Abstract
Aim
The aim of this study was to assess the effect of intracameral air on the endothelial cell morphometrics.
Patients and methods
This is a retrospective controlled interventional cohort study of 26 patients (18 males and 8 females) who underwent unilateral deep anterior lamellar keratoplasty (DALK) for moderate keratoconus. The DALK patients were divided into two groups: a treatment group (14), which had micro perforations of the Descemet Membrane (DM) intraoperatively and received intracameral air at the end of the surgery; and an independent control group (12), which had no micro perforation and thus no intracameral air was injected. Postoperative best corrected visual acuity (BCVA), sphere, cylinder, spherical equivalent (SEQ), central corneal thickness, and endothelial cell morphometric features consisted of the endothelial cell density (ECD), polymegathism, and pleomorphism were compared between treatment and control groups.
Results
The mean BCVA was 0.36 ± 0.36 logMAR in the treatment group and 0.17 ± 0.11 logMAR in the control group (p = 0.081), and the mean corneal thickness was 507.86 ± 62.69 μm in the treatment group and 525.67 ± 37.54 μm in the control group air (p = 0.399). Furthermore, the mean sphere was −5.14 ± 4.17D and −1.02 ± 3.29D, the mean cylinder was −3.16 ± 2.20D and −2.88 ± 1.21D, and the mean SEQ was −6.72 ± 4.66D and −2.46 ± 3.14D and in the treatment and control groups respectively (p = 0.011, 0.693, and 0.013). As to morphometric features, the mean ECD was 2176.76 ± 549.18 cell/mm2 and 2257.30 ± 436.12 cell/mm2 in the treatment and control groups respectively (p = 0.686), and the mean pleomorphism 0.48 ± 0.09 and 0.54 ± 0.10 in the treatment and control groups respectively (p = 0.139). In contrast, the mean polymegathism was 0.37 ± 0.06 and 0.31 ± 0.05 in the treatment and control groups respectively (p = 0.009).
Conclusion
The presence of air inside the anterior chamber for a short term may not cause further endothelial cell loss and can be safely performed to prevent postoperative Descemet Membrane detachment in case of micro perforations.
Keywords: Keratoconus, Keratoplasty, Endothelial cell density, Polymegathism, Pleomorphism
Introduction
Endothelial Cell Density (ECD) plays a major role in the survival of keratoplasty procedures, whether therapeutic1 or optical, penetrating or partial thickness and anterior2, 3, 4 or posterior.5 Hence, much research has focused on ECD as an important factor when comparing survival of various types of keratoplasties.6, 7, 8 Deep Anterior Lamellar Keratoplasty (DALK) is a relatively newer surgical technique as compared to Penetrating Keratoplasty (PKP), usually performed in diseases of the cornea with stromal disease and a healthy endothelium. Various studies have demonstrated a low rate of endothelial cell loss in DALK compared to PKP.7, 9, 10, 11, 12, 13, 14 However, the surgical technique of DALK is very delicate and, surgery may be complicated by micro perforation of the DM in 9.3% up to 32% of the cases.15, 16 Micro perforation may occur during dissection of the host cornea, or while suturing of the donor graft. The micro perforation of the DM during DALK is often salvaged by intracameral injection of sterile air,17 20% sulfur hexafluoride (SF6),18 perfluoropropane (C3F8),19 or room air at the end of the surgery, which might prevent the DM detachment postoperatively in many cases. However, in spite of sealing of micro perforation, use of intracameral air could increase the risk of further loss of endothelial cells. In other circumstances, macroperforation of the DM due to excessive air injection or improper surgical maneuvers may warrant conversion of the DALK into PKP.20
A few studies have compared features of endothelial cells in Patients that had DALK with and without intracameral air. Moreover, endothelial cell morphometrics have remained unstudied in the literature.
The aim of our study was to compare endothelial cell morphometric changes between Patients that underwent DALK with intracameral air for micro perforation and those that had surgery without any air injection.
Materials and methods
In this controlled retrospective interventional cohort study at a tertiary care eye hospital, patients that had unilateral DALK by the first author between October 2010 and June 2014 were evaluated. The preoperative indication for DALK was moderate keratoconus. This study was approved by Institutional Review Board of the hospital and adhered to the principles of the Declaration of Helsinki.
The DALK patients were divided into two groups based on whether micro perforation of the DM occurred and intracameral air was injected at the end of the surgery (Treatment group) or no micro perforation occurred and thus no intracameral air was injected (Control group).
