Abstract
Background
Cerebrotendinous xanthomatosis (CTX) is a rare genetic disorder of bile acid synthesis that can cause progressive neurological damage and premature death. Detection of CTX in the newborn period would be beneficial since an effective treatment is available. We previously described a liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) test with potential to screen newborn dried bloodspots (DBS) for CTX. We report here modifications to the methodology and application of the modified test to analysis of DBS from a CTX-affected and unaffected newborns.
Methods
The testing methodology utilizes keto derivatization to enable sensitive LC-ESI-MS/MS measurement of elevated 7α,12α-dihydroxy-4-cholesten-3-one (7α12αC4) in CTX newborn DBS. We report here method modifications, including use of a DBS extraction procedure used in newborn screening laboratories and a reduced analysis time of 2 min per sample.
Results
Rapid isotope-dilution LC-ESI/MS/MS quantification of the ketosterol bile acid precursor 7α12αC4 provides a test that could readily discriminate a CTX positive newborn DBS sample (with a concentration of 104.4 ng/ml) from unaffected newborn samples (with a mean concentration of 4.1 ± 3.4 ng/ml; range 0.2–15.6 ng/ml, n = 39) analyzed in a blinded manner.
Conclusions
We provide additional evidence suggesting 7α12αC4 may be a promising test marker to screen newborn DBS for CTX. Early detection and intervention through newborn screening would greatly benefit those affected with CTX, preventing morbidity and mortality.
Abbreviations: CTX, cerebrotendinous xanthomatosis; ESI-MS/MS, liquid chromatography-electrospray ionization-tandem MS; DBS, dried bloodspots; 7α12αC4, 7α,12α-dihydroxy-4-cholesten-3-one; CDCA, chenodeoxycholic acid; GC–MS, gas chromatography–mass spectrometry; IRB, institutional Review Board; QAO, quaternary amonoxy; MRM, multiple reaction monitoring; LLOQ, lower limit of quantification; S/N, signal-to-noise; RSD, relative standard deviation; QCs, quality control samples
Keywords: Leukodystrophy, CYP27A1, Bile acid synthesis, Ketosterols, Newborn screening, LC-ESI-MS/MS
1. Introduction
Cerebrotendinous xanthomatosis (CTX; OMIM#213700) is an autosomal recessive childhood-to-adult onset leukodystrophy associated with deficient sterol 27-hydroxylase (CYP27A1), an enzyme important in conversion of cholesterol to the bile acids cholic and chenodeoxycholic acid (CDCA). CTX is difficult to diagnose; for most affected individuals it is not clinically obvious at birth, although neonatal cholestatic jaundice may be a presenting sign in some infants [1], [2]. While symptoms of CTX may present in childhood and adolescence, the disorder is often not recognized until symptoms have progressed. A mean age of first symptom onset ranging between 14 and 19 years old and a mean delay in diagnosis ranging between 17–19 years has been reported [3], [4], [5]. Symptoms in children can include diarrhea, juvenile cataracts and developmental delay [3]. Adolescent-to-adult onset symptoms may include tendon and cerebral xanthomas. In 95–97% of patients neurological symptoms have developed at the time of diagnosis [4], [5]; these may include cognitive impairment, cerebellar signs (for example ataxia) and pyramidal signs (for example spasticity). As the disorder progresses patients can become incapacitated with motor dysfunction, with premature death often occurring due to advancing neurological deterioration. Although only around three hundred cases of CTX have been described worldwide [6], relatively large series of patients have been identified by physicians familiar with the disorder, suggesting CTX may often be under or misdiagnosed.
A simple, effective oral therapy for CTX is available in the form of CDCA, the main bile acid deficient in CTX. Treatment with CDCA has been shown to normalize the biochemical phenotype and halt progression of disease in most cases [7], [8]. Generally treatment of patients with advanced neurological disease does not reverse the impairment [8]. A recent study in a cohort of 16 CTX patients demonstrated those who began CDCA treatment after age 25 years were significantly more limited in ambulation and more cognitively impaired compared to those who started treatment earlier [9]. Therefore it is essential to diagnose and treat CTX as early as possible.
