechanisms of FFA3 paying particular attention to the Gi/o pathway using PTX in mucosa–submucosa preparations. Serosal application of a submaximal concentration of MQC (30 μm) decreased CCh‐evoked ΔI sc by 42%. Pretreatment with serosal PTX (500 ng ml−1) for 45 min before MQC application restored the response to CCh in the presence of MQC (Fig. 4 F), indicating that the anti‐secretory effect of FFA3 activation is mediated by the Gi/o pathway.
Effect of serosal SCFAs on CCh‐induced I sc increase
We further examined whether serosal SCFAs mimic the inhibitory effect of MQC on CCh‐induced I sc increase. The sodium salts of the endogenous FFA3 ligands propionate (NaP) and acetate (NaAc), pH adjusted to 7.4 with 1 n HCl, were serosally applied in mucosa–submucosa preparations. Pretreatment with 1 mm NaP or NaAc suppressed CCh‐induced Cl− secretion as did MQC (Fig. 4 G). Conversely, 3‐chloropropionate, an inactive propionate derivative (Yajima et al. 2011 a), did not affect CCh‐induced ΔI sc (Fig. 4 H).
Effect of FFA3 antagonists on CCh‐induced I sc increase
To confirm the specificity of FFA3 agonist MQC, we tested the synthetic FFA3 antagonist CF3‐MQC (Ulven, 2012) in mucosa–submucosa preparations. Serosal application of CF3‐MQC (1–100 μm) did not affect basal I sc or the response to CCh (Fig. 4 I). Pretreatment with CF3‐MQC (100 μm) prevented the inhibitory effect of serosal MQC (30 μm) on CCh‐induced Cl− secretion (Fig. 4 I, +MQC), suggesting that MQC acts as an FFA3 agonist.
Effect of MQC on luminal NaP‐induced I sc increase
Because luminal NaP evokes epithelial ACh release followed by colonic Cl− secretion (Yajima et al. 2011 a), we examined the effect of MQC on NaP‐induced secretion in rat proximal colon. In mucosa–submucosa preparations, luminal NaP increased I sc, accompanied by increased G t, peaking at ∼1.5 min, and subsequently returning to basal levels (Fig. 5 A). NaP‐induced ΔI sc was reduced 65% by pre‐treatment with TTX or with hexamethonium, and by 97% with atropine (Fig. 5 B). Removal of the submucosa mimicked the inhibitory effect of TTX and hexamethonium (Fig. 5 B), suggesting that the TTX‐sensitive neural pathway is reliant on nicotinic receptors expressed in the submucosal plexus and that TTX‐sensitive and ‐insensitive cholinergic pathways mediate NaP‐induced Cl− secretion. Serosally or mucosally applied MQC (1 mm) decreased the response to NaP (Fig. 5 C and D), suggesting that luminal NaP stimulates the mucosal plexus, which is inhibited by pre‐activation of FFA3.
Figure 5. Effect of MQC on NaP‐ or FK‐evoked ΔIsc .