Retrieved data consisted of age at the time of surgery, gender, intraoperative injection of intracameral air for micro perforation of the DM, preoperative and postoperative sphere, cylinder, spherical equivalent (SEQ), and best corrected distant visual acuity (BCVA) (Converted to LogMAR). In addition, optical central corneal thickness (CCT) and endothelial cell morphometrics of both Patients were collected postoperatively which included ECD, cell size, coefficient of variation (related to polymegathism: a variation in size), and percent of hexagonal cells or hexagonality (inversely related to pleomorphism: a variation in shape), together with the time of endothelium specular microscopy. The mean endothelial cell area is related to ECD via the equation: 106/ECD; consequently, a loss in the ECD causes enlargement of the mean endothelial cell area. On the other hand, the equation used to calculate pleomorphism is standard deviation divided by mean area of the endothelial cells. Patients with a reliable specular photomicrograph and sample size of more than 30 were included in the study. Endothelial cell microscopy and optical pachymetry of CCT were conducted using noncontact semi-automated specular microscopy (SP-3000P, Topcon Medical Systems, Oakland, NJ, USA) by moving the instrument forward to backward and right to left to get three-dot mires in focus; then the instrument would take the endothelial image and analyze it automatically. In specular microscopy, the light does not pass through the cornea; instead, the light is reflected from the cornea in a mirror-like fashion. The examined eye is not touch in the noncontact microscopy; therefore, it is comfortable for the patient. Nonetheless, the noncontact microscopy yields a lower magnification compared to the contact microscopy but a larger field which makes non-contact microscopy suitable for counting cells.
Surgical technique
All patients signed informed consent to undergo DALK with slight modifications by the first author. All surgeries were performed under general anesthesia. Trephination was carried out with Hessburg-Barron trephines (Katena Products, Denville, NJ, USA). Donor was punched with diameter 0.25 mm more than the recipient diameter. Recipient was trephined from 60% to 80% of its thinnest corneal thickness. More than 50% of the corneal stroma was removed using a crescent blade. A Sarnicola blunt spatula (Asico Westmont, IL United States) was used to dissect corneal stroma and make a stromal track for air injection. A Sarnicola cannula was then used to inject room air using the same track made by the blunt spatula to form a big bubble, as originally described by Sarincola and Toro.23 Injection was stopped as air approached the trephination site. Peripheral paracentesis was performed to lower the intraocular pressure raised by the presence of the big bubble in the anterior chamber. Superficial cut was performed to collapse the bubble and the stroma was excised with corneal scissors. The punched donor tissue was prepared by manually removing the DM with the help of trypan blue dye and dry Weck-Cel sponges. The graft was secured via 16 interrupted 10-0 nylon sutures or 8 bite continuous and 8 interrupted suture combinations. In the intracameral air DALK group, two cases received interrupted sutures and twelve cases received combination of interrupted and continuous sutures. In non-intracameral air DALK group, six cases received interrupted sutures, four cases received combination of interrupted and continuous sutures, and two cases were not documented. The Big bubble was achieved in all cases and manual dissection to reach the Descemet Membrane was not performed in any case.
If micro perforation of the DM was noticed during dissection of anterior lamella, the surgery was continued and completed as usual. However, unfiltered room air was injected to fill approximately 60% of the anterior chamber with an air bubble to help seal the perforation. In cases of macroperforation of the DM, where a gush of aqueous was released and anterior chamber shallowed, the surgery was converted to PKP. Such cases were excluded from the study. Micro perforations that occurred during suturing were also treated in the same manner.
Postoperative management
All patients received the following topical combination of antibiotics: Moxifloxacin 0.5% (Vigamox, Alcon-Couvreur N.V., Belgium) and steroid: Prednisolone Acetate 1% (Pred Forte, Allergan Pharmaceuticals Ireland, Westport, Co Mayo, Ireland) 6 times a day. In addition patients who had intracameral air injected for micro perforation received acetazolamide 250 mg tablets four times a day (Diamox, Remedica Ltd, Cyprus) for 3 days to decrease the incidence of intraocular pressure spike. Such patients were also required to adapt a supine position overnight. Postoperative visits were at 1, 7, and 30 days and then every 1–2 months for 12 months. The steroid was tapered over 3–6 months. Selective suture removal was performed to relax steep meridian and reduce astigmatism. Sutures were completely removed one year postoperatively. This decision was based on patient’s visual acuity and healing of corneal graft–host junction.