As the mean age of CTX diagnosis is currently estimated as between 35 and 37 years old [3], [4], [5], we believe screening newborns for CTX will be the best way to achieve early identification and intervention for this disorder. Although CTX fulfills the majority of criteria required for a disorder to be screened for in newborns, there has been no suitable test available to screen newborn dried bloodspots (DBS) for CTX. Blood testing for diagnostic confirmation of CTX is routinely performed using gas chromatography–mass spectrometry (GC–MS) measurement of 5α-cholestanol which is elevated in affected individuals [10], [11]. A focus of our research has been to develop high-throughput amenable electrospray ionization-tandem mass spectrometry (ESI-MS/MS) based blood tests with utility to screen DBS for CTX [12], [13]. We recently described LC-ESI-MS/MS methodology that utilizes keto moiety derivatization to enable highly sensitive isotope dilution quantification of 7α,12α-dihydroxy-4-cholesten-3-one (7α12αC4) in DBS from CTX affected adults and newborns. Quantification of 7α12αC4 provided improved discrimination between CTX affected and unaffected samples compared to 5α-cholestanol, such that it could serve as an improved DBS test for CTX [14]. We demonstrated for the first time that elevated 7α12αC4 could be measured in DBS obtained from CTX affected newborns [14]. With the limitation that we were only able to retrieve and analyze two stored newborn DBS, there appeared to be no overlap between concentrations of 7α12αC4 in DBS from CTX affected and unaffected newborns [14]. We describe here modifications to the testing methodology that make it more amenable for use in newborn screening laboratories and provide further test validation data generated through the blinded analysis of an additional CTX positive newborn DBS, along with unaffected newborn DBS.
2. Materials and methods
2.1. Human subject research considerations
The CTX positive newborn DBS used to perform the blinded analysis was obtained through the Dutch Newborn Screening Program with the consent of the newborn's parents. Anonymized residual DBS from unaffected Dutch newborns were also used with approval of the Dutch National Institute for Public Health and the Environment (RIVM, Bilthoven, The Netherlands; responsible for the Dutch Newborn Screening Program) and the OHSU Institutional Review Board (IRB). Adult CTX positive DBS were collected from participants enrolled in an IRB-approved study at OHSU. Written informed consent was obtained for all OHSU study participants. De-identified DBS samples submitted to the Sterol Analysis Diagnostic laboratory at OHSU were used with IRB approval. For all CTX-positive samples diagnostic confirmation was performed by mutation analysis of CYP27A1. Residual de-identified whole blood was provided by the Oregon Clinical and Translational Research Institute (OCTRI).
2.2. Chemicals and reagents
Authentic 7α,12α-dihydroxy-4-cholesten-3-one (7α12αC4) and 7α-hydroxy-4-cholesten-3-one-d7 (7αC4-d7) were from Toronto Research Chemicals (Toronto, Ontario). Methanol, water and acetonitrile (LC-MS grade) were from Burdick and Jackson (Muskegon, MI). Formic acid (90%) was J.T. Baker brand. Glacial acetic acid (99.99%), hydrazine and oxalic acid were from Sigma-Aldrich (St Louis, MO). Quaternary amonoxy (QAO) reagent (O-(3-trimethylammoniumpropyl) hydroxylamine) bromide is commercially available as Amplifex™ Keto reagent from http://www.sciex.com. The QAO-d3 reagent was provided by SCIEX. Protein Saver 903 filter-paper was obtained from Whatman (Miami, FL).
2.3. Preparation of calibrators and samples for LC-MS/MS measurement of 7α12αC4
We have previously described QAO derivatization for LC-ESI-MS/MS measurement of elevated ketosterols in plasma or DBS [14]. In brief, DBS method calibrators were generated using dilutions of 7α12αC4 spiked into whole blood spotted onto filter-paper, dried and stored desiccated at − 80 °C. The methodology for quantification of 7α12αC4 was modified to use a DBS extraction procedure used in most newborn screening laboratories [15]. DBS calibrators or sample punches (3.2 mm) were extracted with a solution of 80% acetonitrile/20% water/0.05% oxalic acid/15 mM hydrazine using shaking at 100 rpm for 45 min at 45 °C. An aliquot of the extract was removed (50 μl) and mixed with 7αC4-d7 internal standard (250 pg) in 5 μl methanol and QAO reagent (10 μl) prepared according to the kit instructions i.e. QAO reagent was diluted 1:1 with diluent. After 2 h at room temperature QAO derivatization was complete. Previously prepared QAO-d3 tagged 7α12αC4 internal standard (150 pg) in 5 μl methanol was added [14] and the samples were heated at 45 °C for 45 min prior to analysis (or stored at 4 °C over-night and heated at 45 °C for 15 min prior to analysis).