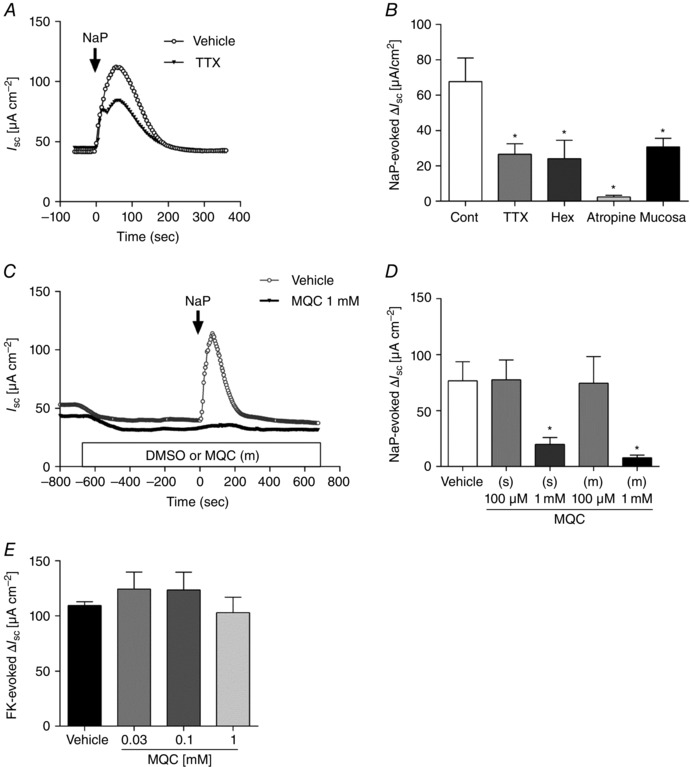
A, representative I sc traces in response to luminal NaP (5 mm) in the presence (solid triangles) or absence (open circles) of TTX (1 μm). B, luminal NaP‐evoked ΔI sc in the presence or absence of TTX (1 μm), hexamethonium (100 μm) or atropine (10 μm), or in the submucosa‐stripped mucosal preparations (Mucosa). C, representative I sc traces in mucosa–submucosa preparations. NaP (5 mm) was added into the luminal bath following luminal DMSO (grey) or MQC (1 mm, black). MQC decreased basal I sc, as did DMSO (vehicle) and inhibited the response to NaP. D, NaP‐evoked ΔI sc in the presence or absence of MQC in the serosal (s) or luminal (m) bath 10 min before the application of NaP (5 mm). *P < 0.05 vs. vehicle. E, FK (5 μm) was added into the serosal bath and ΔI sc was measured in the presence or absence of individual concentrations of serosal MQC.
Effect of MQC on FK‐induced I sc increase
To examine whether neural FFA3 activation suppresses other secretory responses in mucosa–submucosa preparations, we used FK, a potent secretagogue that activates the cAMP pathway. Serosal application of FK (5 μm) consistently increased I sc in the presence or absence of MQC (30 μm–1 mm), indicating that FFA3 activation is not involved in cAMP‐mediated secretory responses and that MQC does not directly damage the mucosa (Fig. 5 E).
Discussion
In this study, we have demonstrated that the SCFA receptor FFA3 was present in the mucosal, submucosal and myenteric plexuses of rat proximal colon. Most of the FFA3‐positive nerves coexpressed the cholinergic neuronal markers, VAChT and CHT1, but did not colocalize with VIP, calbindin or α‐CGRP that innervated the colonic wall. In Ussing chambered mucosal and mucosa–submucosa preparations, synthetic and natural FFA3 agonists inversely regulated nicotinic ACh receptor‐mediated Cl− secretion via TTX‐ and PTX‐sensitive pathways. Our findings suggest that SCFAs, via neural FFA3 activation, have a novel anti‐secretory function.