Statistical analysis
The independent t-test was performed for parameters found normally distributed and Mann–Whitney U test for parameters found non-normally distributed using Statistical Package for Social Sciences (SPSS v.20, Inc, Chicago, IL, USA). P-value less than 0.05 was deemed statistically significant.
Results
Twenty-six patients met the inclusion criteria: 18 males and 8 females. The median age (range) of these patients was 27.93 (16.99–43.96) years at the time of surgery. The median postoperative time to endothelium specular microscopy was 12.90 months.
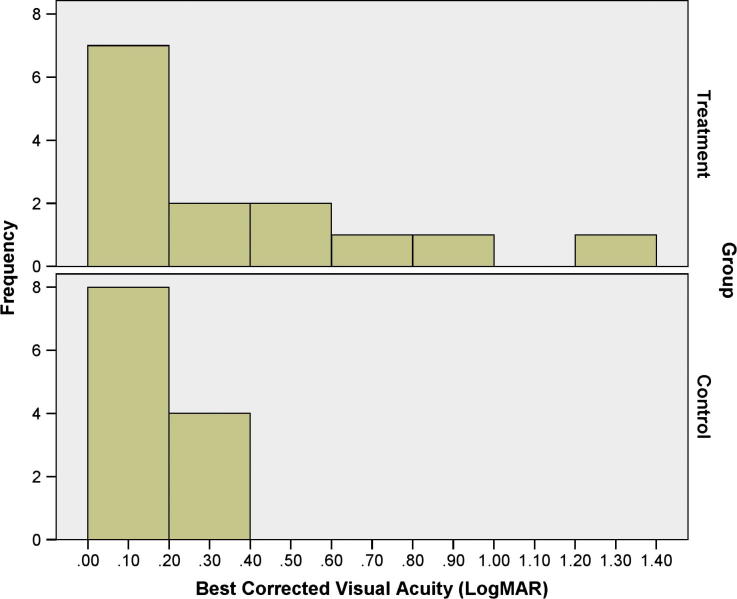
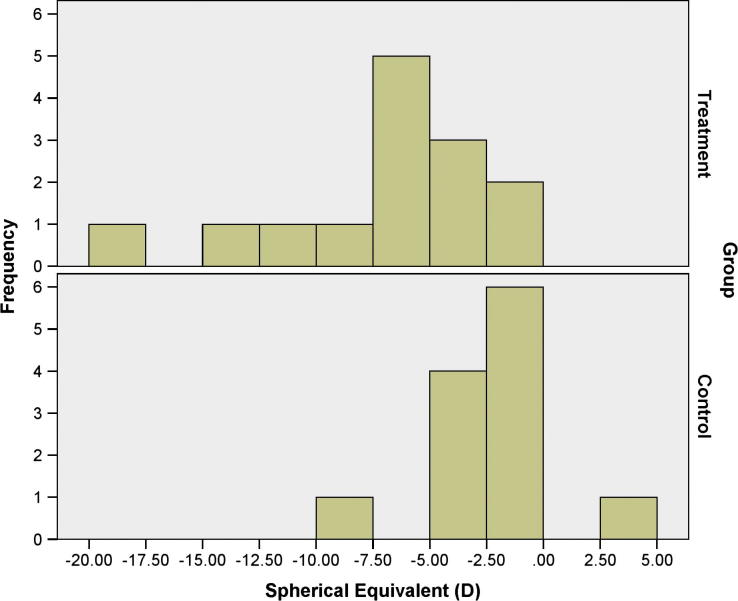
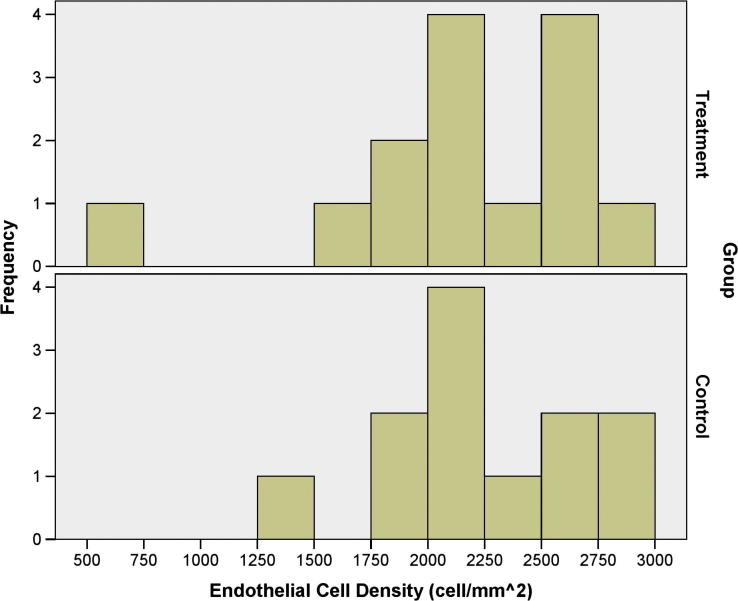
Figure 1, Figure 2 show the distributions of the BCVA and SEQ; it is evident therein that treatment group had more eyes with BCVA less than 0.40 logMAR and surplus myopia than control group; regardless, both study groups had relatively similar distribution of the ECD according to Fig. 3.