2.4. Preparation of QAO-d3 7α12αC4 internal standard
In brief, 7α12αC4 was tagged with isotopically enriched QAO reagent to prepare internal standard by derivatization of 30 ng of 7α12αC4 using 942 μl QAO-d3 reagent (2.8 mg/ml) in methanol at 5% acetic acid. This reaction was kept at room temperature for 18 h and the underivatized ketosterol was monitored to ensure > 98% derivatization. This mixture was frozen without purification and aliquots of QAO-d3 tagged ketosterol internal standard was added to DBS samples after 2 h of derivatization with QAO reagent.
2.5. LC-ESI-MS/MS method
LC-ESI-MS/MS analyses were performed using a QTRAP® 5500 triple-quadrupole hybrid mass spectrometer with linear ion trap functionality (SCIEX, Framingham, MA), equipped with a TurboIonSpray® ESI source. The ionization interface was operated in the positive mode with the following settings; CUR 30, TEM 550, GS1 30, GS2 50, IS 4500 and CAD HIGH. Multiple reaction monitoring (MRM) transitions monitored for quantification of ketosterols were as follows: for QAO 7α12αC4 m/z 531.7 → 152.2, for QAO-d3 7α12αC4 m/z 534.7 → 152.1 and for QAO 7αC4-d7 m/z 522.7 → 152.2. The QTRAP® 5500 was coupled to a Shimadzu UPLC system (Columbia, MD) composed of a SIL-20ACXR auto-sampler and two LC-20ADXR LC pumps. QAO derivatives were resolved using a 50 × 2.1 (i.d.) mm, 5.0 μm Luna C8-HPLC column with guard (Phenomenex; Torrance, CA). The 2 min binary gradient method used mobile phase delivered at a flow rate of 0.8 ml/min with the A solvent 98% water:2% acetonitrile:0.1% formic acid mobile phase and the B solvent 10% water:90% acetonitrile:0.1% formic acid. Solvent B was increased from 10% to 65% over 0.5 min, was kept at 65% for 0.5 min, was increased to 100% over 0.1 min, was kept at 100% for 0.4 min, was decreased to 10% over 0.1 min and was kept at 10% for 0.4 min to equilibrate the column. The HPLC column temperature was kept at 35 °C using a Shimadzu CTO-20AC column oven. The sample injection volume was 10 μl.
2.6. Data analysis and method performance
Calibration curves were generated by performing a least-squares linear regression for peak area ratios (QAO-d0 7α12αC4 analyte/QAO-d3 7α12αC4 or QAO-d0 7αC4-d7 internal standard) plotted against specified calibrant concentration in whole blood (ng/ml) [13]. The lower limit of quantification (LLOQ) was determined as the lowest spiked concentration in matrix for which the signal-to-noise (S/N) ratio was ≥ 40:1 and the within-day reproducibility for calculated concentration was ≤ 20% relative standard deviation (RSD). Within-day reproducibility was determined using calculated concentrations for calibrants generated using 7α12αC4 spiked in whole blood from unaffected individuals or quality control samples (QCs) generated using whole blood from untreated CTX affected individuals spotted onto filter-paper. Potential method interference was evaluated by examination of the peak shape, peak shoulder, and peak area ratio of two MRM transitions (quantifier and qualifier) acquired for 7α12αC4. The qualifier MRM transition was as follows: QAO 7α12αC4 m/z 531.7 → 454.5. Carry-over from the auto-sampler was determined by assessing carry-over from CTX samples with 7α12αC4 concentrations > 1000 ng/ml.