Mucosal and submucosal cholinergic‐mediated pathways are important for regulating colonic ion secretion (Diener et al. 1989). In colonocytes, ACh and VIP are Ca2+‐ and cAMP‐dependent secretagogues, respectively, synergistically potentiating epithelial ion secretion (Neunlist et al. 1998). Consistent with the lack of colocalization with ChAT and VIP in mice intestine (Foong et al. 2014), our whole‐mount immunohistochemistry demonstrated that CHT1 colocalized with FFA3 and not with VIP in the branching nerves surrounding crypts in rat proximal colon, suggesting that FFA3‐positive cholinergic nerves are separate from the VIP‐ergic nerves. On the other hand, in rat small intestine, ChAT labels the majority of submucosal neurons, of which half are immunoreactive for NPY, VIP and calbindin (Mann et al. 1999). In this study, CHT1 detected only 20% (21 plexuses from two rats) of PGP9.5‐IR submucosal neurons in the proximal colon, suggesting that neurotransmitter distribution differs among animal species and intestinal regions. From double‐staining studies of FFA3 with calbindin, FFA3‐IR was present only in nerve fibres and scarcely detected in the neuronal somata. Although CGRP‐ergic secretomotor neurons are identified in rat small intestine and distal colon (Buchan, 1991; Mitsui, 2010), we failed to detect α‐CGRP in the submucosal neurons. Rat myenteric and submucosal neurons express β‐CGRP, which is a gene product almost homologous to, but distinct from, α‐CGRP, rather than α‐CGRP (Sternini & Anderson, 1992). Our antibody directed against α‐CGRP might not recognize β‐CGRP under the experimental conditions used in the present study. By contrast, α‐CGRP expression is greater than β‐CGRP expression in the dorsal root and nodose ganglia (Sternini & Anderson, 1992). Accordingly, our data suggest that α‐CGRP‐IR nerves represent extrinsic afferent neurons and do not express FFA3, at least in rat proximal colon. FFA3‐positive cholinergic nerve fibres and endings probably originated from myenteric neurons and/or extrinsic neurons. Cholinergic vagal nerve endings are present in rat proximal colonic ganglia (Berthoud et al. 1991) and ACh production is present in rat nodose and dorsal root ganglia (Palouzier et al. 1987; Bellier & Kimura, 2007), which include FFA3‐positive afferent neurons (Nohr et al. 2015). Enteric neural circuits, including internal primary afferent neurons and/or interneurons that secondarily excite secretomotor neurons, are proposed to be present in guinea‐pig ileum and in rat distal colon (Christofi et al. 2004; Reed & Vanner, 2007). Therefore, further investigations are required to identify the origin of the FFA3‐IR nerves, as well as their contribution to the neural circuit, which innervate and terminate in mucosal and submucosal plexuses in rat proximal colon.
Our Ussing chamber study demonstrated that the selective FFA3 agonists MQC or AR420626 reduced cholinergic‐mediated secretion via the submucosal plexus. The anti‐secretory effect of MQC was concentration‐dependent and PTX‐sensitive, consistent with Gi/o‐coupled FFA3 activation. TTX reduced CCh‐evoked responses by half, as did hexamethonium, AR420626, MQC or a combination of TTX and MQC, suggesting that FFA3 activation suppresses a nicotinic receptor‐mediated response. Because atropine abolished CCh‐induced secretion, muscarinic receptors were the end target of cholinergic‐mediated secretion via direct and indirect pathways. Pre‐treatment with MQC consistently inhibited nicotine‐induced secretion but did not affect bethanechol‐induced secretion in mucosa–submucosa preparations. Furthermore, neither MQC, nor TTX altered CCh‐evoked secretion in the mucosal preparation, where submucosal neurons were eliminated, suggesting that MQC has no direct interaction with muscarinic receptors expressed on epithelial cells. Because ligands activate nicotinic receptors at the site of neurotransmitter release via a TTX‐sensitive mechanism (Schneider et al. 2000), cholinergic neuronal axons expressing FFA3, which emanate from the myenteric neurons located in the submucosal plexus, are possibly activated by CCh and nicotine, and release ACh. In addition to the synthetic agonists, serosal SCFAs suppressed CCh‐evoked Cl− secretion. The physiological concentration of SCFAs in the subepithelial and submucosal space is unknown; however, it is probably in the micromolar to millimolar range as a result of the absorption of luminal SCFAs via enterocyte MCTs followed by transcellular transport into the submucosal space. Therefore, cholinergic‐mediated Cl− secretion may be physiologically controlled by luminal SCFAs via FFA3 activation. By contrast, 3‐chloropropionate did not alter basal I sc and CCh‐evoked I sc increase. This analogue inhibits NaP‐induced ACh production and subsequent anion secretion in rat distal colon (Yajima et al. 2011 a) and also inhibits lactate transport in rabbit renal brush border membrane (Nord et al. 1983). These observations suggest that 3‐chloropropionate is probably competitive with SCFAs for MCT, but is not itself an FFA3 ligand, and also that the serosal SCFA‐induced anti‐cholinergic effect is not a result of MCT inhibition.