Figure 1.
The distribution of the best corrected visual acuity in the treatment and the control groups.
Figure 2.
The distribution of the spherical equivalent in the treatment and the control groups.
Figure 3.
The distribution of the endothelial cell density in the treatment and the control groups.
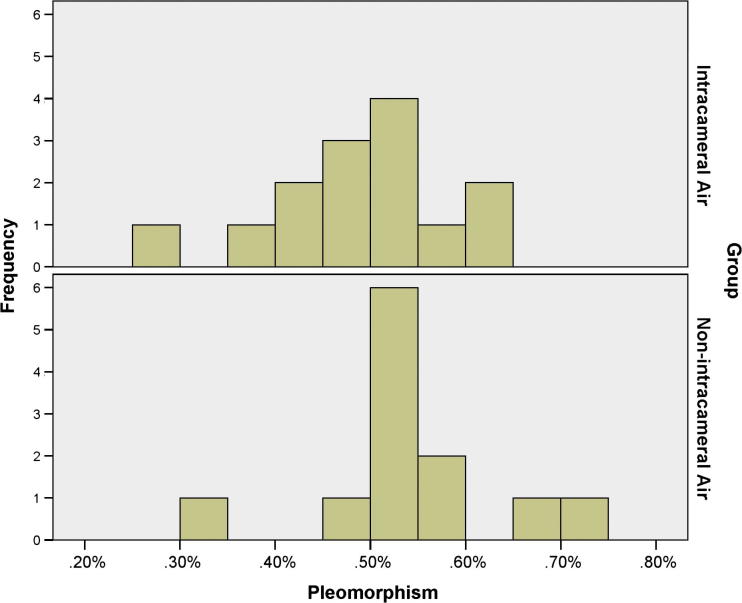
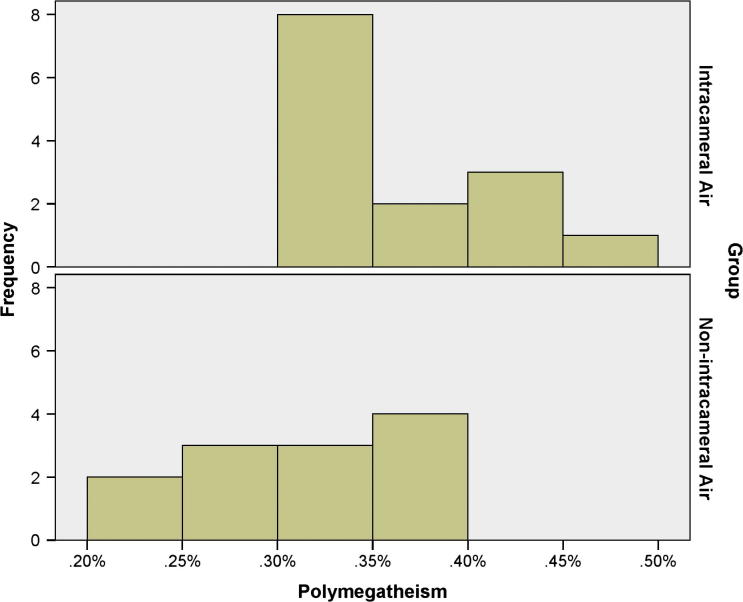
Table 1 shows and compares BCVA, sphere, cylinder, SEQ, CCT, ECD, polymegathism, and pleomorphism between both study groups. The BCVA, cylinder, and the CCT were comparable between the treatment and control groups (p = 0.810, 0.693, and 0.399 respectively); conversely, the sphere and SEQ differed significantly between both study groups (p = 0.011 and 0.013 respectively), in which the treatment group exhibited an excess of myopia. As to the endothelial cell morphometrics, the ECD and pleomorphism were equally comparable between both study groups (p = 0.686 and p = 0.139 respectively); in contrast, the polymegathism differed significantly (p-value = 0.009). Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 compare distributions of BCVA, SEQ, ECD, polymegathism and pleomorphism between both study groups (see Table 2).