3. Results
3.1. LC-ESI-MS/MS method performance studies
DBS calibration curves created for QAO tagged 7α12αC4, using either QAO-d3 tagged 7α12αC4 or QAO tagged 7αC4-d7 internal standard, demonstrated acceptable linearity (correlation coefficients across the range 50–500 ng/ml possessed r2 values > 0.990). Optimally stable-isotope labeled 7α12αC4 internal standard would be used to correct for variability in derivatization; as this is not commercially available we also utilize and provide data for 7αC4-d7 internal standard. Satisfactory within-run and between-run accuracy and precision data was also obtained for calculated concentrations of QAO tagged 7α12αC4 calibrants between 50 and 500 ng/ml (using QAO-d3 tagged 7α12αC4 internal standard the accuracy was within ± 21% and precision < 15.1% RSD, using QAO tagged 7αC4-d7 the accuracy was within ± 16% and precision < 10.4% RSD, see Table 1). For this study we used a 7α12αC4 LLOQ of 50 ng/ml (with concentrations of 7α12αC4 in all unaffected samples < 50 ng/ml and all CTX-positive samples > 50 ng/ml). Between injection carry-over for 7α12αC4 was assessed and determined to be < 20% the LLOQ peak area.
Table 1.
Accuracy and precision data for calculated 7α12α-dihydroxy-4-cholesten-3-one (QAO-d0 12α7αC4) concentrations in DBS.
| Internal standard used | Nominal conc. (ng/ml) 7α12αC4 |
Calculated conc. (ng/ml) 7α12αC4 |
Accuracy (%) |
RSD (%) |
Calculated conc. (ng/ml) 7α12αC4 |
Accuracy (%) |
RSD (%) |
|---|---|---|---|---|---|---|---|
| Within-run (n = 3) | Between-run (n = 8 over 5 days) | ||||||
| QAO-d3 7α12αC4-d0 | 50.0 | 60.7 | 121 | 9.0 | 55.8 | 112 | 9.9 |
| 100 | 92.9 | 93 | 6.8 | 88.1 | 88 | 9.3 | |
| 500 | 461 | 92 | 15.1 | 492 | 98 | 10.4 | |
| QAO-d0 7αC4-d7 | 50.0 | 53.2 | 106 | 7.2 | 57.8 | 116 | 8.6 |
| 100 | 97.5 | 98 | 4.0 | 90.6 | 91 | 9.5 | |
| 500 | 525 | 105 | 6.6 | 511 | 102 | 4.8 | |
3.2. Quantification of 7α12αC4 in newborn DBS as a test for CTX
We previously reported that the concentration range of 7α12αC4 in two CTX positive newborn DBS (120 and 214 ng/ml; that were stored for 30 and 15 years respectively) was around 10-fold higher than the mean concentration in unaffected newborn DBS (16.4 ± 6.0 ng/ml), such that quantification of this ketosterol bile acid precursor provides a test with the potential to screen newborn DBS for CTX [14]. We were able to modify the LC-ESI-MS/MS methodology for quantification of 7α12αC4 to use a DBS extraction technique performed in newborn screening laboratories and to use a reduced LC-ESI-MS/MS analysis time of 2 min per sample. To generate additional method validation data we used the modified methodology to perform a blinded analysis of an additional CTX positive newborn DBS that had been stored for 3 years, along with 39 unaffected newborn DBS. The CTX newborn DBS was from a Dutch infant diagnosed with CTX at 2 weeks old after a diagnostic workup for convulsions caused by a parechovirus encephalitis [16]. The diagnosis of CTX was confirmed with CYP27A1 mutation analysis.