Although luminal NaP stimulates epithelially‐derived ACh release (Yajima et al. 2011 a), FFA3‐selective agonists in the concentrations that we tested did not directly evoke Cl− secretion even with luminal application. Luminal NaP‐induced secretion was inhibited by TTX, hexamethonium or removal of the submucosa, and abolished by atropine in rat proximal colon, which is consistent with a study of secretion in rat distal colon (Yajima, 1988). Similar to the effect of CCh, luminal NaP stimulates epithelial muscarinic receptors and a TTX‐sensitive pathway, which is suppressed by FFA3 activation. Because higher concentrations of MQC were needed to reduce the response to luminal NaP than to serosal CCh, luminal NaP probably releases ACh, which diffuses to the lamina propria where it stimulates mucosal cholinergic axons, followed by neuronal nicotinic receptor activation and activation of the submucosal neural reflex, resulting in the release of additional ACh, which in turn stimulates Cl− secretion. Our results suggest that activation of mucosal cholinergic nerves by luminal NaP is suppressed by neuronal FFA3 activation. Gq/11‐coupled GPCRs expressed on the apical membrane of colonocytes are probably SCFA sensors that stimulate epithelial ACh release (Yajima et al. 2011 b). Therefore, Gi/o‐coupled FFA3 is probably not involved in the stimulatory effects of luminal SCFAs.
Luminal SCFAs stimulate anion secretion via the cholinergic pathway, whereas basolateral SCFAs suppress cholinergic‐mediated secretion via FFA3 activation. This apparently contradictory response to SCFAs might represent a negative feedback system that homeostatically regulates colonic anion secretion during SCFA absorption. From a pharmacological standpoint, SCFAs may have a novel anti‐cholinergic function via FFA3 activation, serving to curtail vagal hyperactivity. Activation of FFA3 suppresses peripheral synaptic transmission by presynaptic Ca2+ influx inhibition in rat prevertebral ganglia (Won et al. 2013). Furthermore, FFA3 activation inhibits glucose‐dependent insulin secretion via Gi/o in vivo and in vitro in mice pancreas (Priyadarshini & Layden, 2015; Tang et al. 2015). FFA3 activation, which modulates transmitter release via inhibition of Ca2+ influx into neurons and endocrine cells, probably exerts its anti‐cholinergic action by presynaptic ACh release.
We also detected FFA3‐IR in intramuscular nerves and in a subpopulation of myenteric neurons. A lack of FFA3 accelerates the intestinal transit rate and decreases the absorption rate of luminal SCFAs (Samuel et al. 2008). In the GI tract, neural FFA3 may be involved in regulation of the rate of nutrient absorption via the slowing of intestinal transit and inhibition of secretion during the digestive phase following a meal. The high concentrations of plasma acetate (∼1 mm) present after alcohol consumption (Korri et al. 1985) or of the ketone β‐hydroxybutyrate present during starvation or diabetic ketoacidosis (6–10 mm) serve as endogenous FFA3 agonists (Won et al. 2013), suggesting that the enteric cholinergic reflex may be disrupted under such conditions. Because ACh availability is increased under stress conditions (Kita et al. 1986) and cholinergic signalling mediates stress‐induced increases in intestinal ion transport and permeability in rats (Saunders et al. 1997), stress‐induced diarrhoea or diarrhoea‐predominant irritable bowel syndrome could be a therapeutic target for the FFA3 agonists.