Table 1.
Comparison of the treatment and the control groups that had deep anterior lamellar keratoplasty.
| Parameter | Treatment group (intracameral air) | Control group (non-intracameral air) | Difference | p-value | 95% Confidence interval |
|
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| BCVA (LogMAR) | 0.36 ± 0.36 | 0.17 ± 0.11 | +0.19 | 0.810 | −0.03 | 0.42 |
| Sphere (D) | −5.14 ± 4.17 | −1.02 ± 3.29 | −4.12 | 0.011 | −7.20 | −1.04 |
| Cylinder (D) | −3.16 ± 2.20 | −2.88 ± 1.21 | −0.29 | 0.693 | −1.76 | 1.19 |
| SEQ (D) | −6.72 ± 4.66 | −2.46 ± 3.14 | −3.56 | 0.013 | −7.54 | −0.99 |
| CCT (μm) | 507.86 ± 62.69 | 525.67 ± 37.54 | −17.81 | 0.399 | −60.58 | 24.96 |
| ECD (cell/mm2) | 2176.76 ± 549.18 | 2257.30 ± 436.12 | −80.54 | 0.686 | −486.95 | 325.86 |
| Polymegathism (%) | 0.37 ± 0.06 | 0.31 ± 0.05 | +0.06 | 0.009 | 0.02 | 0.10 |
| Pleomorphism (%) | 0.48 ± .09 | 0.54 ± 0.10 | −0.06 | 0.139 | −0.13 | 0.02 |
Note: BCVA denotes best corrected visual acuity; SEQ denotes spherical equivalent; CCT denotes central corneal thickness; ECD denotes endothelial cell density.
Figure 4.
The distribution of the pleomorphism in the treatment and the control groups.
Figure 5.
The distribution of the polymegathism in the treatment and the control groups.
Table 2.
Postoperative refraction, central corneal thickness, and endothelium cell morphometrics post deep anterior lamellar keratoplasty (DALK) with and without Intracameral air injection for Descemet Membrane micro-perforation.
| Group | Case # | Age (year) | Gender | BCVA (LogMAR) | Sphere (D) | Cylinder (D) | SEQ (D) | CCT (μm) | ECD (cell/mm2) | Polymegathism (%) | Pleomorphism (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group (intracameral air) | 1 | 30.8 | Female | 0.52 | −1.25 | −1.50 | −2.00 | 610 | 2538.10 | 49.40 | 41.00 |
| 2 | 31.5 | Female | 0.52 | −16.00 | −4.00 | −18.00 | 558 | 2208.00 | 34.60 | 51.70 | |
| 3 | 25.6 | Male | 0.15 | −2.50 | −2.75 | −3.88 | 551 | 2203.00 | 33.60 | 50.50 | |
| 4 | 44.0 | Male | 1.30 | −11.00 | −3.50 | −12.75 | 497 | 1649.50 | 34.00 | 62.00 | |
| 5 | 25.4 | Male | 0.81 | −6.00 | −1.00 | −6.50 | 460 | 2240.00 | 36.90 | 48.90 | |
| 6 | 28.9 | Male | 0.10 | −2.75 | −1.00 | −3.25 | 495 | 708.20 | 33.70 | 51.00 | |
| 7 | 23.2 | Male | 0.22 | −4.00 | −3.50 | −5.75 | 436 | 2580.70 | 43.00 | 48.00 | |
| 8 | 32.2 | Male | 0.15 | −5.00 | −1.50 | −5.75 | 367 | 2097.10 | 41.90 | 27.00 | |
| 9 | 37.7 | Female | 0.15 | −3.00 | −4.50 | −5.25 | 458 | 2820.70 | 31.00 | 55.00 | |
| 10 | 19.6 | Female | 0.10 | −2.00 | −2.00 | −3.00 | 548 | 2349.40 | 32.30 | 48.00 | |
| 11 | 25.6 | Male | 0.03 | −.50 | −3.25 | −2.13 | 529 | 1830.80 | 30.60 | 61.00 | |
| 12 | 31.5 | Female | 0.62 | −4.50 | −1.75 | −5.38 | 570 | 2711.10 | 39.90 | 39.00 | |
| 13 | 30.3 | Male | 0.02 | −5.75 | −4.50 | −8.00 | 506 | 1873.00 | 32.20 | 43.00 | |
| 14 | 36.2 | Female | 0.40 | −7.75 | −9.50 | −12.50 | 525 | 2665.00 | 40.90 | 52.00 | |
| Control group (non-intracameral air) | 1 | 18.4 | Male | 0.10 | −2.25 | −2.75 | −3.63 | 590 | 2623.80 | 27.70 | 54.00 |