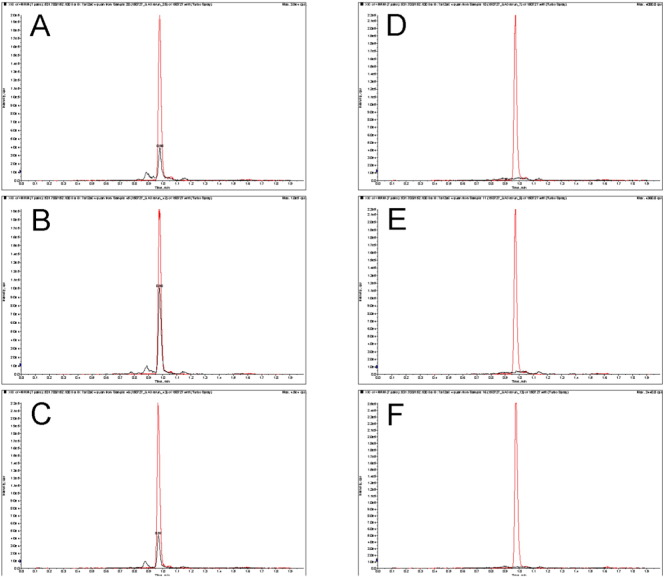
Modification of the previously reported methodology for quantification of 7α12αC4 in DBS [14] did not impact satisfactory measurement of 7α12αC4 and ready identification of a CTX positive newborn DBS (see detection of QAO tagged 7α12αC4 in the Dutch CTX positive newborn DBS, as well as in the previously analyzed CTX positive newborn DBS [14], compared to representative unaffected newborn DBS in Fig. 1). The mean concentration of test marker, QAO tagged 7α12αC4 in newborn DBS, calculated from analysis of all three CTX positive newborn DBS was 172 ± 104 ng/ml, around 40-fold greater than the mean concentration in unaffected newborn DBS (4.1 ± 3.4 ng/ml, see Table 2). The test marker provided perfect separation between the DBS from CTX affected and unaffected newborns under study. That is, the marker concentrations for CTX affected newborns ranged from 104 to 291 ng/ml, while the values for the unaffected newborns ranged from 0.2 to 15.6 ng/ml; these intervals do not overlap (Table 2).
Fig. 1.
Rapid LC-ESI-MS/MS analysis of 7α12αC4 in CTX positive and unaffected newborn DBS The left panels are extracted ion chromatograms for MRM detection of QAO tagged 7α12αC4 and QAO-d3 tagged 7α12αC4 internal standard in CTX positive DBS samples (black and red traces, respectively; QAO tagged 7α12αC4 is the peak eluting at 0.98 min). Panel A is the analysis of a Dutch newborn sample stored for 3 years, panels B and C are the analysis of newborn samples stored for 15 years and 30 years respectively (analysis of these DBS with a 6 min run time was described previously [14]). The right panels are extracted ion chromatograms for MRM detection of QAO tagged 7α12αC4 and QAO-d3 tagged 7α12αC4 internal standard in representative unaffected newborn samples.
Table 2.
7α12αC4 as a marker for CTX in DBS.
| Concentration of QAO tagged 7α12α-dihydroxy-4-cholesten-3-one (ng/ml)a |
||
|---|---|---|
| Calculated using QAO-d3 7α12αC4 internal standard | Calculated using QAO 7αC4-d7 internal standard | |
| Untreated CTX-affected adult DBS (n = 4) | 2869 ± 802b [1979–3910] |
3236 ± 916b [2095–4259] |
| Untreated CTX-affected newborn DBS (n = 3) | 172 ± 104c [104–291] |
176 ± 101c [101–291] |
| Unaffected newborn DBSd (n = 39) | 4.1 ± 3.4e [0.2–15.6] |
4.8 ± 3.5e [1.2–14.9] |
Mean concentration ± S.D. where possible and [range of results] are given.
Non-hydrolyzed sterol.
Calculated outside the analytical measurement range.
7α12αC4 concentrations for the two older CTX-positive newborn DBS were reported previously to range from 120 to 214 ng/ml. Concentrations obtained on re-analysis were within ± 30% of previously reported concentrations.
Unaffected newborn DBS from premature and full term infants were analyzed with no significant differences in concentration noted.
7α12αC4 concentrations in n = 6 unaffected newborn DBS were reported previously to range from 12.3–26.3 ng/ml [14].