In conclusion, neural FFA3 activation counters cholinergic secretion in the mucosal and submucosal plexuses of rat proximal colon. FFA3, which senses luminal bacteria‐derived SCFA probably ‘fine‐tunes’ the activity of the enteric nervous system. We propose that FFA3 is a key modifier of the cholinergic reflex that helps maintain physiological levels of secretion and motility.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
IK, YA and JDK were responsible for the study concept and design, and for the original draft. IK, KK, MW and TI were responsible for antibody production. AK and KI were responsible for chemical design and synthesis. IK, YA and SK were responsible for collection, assembly and analysis of data. IK, YA, MW, TI, AK and JDK were responsible for data interpretation. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by a Department of Veterans Affairs Merit Review Award and NIH R01 DK54221. Antibody production was supported by a project of Comprehensive Brain Science Network (CBSN) in Japan.
Acknowledgements
We thank Dr Paul H. Guth and Dr Eli Engel for useful discussions, as well as Stacey S. Jung for her assistance with the preparation of the manuscript.
References
- Akiba Y, Inoue T, Kaji I, Higashiyama M, Narimatsu K, Iwamoto K, Watanabe M, Guth PH, Engel E, Kuwahara A & Kaunitz JD (2015). Short‐chain fatty acid sensing in rat duodenum. J Physiol 593, 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellier JP & Kimura H (2007). Acetylcholine synthesis by choline acetyltransferase of a peripheral type as demonstrated in adult rat dorsal root ganglion. J Neurochem 101, 1607–1618. [DOI] [PubMed] [Google Scholar]
- Bergman EN (1990). Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev 70, 567–590. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Carlson NR & Powley TL (1991). Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol Regul Integr Comp Physiol 260, R200–R207. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A & Dowell SJ (2003). The Orphan G protein‐coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278, 11312–11319. [DOI] [PubMed] [Google Scholar]
- Buchan AM (1991). Neurofilament M and calbindin D28k are present in mutually exclusive subpopulations of enteric neurons in the rat submucous plexus. Brain Res 538, 171–175. [DOI] [PubMed] [Google Scholar]
- Cani PD & Delzenne NM (2011). The gut microbiome as therapeutic target. Pharmacol Ther 130, 202–212. [DOI] [PubMed] [Google Scholar]
- Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G & Galmiche JP (1998). Short‐chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol Gastrointest Liver Physiol 275, G1415–G1422. [DOI] [PubMed] [Google Scholar]
- Chiocchetti R, Poole DP, Kimura H, Aimi Y, Robbins HL, Castelucci P & Furness JB (2003). Evidence that two forms of choline acetyltransferase are differentially expressed in subclasses of enteric neurons. Cell Tissue Res 311, 11–22. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Wunderlich J, Yu JG, Wang YZ, Xue J, Guzman J, Javed N & Cooke H (2004). Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. J Comp Neurol 469, 16–36. [DOI] [PubMed] [Google Scholar]
- Dass NB, John AK, Bassil AK, Crumbley CW, Shehee WR, Maurio FP, Moore GB, Taylor CM & Sanger GJ (2007). The relationship between the effects of short‐chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil 19, 66–74. [DOI] [PubMed] [Google Scholar]
- Diener M, Knobloch SF, Bridges RJ, Keilmann T & Rummel W (1989). Cholinergic‐mediated secretion in the rat colon: neuronal and epithelial muscarinic responses. Eur J Pharmacol 168, 219–229. [DOI] [PubMed] [Google Scholar]
- Foong JP, Tough IR, Cox HM & Bornstein JC (2014). Properties of cholinergic and non‐cholinergic submucosal neurons along the mouse colon. J Physiol 592, 777–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Tsujita M, Yamazaki M, Kushiya E, Abe M, Akashi K, Natsume R, Kano M, Kamiya H, Watanabe M & Sakimura K (2006). Abundant distribution of TARP gamma‐8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur J Neurosci 24, 2177–2190. [DOI] [PubMed] [Google Scholar]