| 2 | 25.6 | Male | 0.40 | −2.00 | −5.00 | −4.50 | 505 | 2228.00 | 35.60 | 51.00 | |
| 3 | 30.7 | Male | 0.30 | −2.00 | −1.00 | −2.50 | 481 | 1290.80 | 27.70 | 50.00 | |
| 4 | 21.9 | Male | 0.15 | .00 | −2.00 | −1.00 | 561 | 2189.60 | 30.80 | 54.00 | |
| 5 | 35.4 | Female | 0.22 | 6.50 | −4.25 | 4.38 | 491 | 2896.20 | 34.50 | 47.00 | |
| 6 | 17.0 | Male | 0.15 | −4.00 | −1.50 | −4.75 | 528 | 2822.80 | 22.50 | 59.00 | |
| 7 | 29.0 | Male | 0.15 | 1.25 | −4.50 | −1.00 | 557 | 1933.70 | 23.40 | 68.00 | |
| 8 | 28.0 | Male | 0.15 | .00 | −2.00 | −1.00 | 578 | 2149.30 | 26.60 | 55.00 | |
| 9 | 27.9 | Male | 0.05 | .00 | −3.00 | −1.50 | 516 | 2112.20 | 35.60 | 70.00 | |
| 10 | 18.2 | Male | 0.30 | −2.50 | −3.00 | −4.00 | 494 | 1973.80 | 35.00 | 53.00 | |
| 11 | 24.5 | Male | 0.05 | .00 | −2.50 | −1.25 | 485 | 2505.30 | 35.40 | 54.00 | |
| 12 | 19.4 | Female | 0.04 | −7.25 | −3.00 | −8.75 | 522 | 2362.10 | 34.70 | 33.00 | |
BCVA, best corrected visual acuity; SEQ, spherical equivalent; CCT, central corneal thickness; ECD, endothelium cell density.
Post DALK, there was no case of pupillary block, fixed dilated pupil (Urrets-Zavalia syndrome) or pseudoanterior chamber formation (double anterior chamber): complications occasionally associated with intracameral air injection.21, 24, 25 The DM was attached in all cases of treatment and control groups. Only two cases in the treatment group and none in the control group developed cataract.
Discussion
Graft survival is linked to the functionality of the endothelial cells26, 27; hence, preventing endothelial cell loss during surgery has always been vital in patients undergoing keratoplasty. The endothelial cell loss is significantly lower in patients undergoing DALK than PKP.7, 14 Moreover, endothelial cell loss that occurs after DALK may be less progressive over time than in PKP.4, 28 As endothelial cell loss occurs, neighboring cells enlarge in a manner related to the causative trauma resulting in polymegathism and pleomorphism. Assessment of all these cells features (density, size, polymegathism, and pleomorphism) might shed more light on the damage to the endothelium.
It has been proposed that leaving an air bubble inside the anterior chamber may cause trauma to the endothelium29 and induce endothelial cell loss.30 In this study, specular microscopy readings post DALK were comparable between both study groups as to the ECD (p = 0.686), pleomorphism (p = 0.139) and central corneal thickness (p = 0.399), yet different as to polymegathism (p = 0.009). Such a difference may be relatively attributed to irregular enlargement of endothelial cells due to the air in the anterior chamber. Together with this, an excess of myopia was found in the treatment group compared to the control group (p = 0.011). There was a trend toward myopia in the intracameral air group. The suturing technique and donor host disparity were consistent between the two groups. Other factors such as pre-operative axial myopia, anterior chamber depth, lens thickness and, temporary presence of intra-cameral air need to be investigated in future studies.