4. Discussion
We have previously described LC-ESI-MS/MS methodology that utilizes keto moiety derivatization with QAO reagent to incorporate a permanent charge and enable highly sensitive isotope-dilution quantification of 7α12αC4 in DBS from CTX affected newborns [14]. We reported that quantification of 7α12αC4 allowed for discrimination between two CTX positive newborn DBS and unaffected newborn DBS when compared to 5α-cholestanol, such that it served as an improved blood test for CTX with the potential to screen newborn DBS for CTX [14]. This report provides further evidence that the previously described LC-ESI-MS/MS methodology is amenable to screening DBS for CTX, including when the methodology is modified to include a DBS extraction technique used in newborn screening laboratories and a reduced LC-ESI-MS/MS analysis time of 2 min per sample (performed using conventional LC instrumentation).
Although neonatal cholestatic jaundice may be more prevalent in newborns with CTX [1], [2], CTX has not been recognized in many infants. In the majority of cases a diagnosis is established much later in life, after destruction of any newborn screening DBS collected. Obtaining CTX positive newborn DBS samples to develop newborn screening for CTX has therefore been challenging. Recently the case of a Dutch infant diagnosed with CTX was described in the literature, with the child identified after a diagnostic workup for an unrelated condition [16]. The newborn DBS from this infant was obtained and was analyzed in a blinded manner, along with 39 DBS from unaffected newborns. Analysis with the modified methodology we describe here allowed ready identification of the CTX positive newborn DBS sample.
Although the methodology appears to work well as a newborn DBS screening test for CTX, with high sensitivity and specificity, the necessity for LC and the cost of QAO derivatization reagent limits its applicability as a first-tier test in many newborn screening laboratories. However, the testing workflow we describe could relatively easily be adopted as a second-tier test, with commercially available QAO reagent and stable-isotope labeled internal standard, as well as rapid 2 min LC-ESI-MS/MS analysis allowing for high-throughput performance. Efforts are underway to develop a screening test for CTX that requires no HPLC or derivatization.
In summary, we have been able to retrieve and analyze an additional CTX positive newborn DBS, to make a total of three CTX positive newborn DBS that have now been subjected to isotope-dilution quantification of 7α12αC4. We provide additional evidence suggesting this ketosterol bile acid precursor may be a promising test marker to screen newborn DBS for CTX, with no overlap between concentrations of 7α12αC4 in CTX positive and unaffected DBS. The test sensitivity and specificity currently remain at 1.0, however further investigation is needed to establish the operating characteristics of this test as well as an optimal cutoff value that defines a positive test. Availability of a satisfactory test to screen newborn DBS for CTX is a requirement to consider implementation of newborn screening for CTX. Early detection and intervention through newborn screening would greatly benefit those affected with CTX, preventing morbidity and mortality.
Acknowledgements
The authors would like to thank M. Star-Weinstock and S. Purkayastha at SCIEX for providing QAO-d3 reagent, the Bioanalytical Shared Resource at OHSU for providing technical assistance and access to analytical instrumentation and the Dutch National Institute for Public Health and the Environment for providing newborn DBS. Research reported in this publication has been supported by grants awarded to AED from Retrophin, Inc. to develop newborn screening for CTX and by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR000152. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Clayton P.T., Verrips A., Sistermans E., Mann A., Mieli-Vergani G., Wevers R. Mutations in the sterol 27-hydroxylase gene (CYP27A) cause hepatitis of infancy as well as cerebrotendinous xanthomatosis. J. Inherit. Metab. Dis. 2002;25:501–513. doi: 10.1023/a:1021211520034. [DOI] [PubMed] [Google Scholar]