- Grider JR & Piland BE (2007). The peristaltic reflex induced by short‐chain fatty acids is mediated by sequential release of 5‐HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol 292, G429–G437. [DOI] [PubMed] [Google Scholar]
- Harrington AM, Hutson JM & Southwell BR (2007). High affinity choline transporter immunoreactivity in rat ileum myenteric nerves. Cell Tissue Res 327, 421–431. [DOI] [PubMed] [Google Scholar]
- Hudson BD, Christiansen E, Murdoch H, Jenkins L, Hansen AH, Madsen O, Ulven T & Milligan G (2014). Complex pharmacology of novel allosteric free fatty acid 3 receptor ligands. Mol Pharmacol 86, 200–210. [DOI] [PubMed] [Google Scholar]
- Iwanaga T & Kishimoto A (2015). Cellular distributions of monocarboxylate transporters: a review. Biomed Res 36, 279–301. [DOI] [PubMed] [Google Scholar]
- Kaji I, Akiba Y, Said H, Narimatsu K & Kaunitz JD (2015. a). Luminal 5‐HT stimulates colonic bicarbonate secretion in rats. Br J Pharmacol 172, 4655–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Akiba Y, Kaunitz JD, Karaki Si & Kuwahara A (2012. a). Differential expression of short‐chain fatty acid receptor FFA2 and FFA3 in foregut. Gastroenterology 142, S494. [Google Scholar]
- Kaji I, Iwanaga T, Watanabe M, Guth PH, Engel E, Kaunitz JD & Akiba Y (2015. b). SCFA transport in rat duodenum. Am J Physiol Gastrointest Liver Physiol 308, G188–G197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Yasuoka Y, Karaki S & Kuwahara A (2012. b). Activation of TRPA1 by luminal stimuli induces EP4‐mediated anion secretion in human and rat colon. Am J Physiol Gastrointest Liver Physiol 302, G690–G701. [DOI] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A & Tsujimoto G (2011). Short‐chain fatty acids and ketones directly regulate sympathetic nervous system via G protein‐coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108, 8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Hata T, Higashiguchi T, Itoh E & Kawabata A (1986). Changes of total acetylcholine content and the activity of related enzymes in SART (repeated cold)‐stressed rat brain and duodenum. Jpn J Pharmacol 40, 174–177. [DOI] [PubMed] [Google Scholar]
- Korri UM, Nuutinen H & Salaspuro M (1985). Increased blood acetate: a new laboratory marker of alcoholism and heavy drinking. Alcohol Clin Exp Res 9, 468–471. [DOI] [PubMed] [Google Scholar]
- Le PE, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van DJ, Parmentier M & Detheux M (2003). Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278, 25481–25489. [DOI] [PubMed] [Google Scholar]
- Mann PT, Furness JB & Southwell BR (1999). Choline acetyltransferase immunoreactivity of putative intrinsic primary afferent neurons in the rat ileum. Cell Tissue Res 297, 241–248. [DOI] [PubMed] [Google Scholar]
- Mitsui R (2010). Immunohistochemical characteristics of submucosal Dogiel type II neurons in rat colon. Cell Tissue Res 340, 257–265. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Frieling T, Rupprecht C & Schemann M (1998). Polarized enteric submucosal circuits involved in secretory responses of the guinea‐pig proximal colon. J Physiol 506, 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW & Moller M (2015). Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 290, 126–137. [DOI] [PubMed] [Google Scholar]
- Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Seier‐Poulsen S, Han S, Jones RM, Offermanns S & Schwartz TW (2013). GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short‐chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinol 154, 3552–3564. [DOI] [PubMed] [Google Scholar]
- Nord EP, Wright SH, Kippen I & Wright EM (1983). Specificity of the Na+‐dependent monocarboxylic acid transport pathway in rabbit renal brush border membranes. J Membr Biol 72, 213–221. [DOI] [PubMed] [Google Scholar]
- Palouzier B, Barrit‐Chamoin MC, Portalier P & Ternaux JP (1987). Cholinergic neurons in the rat nodose ganglia. Neurosci Lett 80, 147–152. [DOI] [PubMed] [Google Scholar]