This study result was in contrast to a study by Leccisotti21 that showed significant endothelial cell loss in intracameral air compared to non-intracameral air. Leccisotti has evaluated only 7 corneas with intraoperative DM perforations, of which three corneas had intracameral air. Another study by Den and colleagues22 investigated ninety-six patients that had DALK with and without DM perforation. Den’s study has suggested a positive association between endothelial cell loss and the presence of the DM perforation; however, macro perforation and micro perforation were compiled in Den’s study and not all corneas with the DM perforation received intracameral air. Unlike this study, Leccisotti and Den have reported several complications: pupillary block, permanent mydriasis, persistent double anterior chamber, and endothelial decompensation. Both this study and Leccisotti’s reported cataract in intracameral air (treatment) group; however, future studies with larger number of patients are required to provide a statistical evidence.
Micro perforation of the DM is common during deep anterior lamellar keratoplasty. Postoperative Descemet Membrane detachment can be prevented by placing air in the anterior chamber to tamponade the micro perforation. Our study shows that temporary presence of air inside the anterior chamber does not cause further endothelial cell loss and can be safely done in such situations, though myopia and polymegathism may be anticipated. These results are encouraging and should be validated in a larger cohort of patients.
Conflict of interest
There is no financial or proprietary interest in any material or method mentioned.
No public or private grant was received for this research.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
Ashbala Khattak, Email: ashbalakhattak@gmail.com.
Fouad R. Nakhli, Email: fouadraja@hotmail.com.
Hussam Mohammad Abdullatif Abouollo, Email: hussamabouollo@hotmail.com.
References
- 1.Wang J., Zhao G., Xie L. Therapeutic effect of deep anterior lamellar keratoplasty for active or quiescent herpetic stromal keratitis. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1187–1194. doi: 10.1007/s00417-012-1947-2. [DOI] [PubMed] [Google Scholar]
- 2.Fontana L., Parente G., Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143(1):117–124. doi: 10.1016/j.ajo.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Sarnicola V., Toro P., Sarnicola C. Long-term graft survival in deep anterior lamellar keratoplasty. Cornea. 2012;31(6):621–626. doi: 10.1097/ICO.0b013e31823d0412. [DOI] [PubMed] [Google Scholar]
- 4.Yanny Y.Y., Visser N., Schouten J.S. Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty versus penetrating keratoplasty: a randomized multicenter clinical trial. Ophthalmology. 2011;118(2):302–309. doi: 10.1016/j.ophtha.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Calvo-de-Mora M., Quilendrino R., Ham L. Clinical outcome of 500 consecutive cases undergoing descemet’s membrane endothelial keratoplasty. Ophthalmology. 2015;122(3):464–470. doi: 10.1016/j.ophtha.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Maier A.K., Gundlach E., Gonnermann J. Retrospective contralateral study comparing Descemet Membrane endothelial keratoplasty with Descemet stripping automated endothelial keratoplasty. Eye (Lond) 2014 doi: 10.1038/eye.2014.280. (Epup ahead of print.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borderie V.M., Sandali O., Bullet J. Long-term results of deep anterior lamellar versus penetrating keratoplast. Ophthalmology. 2012;119(2):249–255. doi: 10.1016/j.ophtha.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 8.Levinger E. Outcome of “mushroom” pattern femtosecond laser-assisted keratoplasty versus conventional penetrating keratoplasty in patients with keratoconus. Cornea. 2014;33(5):481–485. doi: 10.1097/ICO.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 9.Romano V., Iovieno A., Parente G. Long-term clinical outcomes of deep anterior lamellar keratoplasty in patients with keratoconus. Am J Ophthalmol. 2015;159(3):505–511. doi: 10.1016/j.ajo.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Sogutlu Sari E., Kubaloglu A., Unal M. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for macular corneal dystrophy: a randomized trial. Am J Ophthalmol. 2013;156(2):267–274. doi: 10.1016/j.ajo.