- 2.Von Bahr S., Bjorkhem I., van't Hooft H.F., Alvelius G., Nemeth A., Sjovall J., Fischler B. Mutation in the sterol 27-hydroxylase gene associated with fatal cholestasis in infancy. J. Pediatr. Gastroenterol. Nutr. 2005;40:481–486. doi: 10.1097/01.mpg.0000150419.23031.2a. [DOI] [PubMed] [Google Scholar]
- 3.Verrips A., van Engelen B.G., Wevers R.A., van Geel B.M., Cruysberg J.R., van den Heuvel L.P., Keyser A., Gabreels F.J. Presence of diarrhea and absence of tendon xanthomas in patients with cerebrotendinous xanthomatosis. Arch. Neurol. 2000;57:520–524. doi: 10.1001/archneur.57.4.520. [DOI] [PubMed] [Google Scholar]
- 4.Verrips A., Hoefsloot L.H., Steenbergen G.C., Theelen J.P., Wevers R.A., Gabreels F.J., van Engelen B.G., van den Heuvel L.P. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 2000;123:908–919. doi: 10.1093/brain/123.5.908. [DOI] [PubMed] [Google Scholar]
- 5.Pilo-de-la-Fuente B., Jimenez-Escrig A., Lorenzo J.R., Pardo J., Arias M., res-Luque A., Duarte J., Muniz-Perez S., Sobrido M.J. Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur. J. Neurol. 2011;18:1203–1211. doi: 10.1111/j.1468-1331.2011.03439.x. [DOI] [PubMed] [Google Scholar]
- 6.Gallus G.N., Dotti M.T., Mignarri A., Rufa A., Da P.P., Cardaioli E., Federico A. Four novel CYP27A1 mutations in seven Italian patients with CTX. Eur. J. Neurol. 2010;17:1259–1262. doi: 10.1111/j.1468-1331.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- 7.Berginer V.M., Salen G., Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N. Engl. J. Med. 1984;311:1649–1652. doi: 10.1056/NEJM198412273112601. [DOI] [PubMed] [Google Scholar]
- 8.Mondelli M., Sicurelli F., Scarpini C., Dotti M.T., Federico A. Cerebrotendinous xanthomatosis: 11-year treatment with chenodeoxycholic acid in five patients. An electrophysiological study. J. Neurol. Sci. 2001;190:29–33. doi: 10.1016/s0022-510x(01)00563-9. [DOI] [PubMed] [Google Scholar]
- 9.Salen G. Cholestanol deposition in cerebrotendinous xanthomatosis. A possible mechanism. Ann. Intern. Med. 1971;75:843–851. doi: 10.7326/0003-4819-75-6-843. [DOI] [PubMed] [Google Scholar]
- 10.Yahalom G., Tsabari R., Molshatzki N., Ephraty L., Cohen H., Hassin-Baer S. Neurological outcome in cerebrotendinous xanthomatosis treated with chenodeoxycholic acid: early versus late diagnosis. Clin. Neuropharmacol. 2013;36:78–83. doi: 10.1097/WNF.0b013e318288076a. [DOI] [PubMed] [Google Scholar]
- 11.Leitersdorf E., Reshef A., Meiner V., Levitzki R., Schwartz S.P., Dann E.J., Berkman N., Cali J.J., Klapholz L., Berginner V.M. Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in Jews or Moroccan origin. J. Clin. Invest. 1993;91:2488–2496. doi: 10.1172/JCI116484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBarber A.E., Connor W.E., Pappu A.S., Merkens L.S., Steiner R.D. ESI-MS/MS quantification of 7alpha-hydroxy-4-cholesten-3-one facilitates rapid, convenient diagnostic testing for cerebrotendinous xanthomatosis. Clin. Chim. Acta. 2010;411:43–48. doi: 10.1016/j.cca.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 13.DeBarber A.E., Sandlers Y., Pappu A.S., Merkens L.S., Duell P.B., Lear S.R., Erickson S.K., Steiner R.D. Profiling sterols in cerebrotendinous xanthomatosis: utility of Girard derivatization and high resolution exact mass LC-ESI-MS(n) analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011;879:1384–1392. doi: 10.1016/j.jchromb.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBarber A.E., Luo J., Star-Weinstock M., Purkayastha S., Geraghty M.T., Chiang J., Merkens L.S., Pappu A.S., Steiner R.D. J. Lipid Res. 2014;55:146–154. doi: 10.1194/jlr.P043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhillon K.S., Bhandal A.S., Aznar C.P., Lorey F.W., Neogi Clin P. Chim. Acta. 2011;412:873–879. doi: 10.1016/j.cca.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Huidekoper H.H., Vaz F.M., Verrips A., Bosch A.M. Hepatotoxicity due to chenodeoxycholic acid supplementation in an infant with cerebrotendinous xanthomatosis: implications for treatment. Eur J Pediatr. 2016;175(1):143–146. doi: 10.1007/s00431-015-2584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]