- Priyadarshini M & Layden BT (2015). FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets 7, e1045182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DE & Vanner S (2007). Mucosal stimulation activates secretomotor neurons via long myenteric pathways in guinea pig ileum. Am J Physiol Gastrointest Liver Physiol 292, G608–G614. [DOI] [PubMed] [Google Scholar]
- Richardson A, Delbridge AT, Brown NJ, Rumsey RD & Read NW (1991). Short chain fatty acids in the terminal ileum accelerate stomach to caecum transit time in the rat. Gut 32, 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M & Gordon JI (2008). Effects of the gut microbiota on host adiposity are modulated by the short‐chain fatty‐acid binding G protein‐coupled receptor, Gpr41. Proc Natl Acad Sci USA 105, 16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders PR, Hanssen NP & Perdue MH (1997). Cholinergic nerves mediate stress‐induced intestinal transport abnormalities in Wistar–Kyoto rats. Am J Physiol Gastrointest Liver Physiol 273, G486–G490. [DOI] [PubMed] [Google Scholar]
- Schneider DA, Perrone M & Galligan JJ (2000). Nicotinic acetylcholine receptors at sites of neurotransmitter release to the guinea pig intestinal circular muscle. J Pharmacol Exp Ther 294, 363–369. [PubMed] [Google Scholar]
- Schwartz MP, Samsom M, Van Berge Henegouwen GP & Smout AJ (2001). Effect of inhibition of gastric acid secretion on antropyloroduodenal motor activity and duodenal acid hypersensitivity in functional dyspepsia. Aliment Pharmacol Ther 15, 1921–1928. [DOI] [PubMed] [Google Scholar]
- Soldavini J & Kaunitz JD (2013). Pathobiology and potential therapeutic value of intestinal short‐chain fatty acids in gut inflammation and obesity. Dig Dis Sci 58, 2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires PE, Rumsey RD, Edwards CA & Read NW (1992). Effect of short‐chain fatty acids on contractile activity and fluid flow in rat colon in vitro. Am J Physiol 262, G813–G817. [DOI] [PubMed] [Google Scholar]
- Sternini C & Anderson K (1992). Calcitonin gene‐related peptide‐containing neurons supplying the rat digestive system: differential distribution and expression pattern. Somatosens Mot Res 9, 45–59. [DOI] [PubMed] [Google Scholar]
- Tang C, Ahmed K, Gille A, Lu S, Grone HJ, Tunaru S & Offermanns S (2015). Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med 21, 173–177. [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F & Gribble FM (2012). Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes 61, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulven T (2012). Short‐chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 3, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Declusin RJ, Soergel KH & Baker RD (1976). The effect of short chain fatty acids on transmural electrical potential across rat small intestine in vivo. Biochim Biophys Acta 433, 654–661. [DOI] [PubMed] [Google Scholar]
- Won YJ, Lu VB, Puhl HL, III & Ikeda SR (2013). β‐Hydroxybutyrate modulates N‐type calcium channels in rat sympathetic neurons by acting as an agonist for the G‐protein‐coupled receptor FFA3. J Neurosci 33, 19314–19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T (1985). Contractile effect of short‐chain fatty acids on the isolated colon of the rat. J Physiol 368, 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T (1988). Luminal propionate‐induced secretory response in the rat distal colon in vitro. J Physiol 403, 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T, Inoue R, Matsumoto M & Yajima M (2011. a). Non‐neuronal release of ACh plays a key role in secretory response to luminal propionate in rat colon. J Physiol 589, 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T, Inoue R, Yajima M, Tsuruta T, Karaki S, Hira T & Kuwahara A (2011. b). The G‐protein on cholesterol‐rich membrane microdomains mediates mucosal sensing of short‐chain fatty acid and secretory response in rat colon. Acta Physiol (Oxf) 203, 381–389. [DOI] [PubMed] [Google Scholar]
- Yajima T & Sakata T (1986). Influences of short‐chain fatty acids on the digestive organs. Bifidobacteria Microflora 6, 7–14. [Google Scholar]