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Kubaloglu A., Sari E.S., Unal M. Long-term results of deep anterior lamellar keratoplasty for the treatment of keratoconus. Am J Ophthalmol. 2011;151(5):760–767. doi: 10.1016/j.ajo.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 12.Huanq T., Zhanq X., Wang Y. Outcomes of deep anterior lamellar keratoplasty using the big-bubble technique in various corneal diseases. Am J Ophthalmol. 2012;154(2):282–289. doi: 10.1016/j.ajo.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 13.Kubaloglu A., Koytak A., Sari E.S. Corneal endothelium after deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus: a four-year comparative study. Indian J Ophthalmol. 2012;60(1):35–40. doi: 10.4103/0301-4738.90490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M.H., Chung T.Y., Chung E.S. A retrospective contralateral study comparing deep anterior lamellar keratoplasty with penetrating keratoplasty. Cornea. 2013;32(4):385–389. doi: 10.1097/ICO.0b013e318254be4e. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y.M., Wu S.Q., Yao Y.F. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14(5):438–450. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y.Y., Visser N., Schouten J.S. Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty versus penetrating keratoplasty: a randomized multicenter clinical trial. Ophthalmology. 2011;118(2):302–309. doi: 10.1016/j.ophtha.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Amaral C.E., Palay D.A. Technique for repair of Descemet Membrane detachment. Am J Ophthalmol. 1999;127(1):88–90. doi: 10.1016/s0002-9394(98)00296-7. [DOI] [PubMed] [Google Scholar]
- 18.Mahmood M.A., Teichman K.D., Tomey K.F. Detachment of Descemet’s membrane. J Cataract Refract Surg. 1998;24(6):827–833. doi: 10.1016/s0886-3350(98)80139-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim T., Hasan S.A. A new technique for repairing Descemet Membrane detachments using intracameral gas injection. Arch Ophthalmol. 2002;120(2):181–183. doi: 10.1001/archopht.120.2.181. [DOI] [PubMed] [Google Scholar]
- 20.Jhanji V.1., Sharma N., Vajpayee R.B. Intraoperative perforation of Descemet’s membrane during “big bubble” deep anterior lamellar keratoplasty. Int Ophthalmol. 2010;30(3):291–295. doi: 10.1007/s10792-009-9334-7. [DOI] [PubMed] [Google Scholar]
- 21.Leccisotti A. Descemet’s membrane perforation during deep anterior lamellar keratoplasty: prognosis. J Cataract Refract Surg. 2007;33(5):825–829. doi: 10.1016/j.jcrs.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Den S., Shimmura S., Tsubota Impact of the descemet membrane perforation on surgical outcomes after deep lamellar keratoplasty. Am J Ophthalmol. 2007;143(5):750–754. doi: 10.1016/j.ajo.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 23.Sarincola V., Toro P. Blunt cannula for descemetic deep anterior lamellar keratoplasty. Cornea. 2011;30(8):895–898. doi: 10.1097/ICO.0b013e3181e848c3. [DOI] [PubMed] [Google Scholar]
- 24.Maurino V., Allan B.D., Stevens J.D. Fixed dilated pupil (Urrets-Zavalia syndrome) after air/gas injection after deep lamellar keratoplasty for keratoconus. Am J Ophthalmol. 2002;133(2):266–268. doi: 10.1016/s0002-9394(01)01308-3. [DOI] [PubMed] [Google Scholar]
- 25.Arslan O.S., Unal M., Tuncer I. Deep anterior lamellar keratoplasty using big-bubble technique for treatment of corneal stromal scars. Cornea. 2011;30(6):629–633. doi: 10.1097/ICO.0b013e3181eeb44a. [DOI] [PubMed] [Google Scholar]
- 26.Armitaqe W.J., Dick A.D., Bourne W.M. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci. 2003;44(8):3326–3331. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- 27.Bourne W.M. Cellular changes in transplanted human corneas. Cornea. 2001;20(6):560–569. doi: 10.1097/00003226-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Acar B.T., Vural E.T., Acar S. Changes in endothelial cell density following penetrating keratoplasty and deep anterior lamellar keratoplasty. Int J Ophthalmol. 2011;4(6):644–647. doi: 10.3980/j.issn.2222-3959.2011.06.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong A., Caldwell M.C., Kuo A.N. Air bubble-associated endothelial trauma in descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2009;148(2):256–259. doi: 10.1016/j.ajo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Landry H., Aminian A., Hoffart L. Corneal endothelial toxicity of air and SF6. Invest Ophthalmol Vis Sci. 2011;52(5):2279–2286. doi: 10.1167/iovs.10-6187. [DOI] [PubMed] [Google Scholar]